Antioxidant Properties and Proximate Composition of Different Tissues of European Beaver
Abstract
1. Introduction
2. Results
2.1. Proximate Composition and Cholesterol Concentration
2.2. Fat-Soluble Vitamins and Total Antioxidant Capacity
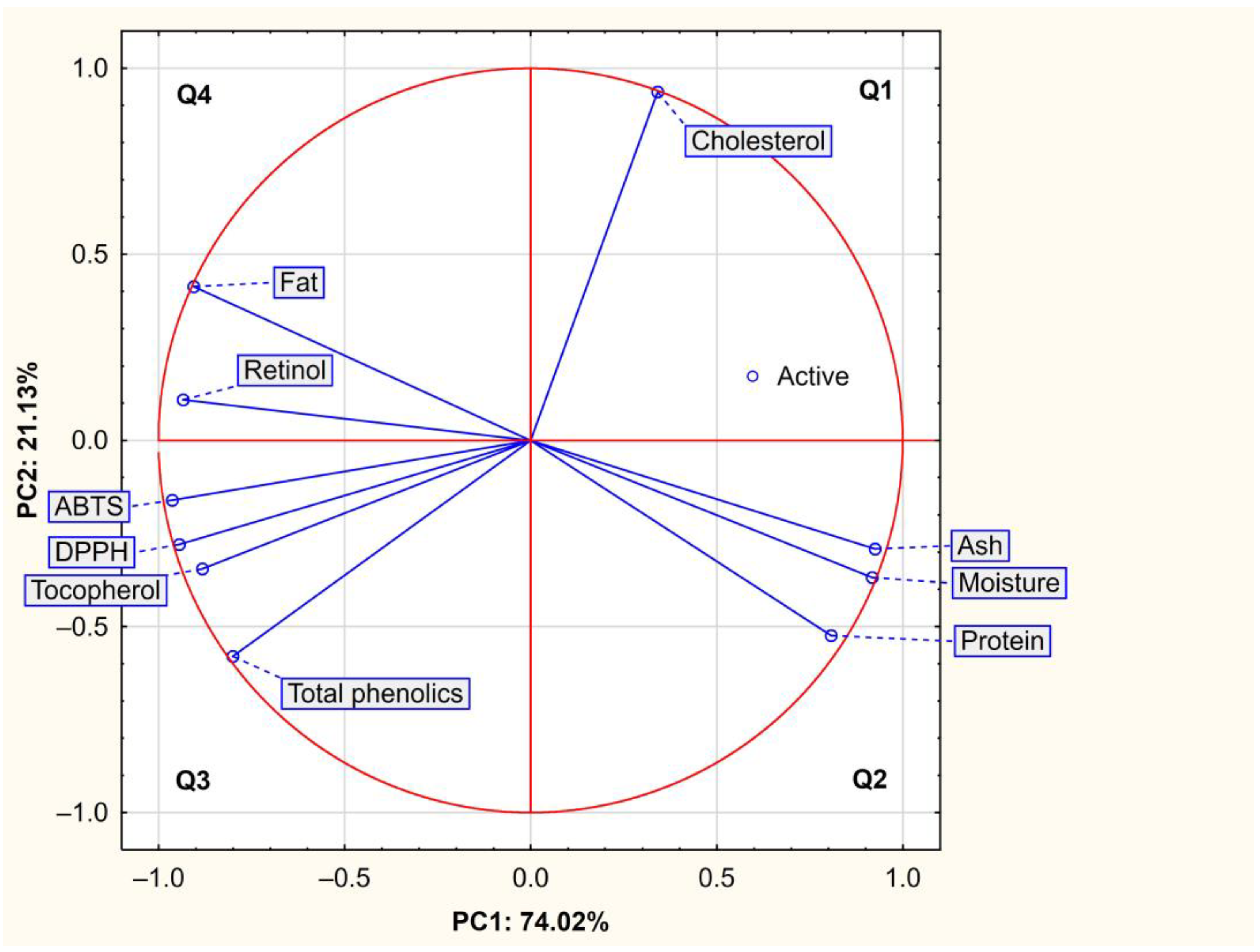
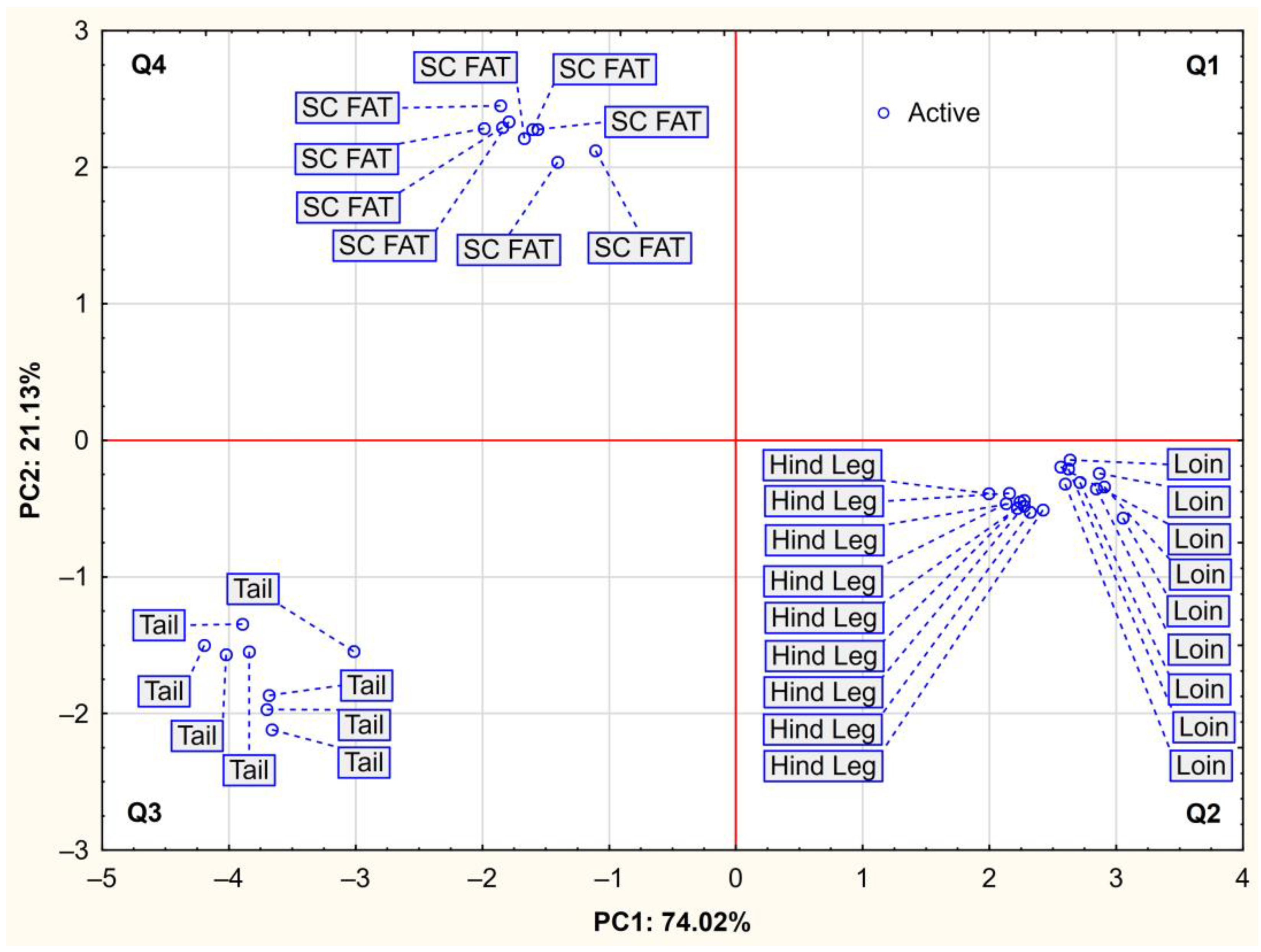
2.3. Correlations and PCA Analysis
3. Discussion
3.1. Proximate Composition and Cholesterol Concentration
3.2. Fat-Soluble Vitamins and Total Antioxidant Capacity
3.3. Correlations and PCA Analysis
4. Materials and Methods
4.1. Animals and Sampling
4.2. Chemical Analyses
4.2.1. Chemical Compounds Studied in This Article
4.2.2. Proximate Composition and Cholesterol Content
4.2.3. Properties of Antioxidant Activity
Preparation of Extracts
Total Phenolic Content
4.2.4. Free Radical Scavenging Activity
DPPH Method
ABTS Method
4.2.5. Alpha Tocopherol and Retinol Contents
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Florek, M.; Drozd, L. Bioactive compounds in deer meat. Med. Weter. 2013, 69, 535–539. [Google Scholar]
- Hoffman, L.C.; Wiklund, E. Game and venison—Meat for the modern consumer. Meat Sci. 2006, 74, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Kaneko, S.; Sogawa, K.; Ahhmed, A.M.; Enomoto, H.; Kawarai, S.; Taira, K.; Mizunoya, W.; Minami, M.; Sakata, R. Isolation, Evaluation, and Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides from Game Meat. Foods 2020, 9, 1168. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, P.; Smulders, F.J.M.; Hilbert, F. Salmonella in meat from hunted game: A Central European perspective. Food Res. Int. 2012, 45, 609–616. [Google Scholar] [CrossRef]
- Hoffman, L.C.; Cawthorn, D. Exotic protein sources to meet all needs. Meat Sci. 2013, 95, 764–771. [Google Scholar] [CrossRef]
- Morrissey, P.A.; Sheehy, P.J.A.; Galvin, K.; Kerry, J.P.; Buckleyh, D.J. Lipid Stability in Meat and Meat Products. Meat Sci. 1998, 49 (Suppl. I), S73–S86. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Gatellier, P.; Mercier, Y.; Renerre, M. Effect of diet finishing mode (pasture or mixed diet) on antioxidant status of Charolais bovine meat. Meat Sci. 2004, 67, 385–394. [Google Scholar] [CrossRef]
- Martínez, J.; Nieto, G.; Ros, G. Total antioxidant capacity of meat and meat products consumed in a reference ‘Spanish standard diet’. Int. J. Food Sci. Technol. 2014, 49, 2610–2618. [Google Scholar] [CrossRef]
- Wu, C.; Duckett, S.K.; Neel, J.P.S.; Fontenot, J.P.; Clapham, W.M. Influence of a finishing systems on hydrophilic and lipophilic oxygen radical absorbance capacity (ORAC) in beef. Meat Sci. 2008, 80, 662–667. [Google Scholar] [CrossRef]
