Biotic Cathode of Graphite Fibre Brush for Improved Application in Microbial Fuel Cells
Abstract
1. Introduction
2. Results
2.1. Power and Polarisation Output
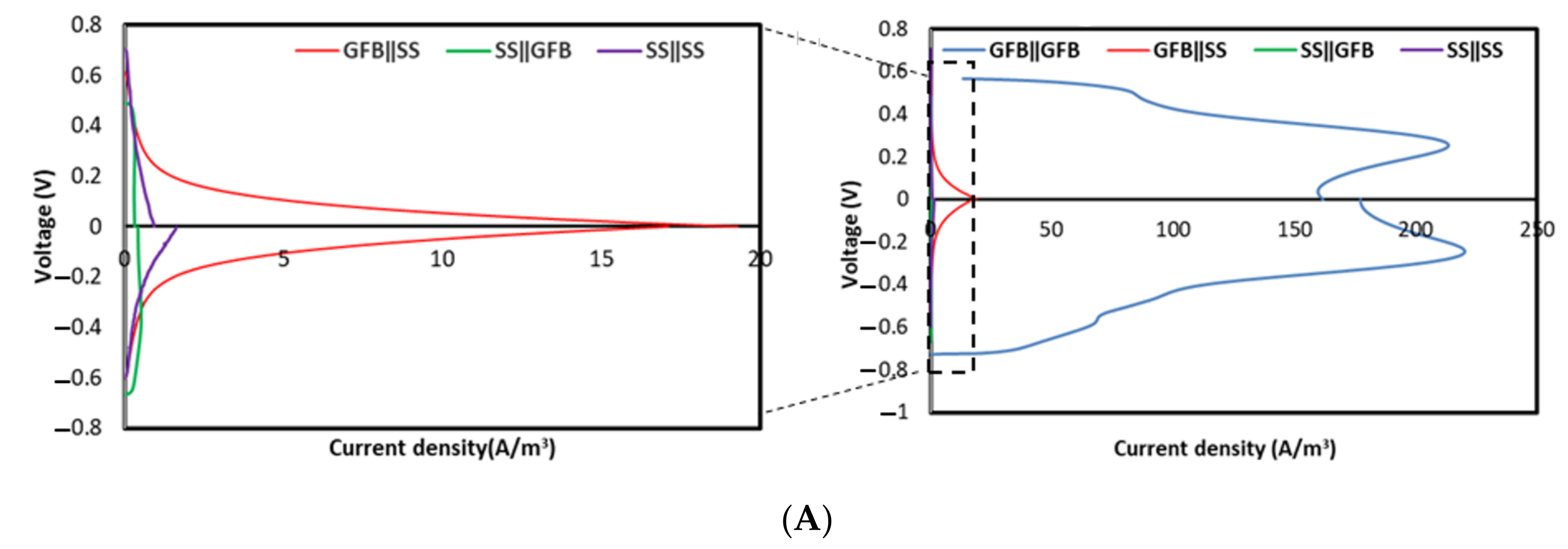
2.1.1. Abiotic Cathode for 60 Days
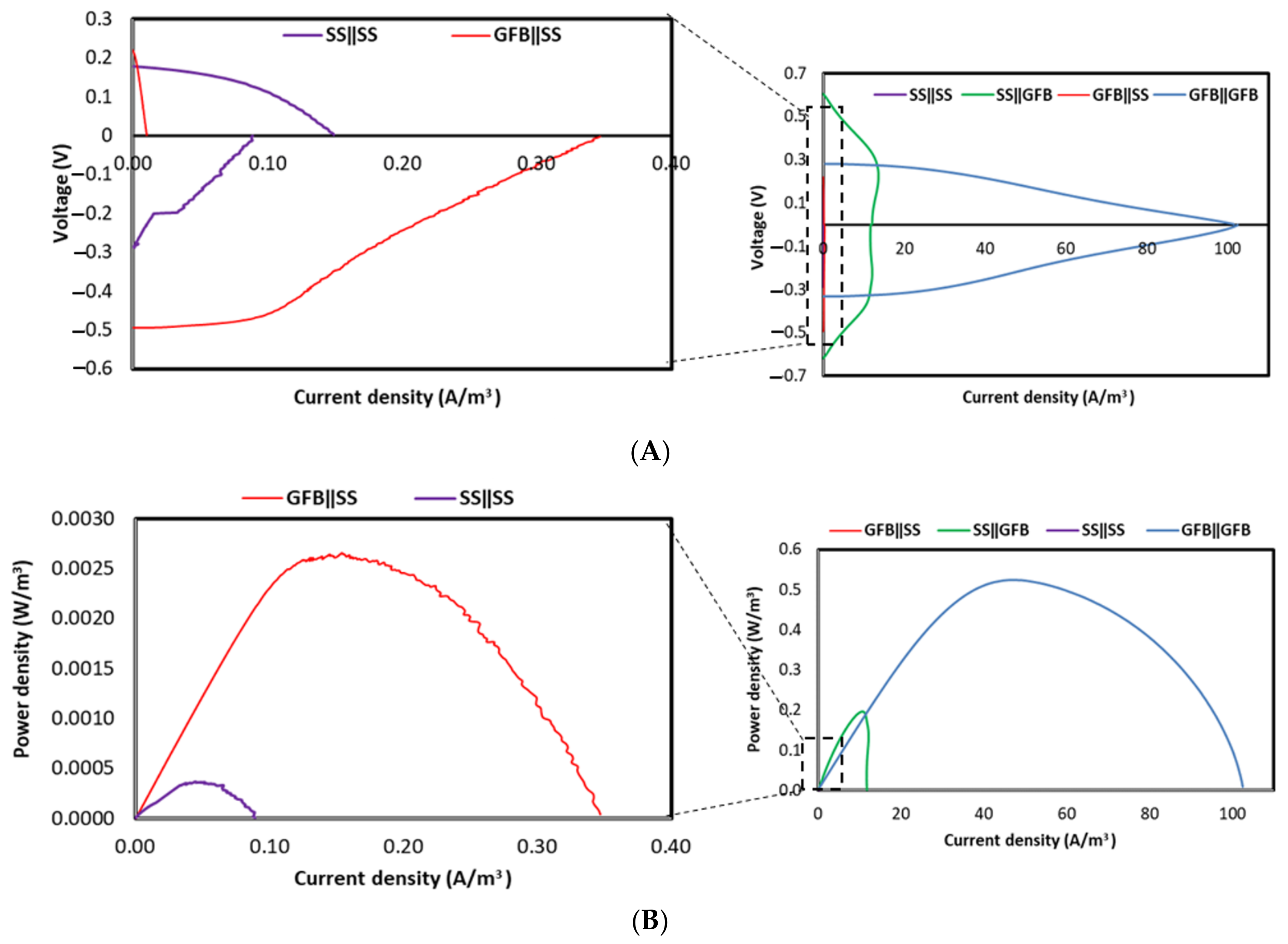
2.1.2. Biotic Cathode
2.2. Chemical Oxygen Requirements
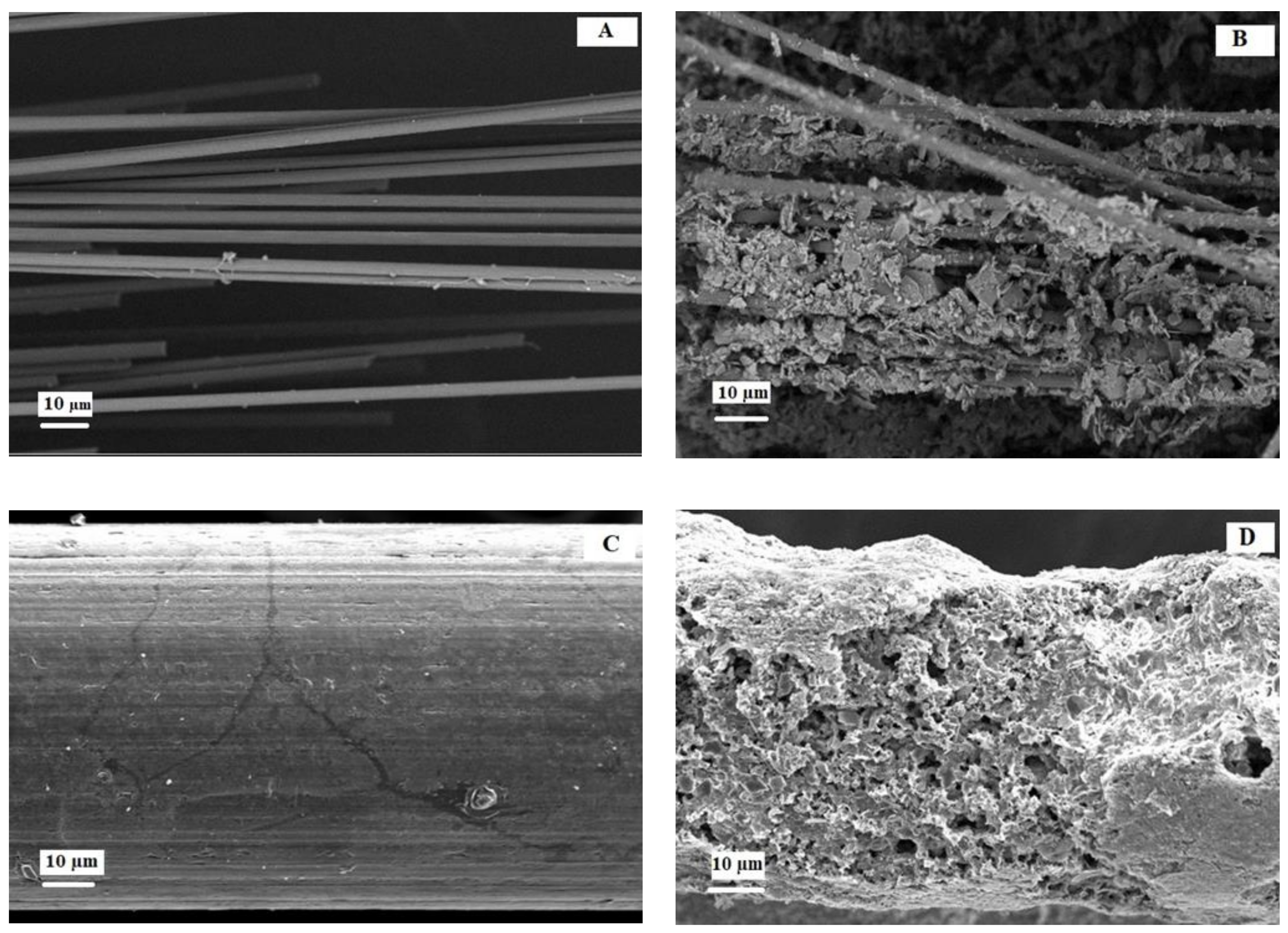
2.3. Morphology Biofilm on Electrodes
3. Discussion
3.1. Electrochemical Performance
3.2. COD Reduction
3.3. Surface Morphology
4. Materials and Methods
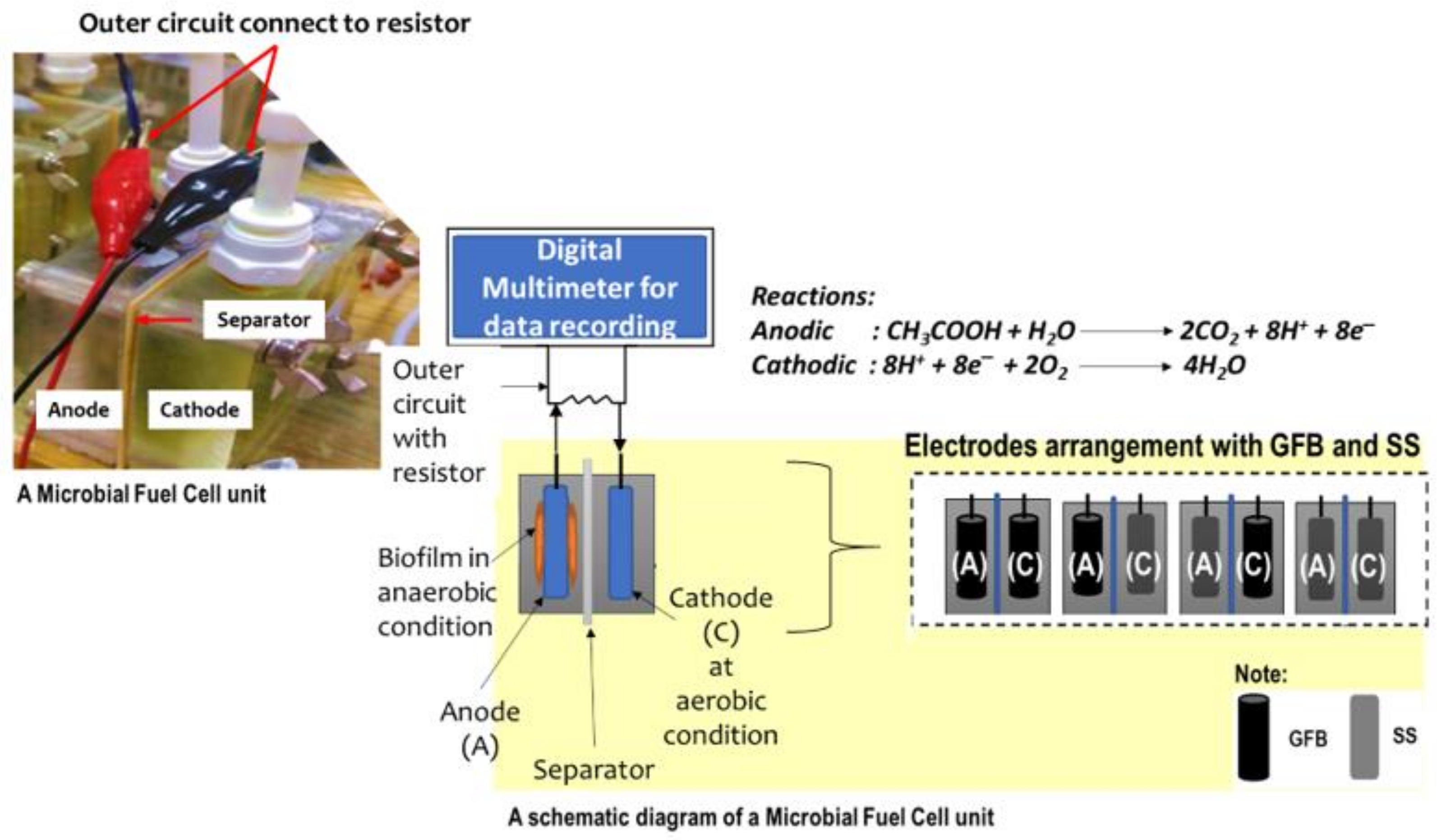
4.1. Preparation of Microbial Fuel Cell Reactors
4.2. Operation of the Microbial Fuel Cell Cathode
4.3. Data Acquisition and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Song, H.-L.; Zhu, Y.; Li, J. Electron transfer mechanisms, characteristics and applications of biological cathode microbial fuel cells—A mini review. Arab. J. Chem. 2019, 12, 2236–2243. [Google Scholar] [CrossRef]
- Daud, S.M.; Daud, W.R.W.; Kim, B.H.; Somalu, M.R.; Bakar, M.H.A.; Muchtar, A.; Jahim, J.M.; Lim, S.S.; Chang, I.S. Comparison of performance and ionic concentration gradient of two-chamber microbial fuel cell using ceramic membrane (CM) and cation exchange membrane (CEM) as separators. Electrochim. Acta 2018, 259, 365–376. [Google Scholar] [CrossRef]
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci. Total Environ. 2021, 754, 142429. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Choudhury, P.; Uday, U.S.P.; Mahata, N.; Tiwari, O.N.; Ray, R.N.; Bandyopadhyay, T.K.; Bhunia, B. Performance improvement of microbial fuel cells for waste water treatment along with value addition: A review on past achievements and recent perspectives. Renew. Sust. Energ. Rev. 2017, 79, 372–389. [Google Scholar] [CrossRef]
