1. Introduction
Kampo medicine is a traditional Japanese medicine that consists of an appropriate combination of multiple crude drugs. Its value has been recognized worldwide based on the accumulation of scientific evidence through extensive use. Generally, during the decoction process of Kampo medicines, multiple crude drugs are co-decocted and expected to exert synergistic effects. However, reports on the significance of combining the crude drugs of Kampo medicines, i.e., the herbal-pair theory, are limited. Previously, we have pioneered the elucidation of the interactions among the crude drugs in orengedokuto, a Kampo medicine, and revealed the chemical interactions [
1,
2] that elicited anti-inflammatory effects [
3,
4]. These studies indicated the importance of easily determining the content of the constituents in the extract. For continuous research and development regarding the interactions between crude drugs, a simple and inexpensive method of quantification is required because not all research institutions have expensive analytical equipment, such as high-performance liquid chromatography (HPLC) and liquid chromatography/mass spectrometry.
Thin-layer chromatography (TLC) is a well-known and simple analytical method that does not require expensive equipment. Recently, quantitative analysis via TLC has become possible owing to improvements in image recognition capability [
5]. Therefore, TLC analysis has attracted attention as a versatile quantitative method. Previously, studies on the quantitative analysis of compounds using TLC have been reported; e.g., berberine from
Coptis teeta [
6] and
Coscinium fenestratum [
7], barakol from
Senna siamea [
8], phyllanthin and gallic acid [
9], and saikosaponins [
10]. However, information on the content of constituents in hot water extracts and their combination which are frequently used in clinical practice is limited.
(
Z)-Ligustilide is a major essential oil bearing phthalide skeleton in Japanese Angelica Root—the root of
Angelica acutiloba or
A. acutiloba var.
sugiyamae (Umbelliferae) stipulated in the Japanese Pharmacopoeia XVIII (JP XVIII) [
11]. Many studies on the pharmacological effects of (
Z)-ligustilide, including antioxidant [
12], analgesic [
13], platelet aggregation reduction [
14], and uterine smooth muscle contraction inhibition [
15], have been reported. (
Z)-Ligustilide is considered the active ingredient in Japanese Angelica Root-containing Kampo medicines such as tokito, tokishakuyakusan, kamishoyosan, juzentaihoto, and kamikihito, which are frequently used in the treatment of gynecological disorders. However, changes in the content of (
Z)-ligustilide extracted from the combination of crude drugs have not been clarified.
