An Edible Oil Enriched with Lycopene from Pink Guava (Psidium guajava L.) Using Different Mechanical Treatments
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
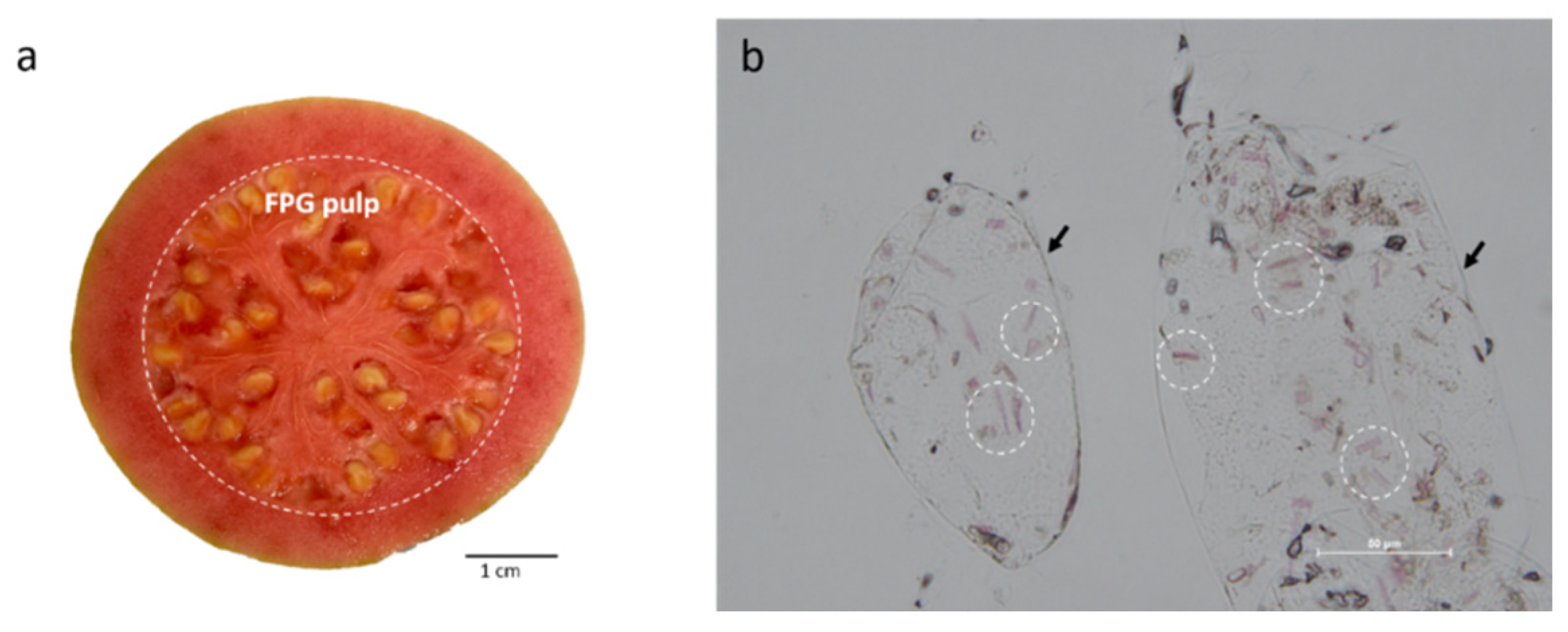
3.1. Characterization of FPG
3.1.1. Quantification of Lycopene in FPG
3.1.2. Light Microscopy of FPG
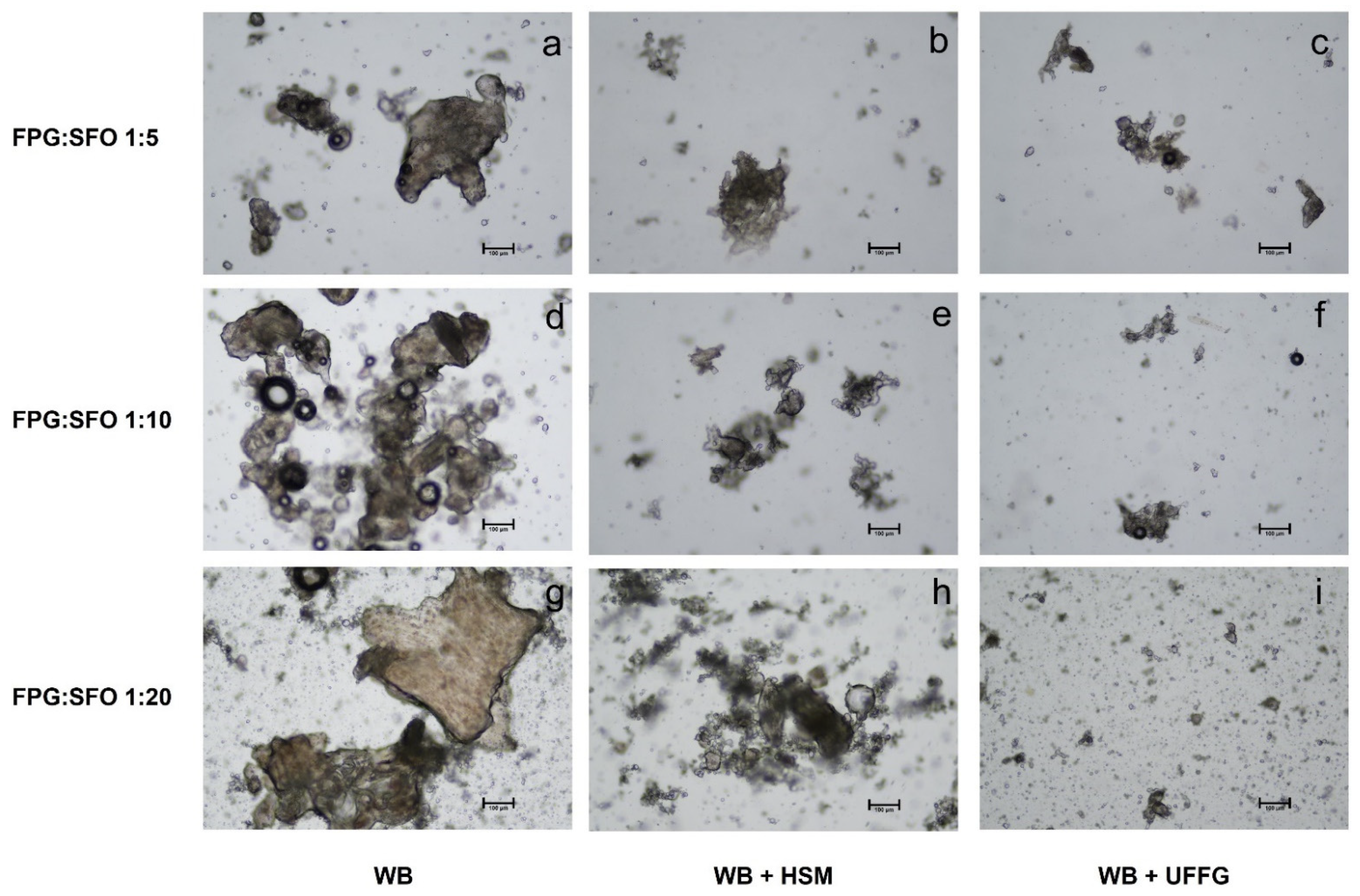
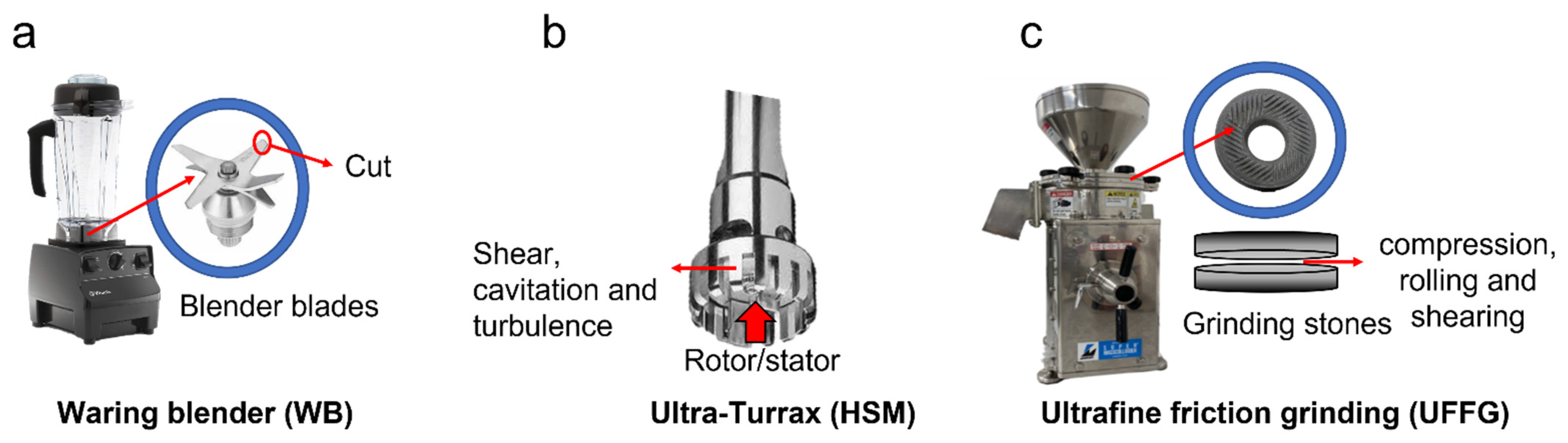
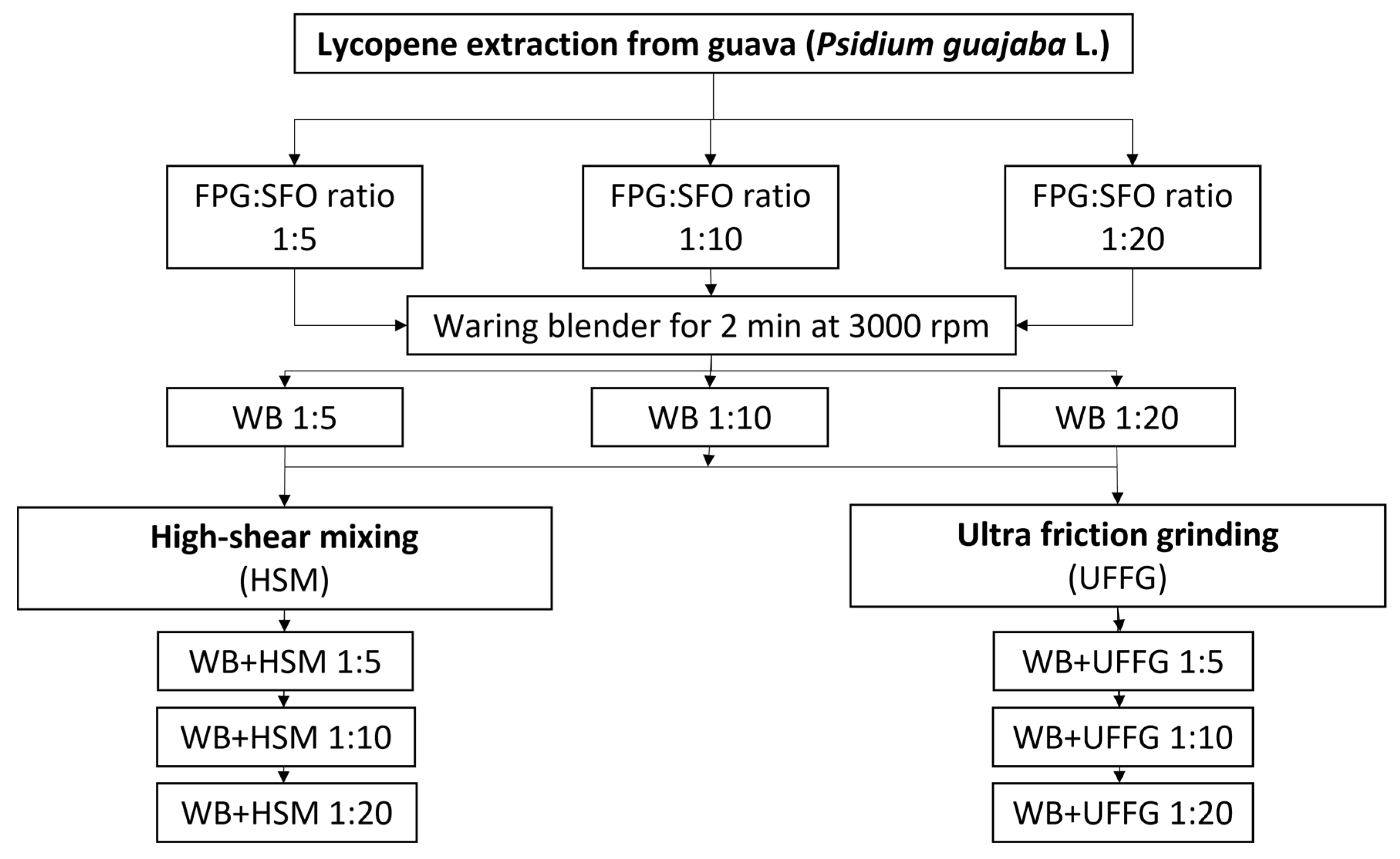
3.2. Lycopene Extraction from FPG
Extraction with SFO