- Palanisamy, G.; Jung, H.-Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.-H. A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Rusli, S.F.N.; Bakar, M.H.A.; Loh, K.S.; Mastar, M.S. Review of high-performance biocathode using stainless steel and carbon-based materials in microbial fuel cell for electricity and water treatment. Int. J. Hydrog. 2019, 44, 30772–30787. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Hu, Y.; Li, S.; Xu, Q. Bio-cathode materials evaluation in microbial fuel cells: A comparison of graphite felt, carbon paper and stainless steel mesh materials. Int. J. Hydrog. 2012, 37, 16935–16942. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, H.; Zhang, C.; Zhang, G.; Yang, F.; Yuan, G.; Gao, F. Simultaneous nitrogen and carbon removal in a single chamber microbial fuel cell with a rotating biocathode. Process Biochem. 2013, 48, 893–900. [Google Scholar] [CrossRef]
- Kargi, F.; Eker, S. Electricity generation with simultaneous wastewater treatment by a microbial fuel cell (MFC) with Cu and Cu–Au electrodes. J. Chem. Technol. Biotechnol. 2007, 82, 658–662. [Google Scholar] [CrossRef]
- Rabaey, K.; Read, S.T.; Clauwaert, P.; Freguia, S.; Bond, P.L.; Blackall, L.L.; Keller, J. Cathodic oxygen reduction catalyzed by bacteria in microbial fuel cells. ISME J. 2008, 2, 519–527. [Google Scholar] [CrossRef] [PubMed]