In this study, we applied TLC analysis to determine the content of constituents in a hot water extract of crude drugs in order to understand the chemical interactions between crude drugs. We first focused on crude drugs, whose constituents were quantified using HPLC methods stipulated in the JP XVIII, and examined them for sufficient analysis using TLC. Next, we analyzed (Z)-ligustilide, a major constituent of Japanese Angelica Root, for which no quantitative method has been described in JP XVIII, and compared the TLC data with HPLC data. In addition, we combined Japanese Angelica Root with crude drugs to analyze (Z)-ligustilide content using TLC to identify the crude drugs that increase the (Z)-ligustilide content.
2. Results
2.1. Comparison of TLC and HPLC Analytical Data
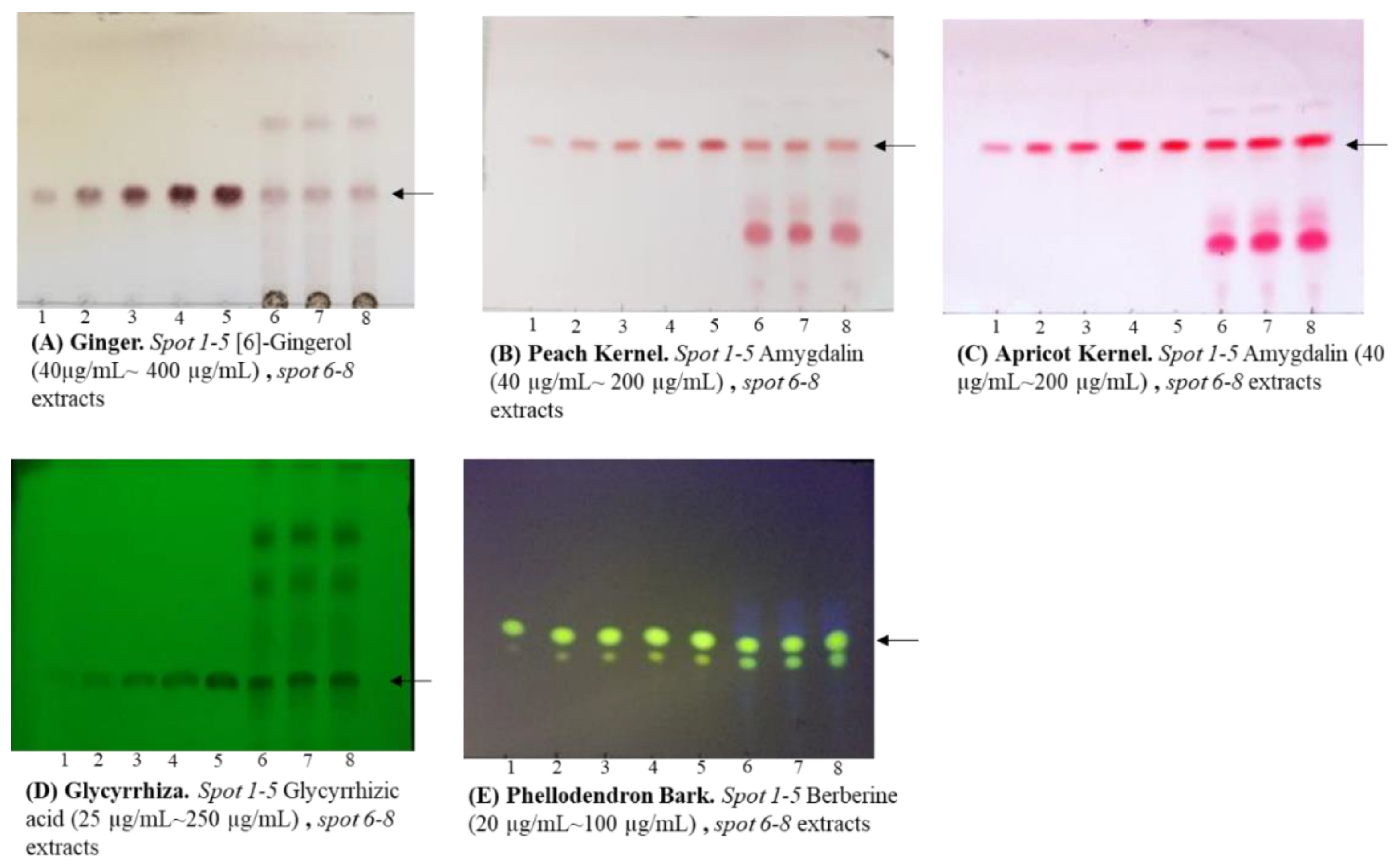
We analyzed marker compounds for the quality control of five crude drug extracts, which are dried powders obtained from decoctions, namely Ginger, Peach Kernel, Apricot Kernel, Glycyrrhiza, and Phellodendron Bark, using the JP XVIII-stipulated HPLC quantification method. In addition, TLC analysis was performed according to modified HPLC conditions, and the TLC and HPLC analytical data were compared (
Table 1). As a result, all crude drugs analyzed using each method satisfied the criteria of the marker compounds, and there was not a significant difference between the TLC and HPLC results. Next, we prepared hot water extracts of the five crude drugs and compared the TLC and HPLC analytical results (
Table 2). No remarkable differences were observed in the results obtained by the different analytical methods; particularly for Phellodendron Bark, no significant differences were found. Furthermore, to confirm the reproducibility of the quantitative TLC analysis, the intra- and inter-day precisions were calculated, resulting in values of less than 3.39% and 7.88%, respectively, for all analyses.
2.2. Analysis of Japanese Angelica Root-Derived (Z)-Ligustilide
Focusing on Japanese Angelica Root, the content of (Z)-ligustilide was determined and compared using HPLC and TLC. First, we confirmed the precisions of (Z)-ligustilide dissolved in methanol by TLC analysis. The intra- and inter-day precisions of (Z)-ligustilide itself for TLC analysis were calculated as 10.9% and 15.8%, respectively. Next, HPLC and TLC pretreatments of the dried powder from hot water extract were performed by dissolution in methanol and by partition with ethyl acetate, respectively, and the extraction recovery tests showed 115.9% and 95.8% recovery, respectively. Using the pretreatment method, the contents of (Z)-ligustilide in the hot water extract were determined to be 0.00388 ± 0.00009% (HPLC) and 0.00219 ± 0.00026% (TLC), showing no markable difference. The coefficients of variation for HPLC and TLC were less than 2.3% and 12%, respectively. The intra- and inter-day precisions of (Z)-ligustilide in the hot water extract for TLC analysis were calculated as 17.0% and 19.8%, respectively. These facts indicated that TLC analysis can be used as a substitute for HPLC analysis of (Z)-ligustilide in the extract.
