Soyauxinine, a New Indolopyridoquinazoline Alkaloid from the Stem Bark of Araliopsis soyauxii Engl. (Rutaceae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Identification of Compounds
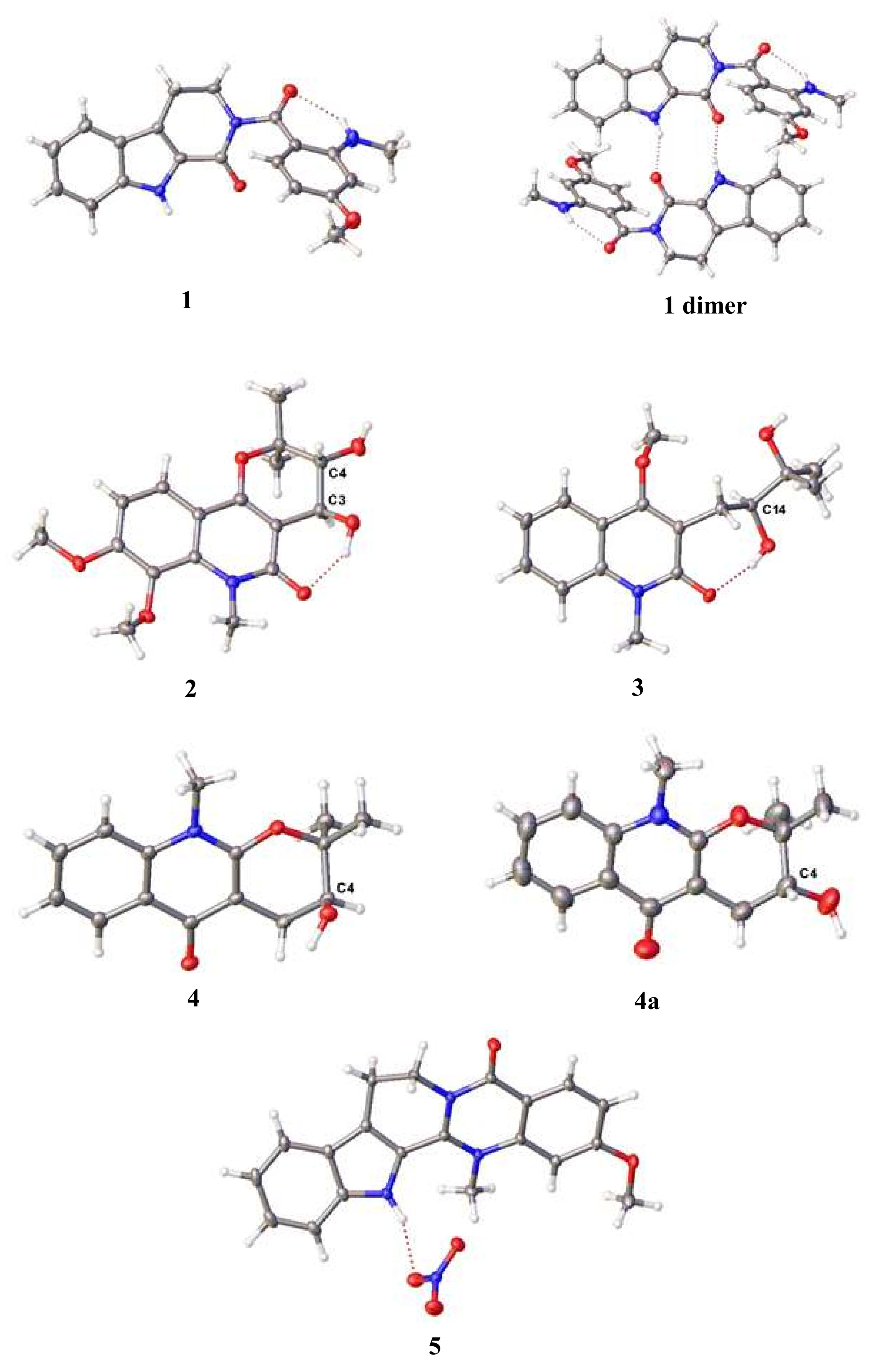
2.2. SC-XRD Analyses
2.3. Semi-Synthesis of Allylated and Acetonitaded Derivatives of Some Naturally Isolated Alkaloids
2.4. Cytotoxic Activity of the Extract, Fractions, and Isolated Compounds
3. Materials and Methods
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. X-ray Diffraction Analyses
3.5. Cytotoxic Activity of the Extract, Fractions, Isolates, and Semi-Synthetic Derivatives
3.6. Preparation of the Semis-Ynthetic Derivatives
3.6.1. Allylation of Edulinine
3.6.2. Acetonidation of Edulinine
3.6.3. Allylation of Ribalinine
3.6.4. Allylation of Soyauxinium Nitrate
3.6.5. Allylation of Arborinine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Noulala, T.C.G.; Fotso, W.G.; Rennert, R.; Lenta, N.B.; Sewald, N.; Arnold, N.; Happi, N.E.; Ngadjui, T.B. Mesomeric form of quaternary indoloquinazoline alkaloid and other constituents from the Cameroonian Rutaceae Araliopsis soyauxii Engl. Biochem. Syst. Ecol. 2020, 91, 104050. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Noulala, T.G.C.; Samba, R.M.A.; Tankeo, B.S.; Abdelfatah, S.; Fotso, W.G.; Happi, N.E.; Ngadjui, T.B.; Beng, P.V.; Kuete, V.; et al. The alkaloid, soyauxinium chloride, displays remarkable cytotoxic effects towards a panel of cancer cells, inducing apoptosis, ferroptosis and necroptosis. Chem. Biol. Interact. 2020, 333, 109334. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Noulala, T.G.C.; Samba, R.M.A.; Tankeo, B.S.; Fotso, W.G.; Happi, N.E.; Ngadjui, T.B.; Beng, P.V.; Kuete, V.; Efferth, T. Cytotoxicity of botanicals and isolated phytochemicals from Araliopsis soyauxii Engl. (Rutaceae) towards a panel of human cancer cells. J. Ethnopharmacol. 2020, 267, 113535. [Google Scholar] [CrossRef]
