Simultaneous Determination and Health Risk Assessment of Four High Detection Rate Pesticide Residues in Pu’er Tea from Yunnan, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Chemicals and Reagents
2.3. Sample Preparation and Analysis
2.3.1. Sample Preparation Method
2.3.2. Instrumental Analysis Method
2.3.3. Method Validation
2.4. Matrix Effect
2.5. Risk Assessment
3. Results
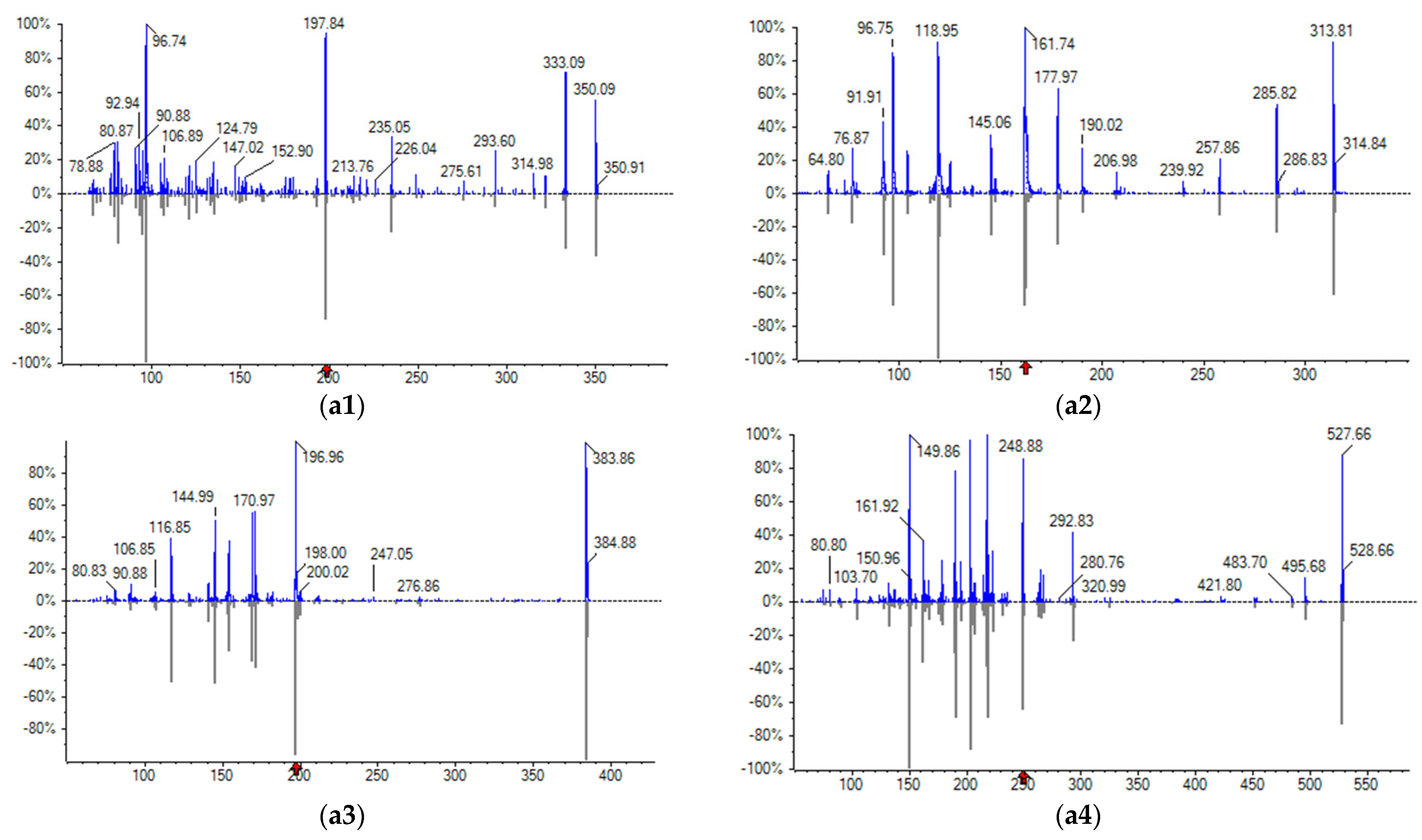
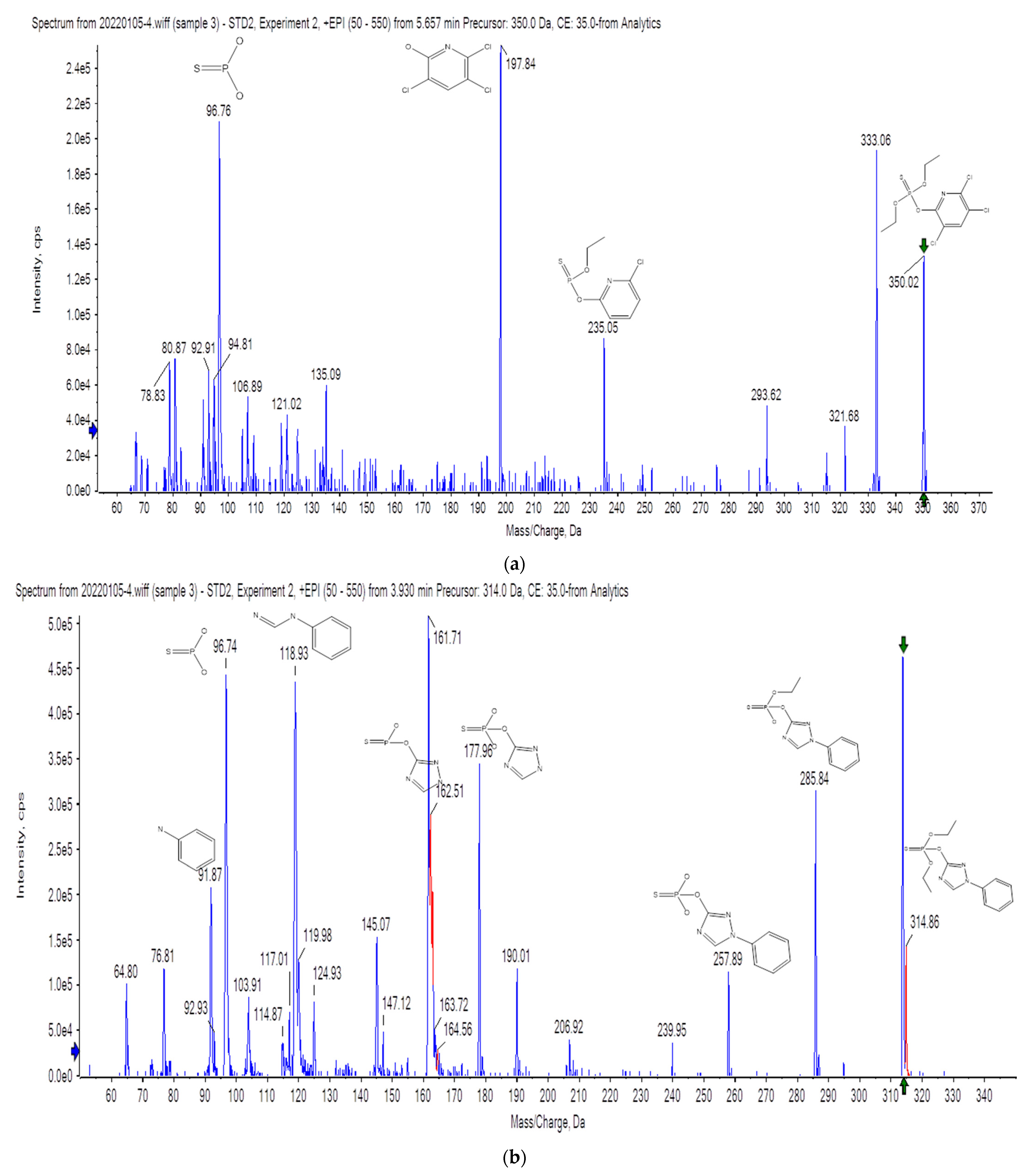
3.1. Optimization of Instrument Conditions
3.2. QTrap System
3.3. Matrix Effects
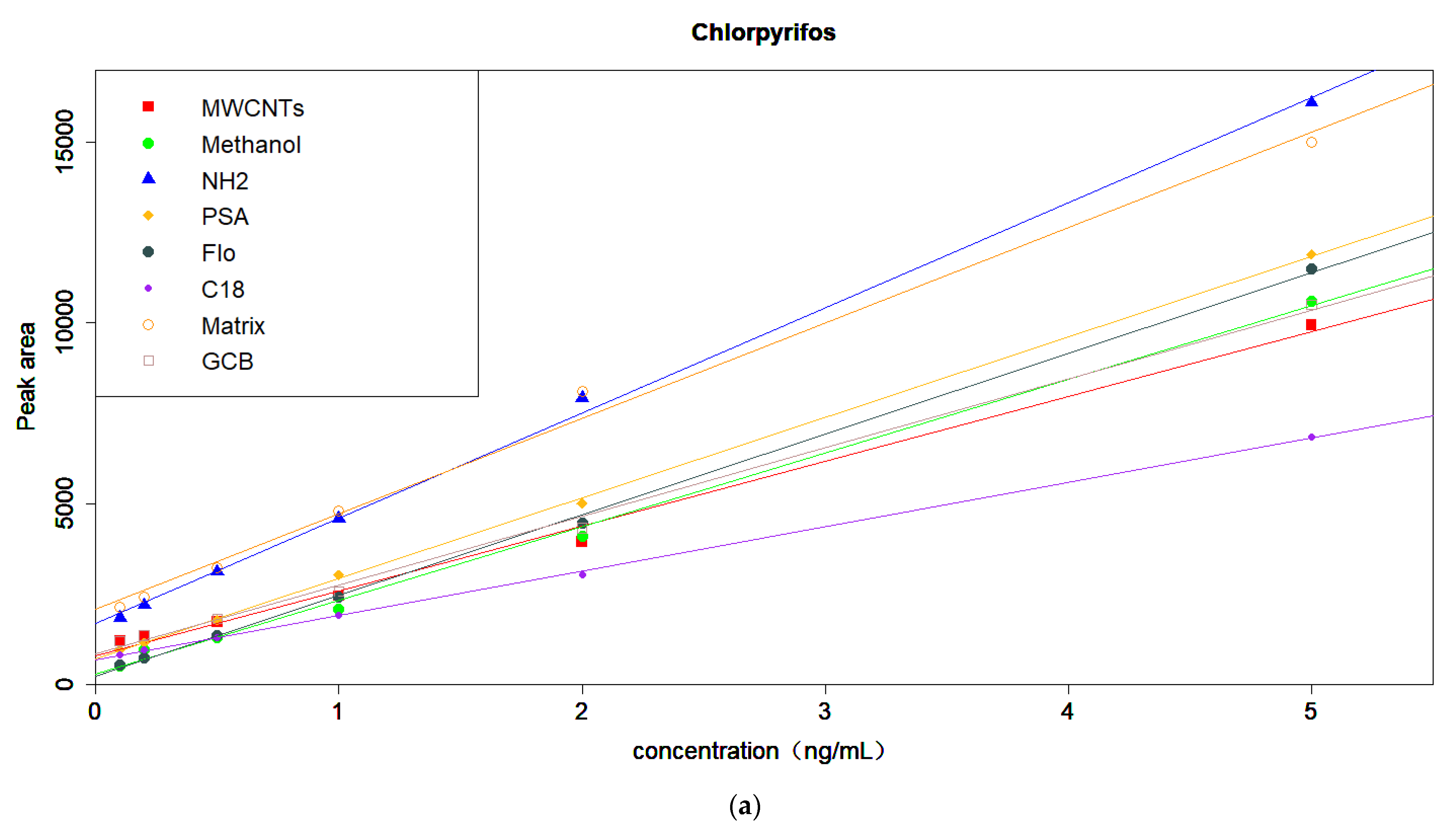
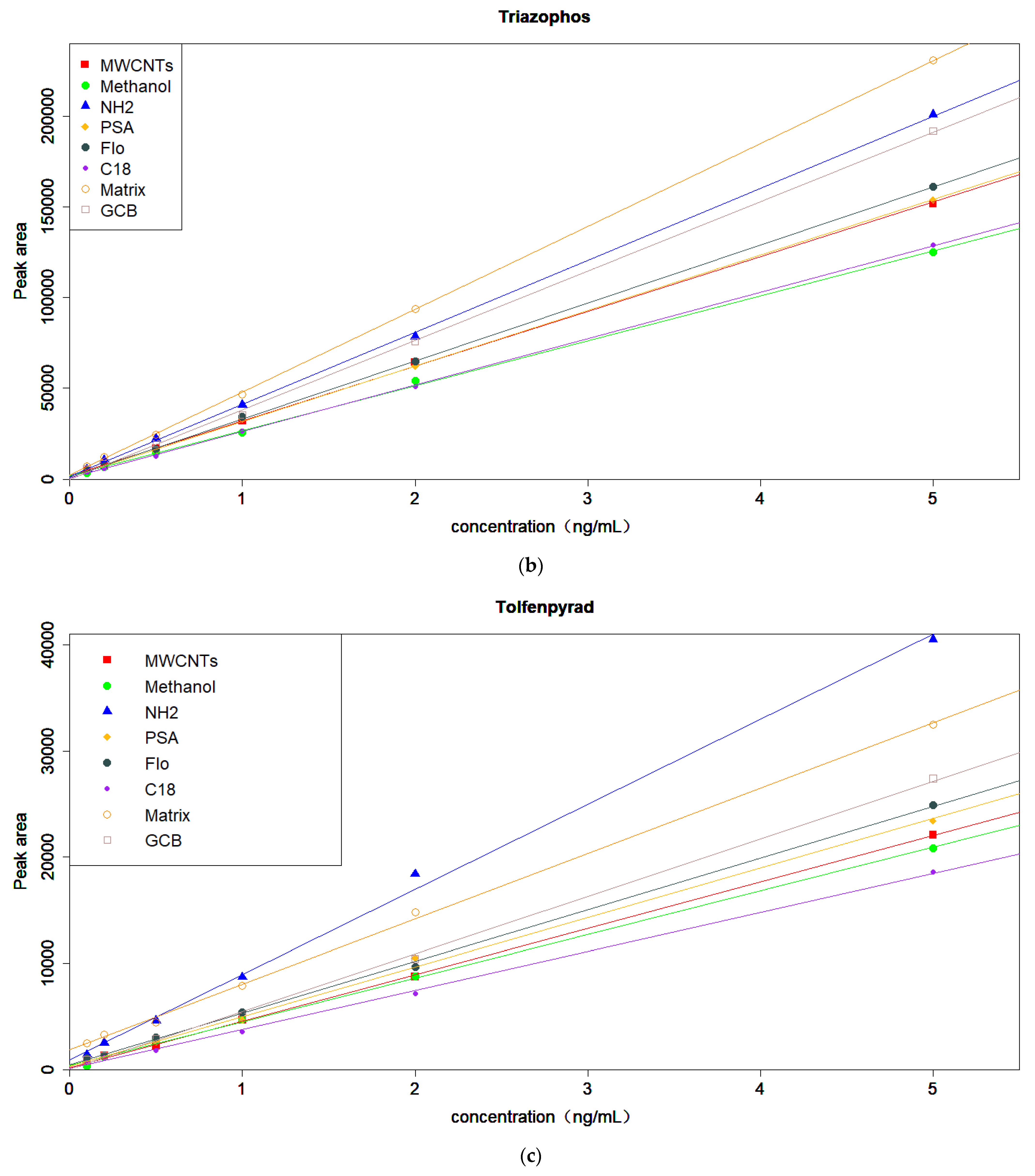
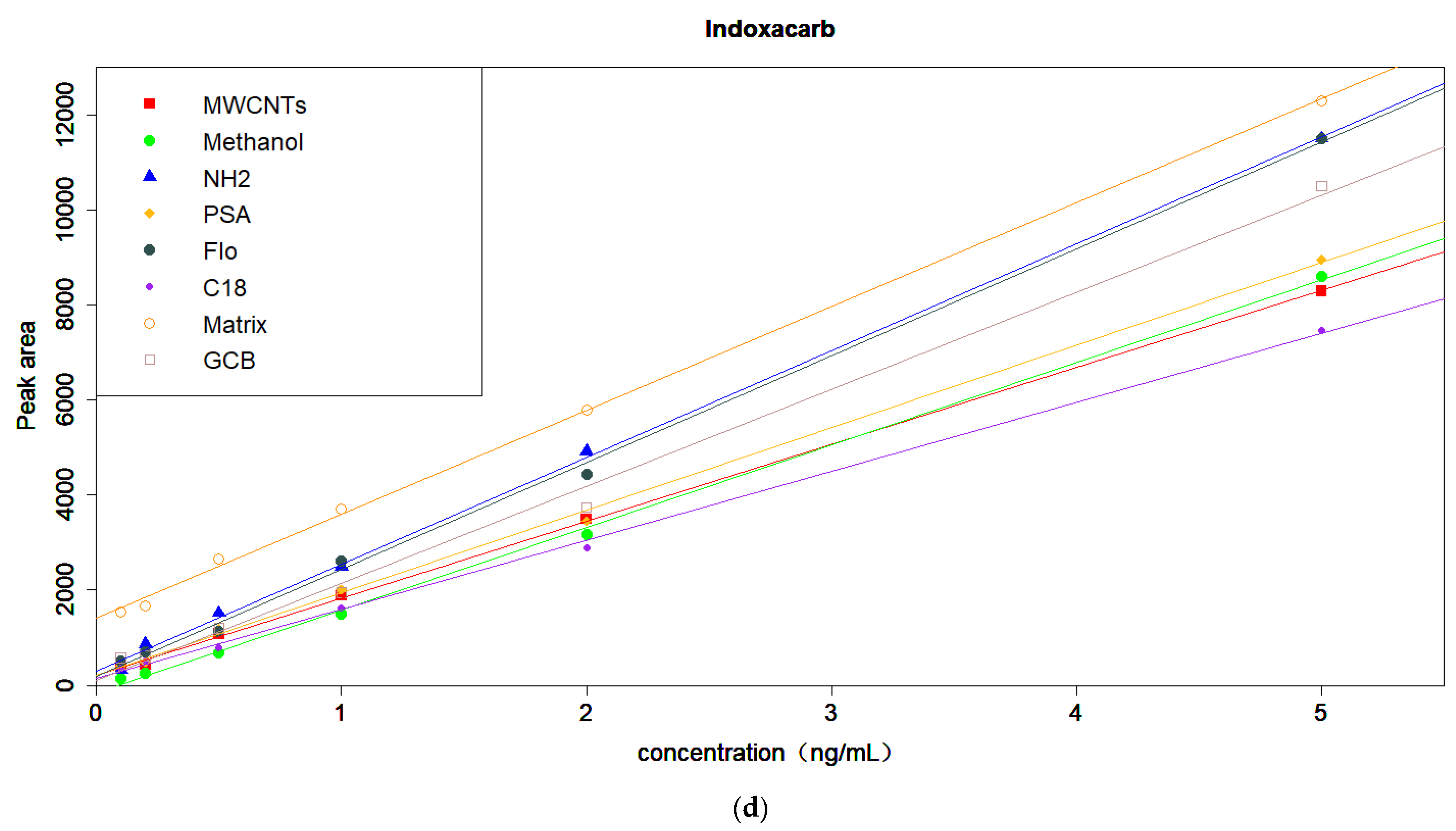
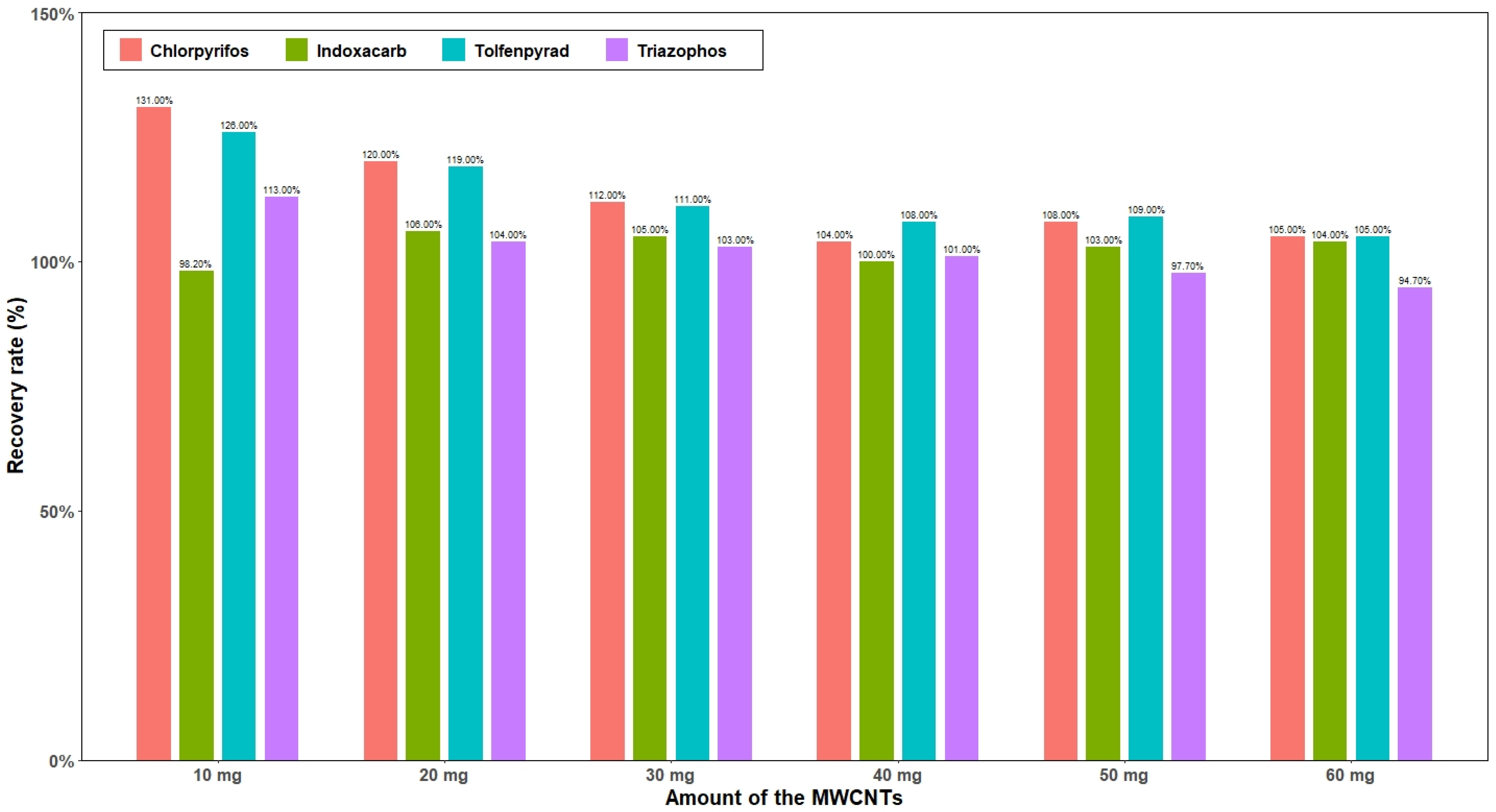
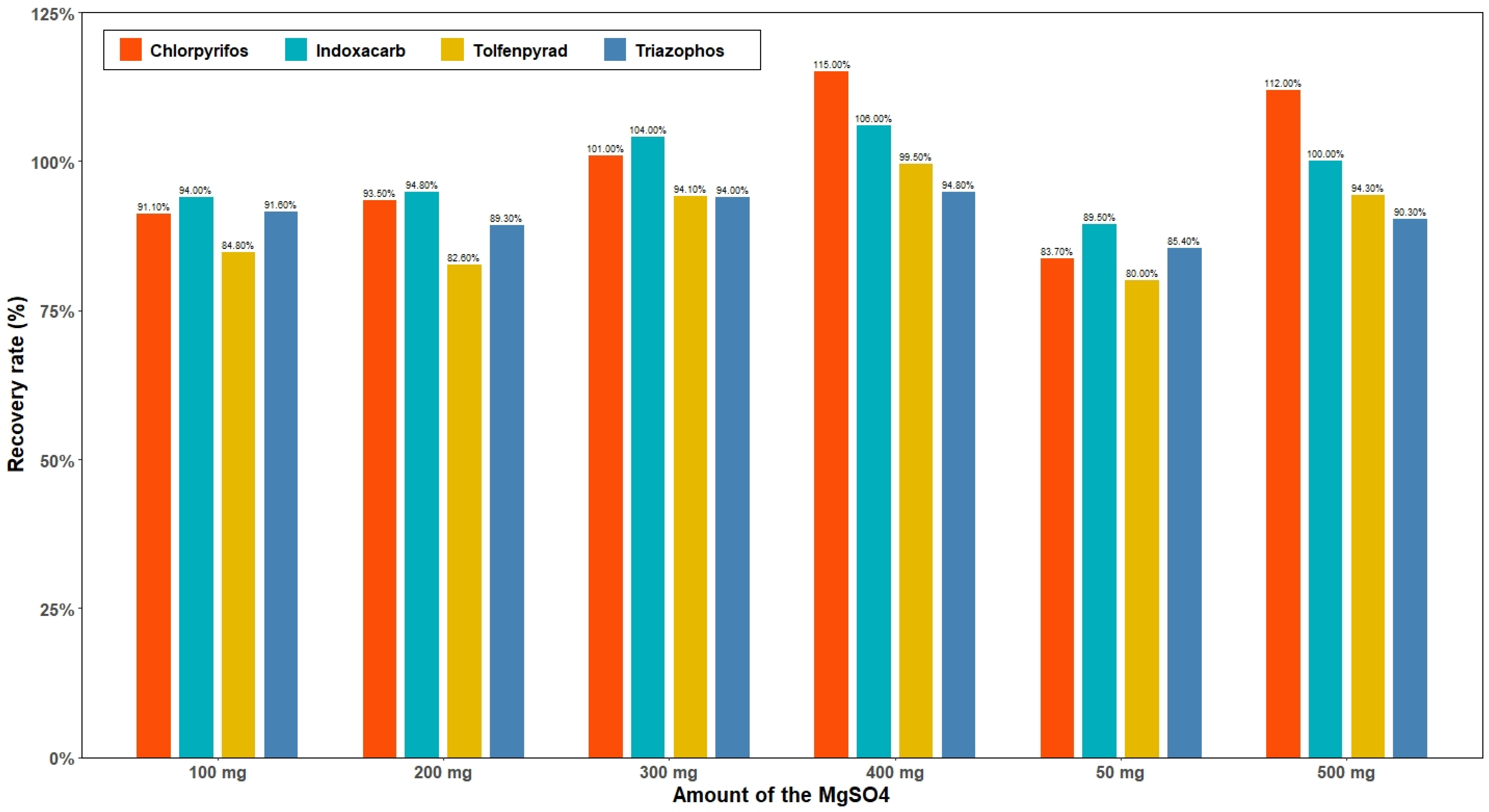
3.4. Optimization of the Amount of MWCNTs and MgSO4
3.5. Method Validation
3.6. Pesticide Residues in Pu’er Tea
3.7. Consumer Exposure Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
References
- Jiang, C.; Zeng, Z.; Huang, Y.; Zhang, X. Chemical compositions of Pu’er tea fermented by Eurotium Cristatum and their lipid-lowering activity. LWT-Food Sci. Technol. 2018, 98, 204–211. [Google Scholar] [CrossRef]
- Qiong, S.; Xishuang, Y. History of Pu’er Tea and comparative study for the effect of its various extracts on lipid-lowering diet. Pak. J. Pharm. Sci. 2014, 27, 1015–1022. [Google Scholar] [PubMed]
- Hsieh, S.-K.; Xu, J.-R.; Lin, N.-H.; Li, Y.-C.; Chen, G.-H.; Kuo, P.-C.; Chen, W.-Y.; Tzen, J.T.C. Antibacterial and laxative activities of strictinin isolated from Pu’er tea (Camellia sinensis). J. Food Drug Anal. 2016, 24, 722–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Zeng, X.; Wu, H.; Shen, S.; Yang, X.; Deng, W.