2.3. Combination with Crude Drugs to Increase the Extracted (Z)-Ligustilide Content
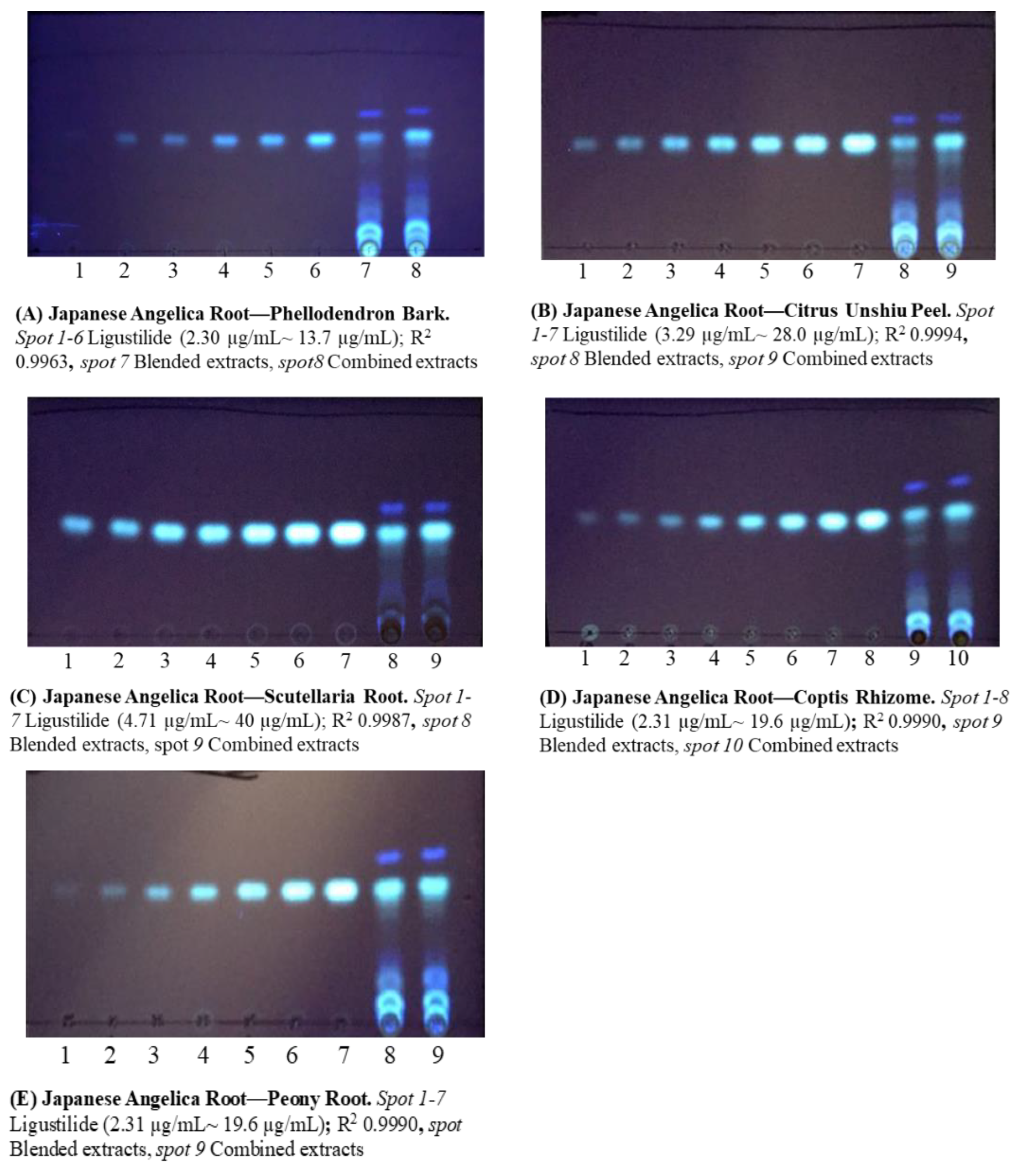
To clarify the chemical interactions between Japanese Angelica Root and other crude drugs, two types of extracts were prepared: a combined formula extract (CF), which is a dried extract of combined crude drugs decocted in hot water, and a blended formula extract (BF) that is a mixture of each dry extract in the corresponding crude drug proportions.
