Abstract
Medicinal plants and herbal preparations are gaining attention in the scientific community today, as they are often used intermittently in the treatment of various diseases. The genus of Polygonum (Polygonaceae), known locally as “madimak”, is an aromatic plant widely used in world flavors. The chemical composition of the essential oils of dried aerial parts of seven of Polygonum was analyzed by GC-MS. These species are Polygonum lapathifolium L., Polygonum persicaria L., Polygonum arenastrum Bor., Polygonum bellardii All., Polygonum arenarium Waldst. Et Kit., Polygonum aviculare L., and Polygonum cognatum Meissn. Qualitative and quantitative differences were found in the essential oil analysis of the seven Polygonum species. The major compounds were determined as (E)-β-farnesene (19. 46%), dodecanal (15.92%), β-caryophyllene (12.95%), in P. aviculare; (E)-β-farnesene (25.00%), dodecanal (20.45%), β-caryophyllene (9.38%), and caryophyllene oxide (8. 26%) in P. persicaria; dodecanal (25.65%), caryophyllene oxide (13.35%), β-caryophyllene (7.95%), and (E)-β-farnesene (6.20%) in P. lapathifolium, and dodecanal (19.65%), (E)-β-farnesene (13.86%), β-caryophyllene (8.06%), and α-terpineol (7.2%) in P. arenarium, dodecanal (16.23%), β-caryophyllene (16.09%), (E)-β-farnesene (12.26%), caryophyllene oxide (7.94%) in P. bellardii, (E)-β-farnesene (20.75%), dodecanal (17.96%), β-caryophyllene (13.01%), α-terpineol (4.97%) in P. arenastrum, (E)-β-farnesene (9.49%), dodecanal (14.01%), β-caryophyllene (11.92%), geranyl acetate (9.49%), and undecanal (7.35%) in P. cognatum. This study is the most comprehensive study conducted to determine the essential oil components of Polygonum species. In addition, a literature review on the composition of the essential oils of these Polygonum taxa was performed. The essential oil components of the species in our study were revealed for the first time with this study.
1. Introduction
The genus Polygonum belonging to the Polygonaceae family includes more than 300 species spread over North and South America, Asia, North Africa, and Europe [1]. They are also widely grown on roadsides, slopes, and cultivated fields [2]. It has been reported that the leaves and young shoots are used to treat various diseases such as abdominal pain, anemia, and diabetes [3,4].
The P. lapathifolium L., P. persicaria L., P. arenastrum Bor., P. bellardii All., P. arenarium Waldst. Et Kit., P. aviculare L., and P. cognatum Meissn. species were the subject of this study. These species were compared morphologically, and interspecies variations emerged [5,6,7]. Therefore, this study aimed to contribute to the literature in terms of evaluating the essential oil content of the Polygonum species, which are widely grown in Turkey, which is rich in endemic species, to reveal alternative sources and to provide data for new biological activity research.
Edible wild plants of different species have played an important role throughout human history. In many parts of the world, Polygonum species are frequently consumed by local people as food, mostly fresh in foods, such as salads and meals [8]. It has been proven by several studies that the Polygonum species have antimicrobial, antioxidant, antifungal, diuretic, insecticide, antidiabetic, antiulcer, and anticancer activities due to the phenolic components, tannins, polyuronides, flavonoid triterpenes, glycosides, and saponins it contains [9,10,11,12,13]. According to a recent study, it was determined that the Polygonum afghanicum and Polygonum aviculare are used as animal feed [14]. It is thought that the Polygonum species, which is consumed as food by people, has a gastroprotective effect through its reducing power and phenolic content [9]. The clinical effects of the Polygonum aviculare on gingivitis were investigated, and it was concluded that the plant extracts used with the mouth rinse method could be used as a supplement in treating gingivitis [15].
P. cognatum, which is cooked and used in food, and P. aviculare are the preferred species among the public [16]. In addition, in meadow and pasture areas, P. arenastrum Bor., P. maritimum L., P. pulchellum Lois., P. convolvulus L., and P. dumetorum L. species are especially common. It was stated that the P. lapathifolium and P. orientale species of the Polygonum genus increase the sensitivity to light in cattle [17,18]. The antioxidant activity, total phenolic compounds, chlorophyll and carotenoids, flavonoids, and chlorophyll and carotenoids content of the leaf’s extracts of Polygonum cognatum Meissn. were investigated. It was further determined that water extracts had high antioxidant activity [11]. It was stated that phenolic compounds, which are also found in high amounts in malt, have allelopathic effects [19,20] and antioxidant and antimicrobial activities [11].
In recent years, people have added wild edible plant species to their food lists due to their medicinal importance and aromatic properties. The fact that the nutritional content of consumed wild vegetables is higher than that of many cultivated vegetables contributed to the increase [8,21]. Many traditional botanical medicines contain bioactive components in their essential oils. Thus, the characterization of herbal essential oils is essential [22,23]. For example, the Malaysian government has listed P. minus in its National Agri-Food Policy to ensure adequate supply and to strengthen the agricultural economy. P. minus has been recognized by the Malaysian government as an essential oil-producing crop in the Herbal Product Scheme [24].
