Fluorination of Terminal Groups Promoting Electron Transfer in Small Molecular Acceptors of Bulk Heterojunction Films
Abstract
1. Introduction
2. Results and Discussion
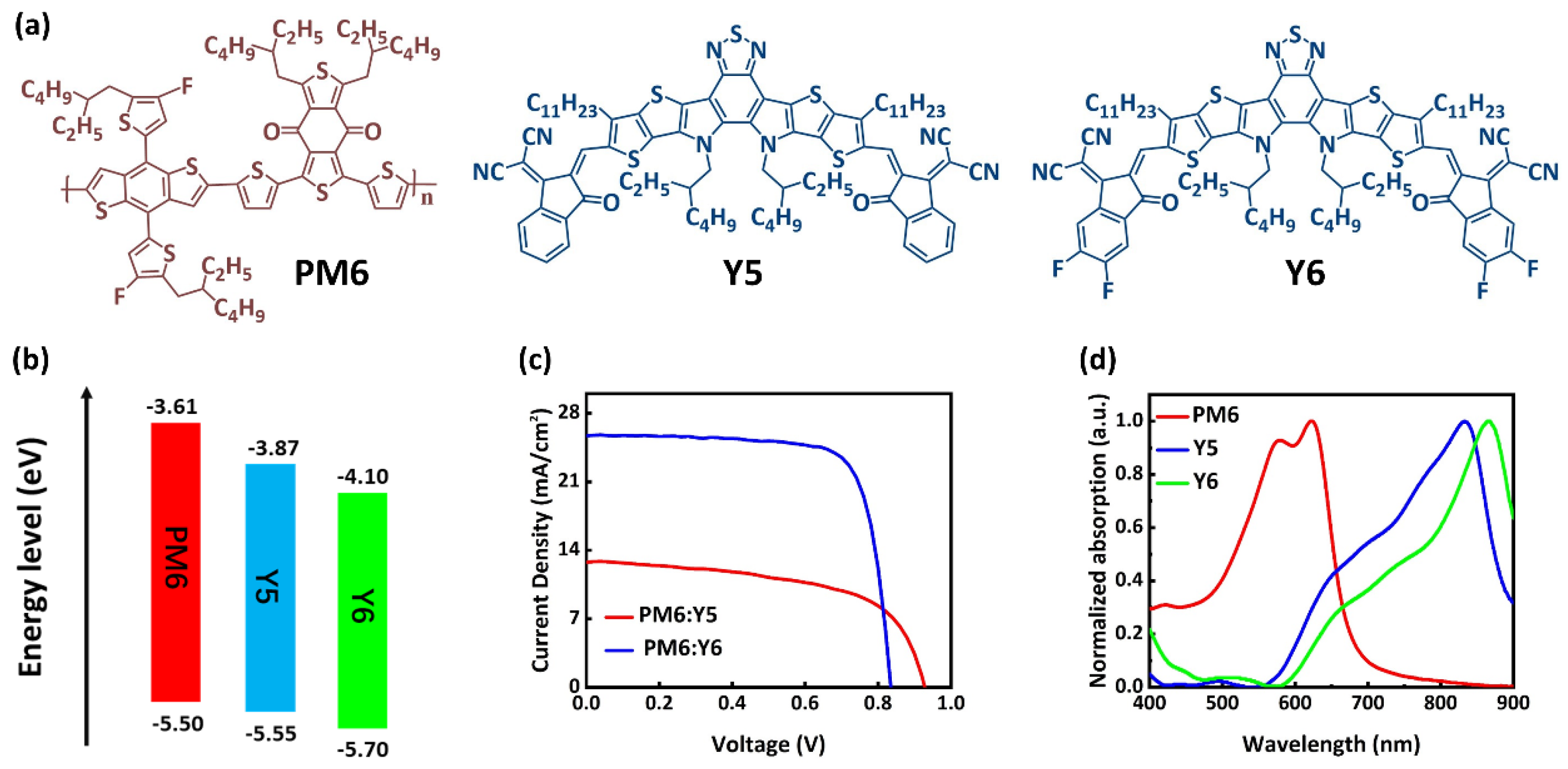
2.1. Optical Properties
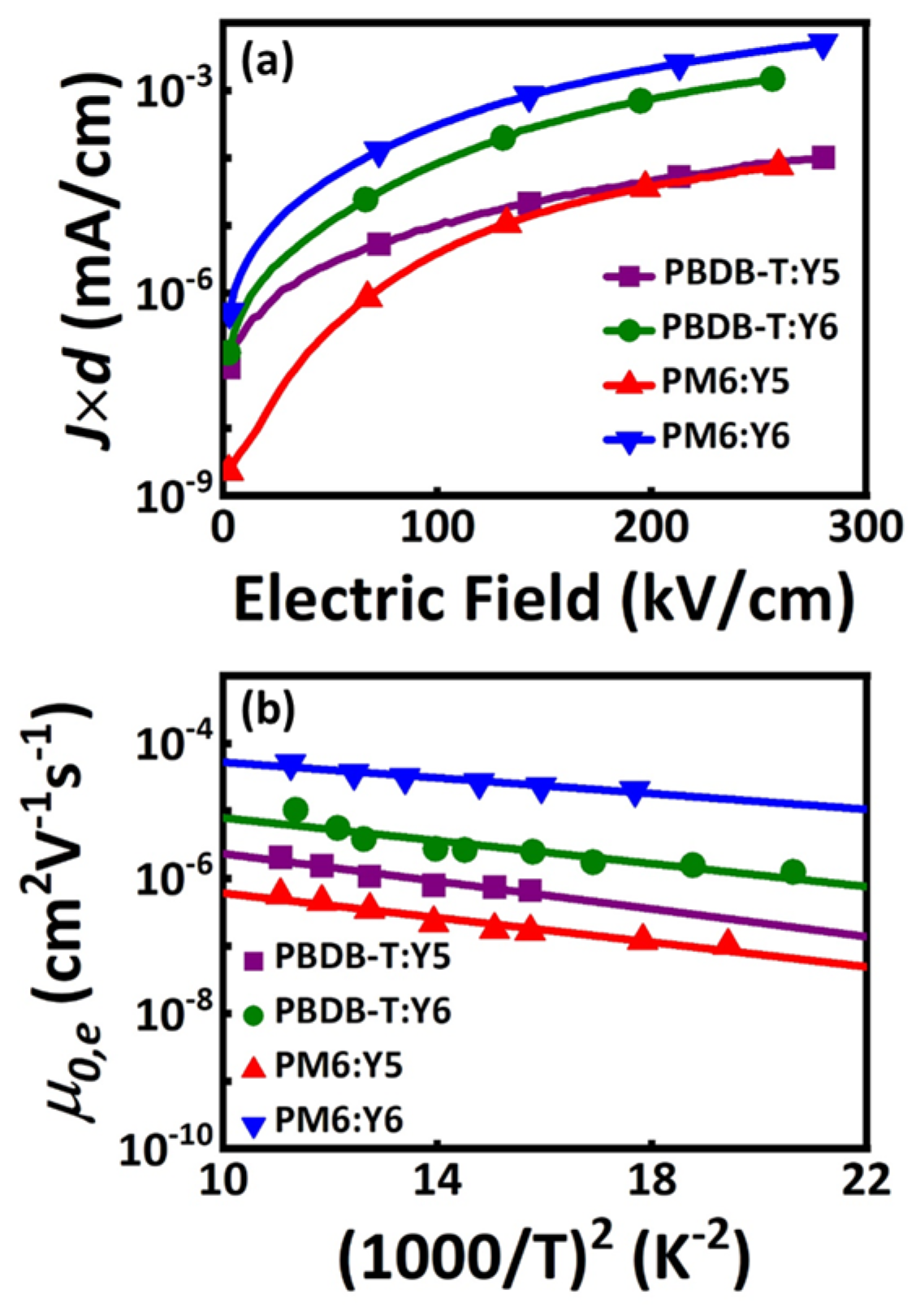
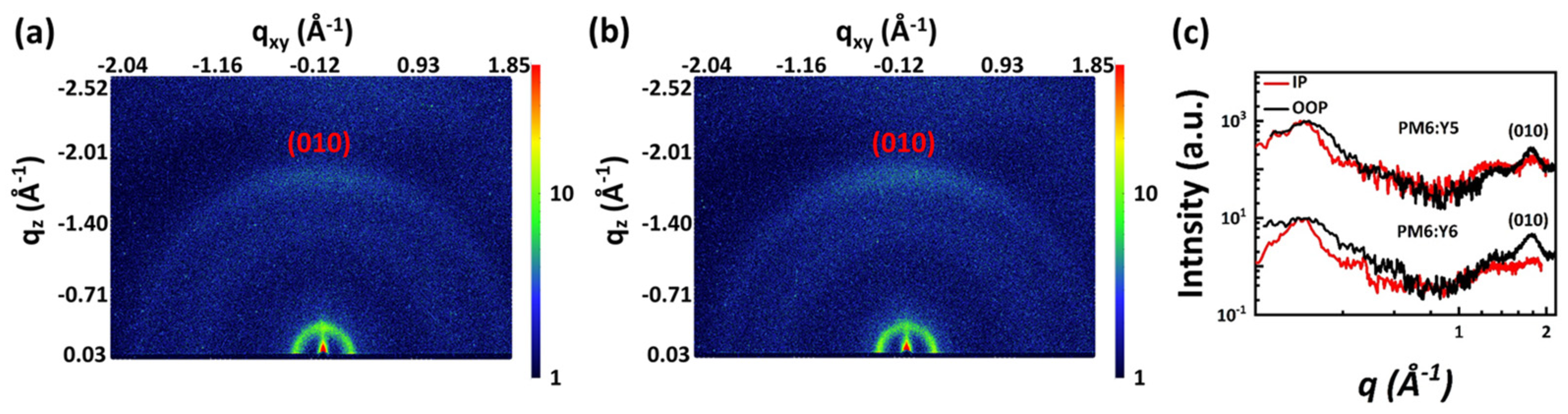
2.2. Charge Transport Properties and Morphology Analysis
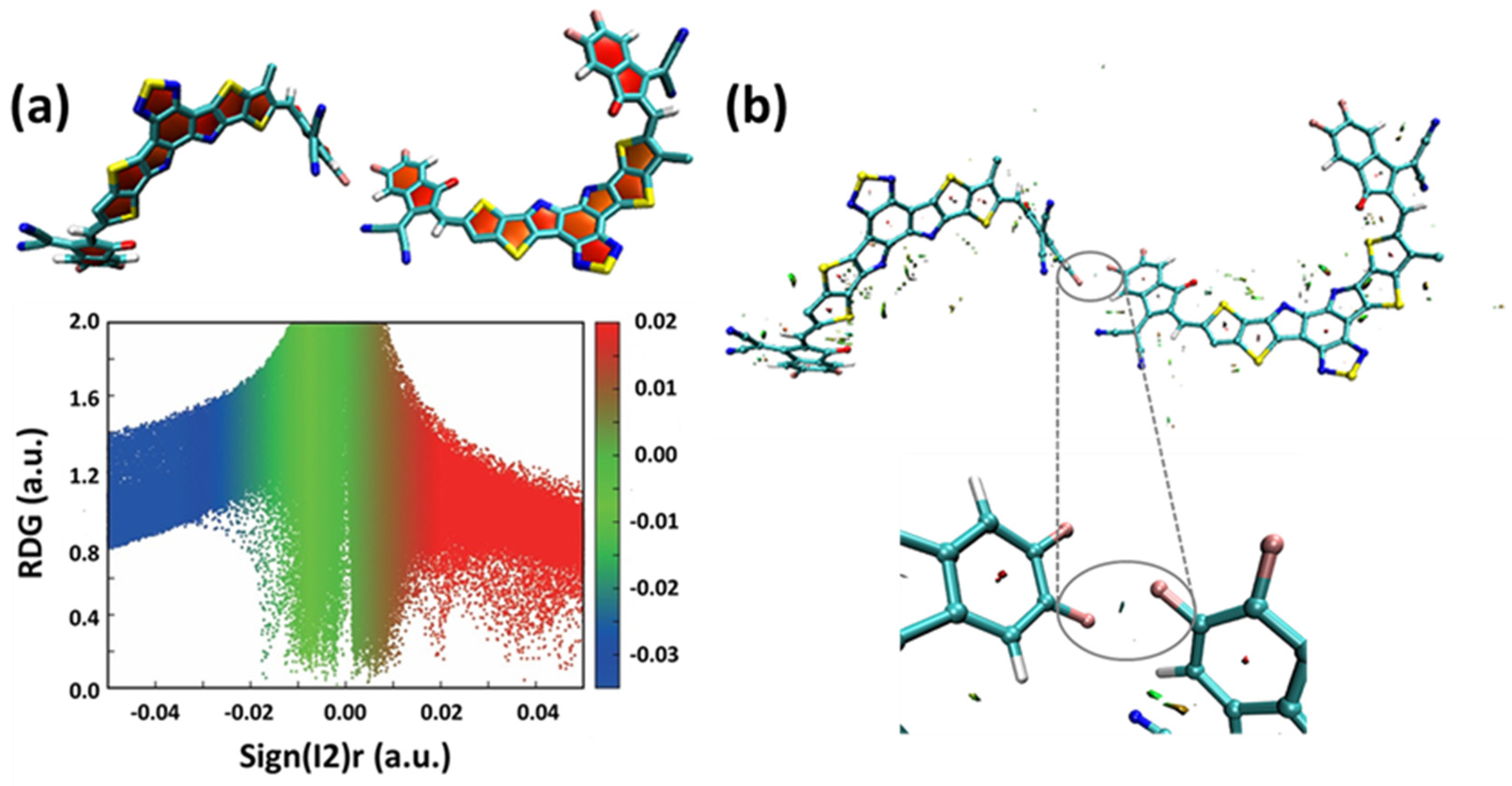
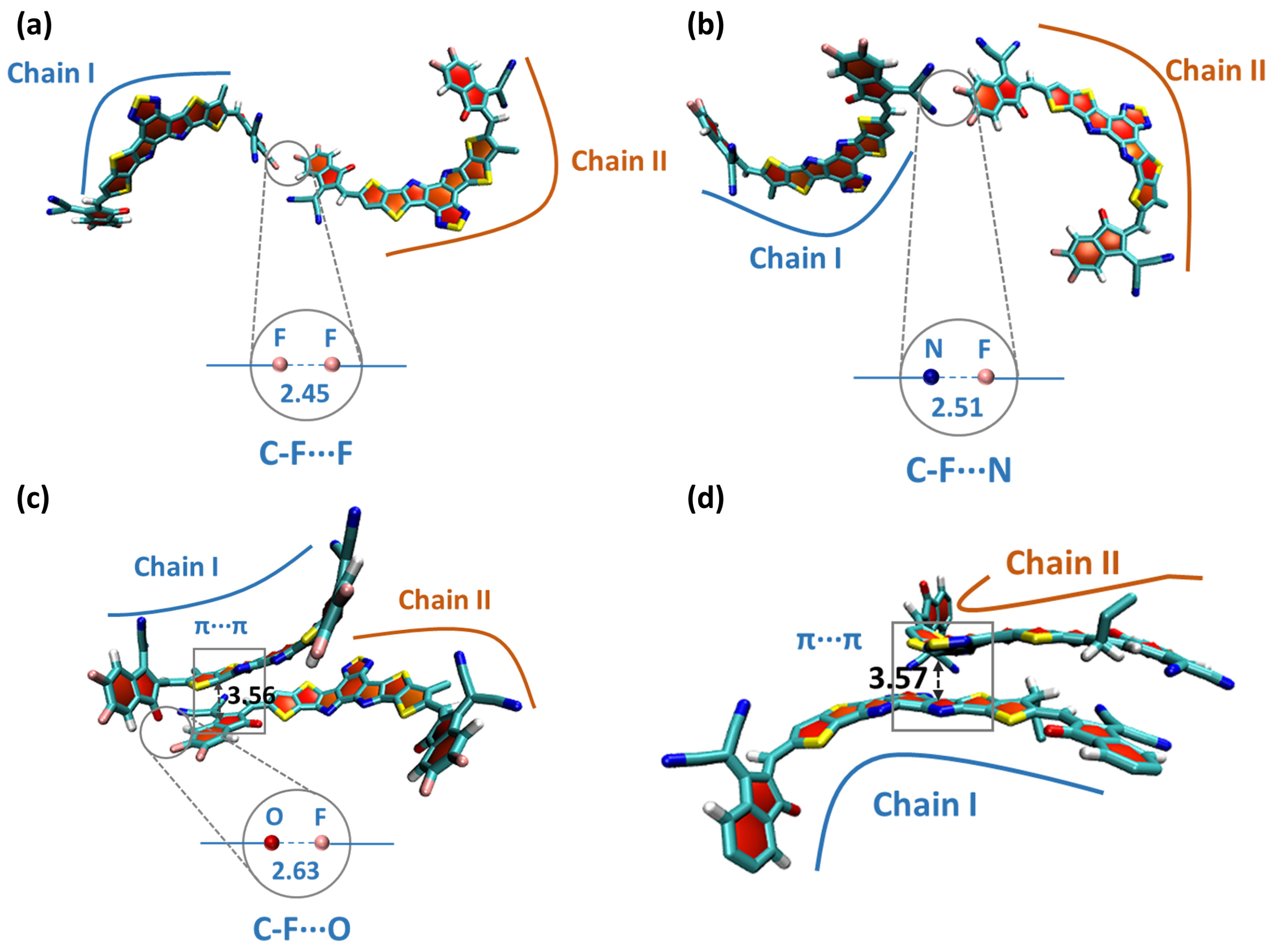
2.3. Molecular Dynamics Simulation
3. Materials and Methods
3.1. Materials
3.2. Computational Methods
3.3. Fabrication of the Electron-Only Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wei, Y.; Chen, Z.; Lu, G.; Yu, N.; Li, C.; Gao, J.; Gu, X.; Hao, X.; Lu, G.; Tang, Z.; et al. Binary Organic Solar Cells Breaking 19% via Manipulating the Vertical Component Distribution. Adv. Mater. 2022, 34, e2204718. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, L.; Meng, H.; Dai, L.; Yan, H.; Li, R.; Peng, Q. Polymer Solar Cells with 18.74% Efficiency: From Bulk Heterojunction to Interdigitated Bulk Heterojunction. Adv. Funct. Mater. 2021, 32, 2108797. [Google Scholar] [CrossRef]
- Li, Y.; Ding, J.; Liang, C.; Zhang, X.; Zhang, J.; Jakob, D.S.; Wang, B.; Li, X.; Zhang, H.; Li, L.; et al. Nanoscale heterogeneous distribution of surface energy at interlayers in organic bulk-heterojunction solar cells. Joule 2021, 5, 3154–3168. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Xian, K.; Zhang, T.; Hong, L.; Wang, Y.; Xu, Y.; Ma, K.; An, C.; et al. Single-Junction Organic Photovoltaic Cells with Approaching 18% Efficiency. Adv. Mater. 2020, 32, e1908205. [Google Scholar] [CrossRef]