The spot area ratios of (
Z)-ligustilide in BF and CF were screened by TLC analysis. The results showed that the extraction efficiency of (
Z)-ligustilide increased by more than 150% when Phellodendron Bark, Citrus Unshiu Peel, Scutellaria Root, Coptis Rhizome, Gardenia Fruit, or Peony Root were combined with Japanese Angelica Root (
Figure S1). We analyzed these combinations using the calibration curve and compared the HPLC and TLC results. In the combinations with Coptis Rhizome and Peony Root, the results of HPLC analysis were similar to those of TLC (
Table 3). In the combination with Gardenia Fruit, an increased extraction efficiency of (
Z)-ligustilide was observed via both HPLC and TLC, although HPLC showed a slightly higher value of 241% compared to 193% obtained by TLC. Furthermore, we compared the HPLC and TLC results of each combination that did not show an increase in the extraction efficiency in the screening test, such as Alisma Tuber, Cnidium Rhizome, and Poria Sclerotium. As a result, there was no remarkable difference between the HPLC and TLC data for each combination (
Table 3). Meanwhile, the HPLC analyses of Citrus Unshiu Peel, Phellodendron Bark, and Scutellaria Root were difficult because of the overlapping peaks of (
Z)-ligustilide and each constituent (data not shown).
3. Discussion
We conducted quantitative analysis by TLC, focusing on crude drugs for which quantitative methods are stipulated in the JP XVIII, and compared the results with HPLC data to verify the validity of the TLC data. The values by both methods were generally correspondent and the precision of the TLC analysis was high, indicating that TLC can be substituted for HPLC, which is commonly used to determine the content of the constituents in crude drugs. However, due to the limited number of crude drugs we compared and the statistically significant differences in some of the comparative data, further studies comparing the HPLC and TLC analytical data for other crude drugs are needed to continue to validate quantitative TLC.
(
Z)-Ligustilide in the hot water extract of Japanese Angelica Root was quantified to be 0.00388% by HPLC and 0.00219% by TLC. Meanwhile, the content percentage of (
Z)-ligustilide from the methanolic solution of Japanese Angelica Root was approximately 0.05–0.3% [
16,
17]. These facts indicated that (
Z)-ligustilide was reduced during the decoction process by hot water. Originally, Japanese Angelica Root was often used in a pulverized powder form of the crude drug itself, not in an extract form, which is a dried powder in hot water extract. Thus, it is desirable to use Japanese Angelica Root in a powder form if the pharmacological effect of Japanese Angelica Root is dependent on (
Z)-ligustilide. To investigate this, it is necessary to quantify and compare each constituent in powder and extract form. In addition, although there are many reports on the quantification of the constituents in crude drug extracts, most of the previous quantitative analyses have been conducted for organic solvent extracts, especially methanolic extracts, and information on the content of constituents in hot water extract used in clinical practice is limited. Therefore, the quantification of constituents in hot water extracts of crude drugs will also need to be achieved.
The extraction efficiency of (
Z)-ligustilide from Japanese Angelica Root increased when combined with Phellodendron Bark, Citrus Unshiu Peel, Scutellaria Root, Coptis Rhizome, Gardenia Fruit, or Peony Root. This result suggested that the π–π interaction between (
Z)-ligustilide and aromatic compounds rich in these crude drugs, increased the solubility of (
Z)-ligustilide. In fact, it has been reported that the combination of several aromatic curcuminoids increased their solubility and enhanced their nematicidal activities compared to single compounds [
18]. Thus, the increased extraction efficiency of (
Z)-ligustilide by its combination with crude drugs may be attributed to the π–π interactions.
We confirmed that TLC could be used to quantify constituents in crude drug extracts and that it could contribute to the elucidation of chemical interactions between crude drugs. Quantitative TLC studies are easier for many researchers who do not specialize in analytical science to conduct quantitative analysis of crude drugs, with the aim of continuing research and development in the interaction of crude drugs in Kampo medicines, thus contributing to enhancing clarity in the herbal-pair theory.
