Analysis of Volatile Compounds from Different Parts of Houttuynia cordata Thunb.
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of Volatile Compounds from Different Parts of H. cordata
2.2. Comparison of Different Extractions
3. Materials and Methods
3.1. Plant Materials
3.2. Analytical Methods
3.2.1. Extraction of Volatile Compounds from Fresh H. cordata by HS-SPME
3.2.2. Extraction of Essential Oil from H. cordata
3.2.3. Gas Chromatography-Flame Ionization Detector (GC-FID)
3.2.4. Gas Chromatography-Mass Spectrometry (GC-MS)
3.2.5. Analysis of Essential Oil from H. cordata
3.2.6. Retention Index (RI) Comparison
3.2.7. Relative Percentage Calculation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fu, J.; Dai, L.; Lin, Z.; Lu, H. Houttuynia cordata Thunb.: A review of phytochemistry and pharmacology and quality control. Chin. Med. 2013, 4, 101–123. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, J.G. Bioactive components and functional properties of Hottuynia cordata and its applications. Pharm. Biol. 2009, 47, 1154–1161. [Google Scholar] [CrossRef]
- Kumar, M.; Prasad, S.K.; Hemalatha, S. A current update on the phytopharmacological aspects of Houttuynia cordata Thunb. Pharmacogn. Rev. 2014, 8, 22–35. [Google Scholar] [CrossRef]
- Hayashi, K.; Kamiya, M.; Hayashi, T. Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, Influenza Virus, and HIV. Planta Med. 1995, 61, 237–241. [Google Scholar] [CrossRef]
- Nuengchamnong, N.; Krittasilp, K.; Ingkaninan, K. Rapid screening and identification of antioxidants in aqueous extracts of Houttuynia cordata using LC–ESI–MS coupled with DPPH assay. Food Chem. 2009, 117, 750–756. [Google Scholar] [CrossRef]
- Yang, L.; Ji, W.; Zhong, H.; Wang, L.; Zhu, X.; Zhu, J. Anti-tumor effect of volatile oil from Houttuynia cordata Thunb. on HepG2 cells and HepG2 tumor-bearing mice. RSC Adv. 2019, 9, 31517–31526. [Google Scholar] [CrossRef]
- Lou, Y.; Guo, Z.; Zhu, Y.; Kong, M.; Zhang, R.; Lu, L.; Wu, F.; Liu, Z.; Wu, J. Houttuynia cordata Thunb. and its bioactive compound 2-undecanone significantly suppress benzo[a]pyrene-induced lung tumorigenesis by activating the Nrf2-HO-1/NQO-1 signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 1–21. [Google Scholar] [CrossRef]
- Li, W.; Yang, F.; Zhan, H.; Liu, B.; Cai, J.; Luo, Y.; Zhou, X. Houttuynia cordata extract ameliorates bladder damage and improves bladder symptoms via anti-inflammatory effect in rats with interstitial cystitis. Evid. Based Complement. Altern. Med. 2020, 2020, 9026901. [Google Scholar] [CrossRef]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Mochona, B.; Qi, X. Biosynthetic factories of essential oils: The aromatic plants. Nat. Prod. Chem. Res. 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An overview of the biological effects of some Mediterranean essential oils on human health. BioMed Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef]
- Manion, C.R.; Widder, R.M. Essentials of essential oils. Am. J. Health Syst. Pharm. 2017, 74, e153–e162. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y. An effective quality control of pharmacologically active volatiles of Houttuynia cordata Thunb. by fast gas chromatography-surface acoustic wave sensor. Molecules 2015, 20, 10298–10312. [Google Scholar] [CrossRef] [PubMed]
- Kosuge, T. Structure of an antimicrobial substance isolated from Houttuynia cordata Thunb. Yakugaku Zasshi 1952, 72, 1227–1231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dong, X.Y.; Jin, Y.; Yang, L.P. Systematic analysis of components and contents in Houttuynia cordata Thunb. Tradit. Med. Res. 2017, 2, 176–188. [Google Scholar] [CrossRef]
- Liang, M.; Qi, M.; Zhang, C.; Zhou, S.; Fu, R.; Huang, J. Gas chromatography–mass spectrometry analysis of volatile compounds from Houttuynia cordata Thunb. after extraction by solid-phase microextraction, flash evaporation and steam distillation. Anal. Chim. Acta 2005, 531, 97–104. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Shi, C.; Fang, J. A comparative study of sodium houttuyfonate and 2-undecanone for their in vitro and in vivo anti-inflammatory activities and stabilities. Int. J. Mol. Sci. 2014, 15, 22978–22994. [Google Scholar] [CrossRef]
- Yang, Z.N.; Luo, S.Q.; Ma, J.; Wu, D.; Hong, L.; Yu, Z.W. GC-MS analyses of the volatiles of Houttuynia cordata Thunb. Pak. J. Pharm. Sci. 2016, 29, 1591–1600. [Google Scholar]
- Asakawa, Y.; Tomiyama, K.; Sakurai, K.; Kawakami, Y.; Yaguchi, Y. Volatile compounds from the different organs of Houttuynia cordata and Litsea cubeba (L. citriodora). J. Oleo Sci. 2017, 66, 889–895. [Google Scholar] [CrossRef]
- Xu, Y.W.; Cai, Q.R.; Zhao, D.; Wu, W. Monoterpene composition of flower and bract from Houttuynia cordata. J. Med. Plants Res. 2011, 5, 3883–3886. [Google Scholar]
- Chen, H.C.; Tsai, Y.J.; Lin, L.Y.; Wu, C.S.; Tai, S.P.; Chen, Y.C.; Chiang, H.M. Volatile compounds from roots, stems and leaves of Angelica acutiloba growing in Taiwan. Nat. Prod. Commun. 2014, 9, 583–586. [Google Scholar] [CrossRef]
- Ogunwande, I.A.; Olawore, N.O.; Adeleke, K.A.; Ekundayo, O.; Koenig, W.A. Chemical composition of the leaf volatile oil of Psidium guajava L. growing in Nigeria. Flavour Fragr. J. 2003, 18, 136–138. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 24 November 2022).
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 24 November 2022).
- The Pherobase. Available online: http://www.pherobase.com (accessed on 24 November 2022).
- Terpenoids Library List. Available online: http://massfinder.com/wiki/Terpenoids_Library_List (accessed on 24 November 2022).