- Descalzo, A.M.; Sancho, A.M. A review of natural antioxidants and their effects on oxidative status, odor and quality of fresh beef produced in Argentina. Meat Sci. 2008, 79, 423–436. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products-a review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Kopec, W.; Wiliczkiewicz, A.; Jamroz, D.; Biazik, E.; Pudlo, A.; Hikawczuk, T.; Skiba, T.; Korzeniowska, M. Antioxidant status of turkey breast meat and blood after feeding a diet enriched with histidine. Poult. Sci. 2016, 95, 53–61. [Google Scholar] [CrossRef]
- Sacchetti, G.; Di Mattia, C.; Pittia, P.; Martino, G. Application of a radical scavenging activity test to measure the total antioxidant activity of poultry meat. Meat Sci. 2008, 80, 1081–1085. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef]
- Mielnik, M.B.; Rzeszutek, A.; Triumf, E.C.; Egelandsdal, B. Antioxidant and other quality properties of reindeer muscle from two different Norwegian regions. Meat Sci. 2011, 89, 526–532. [Google Scholar] [CrossRef]
- Triumf, E.C.; Purchas, R.W.; Mielnik, M.; Maehre, H.K.; Elvevoll, E.; Slinde, E.; Egelandsdal, B. Composition and some quality characteristics of the longissimus muscle of reindeer in Norway compared to farmed New Zealand red deer. Meat Sci. 2012, 90, 122–129. [Google Scholar] [CrossRef]
- Quaresma, M.A.G.; Trigo-Rodrigues, I.; Alves, S.P.; Martins, S.I.V.; Barreto, A.S.; Bessa, R.J.B. Nutritional evaluation of the lipid fraction of Iberian red deer (Cervus elaphus hispanicus) tenderloin. Meat Sci. 2012, 92, 519–524. [Google Scholar] [CrossRef]
- Decker, E.A.; Xu, Z. Minimizing rancidity in muscle foods. Food Technol. 1998, 52, 54–59. [Google Scholar]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Moon, J.K.; Shibamoto, T. Antioxidant Assays for Plant and Food Components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, R.; Haenen, G.R.M.M.; Van den Berg, H. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Thompson, S.; Vehkaoja, M.; Pellikka, J.; Nummi, P. Ecosystem services provided by beavers Castor spp. Mammal Rev. 2021, 51, 25–39. [Google Scholar] [CrossRef]
- Korzeniowski, W.; Kwiatkowska, A.; Janowska, B.; Żmijewski, T. Characterization of reserve fatty tissues in European beaver (Castor fiber L.) bred in farm conditions. Nat. Sci. 2000, 6, 192–199. [Google Scholar]