Among Polygonum species, the roots of the P. cuspidatum are frequently used as a source of resveratrol. Most resveratrol capsules sold as food supplements in the United States contain P. cuspidatum extracts, while the rest contain red wine or red grape extracts. China is one of the leading countries that obtain trans-resveratrol from P. cuspidatum root extracts. Many Chinese companies obtain trans-resveratrol with varying degrees of purity [25]. In a reviewed study, 2.18% trans-resveratrol and 1.07% trans-piseit were detected in P. cuspidatum roots using high-speed counter-current chromatography (HSCCC) [26]. In another study, different parts of the P. cuspidatum plant were evaluated for resveratrol content by using high-performance liquid chromatography. It was observed that the resveratrol content of its perennial root, leaf, stem, and annual root was quite high [27].
The essential oil composition of seven species belonging to the Polygonum genus, which is popularly consumed among the public in many parts of the world, was determined to provide basic data for bioactivity studies. As part of the author’s ongoing research on plants and to improve knowledge on the genus Polygonum, this study aimed to investigate the chemical composition of seven unanalyzed taxa of Polygonum. The essential oils of these Polygonum species were analyzed for the first time in this study. This study represents the first time that such a comprehensive essential oil study has been carried out on Polygonum species.
2. Results
In the essential oil analysis of the seven Polygonum species, qualitative and quantitative differences were found. In the essential oils of P. aviculare L. and P. cognatum Meissn. 33 components were identified representing 88.19% and 98.87% of the oils, respectively. The aerial part of P. aviculare and P. cognatum were hydrodistilled, obtaining yields of 0.9% and 0.8 (v/w) of light yellowish oils, respectively. The aerial parts of the P. lapathifolium L. and P. bellardii All. were hydrodistilled, obtaining yields of 0.9% and 0.8% (v/w) of light yellowish oils, respectively. In the essential oils of this species, 31 and 32 components were identified representing 91.8% and 95.72% of the oils, respectively. P. arenarium has 34 components (0.6 v/w). In the essential oils of P. persicaria L. 29 components and P. arenastrum were identified by 31 components. Additionally, this species was representing 85.30% and 90.02% of the oils, respectively. The aerial parts of the P. persicaria L. obtained yields of 0.8% of yellowish oils and the aerial parts of the P. arenastrum obtained yields of 0.9% (v/w) of yellowish oils.
The major compounds were (E)-β-farnesene (19.46%), dodecanal (15.92%), and β-caryophyllene (12.95%), in P. aviculare; (E)-β-farnesene (25.00%), dodecanal (20.45%), β-caryophyllene (9.38%) and caryophyllene oxide (8.26%) in P. persicaria; dodecanal (25.65%), caryophyllene oxide (13.35%), β-caryophyllene (7.95%) and (E)-β-farnesene (6.20%) in P. lapathifolium; dodecanal (19.65%), (E)-β-farnesene (13.86%), β-caryophyllene (8.06%) and α-terpinene (7.01%) in P. arenarium; dodecanal (16.23%), β-caryophyllene (16.09%), (E)-β-farnesene (12.26%), caryophyllene oxide (7.94%) in P. bellardii; (E)-β-farnesene (20.75%), dodecanal (17.96%), β-caryophyllene (13.01%), α-terpineol (4.97%) in P. arenastrum, (E)-β-farnesene (9.49%), dodecanal (14.01%), β-caryophyllene (11.92%), geranyl acetate (9.49%), 2-methyl-4-pentenal (9.49%), and undecanal (7.35%) in P. cognatum. The compositions of seven of the Polygonum essential oils are listed in Table 1.

Table 1.
Essential oils chemical composition of seven the Polygonum species.
3. Discussion
The result of this study is important regarding the usability of dodecanal and (E)-β-farnesene, which are the major components of the Polygonum species. Ullah et al. reported that the compound dodecanal (43.29%) was found to be the major component of the P. minus essential oil followed by 1-decanol (15.13%), isobornyl acetate (15.13%), n-decanoic acid (5.72%) [38]. In the present study, dodecanal was found in P. aviculare (15.92%), P. persicaria (20.45%), P. lapathifolium (25.65%), P. arenarium (19.65%), P. bellardii (16.23%), P. arenastrum (17.96%), and P. cognatum (14.01%), respectively.
Dodecanal, which was a major component in the seven species in this study, is a tyrosinase inhibitor. Tyrosinase is the enzyme involved in the formation of melanin [39]. The increase in tyrosinase activity in cancerous cells in some cancer types has drawn attention to the importance of the use of tyrosinase in the treatment of these types of cancer. Tyrosinase is a key enzyme in the food industry in maintaining the economic value of fruits and vegetables. It is responsible for the enzymatic browning of vegetables and fruits. In this context, strong tyrosinase inhibitors are needed in agriculture and food [40,41,42]. These results present new opportunities for effective tyrosinase inhibitors. On the other hand, (E)-β-farnesene, which has an important place among the major components of this study, is a group of sesquiterpene isomers found in essential oils. It was reported that this compound was used by aphids to warn other individuals and to repel insects [43,44]. The determination of high levels of (E)-β-farnesene in the essential oil content of the studied Polygonum species we studied provided basic data for future studies in the field of bioactivity research.