- Haghighi, T.M.; Saharkhiz, M.J.; Khosravi, A.R.; Fard, F.R.; Moein, M. Essential oil content and composition of Vitex pseudo-negundo in Iran varies with ecotype and plant organ. Ind. Crops Prod. 2017, 109, 53–59. [Google Scholar] [CrossRef]
- Zribi, I.; Bleton, J.; Moussa, F.; Abderrabba, M. GC-MS analysis of the volatile profile and the essential oil compositions of Tunisian Borago Officinalis L.: Regional locality and organ dependency. Ind. Crops Prod. 2019, 129, 290–298. [Google Scholar] [CrossRef]
- Farag, M.A.; Wessjohann, L.A. Volatiles profiling in medicinal licorice roots using steam distillation and solid-phase microextraction (SPME) coupled to chemometrics. J. Food Sci. 2012, 77, C1179–C1184. [Google Scholar] [CrossRef]
- Peng, L.W.; Sheu, M.J.; Lin, L.Y.; Wu, C.T.; Chiang, H.M.; Lin, W.H.; Lee, M.C.; Chen, H.C. Effect of heat treatments on the essential oils of kumquat (Fortunella margarita Swingle). Food Chem. 2013, 136, 532–537. [Google Scholar] [CrossRef]
- Gao, X.; Lv, S.; Wu, Y.; Li, J.; Zhang, W.; Meng, W.; Wang, C.; Meng, Q. Volatile components of essential oils extracted from Pu-erh ripe tea by different extraction methods. Int. J. Food Prop. 2017, 20, S240–S253. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, Y.; Liu, B.; Fang, X.; Yang, W.; Xu, J. Comparison of headspace solid-phase microextraction with conventional extraction for the analysis of the volatile components in Melia azedarach. Talanta 2011, 86, 356–361. [Google Scholar] [CrossRef]
- Kung, T.L.; Chen, Y.J.; Chao, L.K.; Wu, C.S.; Lin, L.Y.; Chen, H.C. Analysis of Volatile Constituents in Platostoma palustre (Blume) Using Headspace Solid-Phase Microextraction and Simultaneous Distillation-Extraction. Foods 2019, 8, 415. [Google Scholar] [CrossRef]