-W.; Ning, J. Characterizing volatile metabolites in raw Pu’er tea stored in wet-hot or dry-cold environments by performing metabolomic analysis and using the molecular sensory science approach. Food Chem. 2021, 350, 129186. [Google Scholar] [CrossRef]
- Peng, W.; Wang, L.; Qian, Y.; Chen, T.; Dai, B.; Feng, B.; Wang, B. Discrimination of Unfermented Pu’er Tea Aroma of Different Years Based on Electronic Nose. Agric. Res. 2017, 6, 436–442. [Google Scholar] [CrossRef]
- Dou, Z.; Ji, M.; Wang, M.; Li, H. Empirical analysis of Pu’er tea price bubble measurement based on GSADF method. Acta. Agr. Scand. B.-S. P. 2021, 71, 81–90. [Google Scholar] [CrossRef]
- Feng, X. Building an ecological tea garden, promoting the sustainable development of Pu’er tea industry. Yunnan Agri. 2017, 10, 72–73. [Google Scholar]
- Bai, A.; Chen, A.; Chen, W.; Liu, S.; Luo, X.; Liu, Y.; Zhang, D. Residue behavior, transfer and risk assessment of tolfenpyrad, dinotefuran and its metabolites during tea growing and tea brewing. J. Sci. Food Agri. 2021, 101, 5992–6000. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Mu, X.-C.; Yu, H.-Y.; Niu, C.-D.; Wang, L.-X.; Zheng, C.; Chen, Z.; Gao, C.-F. Susceptibility of Empoasca vitis (Hemiptera: Cicadellidae) populations from the main tea-growing regions of China to thirteen insecticides. Crop. Prot. 2017, 96, 204–210. [Google Scholar] [CrossRef]
- Liu, T.-X.; Sparks, A.N., Jr.; Chen, W.; Liang, G.-M.; Brister, C. Toxicity, persistence, and efficacy of indoxacarb on cabbage looper (Lepidoptera: Noctuidae) on cabbage. J. Econ. Entomol. 2002, 95, 360–367. [Google Scholar] [CrossRef]