β-caryophyllene is a bicyclic sesquiterpene that is abundantly detected in the essential oils of many plants such as cloves, thyme, cinnamon, rosemary, and Copaiba oil [45,46,47]. It was shown to have antioxidant, anti-inflammatory, and anti-cancer properties. A former study revealed that β-caryophyllene has remedial eventuality as a hepatoprotective effect in fibrosis [48]. In another study, β-caryophyllene was shown to have protective effects against liver failure in mice [49].
Moreover, β-caryophyllene also effectively treated cisplatin-induced nephrotoxicity and decreased the pro-inflammatory cytokine expression in rats [50]. Interestingly, the P. minus essential oil was set up to have a high attention of β-caryophyllene [51]. Previous studies have shown that essential oil has hepato-protective properties [52,53]. P. minus contains various medicinal values and yields high levels of essential oils, mainly composed of terpenoids, organic acids, and aliphatic aldehydes [22]. A study by Rashid et al. showed that essential oils of the P. minus species could protect against cisplatin-induced hepatotoxicity through the inhibition of oxidative stress, inflammation, and apoptosis. However, the P. minus essential oils used in higher concentration showed pro-oxidant, pro-inflammatory, and pro-apoptotic effects. Therefore, it has been recommended not to use higher doses to avoid any harmful effects [54]. β-caryophyllene was detected in the studied Polygonum species at the rates of “P. aviculare (12.95%), P. persicaria (9.38%), P. lapathifolium (7.95%), P. arenarium (8.06%), P. bellardii (16.09%), P. arenastrum (13.01%), and P. cognatum (11.92%)”.
The components of the essential oils from the sprouts of the Polygonum hydropiper were analyzed using capillary GC and GC–MS. Fifty-three components were identified, representing 91.6% of the total oils. The main constituents of the essential oils were (E)-β-farnesene (44.1%), phytol (10.8%), (E)-caryophyllene (9.3%), and (E)-nerolidol (6.9%). The essential oil from P. hydropiper contained a high content of sesquiterpenoids [55]. The study’s findings showed that (E)-β-farnesene percentages were as follows, 19.46% for P. aviculare, 25.00% for P. persicaria, 6.20% for P. lapathifolium, 13.86% for P. arenarium, 12.26% for P. bellardii, 20.75% for P. arenastrum, and 9.49% for P. cognatum. The fact that the main components of the Polygonum species collected from Turkey, which were used in this study, showed similar results to the main components of the P. hydropiper species collected from Japan is important for the results of the present study.
Yao et al. [56] identified that the major constituents were phellandrene (13.6%), 1-methyl-4-isopropenylbenzene (7.2%), zingiberene (4.9%), and α-thujene (4.5%) while Dung et al. [57] reported that the major components were α-humulene (7.0%, 7.1%), curcumene (2.5%, 2.5%), and α-zingiberene (2.4%, 2.2%) in the stem and leaf oils from Polygonum hydropiper. In this study, the major components in all Polygonum species were (E)-β-farnesene, dodecanal, β-caryophyllene, and caryophyllene oxide.
Another study reported that the analysis of oil extracted from the leaves of the Polygonum hydropiper revealed the presence of the following six constituents: acetic acid (36.03%), propanoic acid, ethyl ester (18.21%), n-propyl acetate (20.67%), and confertifolin (22.91%) [58]. In the study of Kima et al., the main volatile components obtained from Polygonum cuspidatum oil as a result of GS-MS were 2-hexenal (73.36%), 3-hexen-1-ol (6.97%), n-hexanal (2.81%), and 1-penten-3-ol (2.55%) [59]. In another study, a systematic relationship was found when comparing lignans isolated from the P. cuspidatum and P. aviculare species [60]. When the results of these studies were compared with the present study, the major components were different due to the differences in the methods studied [61].
In a study made from the aboveground and underground parts of Polygonum species, it was determined that Polygonum extracts (P. aviculare, P. cognatum, P. patulum, and P. setosum) have an inhibitory effect against cosmetic elastase and collagenase enzymes, and that catechin and quercetin glycosides, which are found at higher rates in extracts prepared from the roots of plants, may be the compounds responsible for the effect [62]. In light of this information, within the scope of this study, it is predicted that Polygonum essential oils are valuable, and they can show high activity in bioactivity studies. With this preliminary study, it is seen that the Polygonum species are promising by increasing efforts to use them both as cosmetics and as preparations.
This study’s results indicate that the seven studied Polygonum species, based on two major essential oil constituents, can be classified into the following three different chemotypes in the eastern Anatolian region of Turkey: dodecanal/caryophyllene oxide chemotype (P. lapathifolium); dodecanal/β-caryophyllene chemotype (P. bellardi and P. cognatum) and dodecanal/(E)-β-farnesene chemotype (P. aviculare, P. arenastrum, P. Arenarium, and P. persicaria).