- Kang, H.; Lee, W.; Oh, J.; Kim, T.; Lee, C.; Kim, B.J. From Fullerene-Polymer to All-Polymer Solar Cells: The Importance of Molecular Packing, Orientation, and Morphology Control. Acc. Chem. Res. 2016, 49, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, Y.; Wang, Z. Tailor-made rylene arrays for high performance n-channel semiconductors. Acc. Chem. Res. 2014, 47, 3135–3147. [Google Scholar] [CrossRef]
- Henson, Z.B.; Mullen, K.; Bazan, G.C. Design strategies for organic semiconductors beyond the molecular formula. Nat. Chem. 2012, 4, 699–704. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Frechet, J.M. Molecular design and ordering effects in pi-functional materials for transistor and solar cell applications. J. Am. Chem. Soc. 2011, 133, 20009–20029. [Google Scholar] [CrossRef]
- Zhang, Q.; Kelly, M.A.; Bauer, N.; You, W. The Curious Case of Fluorination of Conjugated Polymers for Solar Cells. Acc. Chem. Res. 2017, 50, 2401–2409. [Google Scholar] [CrossRef]
- Tang, M.L.; Bao, Z. Halogenated Materials as Organic Semiconductors. Chem. Mater. 2010, 23, 446–455. [Google Scholar] [CrossRef]