- Hikiji, W.; Yamaguchi, K.; Saka, K.; Hayashida, M.; Ohno, Y.; Fukunaga, T. Acute fatal poisoning with Tolfenpyrad. J. Forensic. Leg. Med. 2013, 20, 962–964. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. List of Prohibited and Restricted Pesticides. Available online: http://www.zzys.moa.gov.cn/gzdt/201911/t20191129_6332604.htm (accessed on 7 January 2022).
- GB 2763-2021National Food safety Standard-In Maximum Residue Limits for Pesticides in FoodChina Agriculture Press: Beijing, China, 2021; pp. 95–97, 281–282, 359–360, 365.
- Dong, X.; Lan, T.; Tian, X.; Li, Y.; Zhao, Y.; Zong, Q.; Liu, S.; Pan, C. Simultaneous determination of 14 pesticide residues in tea by multi-plug filtration cleanup combined with LC-MS/MS. J. Environ. Sci. Heal. B. 2021, 56, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, T.; Luo, X.; Xiong, H.; Min, F.; Chen, Y.; Nie, S.; Xie, M. Determination of multi-pesticide residues in green tea with a modified QuEChERS protocol coupled to HPLC-MS/MS. Food Chem. 2019, 275, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Harischandra, N.R.; Pallavi, M.S.; Bheemanna, M.; PavanKumar, K.; Chandra Sekhara Reddy, V.; Udaykumar, N.R.; Paramasivam, M.; Yadav, S. Simultaneous determination of 79 pesticides in pigeonpea grains using GC–MS/MS and LC–MS/MS. Food Chem. 2021, 347, 128986. [Google Scholar] [CrossRef]