- Zalewski, K.; Martysiak-Żurowska, D.; Chylińska-Ptak, M.; Nitkiewicz, B. Characterization of Fatty Acid Composition in the European Beaver (Castor fiber L.). Pol. J. Environ. Stud. 2009, 18, 493–499. [Google Scholar]
- Appavoo, D.M.; Kubow, S.; Kuhnlein, H.V. Lipid Composition of Indigenous Foods Eaten by the Sahtú (Hareskin) Dene-Metis of the Northwest Territories. J. Food Comp. Anal. 1991, 4, 107–119. [Google Scholar] [CrossRef]
- Żochowska-Kujawska, J.; Lachowicz, K.; Sobczak, M.; Bienkiewicz, G. Compositional characteristics and nutritional quality of European beaver (Castor fiber L.) meat and its utility for sausage production. Czech J. Food Sci. 2016, 34, 87–92. [Google Scholar] [CrossRef]
- Florek, M.; Drozd, L.; Skałecki, P.; Domaradzki, P.; Litwińczuk, A.; Tajchman, K. Proximate composition and physicochemical properties of European beaver (Castor fiber L.) meat. Meat Sci. 2017, 123, 8–12. [Google Scholar] [CrossRef]
- Klupsaite, D.; Buckiuniene, V.; Sidlauskiene, S.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Klementaviciute, J.; Viskontaite, E.; Bartkiene, E. Comparison studies of the chemical, physical, technological, and microbiological characteristics of the European roe deer, boar, red deer, and beaver hunted wild game meat. Anim. Sci. J. 2020, 91, e13346. [Google Scholar] [CrossRef]
- Domaradzki, P.; Florek, M.; Skałecki, P.; Litwińczuk, A.; Kędzierska-Matysek, M.; Wolanciuk, A.; Tajchman, K. Fatty acid composition, cholesterol content and lipid oxidation indices of intramuscular fat from skeletal muscles of beaver (Castor fiber L.). Meat Sci. 2019, 150, 131–140. [Google Scholar] [CrossRef]
- Razmaitė, V.; Pileckas, V.; Juškiene, V. Effect of muscle anatomical location on fatty acid composition of beaver (Castor fiber) females. Czech J. Food Sci. 2019, 37, 106–111. [Google Scholar] [CrossRef]
- Razmaitė, V.; Šveistienė, R.; Švirmickas, G.J. Compositional characteristics and nutritional quality of Eurasian beaver (Castor fiber) meat. Czech J. Food Sci. 2011, 29, 480–486. [Google Scholar] [CrossRef]
- Strazdina, V.; Sterna, V.; Jemeljanovs, A.; Jansons, I.; Ikauniece, D. Investigation of beaver meat obtained in Latvia. Agron. Res. 2015, 13, 1096–1103. [Google Scholar]
- Florek, M.; Domaradzki, P.; Drozd, L.; Skałecki, P.; Tajchman, K. Chemical composition, amino acid and fatty acid contents, and mineral concentrations of European beaver (Castor fiber L.) meat. J. Food Meas. Charact. 2017, 11, 1035–1044. [Google Scholar] [CrossRef]