- Curvers, J.; Rijks, J.; Cramers, C.; Knauss, K.; Larson, P. Temperature Programmed Retention Indices: Calculation from Isothermal Data Part 1: Theory. J. High Resolut. Chromatogr. Chromatogr. Commun. 1985, 8, 607–610. [Google Scholar] [CrossRef]
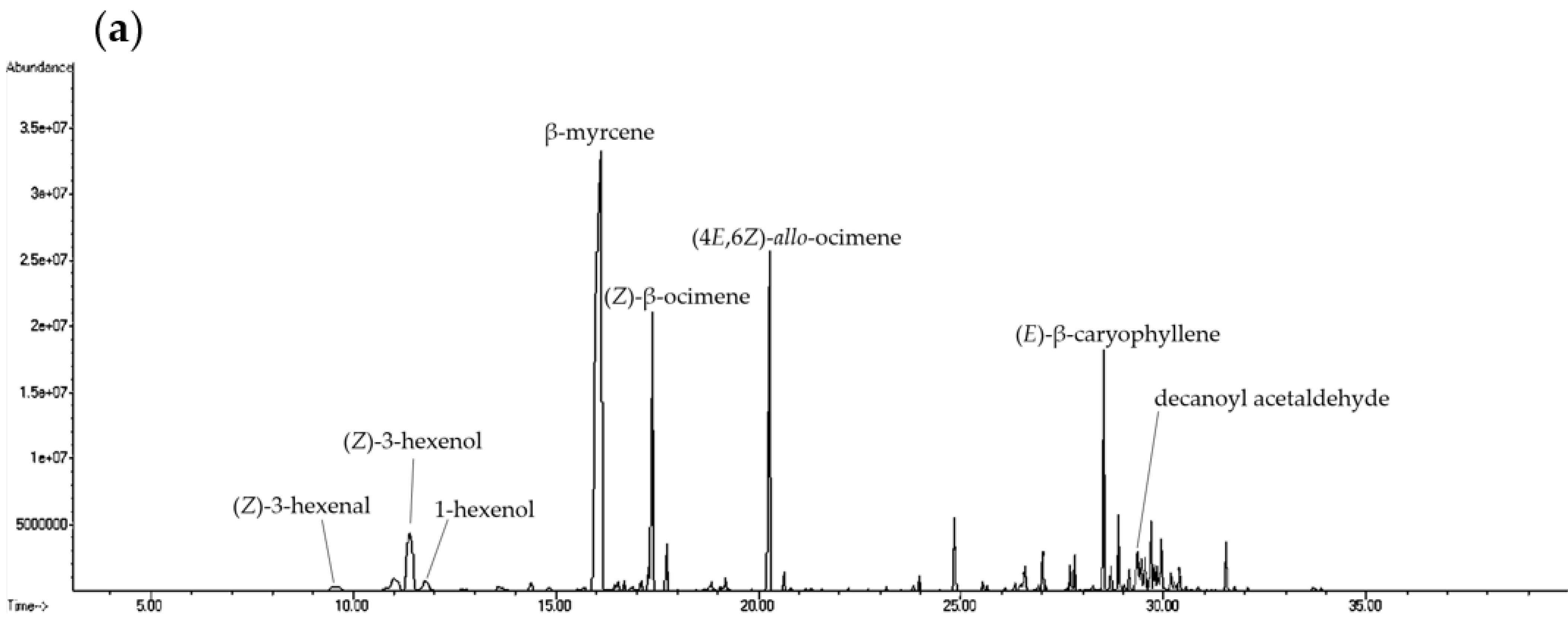
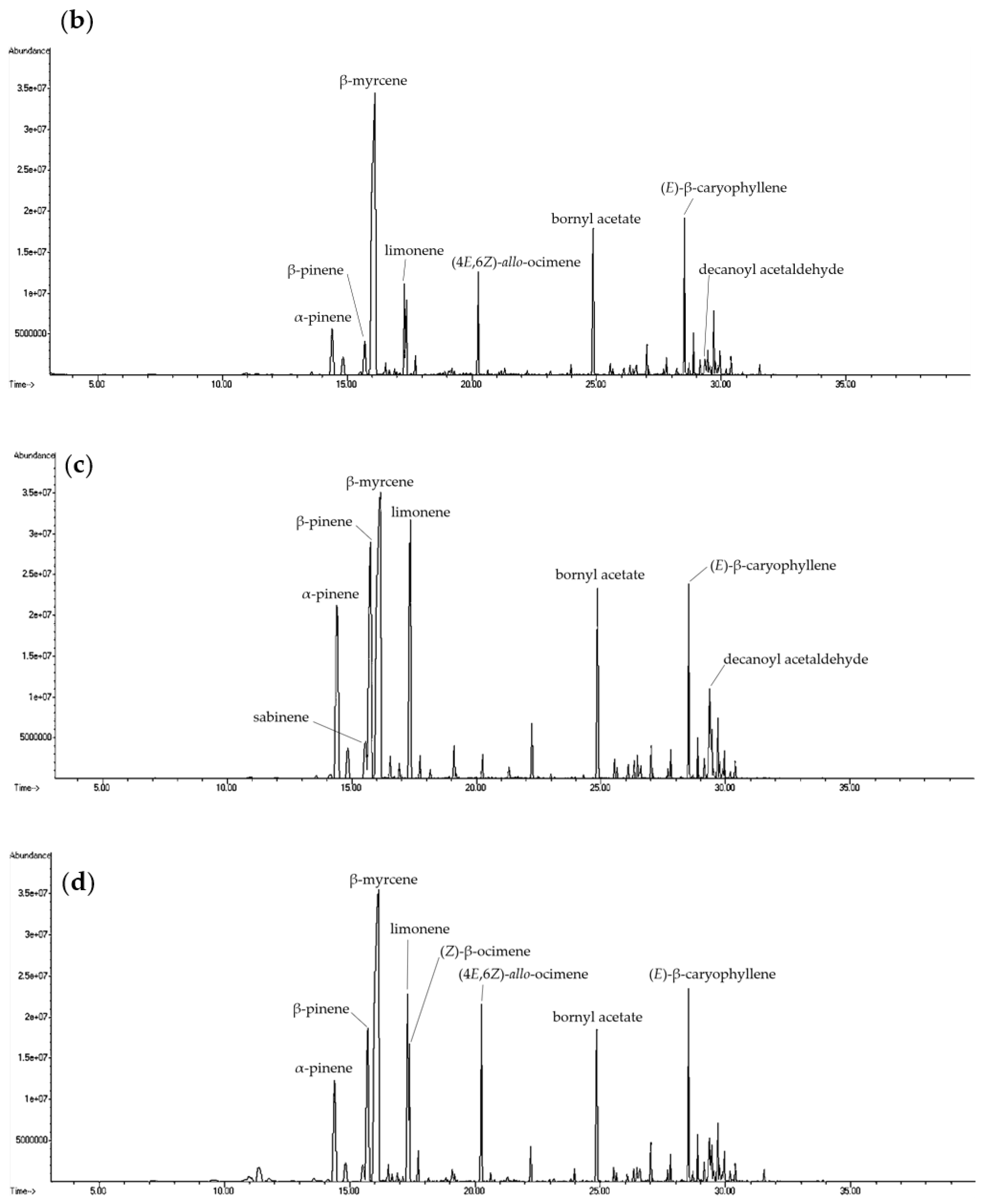
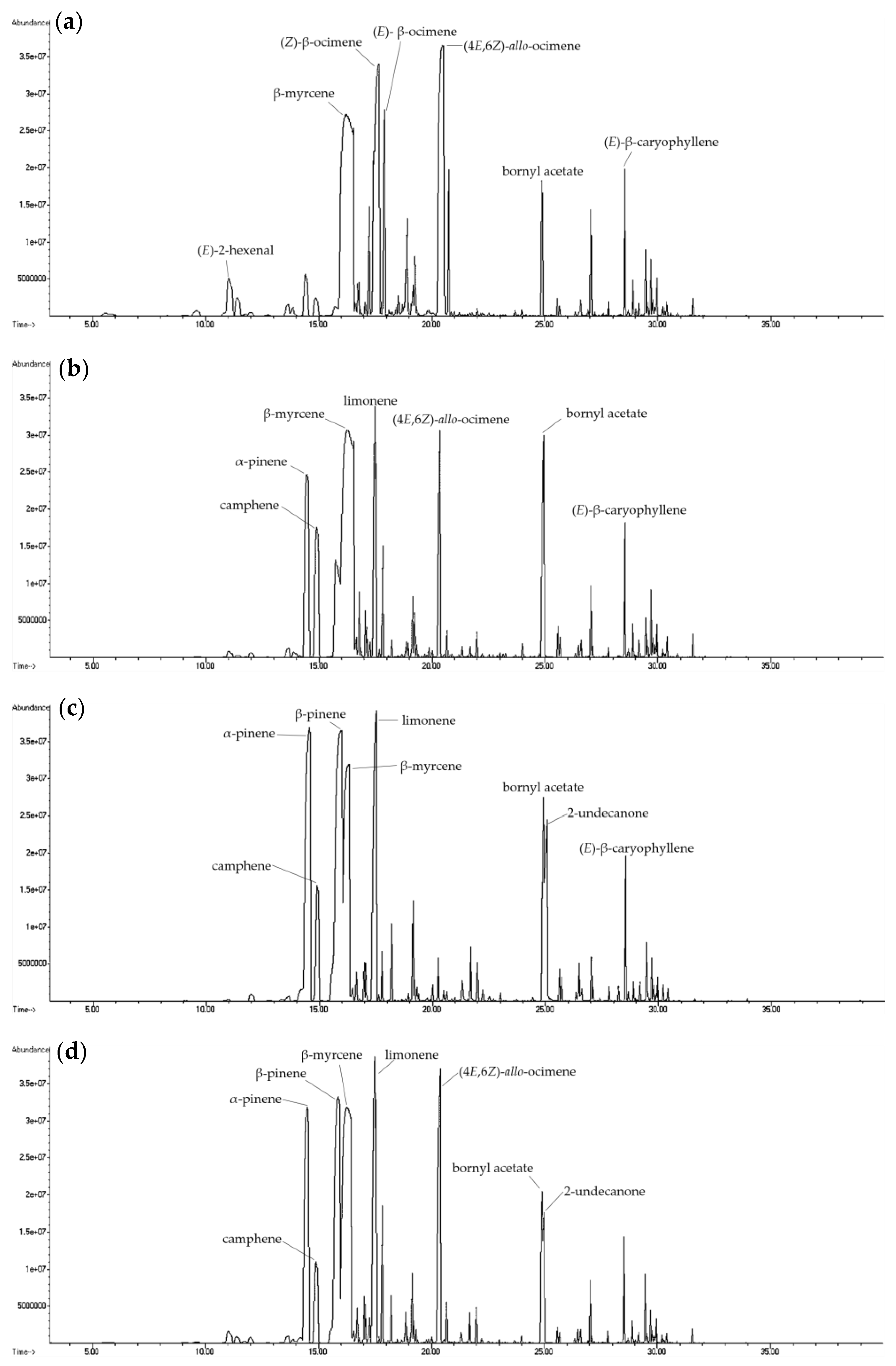
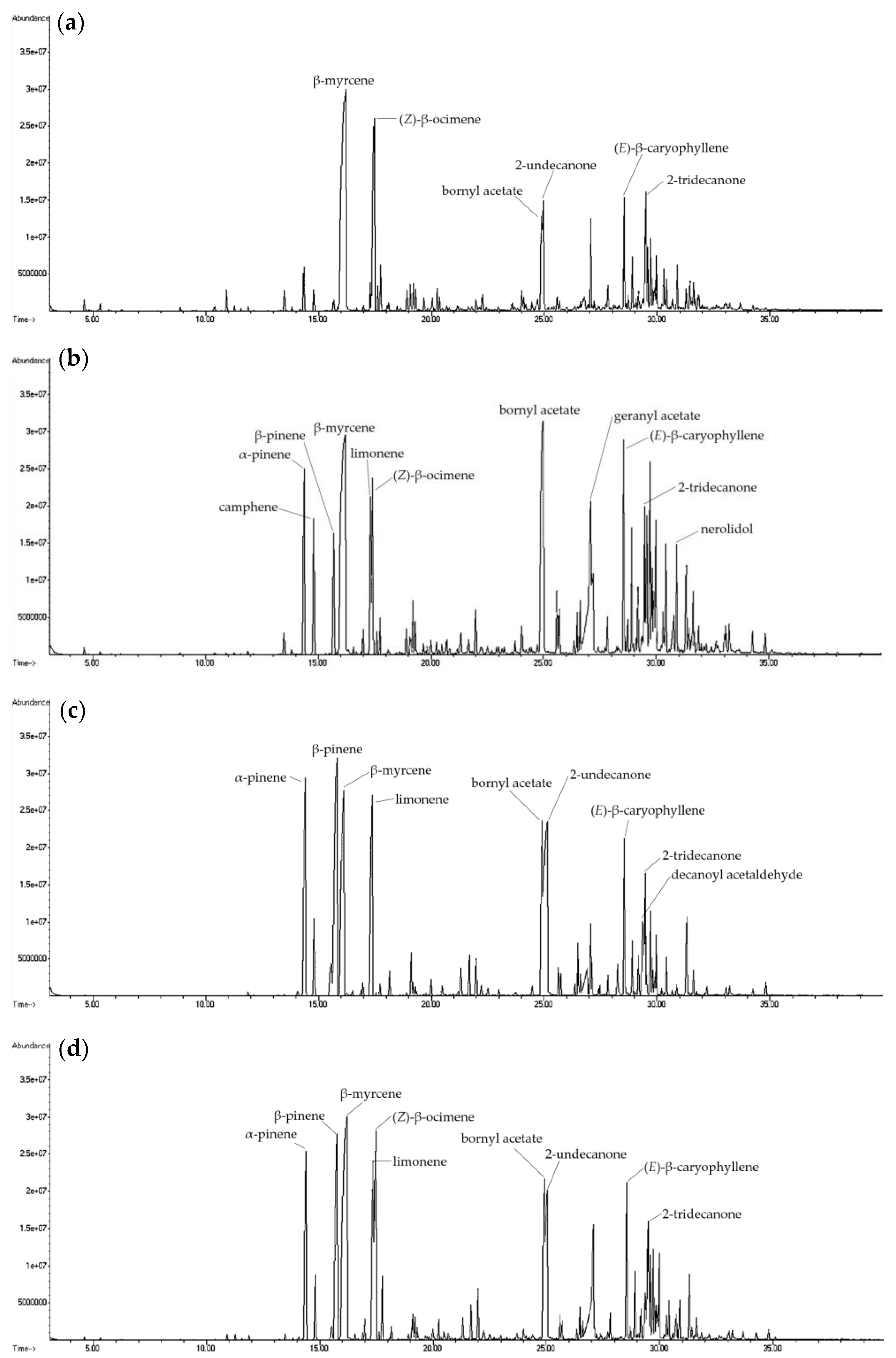
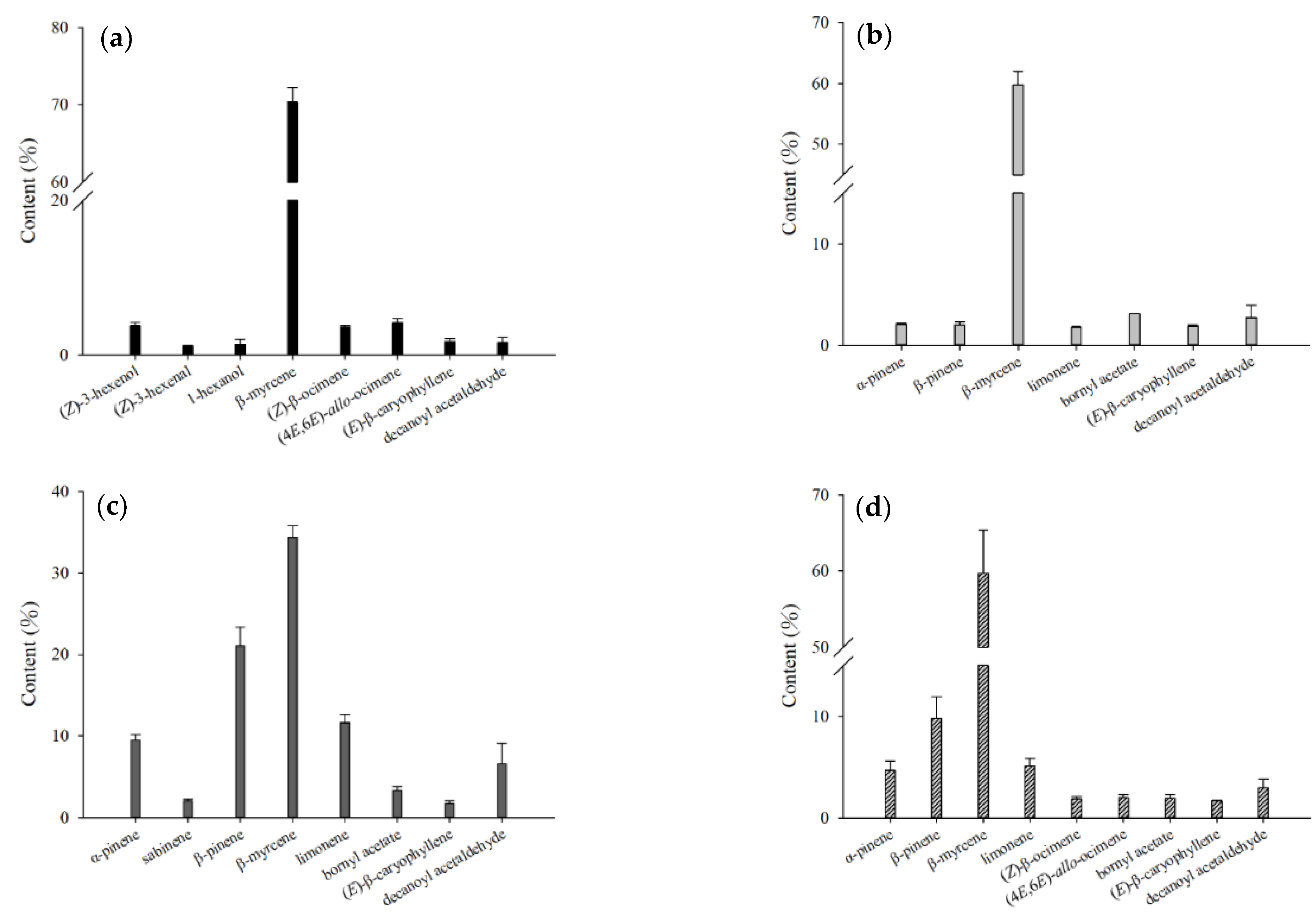
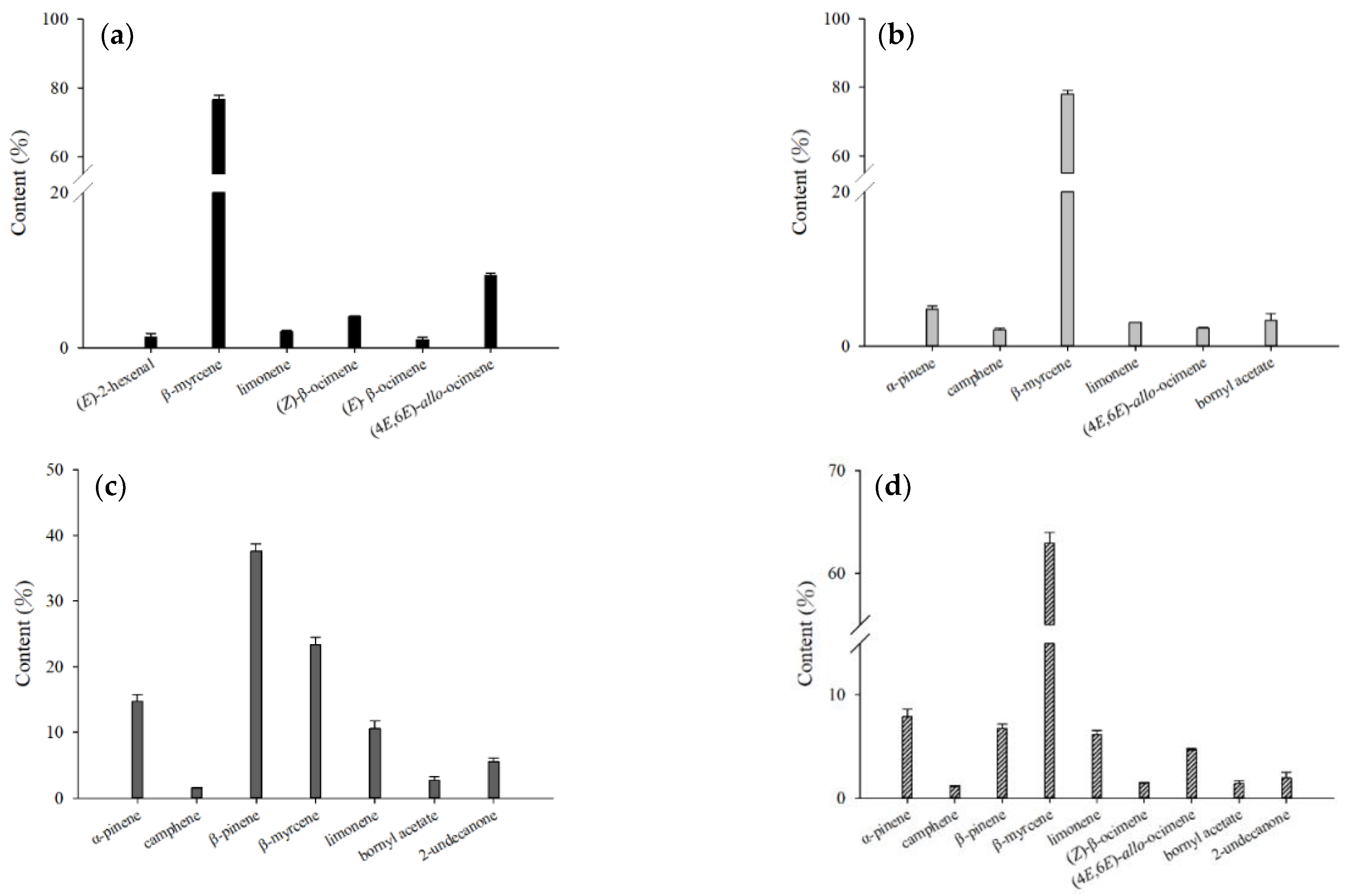
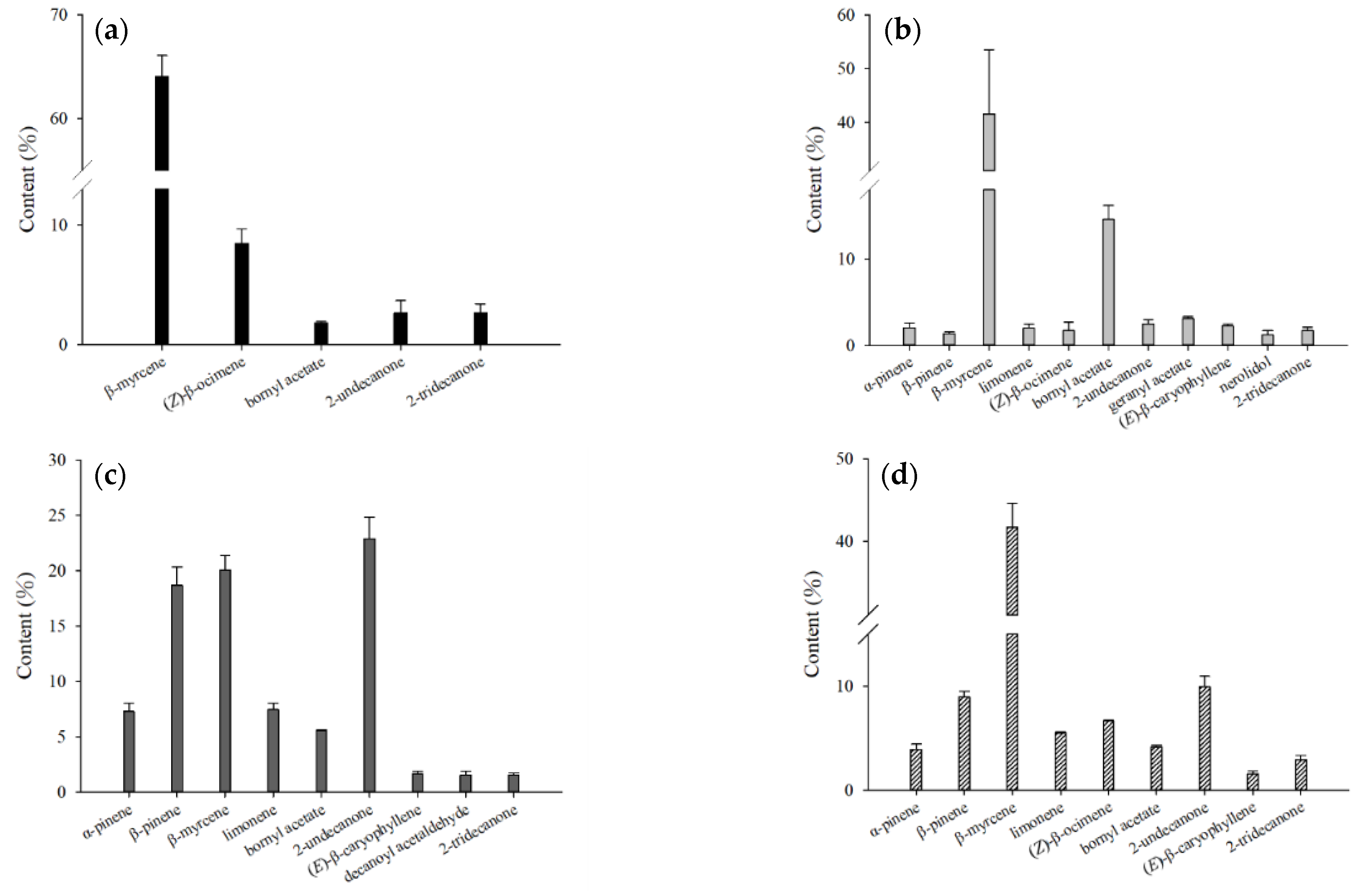
| Compound | CAS Number | Molecular Formula | RI a | RI b | Relative Content (%) c | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HS-SPME | Essential Oil HS-SPME | Essential Oil Direct Injection | ||||||||||||||
| Leaf | Stem | Rhizome | Whole d | Leaf | Stem | Rhizome | Whole | Leaf | Stem | Rhizome | Whole | |||||
| Aliphatic Alcohols | ||||||||||||||||
| prenol | 556-82-1 | C5H10O | 751.5 | 745 | 0.04 ± 0.01 | |||||||||||
| (Z)-3-hexenol | 928-96-1 | C6H12O | 840 | 830 | 3.76 ± 0.45 | 0.47 ± 0.37 | 0.75 ± 0.07 | 0.08 ± 0.01 | 0.29 ± 0.06 | 0.08 ± 0.05 | 0.02 ± 0.01 | 0.07 ± 0.02 | ||||
| 1-hexanol | 111-27-3 | C6H14O | 854 | 849 | 1.38 ± 0.62 | 0.23 ± 0.09 | ||||||||||
| (E)-p-menth-2-en-1-ol | 29803-81-4 | C10H18O | 1112 | 1101 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.14 ± 0.03 | 0.14 ± 0.01 | 0.11 ± 0.01 | |||||||
| 1-nonanol | 143-08-8 | C9H20O | 1157 | 1147 | <0.01 | 0.05 ± 0.04 | 0.01 ± 0.001 | 0.27 ± 0.10 | 0.02 ± 0.004 | |||||||
| 1-decanol | 112-30-1 | C10H22O | 1257 | 1256 | 0.01 ± 0.002 | 0.03 ± 0.004 | 0.08 ± 0.01 | |||||||||
| Aliphatic Aldehydes | ||||||||||||||||
| (Z)-3-hexenal | 6789-80-6 | C6H10O | 769.5 | 764 | 1.05 ± 0.12 | 0.16 ± 0.04 | 0.59 ± 0.31 | 0.09 ± 0.02 | 0.04 ± 0.01 | 0.02 ± 0.004 | ||||||