In the cluster analysis based on major components (≥1%), the outermost species was P. lapathifolium (Figure 1). Among these major components, the amounts of dodecanal and caryophyllene were the highest and the amounts of (E)-β-farnesene and β-caryophyllene were the lowest compared to the other investigated species.
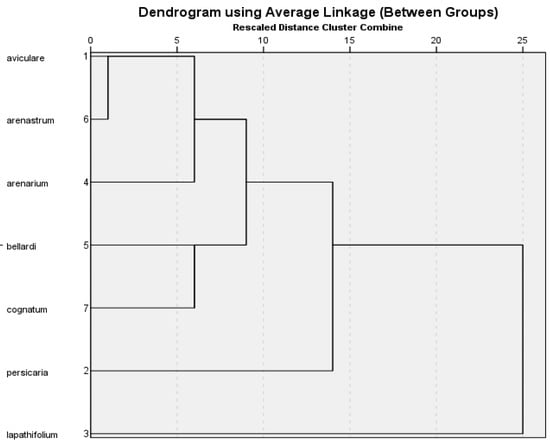
Figure 1.
Clustering analysis of seven Polygonum species to essential oil components.
P. persicaria was close to P. lapathifolium and distant to the other five species in terms of their levels of caryophyllene oxide. In addition, the P. aviculare and P. arenastrum species were the closest species in terms of chemical characteristics, they were similar, and they were included in the same clade. When the groupings were examined, it was determined that some species (P. lapathifolium, P. persicaria, P. arenastrum, P. bellardii, and P. arenarium) belonging to the Polygonum genus were compatible with the morphological description [5].
Multivariate analysis was used based on previously reported studies [38]. Principle component analysis (PCA) and cluster analysis (CA) were performed to identify the compounds for the different samples. The PCA was then performed with Varimax rotation using the matrix correlation configuration. The main components of the principal component analysis were PC1 with 38.19% and with PC2 16.16%, respectively. The total load of PC1 and PC2 was 54.35%. The Kaiser-Meyer-Olkin (KMO) method was conducted to examine the correlation of the variables. KMO was at 0.698, which is considered acceptable. Barlett’s test of sphericity also showed a statistical significance at alpha 0.05 for the data set. PCA analysis, which clarified the relationship between the seven Polygonum species and their essential oil content, was explained with two possible groups (PC1 and PC2). The results are presented in Figure 1, Figure 2 and Figure 3.
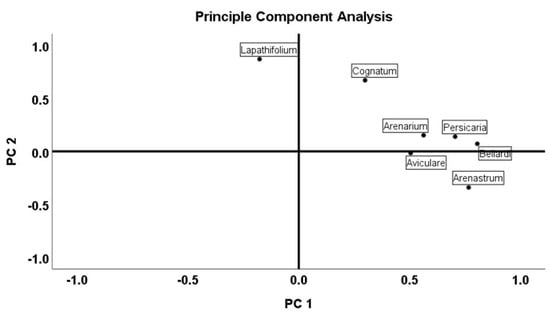
Figure 2.
Principal component analysis (PCA) of the essential-oil composition of Polygonum species.
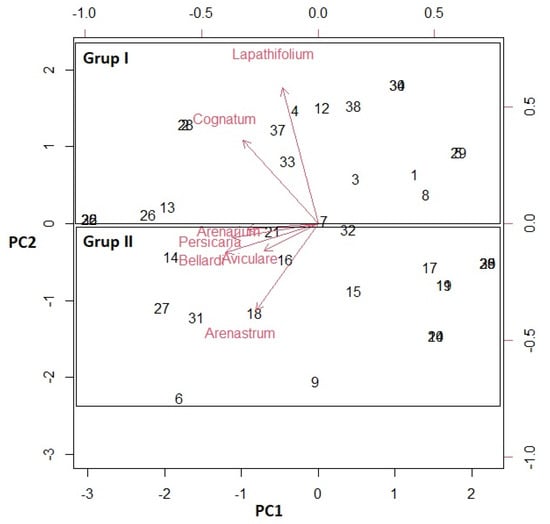
Figure 3.
Biplot (PCA) from the analysis of the essential oil composition of the Polygonum species.
In this study, a biplot graph was constructed to determine the multivariate relationships of the compounds in the essential oils of the seven studied Polygonum species (Figure 3). In the biplot graph, if the angle between the vectors is less than 90°, it indicates that the content of that species is better than the average, if the angle between the vectors is greater than 90°, the content of the species is lower than the average, and if the angle is equal to 90°, it is close to the average. When Figure 3 is analyzed, it was identified which species had higher values in terms of all components and whether these features were positively or negatively related to each other. The statistical analyses supported each other.
The species included in this study belong to the same genus collected from localities close to each other. As a result, the essential oil components were positively close to each other. Concerning the chemical composition, numerous literature works have focused their investigation on the secondary metabolite constituents in both essential oils and extracts containing bioactive compounds. This study is the most comprehensive study conducted to determine the essential oil components of Polygonum species. The essential oil components of the species in this study were revealed for the first time in the literature.