- Ziomek, M.; Drozd, Ł.; Gondek, M.; Pyz-Łukasik, R.; Pedonese, F.; Florek, M.; Domaradzki, P.; Skałecki, P. Microbiological changes in meat and minced meat from beavers (Castor fiber L.) during refrigerated and frozen storage. Foods 2021, 10, 1270. [Google Scholar] [CrossRef] [PubMed]
- Florek, M.; Domaradzki, P.; Skałecki, P.; Ryszkowska-Siwko, M.; Ziomek, M.; Tajchman, K.; Gondek, M.; Pyz-Łukasik, R. Content and solubility of collagen and their relationships to proximate composition and shear force of meat from different anatomical location in carcass of European beaver (Castor fiber). Foods 2022, 11, 1288. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, B.; Żmijewski, T.; Kwiatkowska, A.; Korzeniowski, W. The composition and properties of beaver (Castor fiber) meat. Eur. J. Wildl. Res. 2005, 51, 283–286. [Google Scholar] [CrossRef]
- Korbelová, J.; Vorel, A.; Uhlíková, J. Biology and Ecology of Beavers. In Handbook for Coexisting with Beavers; Vorel, A., Korbelová, J., Eds.; Czech University of Life Sciences Prague: Prague, Czech Republic, 2016; pp. 98–117. [Google Scholar]
- Yang, A.; McLennan, S.R.; Armstrong, J.; Larsen, T.W.; Shaw, F.D.; Tume, R.K. Effect of short-term grain feeding on bovine body fat colour: A cautionary note. Aust. J. Agric. Res. 1993, 44, 215–220. [Google Scholar] [CrossRef]
- Girardi, F.; Cardozo, R.M.; de Souza, V.L.F.; de Moraes, G.V.; dos Santos, C.R.; Visentainer, J.V.; Zara, R.F.; de Souza, N.E. Proximate composition and fatty acid profile of semi confined young capybara (Hydrochoerus hydrochaeris hydrochaeris L. 1766) meat. J. Food Comp. Anal. 2005, 18, 647–654. [Google Scholar] [CrossRef]
- Pinto, M.F.; Ponsano, E.H.; Almeida, A.D.; Heinemann, R.J.; de Souza, W.M. Characteristics and technological potential of the capybara meat. Cienc. Rural 2007, 37, 868–872. [Google Scholar] [CrossRef]
- Sampels, S.; Wiklund, E.; Pickova, J. Influence of diet on fatty acids and tocopherols in M. Longissimus Dorsi from reindeer. Lipids 2006, 41, 463–472. [Google Scholar] [CrossRef]
- Pedrazzoli, M.; Dal Bosco, A.; Castellini, C.; Ranucci, D.; Mattioli, S.; Pauselli, M.; Roscini, V. Effect of age and feeding area on meat quality of wild boars. Ital. J. Anim. Sci. 2017, 16, 353–362. [Google Scholar] [CrossRef]
- Tejerina, D.; García-Torres, S.; Cabeza De Vaca, M.; Vázquez, F.M.; Cava, R. Effect of production system on physical-chemical, antioxidant and fatty acids composition of Longissimus dorsi and Serratus ventralis muscles from Iberian pig. Food Chem. 2012, 133, 293–299. [Google Scholar] [CrossRef]