4. Materials and Methods
4.1. Experimental Materials
4.1.1. Crude Drugs
Japanese Angelica Root (Lot. 6F08M), Peony Root (Lot. 6F16M), and Rehmannia Root (Lot. EB0025) were purchased from Daiko Shoyaku Co., Ltd. (Nagoya, Japan). Japanese Angelica Root (Lot. G0U0330), Glycyrrhiza (Lot. 9AE0114, I940151), Peach Kernel (Lot. B420320), Apricot Kernel (Lot. EBT0123, I180123), Ginseng (Lot. E1E0403), Atractylodes Lancea Rhizome (Lot. F500239), Cinnamon Bark (Lot. F2T0129), Scutellaria Root (Lot. B420015), Cnidium Rhizome (Lot. F6F0233), Ginger (Lot. F4J0227), Citrus Unshiu Peel (Lot. G830312), Coptis Rhizome (Lot. K7T0018), Gardenia Fruit (Lot. 8151104), Ephedra Herb (Lot. 05B0601), Alisma Tuber (Lot. E8T0304), Asiasarum Root (Lot. F1T0202), Astragalus Root (Lot. A2T0014), Pueraria Root (Lot. F4T0108), Perilla Herb (Lot. 7A09M), Aconite Root (Lot. F990243), Pinellia Tuber (Lot. 6C03M), Moutan Bark (Lot. F450521), Glehnia Root and Rhizome (Lot. H5F0518), Poria Sclerotium (Lot. 122404), and Phellodendron Bark (Lot. ABD0016) were purchased from Uchida Wakanyaku Ltd. (Tokyo, Japan).
4.1.2. Materials
The following compounds were purchased from FUJIFILM Wako Pure Chemical Co. (Osaka, Japan): [6]-gingerol (Lot. APQ6161), amygdalin (Lot. KPM0958), glycyrrhizin (Lot. CAH4202), berberine (Lot. PTM0089), and (Z)-ligustilide (Lot. ESJ5922). The solvents used for HPLC and TLC analyses were of research grade. The TLC plate (Silica Gel 60 F254) was purchased from Merck Co. (Darmstadt, Germany).
4.1.3. Equipment
The HPLC equipment (Shimadzu Co., Kyoto, Japan) comprised the following components: Degasser, DGU-20A3R, Pump; LC-20AD, Autosampler; SIL-20A, column oven; CTO-20A, UV-VIS Detector; SPD-20A.
The Kubota 5200 (KUBOTA Co., Tokyo, Japan) centrifuge was used.
4.1.4. Preparation of Hot Water Extracts
A total of 20 g of a cut single crude drug or a combination of two mixed crude drugs with a crude drug ratio of 1:1 (combined formula extract: CF) was weighed, placed in a decoction pot (HARIO Co., Ltd., Tokyo, Japan), and decocted with 400 mL of purified water until the volume was reduced to approximately half of the original volume. The extract was filtered and centrifuged at 3000 rpm for 10 min, and the supernatant was freeze-dried to obtain a dried extract.
The dried extracts were blended in a 1:1 ratio of crude drugs; the extract weight per gram of crude drug was determined, and the mixture was used as a blended formula extract (BF).
4.2. Quantitative Analysis
4.2.1. Marker Compounds for Quality Control Stipulated by JP XVIII
HPLC
We used this method of quantification according to JP XVIII (
Table 4 and
Figure S2).
TLC
We used a modified method of analysis according to the identification test described in JP XVIII (
Figure 1,
Table 5) and the TLC images were analyzed using the software JUST TLC
® (Sweday Co., Södra Sandby, Sweden).
4.2.2. (Z)-Ligustilide
HPLC
In a single hot water extract, blended formula extract, and combined formula extract, a content of each dried extract equivalent to 200 mg of Japanese Angelica Root extract was weighed, methanol was added to adjust the volume to 20 mL using a volumetric flask, and then the solution was filtered. The filtrate was used as the analytical sample.
The analytical conditions for (
Z)-ligustilide were as follows: column, Intersil ODS-3 (
φ 4.6 × 150 mm, 5 µm), solvent; (A) H
2O; (B) 0.1% HCOOH in CH
3CN; flow rate, 1 mL/min; gradient mode, B 50% → 100% (30 min) → 100% (45 min) → 50% (45.01 min) → 50% (60 min). The oven temperature was 40 °C, injection volume was 10 µL, and wavelength was 326 nm. For detailed information, see
Figure S3.