- Yao, H.; Wang, J.; Xu, Y.; Zhang, S.; Hou, J. Recent Progress in Chlorinated Organic Photovoltaic Materials. Acc. Chem. Res. 2020, 53, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Xu, L.; Yin, H.; Cui, B.; Zhang, L.; Hao, X.; Gao, K. Synergistic effect of incorporating intra- and inter-molecular charge transfer in nonfullerene acceptor molecules for highly-efficient organic solar cells. J. Mater. Chem. A 2021, 9, 16834–16840. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, H.; An, Q.; Jiang, M.; Mahmood, A.; Jee, M.H.; Bai, H.R.; Zhi, H.F.; Zhang, S.; Woo, H.Y.; et al. Regioisomer-Free Difluoro-Monochloro Terminal-based Hexa-Halogenated Acceptor with Optimized Crystal Packing for Efficient Binary Organic Solar Cells. Angew. Chem. Int. Ed. Engl. 2022, 134, e202209454. [Google Scholar] [CrossRef]
- Sun, G.; Jiang, X.; Li, X.; Meng, L.; Zhang, J.; Qin, S.; Kong, X.; Li, J.; Xin, J.; Ma, W.; et al. High performance polymerized small molecule acceptor by synergistic optimization on pi-bridge linker and side chain. Nat. Commun. 2022, 13, 5267. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Liang, Z.; Huang, G.; Chen, W.; Zheng, N.; Yang, R. Employing structurally similar acceptors as crystalline modulators to construct high efficiency ternary organic solar cells. J. Mater. Chem. A 2019, 7, 7760–7765. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, Y.; Zhu, J.; Hou, J. Over 14% Efficiency in Polymer Solar Cells Enabled by a Chlorinated Polymer Donor. Adv. Mater. 2018, 30, e1800868. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef]