- Realini, C.E.; Duckett, S.K.; Brito, G.W.; Dalla Rizza, M.; De Mattos, D. Effect of pasture vs. concentrate feeding with or without antioxidants on carcass characteristics, fatty acid composition, and quality of Uruguayan beef. Meat Sci. 2004, 66, 567–577. [Google Scholar] [CrossRef]
- Gerber, N.; Scheeder, M.R.L.; Wenk, C. The influence of cooking and fat trimming on the actual nutrient intake from meat. Meat Sci. 2009, 81, 148–154. [Google Scholar] [CrossRef]
- Kaliniak-Dziura, A.; Domaradzki, P.; Kowalczyk, M.; Florek, M.; Skałecki, P.; Kędzierska-Matysek, M.; Stanek, P.; Dmoch, M.; Grenda, T.; Kowalczuk-Vasilev, E. Effect of heat treatments on the physicochemical and sensory properties of the longissimus thoracis muscle in unweaned Limousin calves. Meat Sci. 2022, 192, 108881. [Google Scholar] [CrossRef]
- Purchas, R.W.; Triumf, E.; Egelandsdal, B. Quality characteristics and composition of the longissimus muscle in the short-loin male and female farmed red deer in New Zealand. Meat Sci. 2010, 86, 505–510. [Google Scholar] [CrossRef]
- Okabe, Y.; Watanabe, A.; Shingu, H.; Kushibiki, S.; Hodate, K.; Ishida, M. Effects of α-tocopherol level in raw venison on lipid oxidation and volatiles during storage. Meat Sci. 2002, 62, 457–462. [Google Scholar] [CrossRef]
- Quaresma, M.A.G.; Alves, S.P.; Trigo-Rodrigues, I.; Pereira-Silva, R.; Santos, N.; Lemos, J.P.C.; Barreto, A.S.; Bessa, R.J.B. Nutritional evaluation of the lipid fraction of feral wild boar (Sus scrofa scrofa) meat. Meat Sci. 2011, 89, 457–461. [Google Scholar] [CrossRef]
- Faustman, C.; Cassens, R.G.; Schaefer, D.M.; Buege, D.R.; Williams, S.N.; Scheller, K.K. Improvement of pigment and lipid stability in Holstein steer beef by dietary supplementation with vitamin E. J. Food Sci. 1989, 54, 858–862. [Google Scholar] [CrossRef]
- Arnold, R.N.; Arp, S.C.; Scheller, K.K.; Williams, S.N.; Schaefer, D.M. Tissue equilibrium and subcellular distribution of vitamin E relative to myoglobin and lipid oxidation in displayed beef. J. Anim. Sci. 1993, 71, 105–118. [Google Scholar] [CrossRef]
- Carpenter, C.E.; Clark, E. Evaluation of methods used in meat iron analysis and iron content of raw and cooked meats. J. Agric. Food Chem. 1995, 43, 1824–1827. [Google Scholar] [CrossRef]
- Kagan, V.E.; Quinn, P.J. The interaction of a-tocopherol and homologues with shorter hydrocarbon chains with phospholipids bilayer dispersions. A fluorescence probe study. Eur. J. Biochem. 1988, 171, 661–667. [Google Scholar] [CrossRef]
- Combs, G.F. The Vitamins: Fundamental Aspects in Nutrition and Health, 3rd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2007; ISBN 9780080561301. [Google Scholar]