- Chaturvedula, P.V.S.; Schilling, J.K.; Miller, J.S.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. New cytotoxic alkaloids from the wood of Vepris punctata from the Madagascar Rainforest. J. Nat. Prod. 2003, 66, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedula, P.V.S.; Schilling, J.K.; Miller, J.S.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. New cytotoxic terpenoids from the wood of Vepris punctata from the Madagascar Rainforest. J. Nat. Prod. 2004, 67, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Kouam, K.A.D.; Kenmogne, S.B.; Lobe, J.S.; Happi, N.E.; Stammler, H.-G.; Waffo, K.F.A.; Sewald, N.; Wansi, J.D. A rotameric tryptamide alkaloid from the roots of Vepris lecomteana (Pierre) Cheek & T. Heller (Rutaceae). Fitoterapia 2019, 135, 9–14. [Google Scholar]
- Kouam, K.A.D.; Bissoue, N.A.; Tcho, T.A.; Happi, N.E.; Waffo, K.F.A.; Sewald, N.; Wansi, J.D. Antimicrobial Furoquinoline Alkaloids from Vepris lecomteana (Pierre) Cheek & T. Heller (Rutaceae). Molecules 2018, 23, 13. [Google Scholar]
- Wang, Q.; Liang, J.; Feng, X. X-ray crystallographic analysis and revision of nmr spectral assignments for rhetsinine. J. China Pharm. 2009, 40, 503–505. [Google Scholar]
- Christopher, E.; Bedira, E.; Dunbar, C.; Khan, I.A.; Okunji, C.O.; Schuster, B.M.; Iwu, M.M. Indoloquinazoline alkaloids from Araliopsis tabouensis. Helv. Chim. Acta 2003, 86, 2914–2918. [Google Scholar] [CrossRef]
- Wabo, H.K.; Tane, P.; Connolly, J.D.; Okunji, C.C.; Schuster, B.M.; Iwu, M. Tabouensinium chloride, a novel quaternary pyranoquinoline alkaloid from Araliopsis tabouensis. Nat. Prod. Res. 2005, 6, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Iqbal, M. Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron. Sustain. Dev. 2007, 27, 45–57. [Google Scholar]
- Barron, D.; Balland, C.; Possety, F.; Ravanel, P.; Desfougères, A. Flavonoïdes prénylés et perméabilité membranaire. Acta Bot. Gall. 1996, 143, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Tameye, J.S.N.; Akak, M.C.; Happi, M.G.; Frese, M.; Stammler, H.-G.; Neumann, B.; Lenta, N.B.; Sewald, N.; Nkengfack, E.A. Antioxidant norbergenin derivatives from the leaves of Diospyros gilletii De Wild (Ebenaceae). Phytochem. Lett. 2020, 36, 63–67. [Google Scholar] [CrossRef]