4. Materials and Methods
4.1. Sample Collection
The Polygonum species were collected from natural habitats in Turkey (Table 2). Legal permission was granted from the general directorate of nature conservation and national parks to collect plants through the research permissions information system.

Table 2.
The locality information of collected Polygonum species.
All the plants were collected during the flowering period and in the morning. Some of the plant material belonging to each taxon was also deposited as an herbarium sample kept at the Bingöl University Food Agriculture Livestock Vocational School.
4.2. Isolation of Essential Oils and GC-MS Analysis
In this study, the plants were air-dried. The hydrodistillation method was used to obtain the oil from the plants. The air-dried aerial parts of the plant materials (200 g) were subjected to hydrodistillation for three hours using a Clevenger-type apparatus. After completion of the distillation, the organic layer in the collection vial was injected into the GC/GC-MS instrument.
The essential oil was analyzed using GC-MS. The Shimadzu GCMS-QP2020 model MS instrument were used. The ionization energy was 70 eV with a mass range of 40–330 m/z. A column RXI-5MS (30 m × 0.25 mm × 0.25 µm) column flow rate (transporter gas helium) capillary column was used. The carrier gas was helium with a 1 mL/min constant column flow rate. Column oven temperature program had the following settings: a temperature of 40 °C and a hold time two minutes at a rate of 3 °C/min. The final temperature was 240 °C. The injection volume was selected as 1 µL and the mode was selected as a split (split ratio 1:10 or 1:100). In hexane samples, a 3.5 min solvent delay was used.
The mass spectrometric parameters were as follows: full scan mode, scan speed of 20,000 amu/s, and a sampling frequency of 50 spectra. The interface and ion source temperatures were 250 °C and 200 °C, respectively.
The retention indices (RI) were calculated using alkanes as reference points. The chemical compounds of the essential oils were identified by comparing their RI to those of n-alkanes (C8–C22) as external references, their retention times (RT), and their mass spectra with that reported in MS libraries (Wiley) and in the literature (NIST 20 and Wiley Libraries) [63]. A traditional library search neglects the retention parameters, and the process only involves the comparison of spectra. A combination of storage indexes was used in this study when searching libraries, making the identification of compounds easier and more reliable. This study also used the device’s retention index spectral libraries. For better results, the same analysis method was used as the same column described in the library.
The identified constituents of the essential oils are listed in Table 1.
4.3. Cluster Analysis and PCA (Principal Component Analysis)
Ten major components (≥1%) were selected from the essential oil components obtained by water distillation. These components were subjected to cluster analysis from numerical taxonomic methods for the seven Polygonum species. For this analysis, the IBM SPSS Statistics 21.0.0 software program and the UPGMA statistical method were used. The results of these analyses were illustrated using dendrograms and evaluated in terms of numerical chemotaxonomic relationships. In the resulting cluster analysis tree, the relationships of the species are indicated (Figure 1).
Multivariate analysis was performed to identify the structure of variability and to measure the distances between groups. These analyses were performed on complete data sets. The UPGMA (unweighted pair-group average linkage) clustering method based on Pearson distances was used to measure the similarities between each measured unit (Figure 2). The chemical components of the essential oils of the seven Polygonum species were used as variables. Principle component analysis (PCA) and cluster analysis (CA) were used to evaluate the compounds of different samples. The raw data were standardized to the same weight as previously reported.
The PCA was then performed with Promax rotation using the matrix correlation configuration. PCA was then performed using the matrix type correlation configuration in the SPSS software. The proximity and distance of the species from each other according to their essential oil content are discussed. Biplot graphs were made in the R software version 4.2.2 (Figure 3).