- Erickson, M.C. Lipid oxidation of muscle foods. In Food Lipids: Chemistry, Nutrition, and Biotechnology; Akoh, C.C., Min, D.B., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 384–430. [Google Scholar]
- Jensen, C.; Lauridsen, C.; Bertelsen, G. Dietary Vitamin E: Quality and Storage Stability of Pork and Poultry. Trends Food Sci. Technol. 1998, 9, 62–72. [Google Scholar] [CrossRef]
- Yang, A.; Brewster, M.J.; Lanari, M.C.; Tume, R.K. Effect of vitamin E supplementation on α-tocopherol and β-carotene concentrations in tissues from pasture- and grain-fed cattle. Meat Sci. 2002, 60, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Parker, R.; Richards, M. Factors affecting lipid oxidation in breast and thigh muscle from chicken, turkey and duck. J. Food Biochem. 2010, 34, 869–885. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M.; Chabani, Z.; Farag, M.A.; Domínguez, R. Measurement of Antioxidant Capacity of Meat and Meat Products: Methods and Applications. Molecules 2021, 26, 3880. [Google Scholar] [CrossRef] [PubMed]
- Sohaib, M.; Butt, M.S.; Shabbir, M.A.; Shahid, M. Lipid stability, antioxidant potential and fatty acid composition of broilers breast meat as influenced by quercetin in combination with α-tocopherol enriched diets. Lipids Health Dis. 2015, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Morcuende, D.; Cava, R. Extensively reared Iberian pigs versus intensively reared white pigs for the manufacture of frankfurters. Meat Sci. 2006, 72, 356–364. [Google Scholar] [CrossRef]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary Polyphenol Supplementation in Food Producing Animals: Effects on the Quality of Derived Products. Animals 2021, 11, 401. [Google Scholar] [CrossRef]
- Nakayama, T.; Kajiya, K.; Kumazawa, S. Interaction of Plant Polyphenols with Liposomes. Adv. Planar Lipid Bilayers Liposomes 2006, 4, 107–133. [Google Scholar] [CrossRef]
- Karonen, M. Insights into Polyphenol–Lipid Interactions: Chemical Methods, Molecular Aspects and Their Effects on Membrane Structures. Plants 2022, 11, 1809. [Google Scholar] [CrossRef]
- Korzeniowska, M.; Króliczewska, B.; Kopeć, W. Carbonyl and sulfhydryl groups of chicken meat proteins after dietary modulation with selenium. Open Chem. 2015, 13, 1293–1302. [Google Scholar] [CrossRef]