TLC
The hot water extract (100 mg) was shaken with 100 mL of water; thereafter, 100 mL of ethyl acetate was added. The solution was then shaken. The ethyl acetate layer was evaporated under reduced pressure, the residue was dissolved in methanol (0.5 mL), and the solution obtained was used as the sample solution.
Twenty microliters of the methanol solution of (
Z)-ligustilide for the standard curve, BF, and CF were applied to a TLC plate, and the plate was developed with
n-hexane/ethyl acetate solution (10:3,
v/
v). After development, the dried plate was irradiated with UV light (
λ 365 nm) (
Figure 2).
A concentration range was set for each analysis, and the spot area of the target compounds was confirmed to be within the range of the calibration curve. The rate of change in the concentration of extracted (Z)-ligustilide was calculated as the concentration ratio of CF/BF.
4.3. Statistics
Results are expressed as the mean ± standard deviation (S.D.) The statistical significance of difference between any two groups was calculated by paired Student’s t-test. The criterion of significance was set as p < 0.001.
5. Conclusions
This study shows that constituents in two extracts that were prepared according to the modified JP XVIII and decocted in hot water can be quantitatively analyzed by TLC, based on the comparison of HPLC analytical data of five crude drugs and the quantitative TLC analysis of (Z)-ligustilide in hot water extract of Japanese Angelica Root, for which quantitative methods are not stipulated in the JP XVIII. Moreover, changes in the extraction efficiency of (Z)-ligustilide depending on the combination of Japanese Angelica Root with 26 crude drugs were analyzed by TLC and HPLC to identify the crude drugs that increased the extraction efficiency by more than 150%. As a result, TLC analysis could satisfy the criteria of the marker compounds and precisely analyze the constituents. No markable differences were observed between the TLC and HPLC analytical data, and good precision results by TLC were obtained. In addition, (Z)-ligustilide content in hot water extract of Japanese Angelica Root was revealed to be 0.00219%, indicating that TLC analysis could quantify extremely small amounts with inter-day precisions of less than 19.8%. Furthermore, each of the 26 crude drugs could be combined with Japanese Angelica Root. In particular, Phellodendron Bark, Citrus Unshiu Peel, Scutellaria Root, Coptis Rhizome, Gardenia Fruit, and Peony Root increased the content of (Z)-ligustilide. Additionally, we found that TLC analysis could quantify even if peaks overlap in HPLC. As suggested by the herbal-pair theory, the determination of constituents in crude drug extracts by TLC which is an inexpensive and simple method, contributes to the elucidation of the chemical interactions between crude drugs consisting of Kampo formulae.
Supplementary Materials
The following are available online. Figure S1: Screening for the extraction efficiency of (Z)-ligustilide in combinations of Japanese Angelica Root with several crude drugs; Figure S2: HPLC chromatograms obtained using JP XVIII-stipulated quantitative methods; Figure S3: HPLC chromatograms of (Z)-ligustilide in combinations of Japanese Angelica Root with crude drugs.
Author Contributions
Conceptualization, N.O. and N.H.; methodology, N.O.; validation, Y.H. and M.N.; formal analysis and investigation, Y.H., M.S., M.N. and K.U.; data curation, N.O.; writing—original draft preparation, N.O.; writing—review and editing, N.H., K.O. and T.H.; supervision, N.H. and T.H.; project administration, N.O. and N.H.; funding acquisition, N.H. and T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Agency for Medical Research and Development (AMED), Grant number JP19mk0101102j0002.