3.3. Characterization of SFO Enriched with Lycopene
3.3.1. Light Microscopy of SFO Enriched with Lycopene
3.3.2. Quantification of Lycopene in SFO
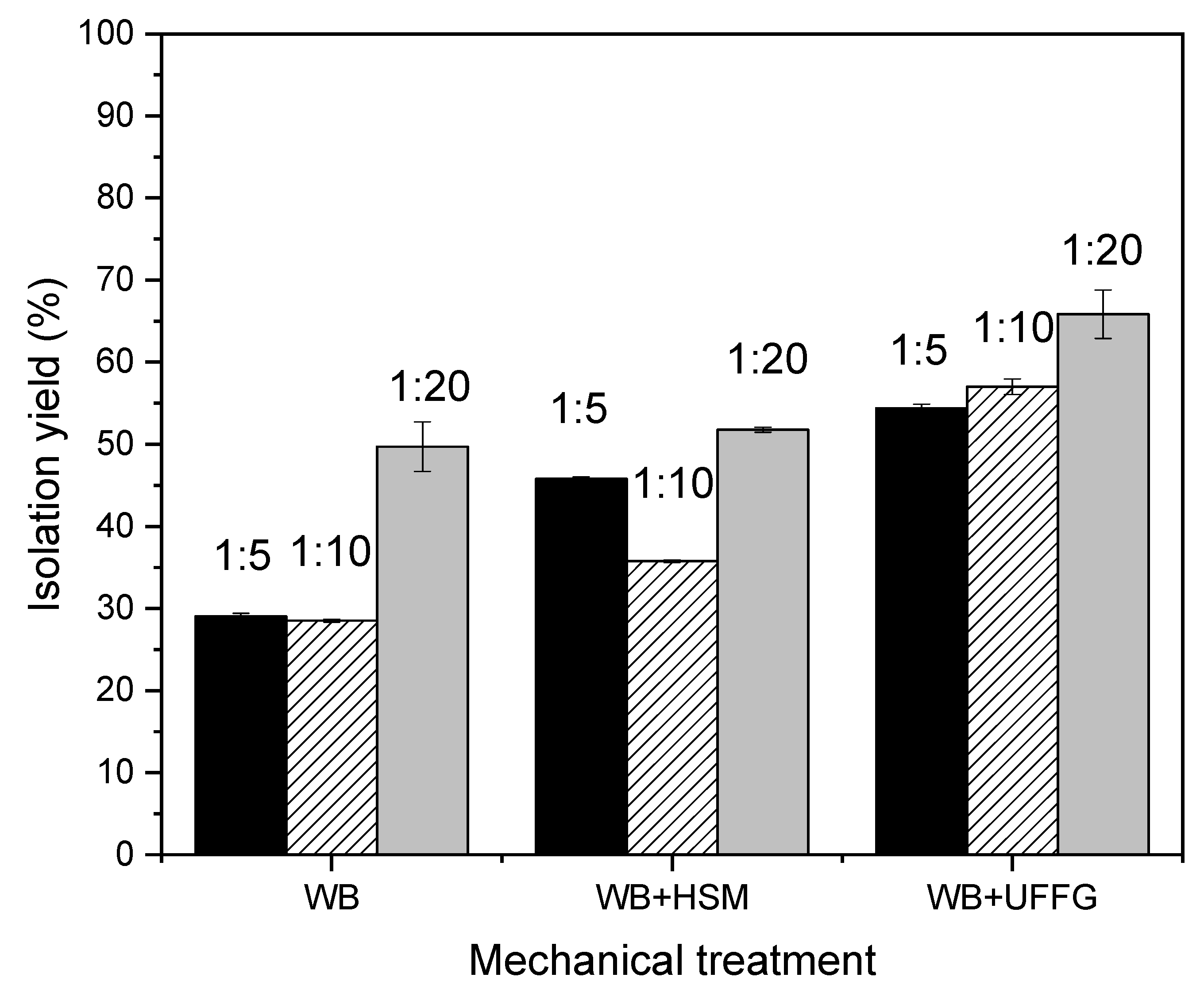
3.3.3. Determination of Lycopene Extraction Yield
3.3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cheng, J.; Miller, B.; Balbuena, E.; Eroglu, A. Lycopene Protects against Smoking-Induced Lung Cancer by Inducing Base Excision Repair. Antioxidants 2020, 9, 643. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Takeshima, M.; Nakano, S. Mechanism of the Anticancer Effect of Lycopene (Tetraterpenoids). In Enzymes; Academic Press: Waltham, MA, USA, 2015; pp. 139–166. [Google Scholar]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; Kuhnleet, G.G.; et al. Extension of use of lycopene (E 160d) to certain meat preparations, meat products and fruit and vegetable preparations. EFSA J. 2017, 15, e05064. [Google Scholar] [CrossRef] [Green Version]
- Anarjan, N. Evaluation the Effects of Ultrasonic Parameters on Simultaneously Extraction and Size Reduction of Lycopene from Tomato Processing Waste. Waste Biomass Valorization 2018, 11, 1929–1940. [Google Scholar] [CrossRef]
- Barba, A.O.; Hurtado, M.C.; Mata, M.S.; Ruiz, V.F.; de Tejada, M.L.S. Application of a UV–vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Periago, M.J.; Rincón, F.; Agüera, M.D.; Ros, G. Mixture Approach for Optimizing Lycopene Extraction from Tomato and Tomato Products. J. Agric. Food Chem. 2004, 52, 5796–5802. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, S.; Yang, X.; Hou, F.; Fan, L.; Wang, W.; Xu, E.; Cheng, H.; Guo, M.; Liu, D. Evaluation of extraction technologies of lycopene: Hindrance of extraction, effects on isomerization and comparative analysis—A review. Trends Food Sci. Technol. 2021, 115, 285–296. [Google Scholar] [CrossRef]
- FDA. Q3C—Tables and List Guidance for Industry. 2017. Available online: https://www.fda.gov/media/71737/download (accessed on 3 October 2021).