- You, S.J.; Ren, N.Q.; Zhao, Q.L.; Wang, J.Y.; Yang, F.L. Power generation and electrochemical analysis of biocathode microbial fuel cell using graphite fibre brush as cathode material. Fuel Cells 2009, 9, 588–596. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Kim, T.G.; Cho, K.-S. Characterization of the COD removal, electricity generation, and bacterial communities in microbial fuel cells treating molasses wastewater. J. Environ. Sci. Health Part A 2016, 51, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Williams, I.; Xu, Z.; Li, B.; Li, B. Energy-positive nitrogen removal using the integrated short-cut nitrification and autotrophic denitrification microbial fuel cells (MFCs). Appl. Energy 2016, 163, 352–360. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, H.; Ma, Y.; Yuan, G.; Yang, F.; Zhang, R. Membrane filtration biocathode microbial fuel cell for nitrogen removal and electricity generation. Enzym. Microb. Technol. 2014, 60, 56–63. [Google Scholar] [CrossRef]
- Cornejo, J.A.; Lopez, C.; Babanova, S.; Santoro, C.; Artyushkova, K.; Ista, L.; Schuler, A.J.; Atanassov, P. Surface modification for enhanced biofilm formation and electron transport in Shewanella anodes. J. Electrochem. Soc. 2015, 162, H597–H603. [Google Scholar] [CrossRef][Green Version]
- Li, C.; Cheng, S. Functional group surface modifications for enhancing the formation and performance of exoelectrogenic biofilms on the anode of a bioelectrochemical system. Crit. Rev. Biotechnol. 2019, 39, 1015–1030. [Google Scholar] [CrossRef]
- Liu, D.; Zheng, T.; Buisman, C.; Heijne, A. Heat-treated stainless steel felt as a new cathode material in a methane-producing bioelectrochemical system. ACS Sustain. Chem. Eng. 2017, 5, 11346–11353. [Google Scholar] [CrossRef]