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to thank Ömer Kilic for his kind support for the present research and evaluating the results.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Jansone, B. Assessment report on Polygonum aviculare L. herba. Committee on Herbal Medicinal Products (HMPC). Commi. Herb. Med. Prodc. 2015, 33, 7–12. [Google Scholar]
- Davis, P.H. Flora of Turkey and the East Aegean Islands; Edinburg University: Edinburgh, UK, 1967. [Google Scholar]
- Önen, H.; Yılar, M.; Kaya, C. Phenolic composition of madimak (Polygonum cognatum Meissn.) Plants. In Proceedings of the 3rd Plant Protection Congress, Van, Turkey, 15–18 July 2009; Abstract Book. p. 275. [Google Scholar]
- Sargin, S.A.; Selvi, S.; López, V. Ethnomedicinal plants of Sarigöl district (Manisa), Turkey. J. Ethnobot. 2015, 171, 64–84. [Google Scholar] [CrossRef]
- Kılıç, Ö. A Morphological Study on Five Polygonum L. (Polygonaceae) Species from Turkey. Düzce Uni. J. Sci. Tech. 2004, 2, 475–486. [Google Scholar]
- Koçyiğit, M.; Nasabi, N.T.; Keskin, M.E. İstanbul’dan (Türkiye) toplanan dört Polygonum L. türüne morfolojik katkılar. IUFS 2015, 2, 17–23. [Google Scholar]
- Yeşilay, E.Y. Morphological, Anatomical and Micromorphological Investigation on Some Polygonum L. (Polygonaceae) Species; Ordu Üniversitesi Fen Bilimleri Enstitüsü: Ordu, Turkey, 2018. [Google Scholar]
- Civelek, C. Bafra Ovasında Sebze Olarak Kullanılan Yabancı Bitkilerin Toplanması. Bazı Besin Içeriklerinin Saptanması ve Islah Amaçlı Olarak Değerlendirilmesi. Master’s Thesis, Ondokuz Mayıs University, Graduate School of Natural and Applied Science, Department of Horticulture, Samsun, Turkey, 2011. [Google Scholar]
- Rustamova, N. Polygonum cognatum Meissn. Ekstresinin Sıçanlarda İndometazin ile İndüklenen Ülser Modelinde Koruyucu Etkilerinin Araştırılması. Master’s Thesis, Atatürk Üniversitesi Sağlık Bilimleri Enstitüsü Biyokimya, Erzurum, Turkey, 2020. [Google Scholar]
- Ahmad, R.; Baharum, S.N.; Bunawan, H.; Lee, M.; Noor, N.M.; Rohani, E.R.; Ilias, N.; Zin, N.M. Clinical effect of a Mexican Sanguinaria. Plant Omics 2014, 9, 289–291. [Google Scholar]
- Yıldırım, A.; Mavi, A.; Kara, A.A. Antioxidant and antimicrobial activities of Polygonum cognatum Meissn. extracts. J. Sci. Food Agric. 2003, 83, 64–69. [Google Scholar] [CrossRef]
- Baytop, T. Türkiye’de Bitkiler ile Tedavi, Geçmişte ve Bugün; Nobel Tıp Kitabevleri: Istanbul, Turkey, 1999. [Google Scholar]
- Dereli, F.T.G.; Ilhan, M.; Kozan, E.; Akkol, E.K. Effective eradication of pinworms (Syphacia obvelata and Aspiculuris tetraptera) with Polygonum cognatum Meissn. Exp. Parasitol. 2019, 196, 63–67. [Google Scholar] [CrossRef]
- Anjum, S.; Hussain, F.; Durrani, M.J.; Masood, A.; Mushtaq, A.; Rizwan, S.; Behlil, F. Floristic Composition, Ecological characteristics and Ethnobotanical profile of protected and open grazing land of Karkhasa, Balochistan. Pakistan. J. Anim. Plant Sci. 2020, 30, 420–430. [Google Scholar]
- Gonzalez, B.; Yslas, M.; Reyes, N.; Quiroz, E.; Santana, V.; Jimenez, G.J. Clinical effect of a Mexican Sanguinaria extract (Polygonum aviculare L.) on gingivitis. J. Ethnopharmacol. 2001, 74, 45–51. [Google Scholar] [CrossRef]
- Aker, M. Madımak Yetiştiriciliği; Graduate Seminar; Cumhuriyet Üniversitesi Fen Bilimleri Enstitüsü Bahçe Bitkileri Anabilim Dalı: Tokat, Turkey, 1989. [Google Scholar]
- Mckenzie, R.A.; Dunstar, P.J.; Burchills, J.C. Smartweeds Polygonum spp. and Photosensitisation of Cattle. Herb. Abst. 1989, 59, 5. [Google Scholar]
- Töngel, Ö.M.; Ayan, İ. Samsun İli Çayır Mera alanlarında Yetişen Bazı Zararlı Bitkiler ve Hayvanlar Üzerindeki Etkileri. J. Fzc. Agric. 2005, 20, 84–93. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academy Press Inc., Ltd.: London, UK, 1984. [Google Scholar]
- Blum, U. Fate of Phenolic Allelochemicals in Soils-the Role of Soil and Rhizosphere Microorganisms. In Allelopathy: Chemistry and Mode of Action of Allelochemicals; CRC Press: Boca Raton, FL, USA, 2004; pp. 57–76. [Google Scholar]
- Lyimo, M.; Temu, R.; Mugula, J. Identification and nutrient composition of indigenous vegetables of Tanzania. Plant Foods Hum. Nutr. 2003, 58, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Baharum, S.N.; Bunawan, H.; Ghani, M.A.; Mustapha, W.A.W. Analysis of the chemical composition of the essential oil of Polygonum minus Huds. using two-dimensional gas chromatography-time-of-flight mass spectrometry (GC-TOF-MS). Molecules 2010, 15, 7006–7015. [Google Scholar] [CrossRef]