- Zhu, B.; Xu, X.; Luo, J.; Jin, S.; Chen, W.; Liu, Z.; Tian, C. Simultaneous determination of 131 pesticides in tea by on-line GPC-GC–MS/MS using graphitized multi-walled carbon nanotubes as dispersive solid phase extraction sorbent. Food Chem. 2019, 276, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Li, J.; Yan, S.a.; Huang, M.; Lin, Q. Occurrence of pesticides in white tea and a corresponding risk exposure assessment for the different residents in Fujian, China. J. Food Sci. 2021, 86, 3743–3754. [Google Scholar] [CrossRef] [PubMed]
- Beneta, A.; Mutavdžić Pavlović, D.; Periša, I.; Petrović, M. Multiresidue GC-MS/MS pesticide analysis for evaluation of tea and herbal infusion safety. Int. J. Environ. An. Chem. 2018, 98, 987–1004. [Google Scholar] [CrossRef]
- Lv, S.; Wu, X.; Guan, J.; Yan, Y.; Ge, M.; Zhu, G. Quantification and Confirmation of Zearalenone Using a LC-MS/MS QTRAP System in Multiple Reaction Monitoring and Enhanced Product Ion Scan Modes. Food Anal. Method. 2021, 14, 1–9. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, Y.; Wang, J.; Chang, J.; Wang, A.; Ren, X.; Liu, B. Quantitative analysis of 17 hypoglycemic drugs in fingerprints using ultra-high-performance liquid chromatography/tandem hybrid triple quadrupole linear ion trap mass spectrometry. Rapid Commun. Mass. Sp. 2022, 36, e9199. [Google Scholar] [CrossRef]
- Sadowska-Rociek, A.; Surma, M.; Cieślik, E. Comparison of different modifications on QuEChERS sample preparation method for PAHs determination in black, green, red and white tea. Environ. Sci. Pollut. R. 2014, 21, 1326–1338. [Google Scholar] [CrossRef]
- Wu, C.-C. Multiresidue method for the determination of pesticides in Oolong tea using QuEChERS by gas chromatography-triple quadrupole tandem mass spectrometry. Food Chem. 2017, 229, 580–587. [Google Scholar] [CrossRef]