- Carrillo, C.; Barrio, Á.; del Mar Cavia, M.; Alonso-Torre, S. Global antioxidant response of meat. J. Sci. Food Agric. 2017, 97, 2358–2365. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Decker, E.A.; Livisay, S.A.; Zhou, S. Mechanisms of endogenous skeletal muscle antioxidants: Chemical and physical aspects. In Antioxidants in Muscle Foods; Decker, E., Faustman, C., Lopez-Bote, C.J., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 2000; pp. 39–47. [Google Scholar]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Zhang, Q.; Tombline, G.; Ablaeva, J.; Zhang, L.; Zhou, X.; Smith, Z.; Zhao, Y.; Xiaoli, A.M.; Wang, Z.; Lin, J.R.; et al. Genomic expansion of Aldh1a1 protects beavers against high metabolic aldehydes from lipid oxidation. Cell Rep. 2021, 37, 109965. [Google Scholar] [CrossRef]
- Stewart, G.; Gosselin, C.; Pandian, S. Selected ion monitoring of tert-butyldimethylsilyl cholesterol ethers for determination of total cholesterol content in foods. Food Chem. 1992, 44, 377–380. [Google Scholar] [CrossRef]
- Bragagnolo, N.; Rodriguez-Amaya, D.B. Total Lipid, Cholesterol, and Fatty Acids of Farmed Freshwater Prawn (Macrobrachium rosenbergii) and Wild Marine Shrimp (Penaeus brasiliensis, Penaeus schimitti, Xiphopenaeus kroyeri). J. Food Comp. Anal. 2001, 14, 359–369. [Google Scholar] [CrossRef]
- Singleton, V.A.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Mouly, P.P.; Gaydou, E.M.; Corsetti, J. Determination of the geographical origin of Valencia orange juice using carotenoid liquid chromatographic profiles. J. Chromatogr. A 1999, 844, 149–159. [Google Scholar] [CrossRef]
| Compound | Loin (Longissimus thoracis and Longissimus lumborum) | Hind Leg (Semimembranosus, Biceps femoris and Semitendinosus) | Tail | Subcutaneous Fat |
|---|---|---|---|---|
| Moisture | 76.16 C ± 0.14 | 75.75 C ± 0.20 | 25.37 B ± 3.28 | 16.46 A ± 0.91 |
| Protein | 22.13 C ± 0.17 | 21.86 C ± 0.29 | 11.65 B ± 1.58 | 6.22 A ± 0.55 |
| Fat | 0.71 A ± 0.10 | 1.54 A ± 0.32 | 61.69 B ± 2.97 | 76.62 C ± 1.11 |
| Ash | 1.13 B ± 0.07 | 1.12 B ± 0.05 | 0.26 A ± 0.03 | 0.23 A ± 0.02 |
| Cholesterol | 49.76 BC ± 0.07 | 49.17 AB ± 0.04 | 44.87 A ± 0.03 | 52.85 C ± 0.05 |
| Compound | Loin (Longissimus thoracis and Longissimus lumborum) | Hind Leg (Semimembranosus, Biceps femoris and Semitendinosus) | Tail | Subcutaneous Fat |
|---|---|---|---|---|
| Retinol (µg/100 g) | 8.61 A ± 0.14 | 9.56 A ± 0.13 | 13.02 B ± 0.10 | 12.23 B ± 0.43 |
| α-Tocopherol (µg/100 g) | 366.29 B ± 0.39 | 356.59 A ± 1.35 | 391.16 ± 0.23 | 371.32 BC ± 0.21 |
| Total phenolics (mg GAE/100 g) | 150.94 A ± 1.10 | 167.33 A ± 1.55 | 543.69 B ± 23.98 | 186.29 A ± 4.35 |
| DPPH (mM TE/100 g) | 2.34 A ± 0.02 | 2.51 AB ± 0.06 | 3.07 C ± 0.07 | 2.68 BC ± 0.01 |