- Guo, K.; Soeriyadi, A.H.; Feng, H.; Prévoteau, A.; Patil, S.A.; Gooding, J.J.; Rabaey, K. Heat-treated stainless steel felt as scalable anode material for bioelectrochemical systems. Bioresour. Technol. 2015, 195, 46–50. [Google Scholar] [CrossRef]
- Yamashita, T.; Ishida, M.; Asakawa, S.; Kanamori, H.; Sasaki, H.; Ogino, A.; Katayose, Y.; Hatta, T.; Yokoyama, H. Enhanced electrical power generation using flame-oxidized stainless steel anode in microbial fuel cells and the anodic community structure. Biotechnol. Biofuels 2016, 9, 62. [Google Scholar] [CrossRef]
- Pocaznoi, D.; Calmet, A.; Etcheverry, L.; Erable, B.; Bergel, A. Stainless steel is a promising electrode material for anodes of microbial fuel cells. Energy Environ. Sci. 2012, 5, 9645–9652. [Google Scholar] [CrossRef]
- Santoro, C.; Guilizzoni, M.; Correa Baena, J.P.; Pasaogullari, U.; Casalegno, A.; Li, B.; Babanova, S.; Artyushkova, K.; Atanassov, P. The effects of carbon electrode surface properties on bacteria attachment and start up time of microbial fuel cells. Carbon 2014, 67, 128–139. [Google Scholar] [CrossRef]
- Mustakeem. Electrode materials for microbial fuel cells: Nanomaterial approach. Mater. Renew. Sustain. Energy 2015, 4, 22. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, Q.; Zhang, J.; Zhang, G.; Lee, D. Electricity generation from bio-treatment of sewage sludge with microbial fuel cell. Bioresour. Technol. 2009, 100, 5808–5812. [Google Scholar] [CrossRef]
- Lawson, K.; Rossi, R.; Regan, J.M.; Logan, B.E. Impact of cathodic electron acceptor on microbial fuel cell internal resistance. Bioresour. Technol. 2020, 316, 123919. [Google Scholar] [CrossRef]