- Jiao, W.; Xiao, Y.; Qian, X.; Tong, M.; Hu, Y.; Hou, R.; Hua, R. Optimized combination of dilution and refined QuEChERS to overcome matrix effects of six types of tea for determination eight neonicotinoid insecticides by ultra performance liquid chromatography–electrospray tandem mass spectrometry. Food Chem. 2016, 210, 26–34. [Google Scholar] [CrossRef]
- Zhang, X.; Mobley, N.; Zhang, J.; Zheng, X.; Lu, L.; Ragin, O.; Smith, C. Analysis of agricultural residues on tea using d-SPE sample preparation with GC-NCI-MS and UHPLC-MS/MS. J. Agri. Food Chem. 2010, 58, 11553–11560. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Gao, F.; Song, D.; Lu, X. Selection of representative matrices for the multiresidue analysis of pesticides in tea by GC-MS/MS. Anal. Methods 2018, 10, 855–866. [Google Scholar] [CrossRef]
- Li, Y.; Pang, G.-F.; Fan, C.-L.; Chen, X. Hierarchical Cluster Analysis of Matrix Effects on 110 Pesticide Residues in 28 Tea Matrixes. J. AOAC Int. 2013, 96, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, G.; Ilyas, F.; Idrees, M.; Mariyam, V. Monitoring and risk assessment due to presence of heavy metals and pesticides in tea samples. Food Sci. Technol. 2018, 38, 625–628. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Qian, Y.; Chen, Q.; Tao, C.; Li, C.; Li, Y. Evaluation of pesticide residues in fruits and vegetables from Xiamen, China. Food Control 2011, 22, 1114–1120. [Google Scholar] [CrossRef]
- GB/T 8302-2013Tea-SamplingChina Agriculture Press: Beijing, China, 2013.