- Yao, H.; Cui, Y.; Yu, R.; Gao, B.; Zhang, H.; Hou, J. Design, Synthesis, and Photovoltaic Characterization of a Small Molecular Acceptor with an Ultra-Narrow Band Gap. Angew. Chem. Int. Ed. Engl. 2017, 56, 3045–3049. [Google Scholar] [CrossRef]
- Shi, R.; Hu, H.; Chen, T.; Gui, R.; Liu, J.; Hao, X.; Yin, H. Extracting energetic disorder in organic solar cells using percolation models. ChemPhysMater 2022. [Google Scholar] [CrossRef]
- Bassle, H. Charge Transport in Disordered Organic Photoconductors. Phys. Status Solidi B 1993, 175, 15–56. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, C.; Hu, H.; Karuthedath, S.; Gao, Y.; Tang, H.; Yan, C.; Cui, L.; Fong, P.W.K.; Zhang, Z.; et al. Highly Crystalline Near-Infrared Acceptor Enabling Simultaneous Efficiency and Photostability Boosting in High-Performance Ternary Organic Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 48095–48102. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Li, S.; Liu, X.; Zhang, S.; Ghasemi, M.; Xiong, Y.; Hou, J.; Ade, H. Quenching to the Percolation Threshold in Organic Solar Cells. Joule 2019, 3, 443–458. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, W.; Zhou, G.; Zhang, M.; Qian, D.; Zhang, J.; Chen, S.; Xu, S.; Yang, C.; Gao, F.; et al. Subtle Molecular Tailoring Induces Significant Morphology Optimization Enabling over 16% Efficiency Organic Solar Cells with Efficient Charge Generation. Adv. Mater. 2020, 32, e1906324. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nan, G.; Yang, X.; Peng, Q.; Li, Q.; Shuai, Z. Computational methods for design of organic materials with high charge mobility. Chem. Soc. Rev. 2010, 39, 423–434. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron transfer reactions in chemistry. Theory and experiment. Rev. Mod. Phys. 1993, 65, 599–610. [Google Scholar] [CrossRef]
- Coropceanu, V.; Cornil, J.; da Silva Filho, D.A.; Olivier, Y.; Silbey, R.; Bredas, J.L. Charge transport in organic semiconductors. Chem. Rev. 2007, 107, 926–952. [Google Scholar] [CrossRef]
- Yin, H.; Ma, L.K.; Wang, Y.; Huang, J.; Yu, H.; Zhang, J.; Chow, P.C.Y.; Ma, W.; So, S.K.; Yan, H. Donor Polymer Can Assist Electron Transport in Bulk Heterojunction Blends with Small Energetic Offsets. Adv. Mater. 2019, 31, e1903998. [Google Scholar] [CrossRef]
- Hu, H.; Mu, X.; Qin, W.; Gao, K.; Hao, X.; Yin, H. Rationalizing charge carrier transport in ternary organic solar cells. Appl. Phys. Lett. 2022, 120, 023302. [Google Scholar] [CrossRef]
- Tang, X.-D.; Liao, Y.; Gao, H.-Z.; Geng, Y.; Su, Z.-M. Theoretical study of the bridging effect on the charge carrier transport properties of cyclooctatetrathiophene and its derivatives. J. Mater. Chem. 2012, 22, 6907–6918. [Google Scholar] [CrossRef]
- Mulliken, R.S. Structures of Complexes Formed by Halogen Molecules with Aromatic and with Oxygenated Solvents. J. Am. Chem. Soc. 1950, 72, 600–608. [Google Scholar] [CrossRef]