- Tabekoueng, B.G.; Akak, M.C.; Happi, G.M.; Langat, M.K.; Frese, M.; Stammler, H.-G.; Neumann, B.; Azebaze, A.G.B.; Waffo, A.F.; Wansi, J.D.; et al. The chemistry of the West and Central African Penianthus zenkeri Diels (Menispermaceae). Phytochem. Lett. 2020, 38, 12–18. [Google Scholar] [CrossRef]
- Happi, G.M.; Kemayou, G.P.M.; Stammler, H.-G.; Neumann, B.; Ismael, M.; Kouam, S.F.; Wansi, J.D.; Tchouankeu,, J.C.; Frese, M.; Lenta, N.B.; et al. Three phragmalin-type limonoids orthoesters and the structure of odoratone isolated from the bark of Entandrophragma candollei (Meliaceae). Phytochemistry 2021, 181, 112537. [Google Scholar] [CrossRef] [PubMed]
- Tsopgni, T.W.D.; Azebaze, A.G.B.; Teinkela, M.J.E.; Lenta, N.B.; Bosyom, F.F.; Ngadjui, T.B.; Vardamides, J.C. New unsaturated fatty acid and other chemical constituents from the roots of Cola rostrata K. Schum. (Malvaceae). Biochem. Syst. Ecol. 2019, 86, 103913. [Google Scholar] [CrossRef]
- Réthy, B.; Zupkó, I.; Minorics, R.; Hohmann, J.; Ocsovszki, I.; Falkay, G. Investigation of cytotoxic activity on human cancer cell lines of arborinine and furanoacridones isolated from Ruta graveolens. Planta Med. 2007, 73, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sammet, B.; Bogner, T.; Nahrwold, M.; Weiss, C.; Sewald, N. Approaches for the synthesis of functionalized cryptophycins. J. Org. Chem. 2010, 75, 6953–6960. [Google Scholar] [CrossRef] [PubMed]

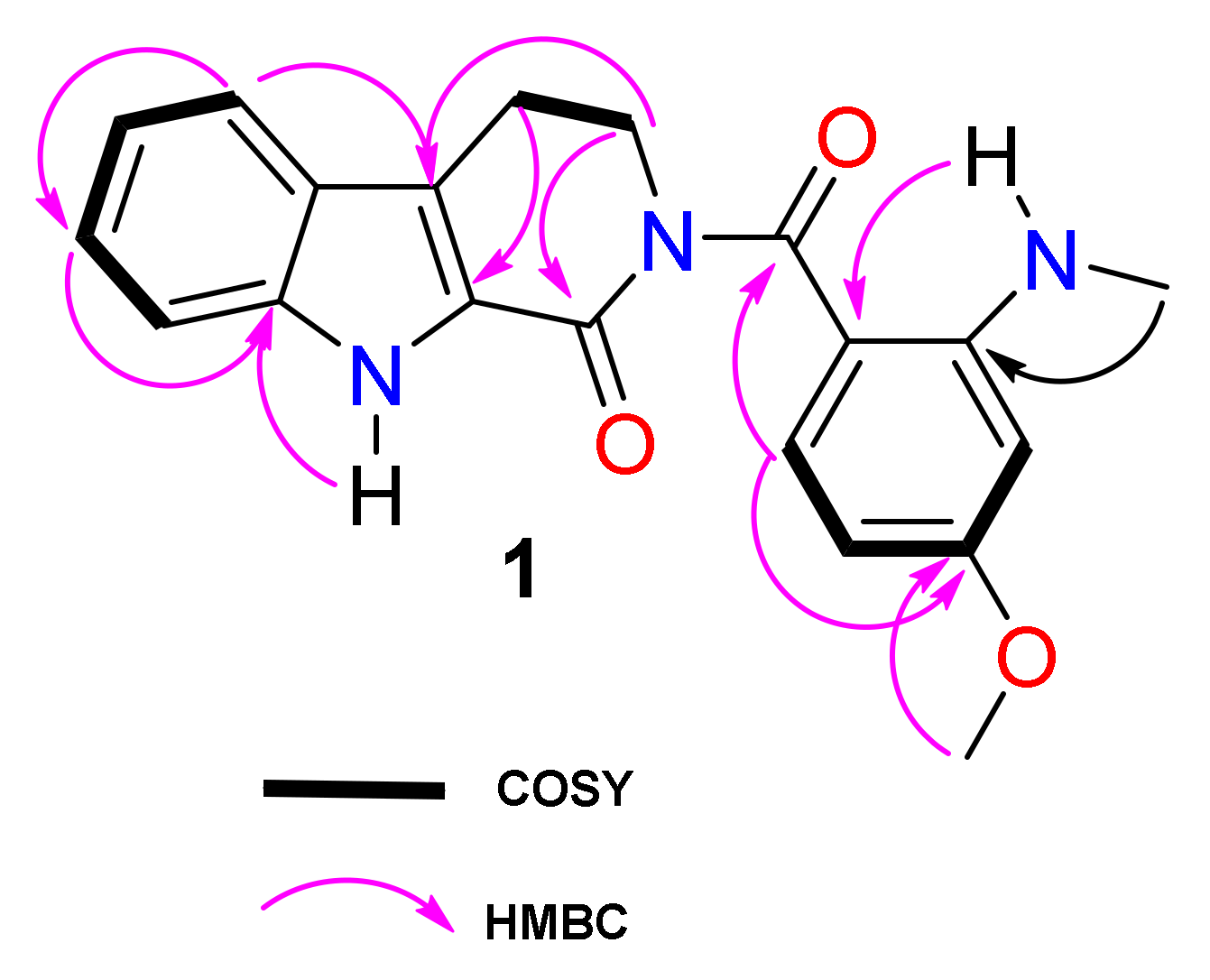



| Position | 1 | ||
|---|---|---|---|
| δc | δH | HMBC | |
| 1 | 154.0 | ||
| 2 | 94.8 | 6.18(1H, d, J = 2.4) | 1, 3 |
| 3 | 165.4 | ||
| 4 | 102.2 | 6.11 (1H, dd; J = 9.0, 2.4) | 3, 5a |
| 5 | 135.5 | 7.45 (1H, d, J = 9.0) | 1, 3, 6 |
| 5a | 109.0 | ||
| 6 | 175.1 | ||
| 7 | 47.8 | 4.15 (2H, t, J = 6.4) | 6, 8a, 13 |
| 8 | 21.3 | 3.20 (2H, t, J = 6.4) | 8a, 13a |
| 8a | 122.3 | ||
| 9a | 125.0 | ||
| 9 | 120.7 | 7.63 (1H, d, J = 8.0) | 12, 12a |
| 10 | 120.7 | 7.16 (1H, t, J = 8.0) | 9a, 12 |
| 11 | 126.0 | 7.28 (1H, t, J = 8.0) | 9, 10, 12 |
| 12 | 113.0 | 7.14 (1H, t, J = 8.0) | 9, 9a, 10 |