| hexanal | 66-25-1 | C6H12O | 776 | 766 | 0.01 ± 0.002 | 0.04 ± 0.01 | 0.01 ± 0.001 | |||||||||
| (E)-2-hexenal | 6728-26-3 | C6H10O | 827 | 819 | 0.74 ± 0.24 | 0.30 ± 0.14 | 1.35 ± 0.51 | 0.10 ± 0.02 | 0.31 ± 0.06 | 0.55 ± 0.12 | 0.01 ± 0.01 | 0.09 ± 0.02 | ||||
| (2E,4E)-hexadienal | 142-83-6 | C6H8O | 878 | 871 | 0.12 ± 0.08 | <0.01 | 0.03 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.003 | 0.01 ± 0.003 | ||||||
| α-campholenal | 4501-58-0 | C10H16O | 1105 | 1098 | <0.01 | 0.01 ± 0.002 | ||||||||||
| decanal | 112-31-2 | C10H20O | 1185 | 1178 | 0.15 ± 0.05 | 0.40 ± 0.02 | 0.69 ± 0.13 | 0.29 ± 0.13 | 0.02 ± 0.002 | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.13 ± 0.01 | 0.09 ± 0.002 | |||
| decanoyl acetaldehyde | 56505-80-7 | C12H22O2 | 1367 | 1.59 ± 0.70 | 2.74 ± 1.23 | 6.55 ± 2.56 | 2.96 ± 0.86 | 0.04 ± 0.01 | 0.16 ± 0.09 | 0.33 ± 0.08 | 1.54 ± 0.33 | 0.62 ± 0.16 | ||||
| Aliphatic Ester | ||||||||||||||||
| n-nonanyl acetate | 143-13-5 | C11H22O2 | 1292 | 1284 | 0.19 ± 0.19 | 0.05 ± 0.03 | ||||||||||
| Aliphatic Ketones | ||||||||||||||||
| sulcatone | 110-93-0 | C8H14O | 964 | 956 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.002 | |||||||||
| 2-nonanone | 821-55-6 | C9H18O | 1072 | 1063 | <0.01 | 0.01 ± 0.003 | 0.01 ± 0.001 | |||||||||
| 2-undecanone | 112-12-9 | C11H22O | 1274 | 1272 | 0.17 ± 0.07 | <0.01 | 5.51 ± 0.61 | 1.93 ± 0.56 | 2.67 ± 1.02 | 2.48 ± 0.47 | 22.89 ± 1.97 | 9.92 ± 1.02 | ||||
| 3-dodecanone | 1534-27-6 | C12H24O | 1370 | 1364 | <0.01 | 0.02 ± 0.001 | ||||||||||
| 2-dodecanone | 6175-49-1 | C12H24O | 1375 | 1367 | <0.01 | <0.01 | <0.01 | <0.01 | 0.08 ± 0.02 | <0.01 | 0.06 ± 0.01 | 0.04 ± 0.004 | ||||
| Compound | CAS Number | Molecular Formula | RI | RI | Relative Content (%) | |||||||||||