- Shen, Q.J.; Pang, X.; Zhao, X.R.; Gao, H.Y.; Sun, H.-L.; Jin, W.J. Phosphorescent cocrystals constructed by 1,4-diiodotetrafluorobenzene and polyaromatic hydrocarbons based on C–I⋯π halogen bonding and other assisting weak interactions. CrystEngComm 2012, 14, 5027–5034. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, C.; Lau, T.K.; Zhang, G.; Lu, X.; Yip, H.L.; So, S.K.; Beaupre, S.; et al. Fused Benzothiadiazole: A Building Block for n-Type Organic Acceptor to Achieve High-Performance Organic Solar Cells. Adv. Mater. 2019, 31, e1807577. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Li, D.; Chen, M.; Mao, Y.; Du, B.; Zhuang, Y.; Tan, W.; Huang, W.; Zhao, Y.; et al. Modulation of J-Aggregation of Nonfullerene Acceptors toward Near-Infrared Absorption and Enhanced Efficiency. Macromolecules 2020, 53, 3747–3755. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Bayly, C.I.; Cieplak, P.; Cornell, W.D.; Kollman, P.A. A Well-Behaved Electrostatic Potential Based Method Using Charge Restraints for Deriving Atomic Charges—The Resp Model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.I.; Shao, Q.; Zhang, J.; Yang, L.; Gao, Y.Q. Enhanced sampling in molecular dynamics. J. Chem. Phys. 2019, 151, 070902. [Google Scholar] [CrossRef]
| Active Layer | VOC (V) | JSC (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| PM6:Y5 | 0.934 ± 0.006 | 12.72 ± 0.20 | 57.47 ± 1.39 | 6.83 ± 0.16 |