- European Commission Directorate-General for Health and Food Safety. European Commission Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues and Analysis in Food and Feed. Document No. SANTE/12682/2019. 2019. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 7 January 2022).
- Stahnke, H.; Kittlaus, S.; Kempe, G.n.; Alder, L. Reduction of matrix effects in liquid chromatography–electrospray ionization–mass spectrometry by dilution of the sample extracts: How much dilution is needed? Anal. Chem. 2012, 84, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- New, L.-S.; Chan, E.C.Y. Evaluation of BEH C18, BEH HILIC, and HSS T3 (C18) Column Chemistries for the UPLC-MS-MS Analysis of Glutathione, Glutathione Disulfide, and Ophthalmic Acid in Mouse Liver and Human Plasma. J. Chromatogr. Sci. 2008, 46, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, M.; Chen, H.; Shi, Z.; Feng, Y.; Rui, W.J.A.M. Rapid and reliable method for analysis of raw and honey-processed astragalus by UPLC/ESI-Q-TOF-MS using HSS T3 columns. Anal. Methods 2014, 6, 8045–8054. [Google Scholar] [CrossRef]
- Han, C.; Hu, B.; Chen, S.; Wang, N.; Hou, J.; Jin, N.; Shen, Y. Determination of Xinjunan pesticide residue in foodstuffs of plant origin by a modified QuEChERS method and ultra performance liquid chromatography-tandem mass spectrometry. LWT 2021, 151, 112101. [Google Scholar] [CrossRef]
- Gao, G.; Chen, H.; Zhu, L.; Chai, Y.; Ma, G.; Wang, C.; Hao, Z.; Liu, X.; Lu, C. Simultaneous determination of bisphenol A and tetrabromobisphenol A in tea using a modified QuEChERS sample preparation method coupled with liquid chromatography-tandem mass spectrometry. Anal. Methods 2017, 9, 6769–6776. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Wang, L.; Ding, L.; Wang, M.; Yan, H.; Li, Y.; Zhu, S. Simultaneous determination of 103 pesticide residues in tea samples by LC-MS/MS. J. Sep. Sci. 2009, 32, 1294–1301. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, L.; Zhang, C.; Zhang, H.; Lian, K. Determination of 12 insect growth regulator residues in foods of different matrixes by modified QuEChERS and UPLC-MS/MS. RSC Adv. 2021, 11, 12162–12171. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, D.; Meng, X.; Shi, Q.; Li, P.; Jin, L.; Zhang, K.; Song, B. Enantioselective degradation of indoxacarb from different commercial formulations applied to tea. Chirality 2015, 27, 262–267. [Google Scholar] [CrossRef]