| HS-SPME | Essential Oil HS-SPME | Essential Oil Direct Injection | ||||||||||||||
| Leaf | Stem | Rhizome | Whole | Leaf | Stem | Rhizome | Whole | Leaf | Stem | Rhizome | Whole | |||||
| 2-tridecanone | 593-08-8 | C13H26O | 1477 | 1466 | 0.06 ± 0.02 | 0.12 ± 0.03 | 0.09 ± 0.01 | 0.17 ± 0.02 | 2.66 ± 0.76 | 1.70 ± 0.39 | 1.57 ± 0.17 | 2.93 ± 0.41 | ||||
| 2-pentadecanone | 2345-28-0 | C15H30O | 1681 | 1671 | <0.01 | 0.14 ± 0.04 | 0.05 ± 0.03 | 0.14 ± 0.04 | ||||||||
| Aromatic Compounds | ||||||||||||||||
| p-cymene | 99-87-6 | C10H14 | 1014 | 1010 | 0.03 ± 0.02 | 0.07 ± 0.002 | 0.06 ± 0.01 | 0.08 ± 0.04 | 0.03 ± 0.001 | 0.09 ± 0.01 | 0.15 ± 0.01 | 0.13 ± 0.02 | 0.04 ± 0.003 | 0.11 ± 0.01 | 0.06 ± 0.01 | 0.11 ± 0.003 |
| p-cymenene | 1195-32-0 | C10H12 | 1073 | 1068 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.004 | ||||||||
| methyl salicylate | 119-36-8 | C8H8O3 | 1172 | 1169 | <0.01 | 0.01 ± 0.002 | 0.01 ± 0.001 | 0.05 ± 0.01 | 0.03 ± 0.003 | |||||||
| Hydrocarbon | ||||||||||||||||
| nonane | 111-84-2 | C9H20 | 900 | 890 | <0.01 | 0.01 ± 0.002 | 0.01 ± 0.002 | 0.01 ± 0.003 | 0.01 ± 0.002 | 0.01 ± 0.005 | 0.01 ± 0.001 | |||||
| Monoterpenes | ||||||||||||||||
| tricyclene | 508-32-7 | C10H16 | 921 | 914 | 0.01 ± 0.001 | <0.01 | <0.01 | |||||||||
| α-thujene | 2867-05-2 | C10H16 | 924 | 917 | 0.13 ± 0.05 | 0.08 ± 0.02 | 0.19 ± 0.02 | 0.06 ± 0.01 | 0.01 ± 0.001 | 0.04 ± 0.01 | 0.02 ± 0.001 | |||||
| α-pinene | 80-56-8 | C10H16 | 933 | 925 | 0.26 ± 0.04 | 2.08 ± 0.11 | 9.45 ± 0.69 | 4.68 ± 0.92 | 0.52 ± 0.03 | 4.78 ± 0.41 | 14.74 ± 1.00 | 7.91 ± 0.72 | 0.44 ± 0.01 | 2.04 ± 0.59 | 7.28 ± 0.76 | 3.90 ± 0.51 |
| α-fenchene | 471-84-1 | C10H16 | 944 | 938 | <0.01 | |||||||||||