| 12a | 138.4 | ||
| 13a | 126.4 | ||
| 13 | 162.1 | ||
| 1-NCH3 | 29.7 | 2.96 (3H, d, J = 5.0) | 1 |
| 1-NHCH3 | 7.80 (1H, q, J = 5.0) | 2 | |
| 3-OCH3 | 55.3 | 3.81(1H, s) | 3 |
| NH | 9.69, brs | 8a, 12a | |
| IC50 (µM) | |
|---|---|
| Samples | KB-3-1 |
| 1 | ˃50 |
| 2 | Inactive |
| 3 | Inactive |
| 3a | Inactive |
| 3b | ˃500 |
| 3c | ˃500 |
| 4 | Inactive |
| 4b | Inactive |
| 5 | Inactive |
| 5a | Inactive |
| 6 | 50 |
| 6a | ˃500 |
| 7 | Inactive |
| 9 | 10 |
| FTAE1 | Inactive |
| FTAE2 | Inactive |
| FTAE3 | Inactive |
| TAE | Inactive |
| Griseofulvin | 17–21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noulala, C.G.T.; Ouete, J.L.N.; Atangana, A.F.; Mbahbou, G.T.B.; Fotso, G.W.; Stammler, H.-G.; Lenta, B.N.; Happi, E.N.; Sewald, N.; Ngadjui, B.T. Soyauxinine, a New Indolopyridoquinazoline Alkaloid from the Stem Bark of Araliopsis soyauxii Engl. (Rutaceae). Molecules 2022, 27, 1104. https://doi.org/10.3390/molecules27031104
Noulala CGT, Ouete JLN, Atangana AF, Mbahbou GTB, Fotso GW, Stammler H-G, Lenta BN, Happi EN, Sewald N, Ngadjui BT. Soyauxinine, a New Indolopyridoquinazoline Alkaloid from the Stem Bark of Araliopsis soyauxii Engl. (Rutaceae). Molecules. 2022; 27(3):1104. https://doi.org/10.3390/molecules27031104
Chicago/Turabian StyleNoulala, Cédric Guy Tchatchouang, Judith Laure Nantchouang Ouete, Albert Fouda Atangana, Gabin Thierry Bitchagno Mbahbou, Ghislain Wabo Fotso, Hans-Georg Stammler, Bruno Ndjakou Lenta, Emmanuel Ngeufa Happi, Norbert Sewald, and Bonaventure Tchaleu Ngadjui. 2022. "Soyauxinine, a New Indolopyridoquinazoline Alkaloid from the Stem Bark of Araliopsis soyauxii Engl. (Rutaceae)" Molecules 27, no. 3: 1104. https://doi.org/10.3390/molecules27031104
APA StyleNoulala, C. G. T., Ouete, J. L. N., Atangana, A. F., Mbahbou, G. T. B., Fotso, G. W., Stammler, H.-G., Lenta, B. N., Happi, E. N., Sewald, N., & Ngadjui, B. T. (2022). Soyauxinine, a New Indolopyridoquinazoline Alkaloid from the Stem Bark of Araliopsis soyauxii Engl. (Rutaceae). Molecules, 27(3), 1104. https://doi.org/10.3390/molecules27031104