- Cao, X.; Liang, P.; Song, X.; Wang, Y.; Qiu, Y.; Huang, X. Trickling filter in a biocathode microbial fuel cell for efficient wastewater treatment and energy production. Sci. China Technol. Sci. 2019, 62, 1703–1709. [Google Scholar] [CrossRef]
- Rusli, S.F.N.; Bakar, M.H.A.; Rani, S.J.A.; Shyuan, L.K.; Master, M.S. Aryl diazonium modification for improved graphite fibre brush in microbial fuel cell. Sains Malays. 2018, 47, 3017–3023. [Google Scholar] [CrossRef]
- Daud, S.M.; Bakar, M.H.A.; Daud, W.R.W.; Kim, B.H.; Jahim, M.J.; Muchtar, A.; Somalu, M.R.; Pak, H.L.; Abdul, P.M. Improvement of microbial fuel cell performance using novel kaolin earthenware membrane coated with a polybenzimidazole layer. Energy Sci. Eng. 2021, 9, 2342–2353. [Google Scholar] [CrossRef]
- Nasruddin, N.I.S.M.; Bakar, M.H.A. Mitigating membrane biofouling in biofuel cell system—A review. Open Chem. 2021, 19, 1202–1215. [Google Scholar] [CrossRef]
- Wetser, K.; Sudirjo, E.; Buisman, C.J.N.; Strik, D.P.B.T.B. Electricity generation by a plant microbial fuel cell with an integrated oxygen reducing biocathode. Appl. Energy 2015, 137, 151–157. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Huang, W.; Zhang, L. Enhanced electricity generation for biocathode microbial fuel cell by in situ microbial-induced reduction of graphene oxide and polarity reversion. Int. J. Hydrog. 2017, 42, 12574–12582. [Google Scholar] [CrossRef]
- Hou, B.; Lu, J.; Wang, H.; Li, Y.; Liu, P.; Liu, Y.; Chen, J. Performance of microbial fuel cells based on the operational parameters of biocathode during simultaneous Congo red decolorization and electricity generation. Bioelectrochemistry 2019, 128, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamun, A.; Jafary, T.; Baawain, M.S.; Rahman, S.; Choudhury, M.R.; Tabatabaei, M.; Lam, S.S. Energy recovery and carbon/nitrogen removal from sewage and contaminated groundwater in a coupled hydrolytic-acidogenic sequencing batch reactor and denitrifying biocathode microbial fuel cell. Environ. Res. 2020, 183, 109273. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Qu, Y.; Dong, Y.; Chen, D.; Liang, D.; Liu, J.; Zhang, J.; Ren, N.; Feng, Y. Simultaneous electricity generation and eutrophic water treatment utilizing iron coagulation cell with nitrification and denitrification biocathodes. Sci. Total Environ. 2021, 782, 146436. [Google Scholar] [CrossRef] [PubMed]
- Rimboud, M.; Etcheverry, L.; Barakat, M.; Achouak, W.; Bergel, A.; Délia, M.-L. Hypersaline microbial fuel cell equipped with an oxygen-reducing microbial cathode. Bioresour. Technol. 2021, 337, 125448. [Google Scholar] [CrossRef] [PubMed]
- Breheny, M.; Bowman, K.; Farahmand, N.; Gomaa, O.; Keshavarz, T.; Kyazze, G. Biocatalytic electrode improvement strategies in microbial fuel cell systems. J. Chem. Technol. Biotechnol. 2019, 94, 2081–2091. [Google Scholar] [CrossRef]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Wang, X.; Logan, B.E. Treatment of carbon fiber brush anodes for improving power generation in air–cathode microbial fuel cells. J. Power Sources 2010, 195, 1841–1844. [Google Scholar] [CrossRef]


| Anode/Cathode | Inoculum Anode/Cathode | Power (W/m2) | Power (W/m3) | Reference |
|---|---|---|---|---|
| Graphite felts | Spartina anglica/Aerobic wastewater | 0.24 | [30] | |
| Carbon cloth/Activated granular carbon | Anaerobic reactor effluent (ARE)/ARE and aerobic active sludge | 1.32 | [19] | |