- Amiri-Rigi, A.; Abbasi, S. Extraction of lycopene using a lecithin-based olive oil microemulsion. Food Chem. 2019, 272, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Kehili, M.; Sayadi, S.; Frikha, F.; Zammel, A.; Allouche, N. Optimization of lycopene extraction from tomato peels industrial by-product using maceration in refined olive oil. Food Bioprod. Process. 2019, 117, 321–328. [Google Scholar] [CrossRef]
- Rahimi, S.; Mikani, M. Lycopene green ultrasound-assisted extraction using edible oil accompany with response surface methodology (RSM) optimization performance: Application in tomato processing wastes. Microchem. J. 2019, 146, 1033–1042. [Google Scholar] [CrossRef]
- Kunthakudee, N.; Sunsandee, N.; Chutvirasakul, B.; Ramakul, P. Extraction of lycopene from tomato with environmentally benign solvents: Box-Behnken design and optimization. Chem. Eng. Commun. 2019, 207, 574–583. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Qian, C.; Martin-Belloso, O.; McClements, D. Modulating β-carotene bioaccessibility by controlling oil composition and concentration in edible nanoemulsions. Food Chem. 2013, 139, 878–884. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on β-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef]
- Pilorgé, E. Sunflower in the global vegetable oil system: Situation, specificities and perspectives. OCL 2020, 27, 34. [Google Scholar] [CrossRef]
- Jurić, S.; Jurić, M.; Ferrari, G.; Jambrak, A.R.; Donsì, F. Lycopene-rich cream obtained via high-pressure homogenisation of tomato processing residues in a water–oil mixture. Int. J. Food Sci. Technol. 2021, 56, 4907–4914. [Google Scholar] [CrossRef]
- Jurić, S.; Ferrari, G.; Velikov, K.P.; Donsì, F. High-pressure homogenization treatment to recover bioactive compounds from tomato peels. J. Food Eng. 2019, 262, 170–180. [Google Scholar] [CrossRef]
- Mert, B. Using high pressure microfluidization to improve physical properties and lycopene content of ketchup type products. J. Food Eng. 2012, 109, 579–587. [Google Scholar] [CrossRef]
- Zuluaga, R.; Putaux, J.L.; Cruz, J.; Vélez, J.; Mondragon, I.; Gañán, P. Cellulose microfibrils from banana rachis: Effect of alkaline treatments on structural and morphological features. Carbohydr. Polym. 2009, 76, 51–59. [Google Scholar] [CrossRef]
- Guerra, A.M.S.; Hoyos, C.G.; Velásquez-Cock, J.A.; Penagos, L.V.; Rojo, P.G.; Acosta, L.V.; Pereira, M.A.; Zuluaga, R. Effect of ultra-fine friction grinding on the physical and chemical properties of curcuma (Curcuma longa L.) suspensions. J. Food Sci. 2019, 85, 132–142. [Google Scholar] [CrossRef]
- Guerra, A.S.; Hoyos, C.G.; Molina-Ramírez, C.; Velásquez-Cock, J.; Vélez, L.; Gañán, P.; Eceiza, A.; Goff, H.D.; Zuluaga, R. Extraction and preservation of lycopene: A review of the advancements offered by the value chain of nanotechnology. Trends Food Sci. Technol. 2021, 116, 1120–1140. [Google Scholar] [CrossRef]
- Gómez Hoyos, C.; Mazo Márquez, P.; Penagos Vélez, L.; Serpa Guerra, A.; Eceiza, A.; Urbina, L.; Velásquez-Cock, J.; Gañán Rojo, P.; Vélez Acosta, L.; Zuluaga, R. Cocoa shell: An industrial by-product for the preparation of suspensions of holocellulose nanofibers and fat. Cellulose 2020, 27, 10873–10884. [Google Scholar] [CrossRef]