| ABTS (mM TE/100 g) | 2.58 A ± 0.02 | 2.78 AB ± 0.04 | 3.33 C ± 0.03 | 3.01 BC ± 0.05 |
| Variable | Retinol | α-Tocopherol | Total Phenolics | DPPH | ABTS |
|---|---|---|---|---|---|
| Moisture | −0.885 ** | −0.692 ** | −0.539 ** | −0.754 ** | −0.815 ** |
| Ash | −0.880 ** | −0.718 ** | −0.578 * | −0.787 ** | −0.831 ** |
| Fat | 0.873 ** | 0.669 ** | 0.497 * | 0.733 ** | 0.800 ** |
| Protein | −0.777 ** | −0.552 ** | −0.330 | −0.617 ** | −0.697 ** |
| Cholesterol | −0.215 | −0.621 ** | −0.810 ** | −0.586 ** | −0.481 * |
| Retinol | – | 0.748 ** | 0.666 ** | 0.858 ** | 0.894 ** |
| α-Tocopherol | – | – | 0.913 ** | 0.886 ** | 0.851 ** |
| Total phenolics | – | – | – | 0.907 ** | 0.852 ** |
| DPPH | – | – | – | – | 0.989 ** |
| Component | PC1 | PC2 |
|---|---|---|
| Eigenvalue | 7.40 | 2.11 |
| Proportion of variance (%) | 74.02 | 21.13 |
| Cumulative of variance (%) | 74.02 | 95.16 |
| Variable | ||
| Moisture (%) | 0.917 | −0.368 |
| Protein (%) | 0.807 | −0.525 |
| Fat (%) | −0.906 | 0.413 |
| Ash (%) | 0.925 | −0.291 |
| Cholesterol | 0.341 | 0.936 |
| Retinol | −0.935 | 0.109 |
| α-Tocopherol | −0.883 | −0.345 |
| Total phenolics | −0.801 | −0.580 |
| DPPH | −0.944 | −0.280 |
| ABTS | −0.964 | −0.160 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florek, M.; Domaradzki, P.; Skałecki, P.; Stryjecka, M.; Tajchman, K.; Kaliniak-Dziura, A.; Teter, A.; Kędzierska-Matysek, M. Antioxidant Properties and Proximate Composition of Different Tissues of European Beaver. Molecules 2022, 27, 8973. https://doi.org/10.3390/molecules27248973
Florek M, Domaradzki P, Skałecki P, Stryjecka M, Tajchman K, Kaliniak-Dziura A, Teter A, Kędzierska-Matysek M. Antioxidant Properties and Proximate Composition of Different Tissues of European Beaver. Molecules. 2022; 27(24):8973. https://doi.org/10.3390/molecules27248973
Chicago/Turabian StyleFlorek, Mariusz, Piotr Domaradzki, Piotr Skałecki, Małgorzata Stryjecka, Katarzyna Tajchman, Agnieszka Kaliniak-Dziura, Anna Teter, and Monika Kędzierska-Matysek. 2022. "Antioxidant Properties and Proximate Composition of Different Tissues of European Beaver" Molecules 27, no. 24: 8973. https://doi.org/10.3390/molecules27248973
APA StyleFlorek, M., Domaradzki, P., Skałecki, P., Stryjecka, M., Tajchman, K., Kaliniak-Dziura, A., Teter, A., & Kędzierska-Matysek, M. (2022). Antioxidant Properties and Proximate Composition of Different Tissues of European Beaver. Molecules, 27(24), 8973. https://doi.org/10.3390/molecules27248973