| camphene | 79-92-5 | C10H16 | 946 | 940 | 0.13 ± 0.01 | 0.73 ± 0.03 | 0.78 ± 0.25 | 0.73 ± 0.10 | 0.31 ± 0.01 | 2.06 ± 0.25 | 1.53 ± 0.11 | 1.10 ± 0.07 | 0.22 ± 0.01 | 1.00 ± 0.31 | 0.77 ± 0.08 | 0.55 ± 0.06 |
| sabinene | 3387-41-5 | C10H16 | 967 | 963 | 0.14 ± 0.02 | 2.07 ± 0.23 | 0.40 ± 0.09 | 0.02 ± 0.002 | 0.20 ± 0.34 | 0.19 ± 0.04 | ||||||
| β-pinene | 127-91-3 | C10H16 | 972 | 965 | 0.13 ± 0.01 | 1.98 ± 0.33 | 21.01 ± 2.32 | 9.79 ± 2.11 | 0.05 ± 0.01 | 0.94 ± 0.12 | 37.61 ± 1.16 | 6.70 ± 0.46 | 0.16 ± 0.004 | 1.39 ± 0.17 | 19.16 ± 2.07 | 8.95 ± 0.57 |
| β-myrcene | 123-35-3 | C10H16 | 983 | 983 | 70.39 ± 1.85 | 59.81 ± 2.25 | 34.34 ± 1.44 | 59.74 ± 5.65 | 76.68 ± 1.24 | 78.00 ± 1.15 | 23.32 ± 1.18 | 62.95 ± 1.03 | 64.03 ± 2.07 | 41.59 ± 12.01 | 20.08 ± 1.31 | 41.69 ± 2.87 |
| α-phellandrene | 99-83-2 | C10H16 | 998 | 992 | 0.06 ± 0.01 | 0.19 ± 0.03 | 0.10 ± 0.02 | 0.03 ± 0.002 | 0.22 ± 0.02 | 0.16 ± 0.01 | 0.01 ± 0.001 | 0.03 ± 0.02 | 0.05 ± 0.01 | |||
| δ-3-carene | 13466-78-9 | C10H16 | 1005 | 1001 | <0.01 | |||||||||||
| α-terpinene | 99-86-5 | C10H16 | 1010 | 1008 | 0.05 ± 0.01 | 0.13 ± 0.03 | 0.07 ± 0.01 | 0.03 ± 0.004 | 0.11 ± 0.003 | 0.14 ± 0.01 | 0.13 ± 0.01 | <0.01 | 0.01 ± 0.01 | 0.04 ± 0.003 | 0.06 ± 0.01 | |
| limonene | 138-86-3 | C10H16 | 1023 | 1014 | 0.18 ± 0.01 | 1.73 ± 0.15 | 11.59 ± 1.01 | 5.12 ± 0.70 | 2.06 ± 0.16 | 3.07 ± 0.04 | 10.57 ± 1.18 | 6.13 ± 0.42 | 0.26 ± 0.01 | 2.00 ± 0.43 | 7.45 ± 0.61 | 5.48 ± 0.11 |
| (Z)-β-ocimene | 3338-55-4 | C10H16 | 1028 | 1020 | 3.54 ± 0.29 | 0.61 ± 0.05 | 1.85 ± 0.25 | 3.99 ± 0.12 | 0.90 ± 0.01 | 1.40 ± 0.10 | 8.49 ± 1.21 | 1.71 ± 0.98 | 6.63 ± 0.15 | |||
| (E)-β-ocimene | 3779-61-1 | C10H16 | 1038 | 1028 | 0.54 ± 0.10 | 0.41 ± 0.04 | 0.21 ± 0.03 | 0.36 ± 0.04 | 1.06 ± 0.25 | 0.48 ± 0.01 | 0.15 ± 0.01 | 0.74 ± 0.07 | 0.35 ± 0.09 | 0.16 ± 0.09 | 0.09 ± 0.003 | 0.58 ± 0.06 |