| PM6:Y6 | 0.828 ± 0.005 | 25.12 ± 0.29 | 76.31 ± 0.68 | 15.87 ± 0.22 |
| Active Layer | Location (Å−1) | d (Å) | FWHM (Å−1) | CCL (Å) |
|---|---|---|---|---|
| PM6:Y5 | 1.76 | 3.57 | 0.27 | 20.94 |
| PM6:Y6 | 1.77 | 3.55 | 0.25 | 22.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Shi, R.; Gui, R.; Hu, H.; Zhang, W.; Zhang, K.; Cui, B.; Yin, H.; Gao, K.; Liu, J. Fluorination of Terminal Groups Promoting Electron Transfer in Small Molecular Acceptors of Bulk Heterojunction Films. Molecules 2022, 27, 9037. https://doi.org/10.3390/molecules27249037
Chen T, Shi R, Gui R, Hu H, Zhang W, Zhang K, Cui B, Yin H, Gao K, Liu J. Fluorination of Terminal Groups Promoting Electron Transfer in Small Molecular Acceptors of Bulk Heterojunction Films. Molecules. 2022; 27(24):9037. https://doi.org/10.3390/molecules27249037
Chicago/Turabian StyleChen, Tao, Rui Shi, Ruohua Gui, Haixia Hu, Wenqing Zhang, Kangning Zhang, Bin Cui, Hang Yin, Kun Gao, and Jianqiang Liu. 2022. "Fluorination of Terminal Groups Promoting Electron Transfer in Small Molecular Acceptors of Bulk Heterojunction Films" Molecules 27, no. 24: 9037. https://doi.org/10.3390/molecules27249037
APA StyleChen, T., Shi, R., Gui, R., Hu, H., Zhang, W., Zhang, K., Cui, B., Yin, H., Gao, K., & Liu, J. (2022). Fluorination of Terminal Groups Promoting Electron Transfer in Small Molecular Acceptors of Bulk Heterojunction Films. Molecules, 27(24), 9037. https://doi.org/10.3390/molecules27249037