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Oshima, N.; Shimazu, T.; Narukawa, Y.; Hada, N.; Kiuchi, F. Quantitative analysis of anti-inflammatory activity of orengedokuto II: Berberine is responsible for inhibition of NO production. J. Nat. Med. 2018, 72, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Okoshi, K.; Uekusa, Y.; Narukawa, Y.; Kiuchi, F. Solubility enhancement of berberine–baicalin complex by the constituents of Gardenia Fruit. J. Nat. Med. 2021, 75, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Oshima, N.; Narukawa, Y.; Hada, N.; Kiuchi, F. Quantitative analysis of anti-inflammatory activity of orengedokuto: Importance of combination of flavonoids in inhibition of PGE2 production in mouse macrophage-like cell line J774.1. J. Nat. Med. 2013, 67, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Shibuya, N.; Narukawa, Y.; Oshima, N.; Hada, N.; Kiuchi, F. Synergistic effect of baicalein, wogonin and oroxylin A mixture: Multistep inhibition of the NF-κB signalling pathway contributes to an anti-inflammatory effect of Scutellaria Root flavonoids. J. Nat. Med. 2018, 72, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Sibug-Torres, S.M.; Padolina, I.D.; Cruz, P.; Garcia, F.C.; Garrovillas, M.J.; Yabillo, M.R.; Enriquez, E.P. Smartphone-based image analysis and chemometric pattern recognition of the thin-layer chromatographic fingerprints of herbal materials. Anal. Methods 2019, 11, 721. [Google Scholar] [CrossRef]
- Goswami, A.K.; Gogoi, N.; Shakya, A.; Sharma, H.K. Development and validation of high-performance thin-layer chromatographic method for quantification of berberine in rhizomes of coptis teeta wall, an endangered species collected from arunachal pradesh, India. J. Chromatogr. Sci. 2019, 57, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Rojsanga, P.; Gritsanapan, W.; Suntornsuk, L. Determination of berberine content in the stem extracts of Coscinium fenestratum by TLC densitometry. Med. Princ. Pract. 2006, 15, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Padumanonda, T.; Suntornsuk, L.; Gritsanapan, W. Quantitative analysis of barakol content in Senna siamea leaves and flowers by TLC-densitometry. Med. Princ. Pract. 2007, 16, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tatiya, A.U.; Patil, R.P.; Sutar, M.P.; Shirkhedkar, A.A.; Surana, S.J. Determination of Phyllanthin and Gallic Acid In Herbal Hepatoprotective Formulation By TLC-Densitometry Analysis. Pharmacogn. J. 2011, 3, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Tian, R.; Xie, P.; Liu, H. Evaluation of traditional Chinese herbal medicine: Chaihu (Bupleuri Radix) by both high-performance liquid chromatographic and high-performance thin-layer chromatographic fingerprint and chemometric analysis. J. Chromatogr. A 2009, 1216, 2150–2155. [Google Scholar] [CrossRef] [PubMed]
- The Ministry of Health, Labour and Welfare of Japan. The Japanese Pharmacopoeia, 18th ed.; The Ministry of Health, Labour and Welfare of Japan: Tokyo, Japan, 2021.
- Wu, Z.; Uchi, H.; Morino-Koga, S.; Shi, W.; Furue, M. (Z)-ligustilide ameliorated ultraviolet B-induced oxidative stress and inflammatory cytokine production in human keratinocytes through upregulation of Nrf2/HO−1 and suppression of NF-κB pathway. Exp. Dermatol. 2015, 24, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Pan, W.; Qian, D.; Duan, J.; Shang, E.; Tang, Y. Analgesic activity of DaChuanXiongFang after intranasal administration and its potential active components in vivo. J. Ethnopharmacol. 2013, 150, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, J.-R.; Wang, J.; Yu, D.-K.; Chen, Y.-S.; He, Y.; Wang, C.-Y. (Z)-ligustilide extracted from Radix angelica sinensis decreased platelet aggregation induced by ADP ex vivo and arterio-venous shunt thrombosis in vivo in rats. Yakugaku Zasshi 2009, 129, 855–859. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Bai, B.; Kuang, X.; Yu, Y.; Wang, C.; Ke, Y.; Xu, Y.; Tzang, A.H.C.; Qian, Z.M. (Z)-ligustilide inhibits spontaneous and agonists- or K+ depolarization-induced contraction of rat uterus. J. Ethnopharmacol. 2006, 108, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Ando, H.; Sasaki, Y. Quality evaluation of Angelicae acutilobae radix: Individual differences and localization of (Z)-ligustilide in Angelica acutiloba root. J. Nat. Med. 2021, 75, 1–10. [Google Scholar] [CrossRef]
- Fukuda, K.; Marutaa, K.; Itoh, K.; Taniguchi, M.; Shibano, M.; Baba, K.; Shiratori, M.; Tani, T.; Matsuda, H. Fibrinolytic acitivity of (Z)-ligustilide and pharmaceutical comparison of Angelica acutiloba roots before and after processing in hot water. J. Tradit. Med. 2009, 25, 210–218. [Google Scholar]
- Kiuchi, F.; Goto, Y.; Sugimoto, N.; Akao, N.; Kondo, K.; Tsuda, Y. Nematocidal activity of turmeric: Synergistic action of curcuminoids. Chem. Pharm. Bull. 1993, 41, 1640–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).