| β-terpinene | 99-84-3 | C10H16 | 1049 | 1046 | 0.02 ± 0.001 | |||||||||||
| Compound | CAS Number | Molecular Formula | RI | RI | Relative Content (%) | |||||||||||
| HS-SPME | Essential Oil HS-SPME | Essential Oil Direct Injection | ||||||||||||||
| Leaf | Stem | Rhizome | Whole | Leaf | Stem | Rhizome | Whole | Leaf | Stem | Rhizome | Whole | |||||
| γ-terpinene | 99-85-4 | C10H16 | 1050 | 1047 | 0.12 ± 0.02 | 0.05 ± 0.01 | 0.01 ± 0.001 | 0.05 ± 0.01 | 0.29 ± 0.05 | 0.14 ± 0.02 | 0.02 ± 0.01 | 0.19 ± 0.02 | 0.07 ± 0.05 | |||
| α-terpinolene | 586-62-9 | C10H16 | 1079 | 1072 | 0.19 ± 0.02 | 0.10 ± 0.01 | 0.43 ± 0.01 | 0.21 ± 0.07 | 0.10 ± 0.001 | 0.21 ± 0.01 | 0.42 ± 0.08 | 0.29 ± 0.04 | 0.15 ± 0.03 | 0.36 ± 0.03 | 0.22 ± 0.03 | |
| (4E,6Z)-allo-ocimene | 7216-56-0 | C10H16 | 1116 | 1107 | 4.22 ± 0.44 | 0.60 ± 0.03 | 0.18 ± 0.01 | 2.00 ± 0.30 | 9.32 ± 0.33 | 2.31 ± 0.08 | 0.13 ± 0.01 | 4.66 ± 0.12 | 0.14 ± 0.003 | 0.05 ± 0.01 | 0.01 ± 0.002 | 0.11 ± 0.01 |
| (4E,6E)-allo-ocimene | 3016-19-1 | C10H16 | 1129 | 1123 | 0.33 ± 0.07 | 0.17 ± 0.05 | 0.03 ± 0.004 | 0.15 ± 0.03 | 0.39 ± 0.003 | 0.17 ± 0.002 | 0.02 ± 0.001 | 0.32 ± 0.02 | ||||
| Sesquiterpenes | ||||||||||||||||
| α-cubebene | 17699-14-8 | C15H24 | 1351 | 1348 | 0.06 ± 0.03 | 0.01 ± 0.001 | <0.01 | <0.01 | 0.05 ± 0.004 | |||||||
| α-copaene | 3856-25-5 | C15H24 | 1375 | 1377 | <0.01 | 0.01 ± 0.001 | 0.04 ± 0.005 | 0.04 ± 0.01 | 0.01 ± 0.001 | |||||||
| β-elemene | 515-13-9 | C15H24 | 1388 | 1386 | 0.19 ± 0.02 | 0.17 ± 0.02 | 0.17 ± 0.04 | 0.01 ± 0.001 | 0.02 ± 0.01 | 0.03 ± 0.003 | 0.03 ± 0.01 | 0.11 ± 0.01 | 0.14 ± 0.03 | 0.11 ± 0.01 | 0.13 ± 0.02 | |
| (Z)-β-caryophyllene | 118-65-0 | C15H24 | 1405 | 1402 | <0.01 | 0.01 ± 0.001 | ||||||||||