| Carbon brush/Carbon cloth with Pt | Influent from wastewater treatment/Aeration tank | 0.29 | [14] | |
| Carbon felts | Activated sludge with graphene oxide | 0.065 | [31] | |
| Carbon papers | Aerobic and anaerobic sludges from municipal wastewater/Aerobic sludge | 0.22 | [32] | |
| Carbon fibre brushes | Effluent from primary sedimentation tank/Mixture of dewatered sludges: activated tank, digester tank, settling basin and nitrifying tank | 7.1 | [33] | |
| Ferum/Carbon graphite fibre brushes | Algae/Domestic wastewater | 6.6 | [34] | |
| Carbon felts | Salt marsh sediment | 0.21 | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusli, S.F.N.; Daud, S.M.; Abu Bakar, M.H.; Loh, K.S.; Masdar, M.S. Biotic Cathode of Graphite Fibre Brush for Improved Application in Microbial Fuel Cells. Molecules 2022, 27, 1045. https://doi.org/10.3390/molecules27031045
Rusli SFN, Daud SM, Abu Bakar MH, Loh KS, Masdar MS. Biotic Cathode of Graphite Fibre Brush for Improved Application in Microbial Fuel Cells. Molecules. 2022; 27(3):1045. https://doi.org/10.3390/molecules27031045
Chicago/Turabian StyleRusli, Siti Farah Nadiah, Siti Mariam Daud, Mimi Hani Abu Bakar, Kee Shyuan Loh, and Mohd Shahbudin Masdar. 2022. "Biotic Cathode of Graphite Fibre Brush for Improved Application in Microbial Fuel Cells" Molecules 27, no. 3: 1045. https://doi.org/10.3390/molecules27031045
APA StyleRusli, S. F. N., Daud, S. M., Abu Bakar, M. H., Loh, K. S., & Masdar, M. S. (2022). Biotic Cathode of Graphite Fibre Brush for Improved Application in Microbial Fuel Cells. Molecules, 27(3), 1045. https://doi.org/10.3390/molecules27031045