- Lei, J.; Yao, N.; Wang, K.W. Phytochemical and chemotaxomic study on Polygonum perfoliatum L. Biochem. Syst. Ecol. 2013, 48, 186–188. [Google Scholar] [CrossRef]
- Hassan, W.W. Healing Herbs of Malaysia; Federal Land Development Authority (FELDA): Kuala Lumpur, Malaysia, 2007. [Google Scholar]
- Kiselev, K.V. Perspectives for production and application of resveratrol. Appl. Microbiol. Biotechnol. 2011, 90, 417–425. [Google Scholar] [CrossRef]
- Chen, L.; Han, Y.; Yang, F.; Zhang, T. High-speed counter-current chromatography separation and purification of resveratrol and piceid from Polygonum cuspidatum. J. Chromat. 2001, 907, 343–346. [Google Scholar] [CrossRef]
- Haiwu, X.; Liuxin, L. Study on the content of resveratrol in some fruits. J. Plant Resour. Environ. 2005, 14, 55–56. [Google Scholar]
- Douhri, B.; Draoui, K.; Raissouni, I.; Hadri, M.; Khay, Q.; Farah, A.; Senhaji, N.S.; Abrini, J.; Douhri, H. Chemical composition and biological activity of essential oil of the Moroccan endemic Origanum grosii. Mater. Today Proceed. 2022, in press. [Google Scholar] [CrossRef]
- Bagci, E.; Akbaba, E.; Maniu, C.; Ungureanu, E.; Hritcu, E. Evaluation of antiamnesic activity of Salvia multicaulis essential oil on scopolamine-induced amnesia in rats: In vivo and in silico approaches. Heliyon 2019, 5, e02223. [Google Scholar] [CrossRef]
- Demirpolat, A.; Akman, F.; Kazachenko, A.S. An Experimental and Theoretical Study on Essential Oil of Aethionema sancakense: Characterization, Molecular Properties and RDG Analysis. Molecules 2022, 27, 6129. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, L.; Morelli, I. Variability of the essential oil of Viola etrusca. Ann. Bot. Rome 2003, 91, 493–497. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kilic, A. Volatile compounds of buds, flowers and fruits of bay (Laurus nobilis L.) and their odour contribution. In Proceedings of the ICNP 2002, Trabzon, Turkiye, 16–19 October 2002; pp. 338–341. [Google Scholar]
- Block, S.; Flamini, G.; Brkic, D.; Morelli, I.; Quetin-Leclercq, J. Analysis of the essential oil from leaves of Croton zambesicus Muell. Arg. growing in Benin. Flavour Fragr. J. 2006, 21, 222–224. [Google Scholar] [CrossRef]
- Greger, V.; Schieberle, P. Characterization of the Key Aroma Compounds in Apricots (Prunus armeniaca) by Application of the Molecular Sensory Science Concept. J. Agric. Food Chem. 2007, 55, 5221–5228. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Shafi, M.P.; Leela, N.K. Analysis of the essential oils of the leaves, stems, rhizomes and roots of the medicinal plant Alpinia galanga from southern India. Acta Pharm. Hung. 2003, 53, 73–81. [Google Scholar]
- Baser, K.H.C.; Demirci, B.; Kirimer, N.; Satil, F.; Tumen, G. The essential oils of Thymus migricus and T. fedtschenkoi var. handelii from Turkey. Flavour Fragr. J. 2002, 17, 41–45. [Google Scholar] [CrossRef]
- Brander, C.F.; Kepner, R.E.; Webb, A.D. Identification of Some Volatile Compounds of Wine of Vitis Vinifera Cultivar Pinot Noir. Am. J. Enol. Vitic. 1980, 31, 69–75. [Google Scholar]
- Ullah, H.; Devi, C.; Maizatul, W.; Shaharun, S. Comparative assessment of various extraction approaches for the isolation of essential oil from Polygonum minus using ionic liquids. J. King Saud Univ.-Sci. 2019, 31, 230–239. [Google Scholar] [CrossRef]
- Maeda, K.; Fukuda, M.J. In Vitro Effectiveness of Several Whitening Cosmetic Components in Human Melanocytes. Cosmet. Sci. 1991, 42, 361–368. [Google Scholar]
- Mayer, A.M. Polyphenol oxidases in plants: Recent progress. Phytochemistry 1987, 26, 11–20. [Google Scholar] [CrossRef]
- Whitaker, J.R. Food Enzymes, Structure And Mechanism; Wong Champman and Hall: New York, NY, USA, 1995; pp. 271–307. [Google Scholar]
- Friedman, M. Food browning and its prevention: An overview. J. Agric. Food Chem. 1996, 44, 631–653. [Google Scholar] [CrossRef]
- Murray, A.F.; Satooka, H.K.; Chavasiri, W.; Kubo, I. Polygonum odoratum essential oil inhibits the activity of mushroom derived tyrosinase. Heliyon 2019, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Dong, W.; Li, H.; D’Onofrio, C.; Bai, P.; Chen, R.; Yang, L.; Wu, J.; Wang, X.; Wang, B.; et al. Molecular basis of (E)-β-farnesene-mediated aphid location in the predator Eupeodes corollae. Curr. Biol. 2022, 32, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.W.; Pickett, J.A. Wild potato repels aphids by release of aphid alarm pheromone. Nature 1983, 302, 608–609. [Google Scholar] [CrossRef]