- Food Codex Data. Available online: https://www.sdtdata.com/fx/fcv1/tsLibIndex (accessed on 7 January 2022).
- Wang, X.; Zhou, L.; Zhang, X.; Luo, F.; Chen, Z. Transfer of pesticide residue during tea brewing: Understanding the effects of pesticide’s physico-chemical parameters on its transfer behavior. Food Res. Int. 2019, 121, 776–784. [Google Scholar] [CrossRef]
- Chen, H.; Pan, M.; Pan, R.; Zhang, M.; Liu, X.; Lu, C. Transfer rates of 19 typical pesticides and the relationship with their physicochemical property. J. Agri. Food Chem. 2015, 63, 723–730. [Google Scholar] [CrossRef]
- Chen, H.; Pan, M.; Liu, X.; Lu, C. Evaluation of transfer rates of multiple pesticides from green tea into infusion using water as pressurized liquid extraction solvent and ultra-performance liquid chromatography tandem mass spectrometry. Food Chem. 2017, 216, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Yan, S.-A.; Li, J.; Huang, M.; Lin, Q. Health risk assessment of 42 pesticide residues in Tieguanyin tea from Fujian, China. Drug Chem. Toxicol. 2020, 1–8. [Google Scholar] [CrossRef]

| Pesticide | Ionization Mode | Precursor Ion/(m/z) | Product Ion/(m/z) | DP/(Volts) | CE/(Volts) |
|---|---|---|---|---|---|
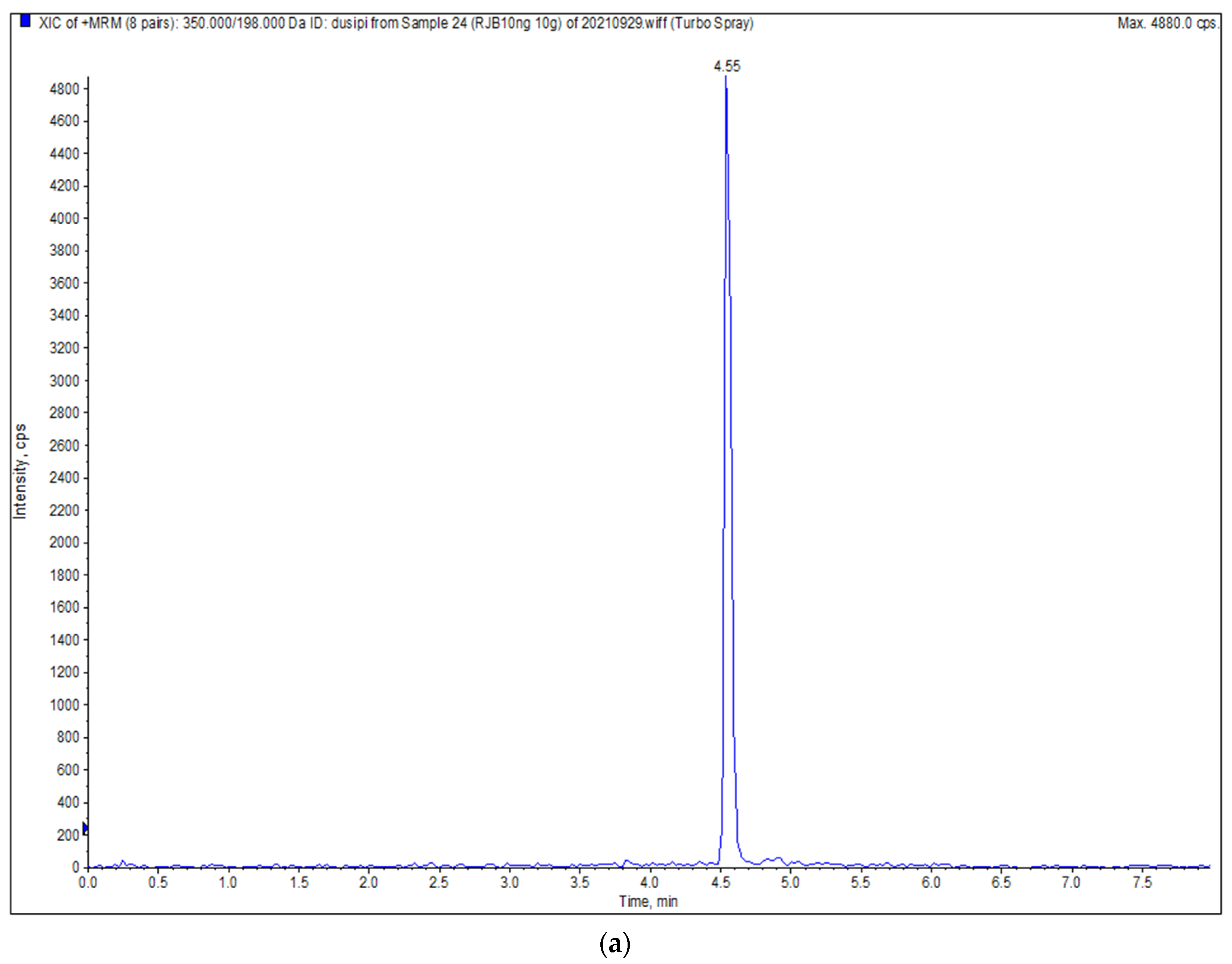
| Chlorpyrifos | ESI+ | 350.0 | 198.0 */96.9 # | 60 | 25/40 |
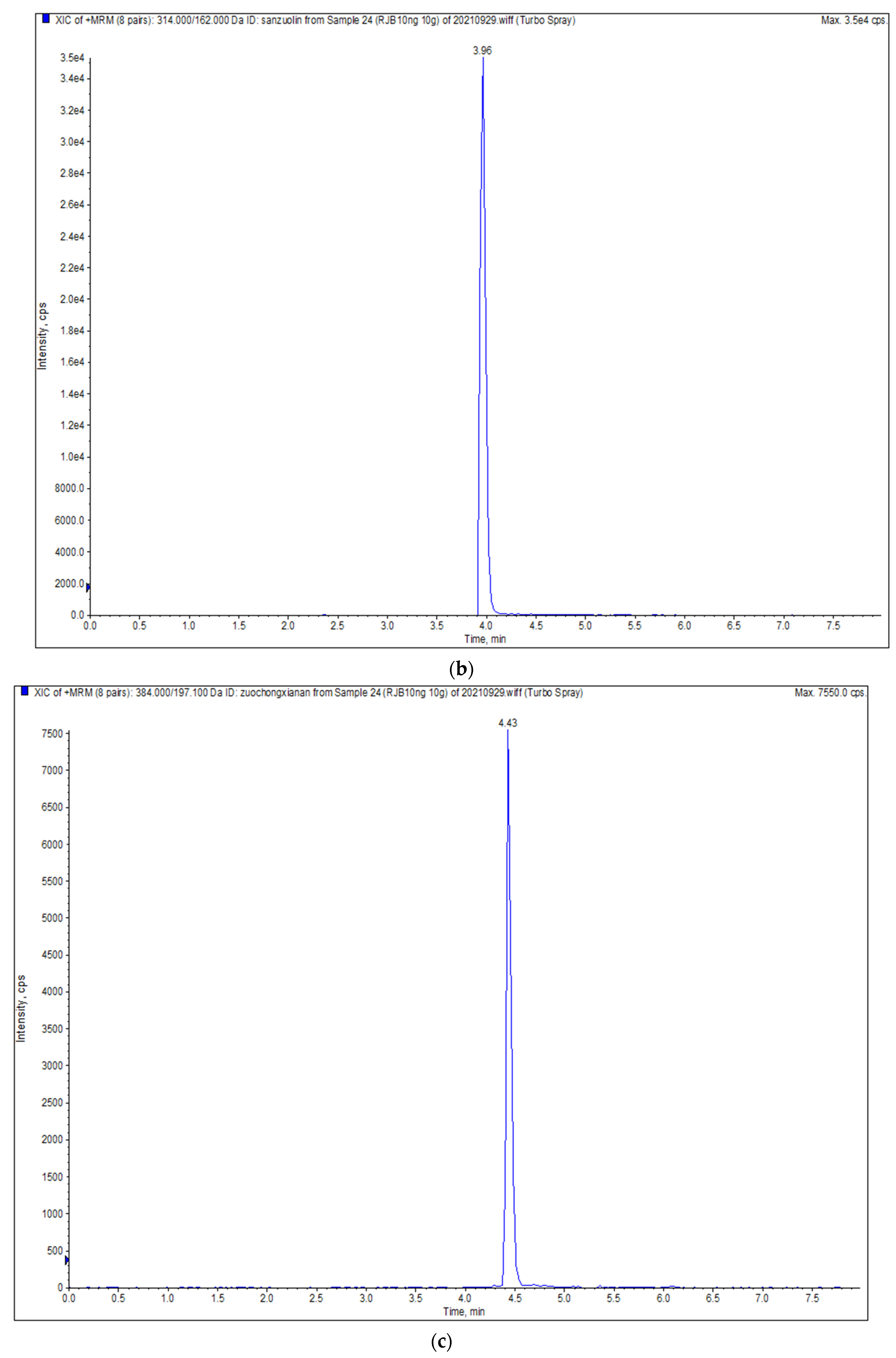
| Triazophos | ESI+ | 314.0 | 162.0 */119.1 # | 55 | 25/50 |
| Tolfenpyrad | ESI+ | 384.0 | 197.1 */154.1 # | 60 | 35/55 |
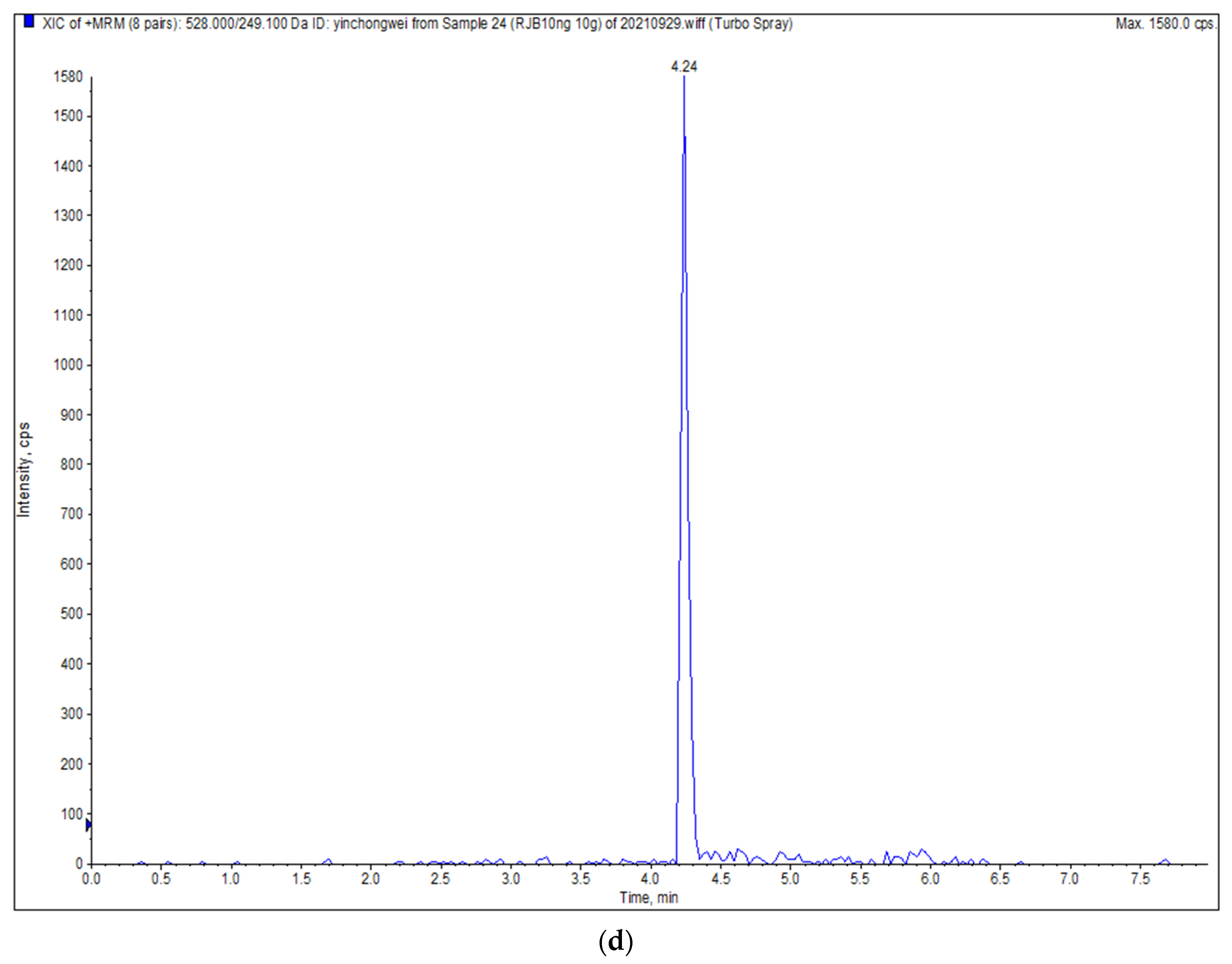
| Indoxacarb | ESI+ | 528.0 | 249.1 */293.1 # | 60 | 25/22 |
| Pesticide | Linear Ranges (μg/L) | R2 | LOQ (μg/kg) | LOD (μg/kg) | ME% |
|---|---|---|---|---|---|
| Chlorpyrifos | 0.01–5.0 | 0.9944 | 0.10 | 0.03 | 13.8 |
| Triazophos | 0.01–5.0 | 0.9997 | 0.50 | 0.15 | −17.8 |
| Tolfenpyrad | 0.01–5.0 | 0.9996 | 0.20 | 0.06 | −5.8 |
| Indoxacarb | 0.01–5.0 | 0.9995 | 0.50 | 0.15 | 7.3 |
| Pesticide | Spiked Amount (μg/kg) | Recovery (%)/RSD (%) | Intraday Precision (%) | Interday Precision (%) |
|---|---|---|---|---|
| Chlorpyrifos | 0.10 | 85.4/5.9 | 4.5 | 5.2 |
| 0.50 | 103.0/6.4 | 4.2 | 5.8 | |
| 1.00 | 105.0/5.3 | 3.7 | 4.7 | |
| Triazophos | 0.50 | 74.8/5.3 | 3.7 | 4.1 |
| 2.50 | 92.9/4.7 | 4.3 | 4.5 | |
| 5.00 | 96.4/3.9 | 3.9 | 3.5 | |
| Tolfenpyrad | 0.20 | 81.3/6.5 | 4.9 | 5.6 |
| 1.00 | 97.3/6.6 | 4.7 | 3.5 | |
| 2.00 | 101.0/5.9 | 3.9 | 4.5 | |
| Indoxacarb | 0.50 | 90.4/6.7 | 4.7 | 3.6 |
| 2.50 | 98.1/5.8 | 3.5 | 4.4 | |
| 5.00 | 96.4/4.2 | 3.6 | 4.6 |