- Velásquez-Cock, J.; Gañán, P.; Posada, P.; Castro, C.; Serpa, A.; Gómez, H.C.; Putaux, J.-L.; Zuluaga, R. Influence of combined mechanical treatments on the morphology and structure of cellulose nanofibrils: Thermal and mechanical properties of the resulting films. Ind. Crop. Prod. 2016, 85, 1–10. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Gleichenhagen, M.; Heller, A.; Esquivel, P.; Schulze-Kaysers, N.; Schieber, A. Carotenoid Profile, Antioxidant Capacity, and Chromoplasts of Pink Guava (Psidium guajava L. Cv. ‘Criolla’) during Fruit Ripening. J. Agric. Food Chem. 2017, 65, 3737–3747. [Google Scholar] [CrossRef]
- Campoli, S.S.; Rojas, M.; Amaral, J.E.P.G.D.; Canniatti-Brazaca, S.G.; Augusto, P.E.D. Ultrasound processing of guava juice: Effect on structure, physical properties and lycopene in vitro accessibility. Food Chem. 2018, 268, 594–601. [Google Scholar] [CrossRef]
- Anese, M.; Mirolo, G.; Beraldo, P.; Lippe, G. Effect of ultrasound treatments of tomato pulp on microstructure and lycopene in vitro bioaccessibility. Food Chem. 2013, 136, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Pan, S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf.). Ultrason. Sonochemistry 2013, 20, 1026–1032. [Google Scholar] [CrossRef]
- Trujillo-Cayado, L.A.; Alfaro, M.C.; García, M.; Muñoz, J. Comparison of homogenization processes for the development of green O/W emulsions formulated with N,N-dimethyldecanamide. J. Ind. Eng. Chem. 2017, 46, 54–61. [Google Scholar] [CrossRef]
- Kang, T.; Paulapuro, H. New mechanical treatment for chemical pulp. J. Process Mech. Eng. 2006, 220, 161–166. [Google Scholar] [CrossRef]
- Kang, T.; Paulapuro, H. Recycle Potential of Externally Fibrillated Chemical Pulp. Prog. Pap. Recycl. 2006, 15, 11–17. [Google Scholar]
- Uetani, K.; Yano, H. Nanofibrillation of Wood Pulp Using a High-Speed Blender. Biomacromolecules 2011, 12, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Sadler, G.; Davis, J.; Dezman, D. Rapid Extraction of Lycopene and?—Carotene from Reconstituted Tomato Paste and Pink Grapefruit Homogenates. J. Food Sci. 1990, 55, 1460–1461. [Google Scholar] [CrossRef]
- Marković, K.; Hruškar, M.; Vahčić, N. Lycopene content of tomato products and their contribution to the lycopene intake of Croatians. Nutr. Res. 2006, 26, 556–560. [Google Scholar] [CrossRef]
- Villaseñor-Aguilar, M.-J.; Padilla-Medina, J.-A.; Botello-Álvarez, J.-E.; Bravo-Sánchez, M.-G.; Prado-Olivares, J.; Espinosa-Calderon, A.; Barranco-Gutiérrez, A.-I. Current Status of Optical Systems for Measuring Lycopene Content in Fruits: Review. Appl. Sci. 2021, 11, 9332. [Google Scholar] [CrossRef]
- Hammud, H.H.; Bouhadir, K.H.; Masoud, M.S.; Ghannoum, A.M.; Assi, S.A. Solvent Effect on the Absorption and Fluorescence Emission Spectra of Some Purine Derivatives: Spectrofluorometric Quantitative Studies. J. Solut. Chem. 2008, 37, 895–917. [Google Scholar] [CrossRef]
- Orozco, F.D.A.; Sousa, A.C.; Araujo, M.C.U.; Domini, C.E. A new flow UV–Vis kinetics spectrophotometric method based on a photodegradative reaction for determining the oxidative stability of biodiesel. Fuel 2020, 262, 116197. [Google Scholar] [CrossRef]
- Lopez-Sanchez, P.; Nijsse, J.; Blonk, H.C.G.; Bialek, L.; Schumm, S.; Langton, M. Effect of mechanical and thermal treatments on the microstructure and rheological properties of carrot, broccoli and tomato dispersions. J. Sci. Food Agric. 2011, 91, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemist. Basic calculations for chemical and biological analyses. In Official Methods of Analysis; AOAC: Arlington, TX, USA, 1996. [Google Scholar]