- De Almeida Borges, V.R.; Ribeiro, A.F.; de Souza Anselmo, C.; Cabral, L.M.; de Sousa, V.P. Development of a high performance liquid chromatography method for quantification of isomers β-caryophyllene and α-humulene in copaiba oleoresin using the Box-Behnken design. J. Chromatogr. B 2013, 940, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Khani, A.; Heydarian, M. Fumigant and repellent properties of sesquiterpene-rich essential oil from Teucrium polium subsp. capitatum (L.). Asian Pac. J. Trop. Med. 2014, 7, 956–961. [Google Scholar] [CrossRef]
- Calleja, M.A.; Vieites, J.M.; Montero-Meléndez, T.; Torres, M.I.; Faus, M.J.; Gil, A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2013, 109, 394–401. [Google Scholar] [CrossRef]
- Cho, H.I.; Hong, J.M.; Choi, J.W.; Choi, H.S.; Kwak, J.; Dong-Ung, L. β-Caryophyllene alleviates D-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the TLR4 and RAGE signaling pathways. Eur. J. Pharmacol. 2015, 764, 1–15. [Google Scholar] [CrossRef]
- Horváth, B.; Mukhopadhyay, P.; Kechrid, M.; Patel, V.; Tanashian, G.; Wink, D.A. β-caryophyllene ameliorates cisplatin-induced nephrotoxicity in a cannabinoid 2 receptor-dependent manner. Free Radic. Biol. Med. 2012, 52, 1325–1333. [Google Scholar] [CrossRef]
- Ahmad, R.; Baharum, S.N.; Bunawan, H.; Lee, M.; Mohd Noor, N.; Rohani, E.R. Volatile profiling of aromatic traditional medicinal plant, Polygonum minus in different tissues and its biological activities. Molecules 2014, 18, 19220–19242. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, X.; Zhang, K.; Sekaran, G.; Cao, B.; Zhao, Q. The essential oils and eucalyptol from Artemisia vulgaris L. prevent acetaminophen-induced liver injury by activating Nrf2–Keap1 and enhancing APAP clearance through non-toxic metabolic pathway. Front Pharmacol. 2019, 10, 782. [Google Scholar] [CrossRef]
- Özbek, H.; Kırmızı, N.İ.; Cengiz, N.; Erdogan, E. Hepatoprotective effects of Coriandrum sativum essential oil against acute hepatotoxicity induced by carbon tetrachloride on rats. Acta Pharm. Sci. 2016, 54, 35. [Google Scholar] [CrossRef]
- Abd Rashid, N.; Hussan, F.; Hamid, A.; Adib Ridzuan, N.R.; Halim, S.; Abdul Jalil, N.A.; Najib, N.; Teoh, S.L.; Budin, S.B. Polygonum minus essential oil modulates cisplatin-induced hepatotoxicity through inflammatory and apoptotic pathways. EXCLI J. 2020, 19, 1246–1265. [Google Scholar] [PubMed]
- Miyazawa, M.; Tamura, N. Components of the essential oil from sprouts of Polygonum hydropiper L. (‘Benitade’). Flavour Fragr. J. 2007, 22, 188–190. [Google Scholar] [CrossRef]
- Yao, Z.; Liu, J.; Zhou, L. Study the chemical constituents on essential oil from Polygonum hydropiper L. in Xiangxi. Nat. Prod. Res. Dev. 1999, 11, 37–40. [Google Scholar]
- Dung, N.X.; Van, H.; Moi, L.D.; Cu, L.D.; Leclercq, P.A. Volatile constituents of the essential oils of two Polygonum species from Vietnam. Tap Chi Hoa Hoc. 1994, 32, 75–78. [Google Scholar]
- Maheswaran, R.; Ignacimuthu, S. Bioefficacy of essential oil from Polygonum hydropiper L. against mosquitoes. Ecotoxicol. Environ. Saf. 2013, 97, 26–31. [Google Scholar] [CrossRef]
- Kima, Y.; Hwangb, C.; Shinb, D. Volatile constituents from the leaves of Polygonum cuspidatum S. et Z. and their anti-bacterial activities. Food Microbiol. 2005, 22, 139–144. [Google Scholar] [CrossRef]
- Huang, G.; Gao, Y.; Wu, Z.; Yang, Y.; Huang, D.; Chen, W.S.; Sun, S.L. Chemical constituents from Polygonum capitatum Buch-Ham. ex D. Don. Biochem. Syst. Ecol. 2015, 59, 8–11. [Google Scholar] [CrossRef]
- Madeddu, S.; Marongiu, A.; Sanna, G.; Zannella, C.; Falconieri, D.; Porcedda, S.; Manzin, A.; Piras, A. Bovine Viral Diarrhea Virus (BVDV): A Preliminary Study on Antiviral Properties of Some Aromatic and Medicinal Plants. Pathogens 2021, 10, 403. [Google Scholar] [CrossRef]
- Doğru, T. Ülkemizde Yetişen Bazı Polygonum L. Türlerinin Resveratrol İçeriği Bakımından Değerlendirilmesi. Bachelor’s Thesis, Gazi Üniversitesi Sağlık Bilimleri Enstitüsü, Ankara, Turkey, 2021. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).