| Pesticide | Detection Rate (%) | Range of Detected Content (mg/kg) | Mean Residue Level (mg/kg) | Median Residue Level (mg/kg) |
|---|---|---|---|---|
| Chlorpyrifos | 12.2 | 1.10–5.28 | 2.14 | 1.62 |
| Triazophos | 10.4 | 0.014–0.103 | 0.049 | 0.046 |
| Tolfenpyrad | 35.7 | 1.02–51.8 | 11.6 | 5.01 |
| Indoxacarb | 5.2 | 1.07–4.89 | 2.84 | 2.96 |
| Pesticide | China (mg/kg) | England (mg/kg) | Japan (mg/kg) | Korea (mg/kg) | European Union (mg/kg) | CAC * (mg/kg) | Canada (mg/kg) | America (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| Chlorpyrifos | 2 | 0.10 | 10 | 2.0 | 2.0 | 2.0 | — | — |
| Triazophos | — | 0.02 | 0.05 | 0.02 | 0.02 | — | — | — |
| Tolfenpyrad | 50 | — | 20 | 30 | 30 | 30 | 30 | |
| Indoxacarb | 5 | — | — | — | 0.05 | 5 | — | — |
| Pesticide | C (mg/kg) | D a (g) | T (%) | Bw b | EDI | ADI (mg/kg bw) [13] | HQ |
|---|---|---|---|---|---|---|---|
| Chlorpyrifos | 2.14 | 10 | 8.6 [41] | 60 | 3.07 × 10−5 | 0.01 | 0.00307 |
| Triazophos | 0.049 | 10 | 27.1 [42] | 60 | 2.21 × 10−6 | 0.001 | 0.00221 |
| Tolfenpyrad | 11.6 | 10 | 4.2 [41] | 60 | 8.12 × 10−5 | 0.006 | 0.0135 |
| Indoxacarb | 2.84 | 10 | 1.6 [43] | 60 | 7.57 × 10−6 | 0.01 | 0.000757 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.; Chen, X.-L.; Guo, J.; Li, M.-X.; Tang, Y.-F.; Li, M.-X.; Li, Y.-G.; Cheng, L.; Liu, H.-C. Simultaneous Determination and Health Risk Assessment of Four High Detection Rate Pesticide Residues in Pu’er Tea from Yunnan, China. Molecules 2022, 27, 1053. https://doi.org/10.3390/molecules27031053
Lin T, Chen X-L, Guo J, Li M-X, Tang Y-F, Li M-X, Li Y-G, Cheng L, Liu H-C. Simultaneous Determination and Health Risk Assessment of Four High Detection Rate Pesticide Residues in Pu’er Tea from Yunnan, China. Molecules. 2022; 27(3):1053. https://doi.org/10.3390/molecules27031053
Chicago/Turabian StyleLin, Tao, Xing-Lian Chen, Jin Guo, Meng-Xia Li, Yu-Feng Tang, Mao-Xuan Li, Yan-Gang Li, Long Cheng, and Hong-Cheng Liu. 2022. "Simultaneous Determination and Health Risk Assessment of Four High Detection Rate Pesticide Residues in Pu’er Tea from Yunnan, China" Molecules 27, no. 3: 1053. https://doi.org/10.3390/molecules27031053
APA StyleLin, T., Chen, X.-L., Guo, J., Li, M.-X., Tang, Y.-F., Li, M.-X., Li, Y.-G., Cheng, L., & Liu, H.-C. (2022). Simultaneous Determination and Health Risk Assessment of Four High Detection Rate Pesticide Residues in Pu’er Tea from Yunnan, China. Molecules, 27(3), 1053. https://doi.org/10.3390/molecules27031053