- Brandt, S.; Pék, Z.; Barna, É.; Lugasi, A.; Helyes, L. Lycopene content and colour of ripening tomatoes as affected by environmental conditions. J. Sci. Food Agric. 2006, 86, 568–572. [Google Scholar] [CrossRef]
- Nagarajan, J.; Krishnamurthy, N.P.; Ramanan, R.N.; Raghunandan, M.E.; Galanakis, C.; Ooi, C.W. A facile water-induced complexation of lycopene and pectin from pink guava byproduct: Extraction, characterization and kinetic studies. Food Chem. 2019, 296, 47–55. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]


| SFO:FPG Ratio | Dmean (μm) | D10 (μm) | D50 (μm) | D90 (μm) |
| WB | ||||
| 1:5 | 55.80 ± 1.96 A,a | 16.12 ± 1.43 A,a | 28.15 ± 0.76 A,a | 71.015 ± 6.49 A,a |
| 1:10 | 46.68 ± 16.51 A,a | 13.713 ± 0.72 A,a | 28,72 ± 4.95 A,a | 96.35 ± 23.53 A,a |
| 1:20 | 84.55 ± 35.03 A,a | 14.51 ± 1.08 A,a | 29.20 ± 5.15 A.a | 92.46 ± 7.69 A,a |
| WB + HSM | ||||
| 1:5 | 48.68 ± 2.68 A,a | 14.82 ± 0.84 A,a | 29.91 ± 6.16 A,a | 81.20 ± 13.40 A,a |
| 1:10 | 61.79 ± 0.83 A,b | 16.78 ± 4.46 A,a | 33.87 ± 7.24 A,a | 134.53 ± 44.12 A,a |
| 1:20 | 66.63 ± 1.75 A,b | 15.34 ± 0.19 A,a | 29.75 ± 0.78 A,a | 138.43 ± 15.12 B,a |
| WB + UFFG | ||||
| 1:5 | 78.35 ± 1.02 B,a | 17.03 ± 1.94 A,a | 34.24 ± 2.87 A,a | 191.65 ± 0.87 B,a |
| 1:10 | 41,85 ± 6.24 A,b | 14.48 ± 0.87 A,ab | 24.67 ± 2.10 A,b | 82.94 ± 7.28 A,b |
| 1:20 | 23.35 ± 0.65 B,c | 10.61 ± 0.13 B,b | 17.68 ± 1.15 B,c | 40.29 ± 6.54 C,c |
| SFO:FPG Ratio | Mechanical Treatment | ||
|---|---|---|---|
| WB | WB + HSM | WB + UFFG | |
| (mg lycopene/g FPG) | |||
| 1:5 | 8.03 ± 0.23 A,a | 7.89 ± 0.11 B,a | 13.75 ± 1.88 C,a |
| 1:10 | 12.67 ± 0.12 A,b | 9.89 ± 0.10 B,b | 14.32 ± 0.19 C,b |
| 1:20 | 15.05 ± 0.30 A,b | 15.77 ± 0.59 A,c | 18.22 ± 1.8 B,c |
| Method Description | Lycopene Source | Extraction Solvent | Lycopene Concentration (mg/g) | Reference |
|---|---|---|---|---|
| WB + UFFG | FPG | SFO | 18.21 ± 1.83 mg/g FPG | Current work |
| High-pressure homogenization water assisted | Fresh tomato residues | Water and ethyl lactate | 4.00 mg/g tomato peel | [18] |
| High-pressure homogenization oil–water emulsion assisted | Fresh tomato | Water, SFO and acetone | 3.94 ± 0.12 mg/g cream (oily phase) | [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoyos, C.G.; Guerra, A.S.; Pérez, S.A.; Velásquez-Cock, J.; Villegas, M.; Gañán, P.; Gallego, R.Z. An Edible Oil Enriched with Lycopene from Pink Guava (Psidium guajava L.) Using Different Mechanical Treatments. Molecules 2022, 27, 1038. https://doi.org/10.3390/molecules27031038
Hoyos CG, Guerra AS, Pérez SA, Velásquez-Cock J, Villegas M, Gañán P, Gallego RZ. An Edible Oil Enriched with Lycopene from Pink Guava (Psidium guajava L.) Using Different Mechanical Treatments. Molecules. 2022; 27(3):1038. https://doi.org/10.3390/molecules27031038
Chicago/Turabian StyleHoyos, Catalina Gómez, Angélica Serpa Guerra, Shaydier Argel Pérez, Jorge Velásquez-Cock, Mariana Villegas, Piedad Gañán, and Robin Zuluaga Gallego. 2022. "An Edible Oil Enriched with Lycopene from Pink Guava (Psidium guajava L.) Using Different Mechanical Treatments" Molecules 27, no. 3: 1038. https://doi.org/10.3390/molecules27031038
APA StyleHoyos, C. G., Guerra, A. S., Pérez, S. A., Velásquez-Cock, J., Villegas, M., Gañán, P., & Gallego, R. Z. (2022). An Edible Oil Enriched with Lycopene from Pink Guava (Psidium guajava L.) Using Different Mechanical Treatments. Molecules, 27(3), 1038. https://doi.org/10.3390/molecules27031038