| (Z)-α-bergamotene | 18252-46-5 | C15H24 | 1413 | 1406 | <0.01 | <0.01 | ||||||||||
| (E)-β-caryophyllene | 87-44-5 | C15H24 | 1419 | 1425 | 1.72 ± 0.34 | 1.89 ± 0.12 | 1.78 ± 0.27 | 1.70 ± 0.05 | 0.13 ± 0.02 | 0.41 ± 0.15 | 0.42 ± 0.04 | 0.32 ± 0.10 | 0.97 ± 0.04 | 2.22 ± 0.24 | 1.70 ± 0.22 | 1.56 ± 0.23 |
| (E)-α-bergamotene | 13474-59-4 | C15H24 | 1432 | 1433 | 0.19 ± 0.04 | 0.30 ± 0.02 | 0.03 ± 0.002 | 0.08 ± 0.004 | 0.01 ± 0.001 | 0.02 ± 0.01 | <0.01 | 0.01 ± 0.003 | 0.17 ± 0.02 | 0.18 ± 0.03 | 0.02 ± 0.003 | 0.10 ± 0.02 |
| (Z)-β-farnesene | 28973-97-9 | C15H24 | 1446 | 1438 | 0.37 ± 0.05 | 0.34 ± 0.03 | 0.20 ± 0.02 | 0.29 ± 0.05 | 0.03 ± 0.001 | 0.08 ± 0.02 | 0.03 ± 0.01 | 0.06 ± 0.01 | 0.42 ± 0.01 | 0.77 ± 0.10 | 0.35 ± 0.06 | 0.45 ± 0.07 |
| α-humulene | 6753-98-6 | C15H24 | 1451 | 1458 | 0.16 ± 0.03 | 0.29 ± 0.01 | 0.17 ± 0.003 | 0.01 ± 0.002 | 0.05 ± 0.02 | 0.04 ± 0.004 | 0.03 ± 0.01 | 0.11 ± 0.01 | 0.35 ± 0.06 | 0.27 ± 0.03 | 0.22 ± 0.03 | |
| alloaromadendrene | 25246-27-9 | C15H24 | 1460 | 1460 | <0.01 | 0.01 ± 0.002 | ||||||||||
| (Z,E)-α-farnesene | 26560-14-5 | C15H24 | 1480 | 1471 | 0.37 ± 0.06 | 0.02 ± 0.002 | ||||||||||
| β-selinene | 17066-67-0 | C15H24 | 1482 | 1480 | 0.47 ± 0.04 | 0.83 ± 0.01 | 0.41 ± 0.05 | 0.16 ± 0.03 | 0.04 ± 0.003 | |||||||
| valencene | 4630-07-3 | C15H24 | 1484 | 1490 | 0.10 ± 0.01 | 0.20 ± 0.003 | 0.08 ± 0.01 | <0.01 | 0.01 ± 0.001 | 0.03 ± 0.01 | 0.01 ± 0.001 | 0.02 ± 0.003 | 0.20 ± 0.03 | 0.10 ± 0.01 | ||
| α-selinene | 473-13-2 | C15H24 | 1490 | 1494 | 0.36 ± 0.03 | 0.52 ± 0.03 | 0.28 ± 0.02 | 0.03 ± 0.01 | 0.09 ± 0.02 | 0.04 ± 0.004 | 0.06 ± 0.01 | 0.44 ± 0.02 | 0.33 ± 0.04 | |||
| β-bisabolene | 495-61-4 | C15H24 | 1500 | 1499 | <0.01 | |||||||||||
| γ-cadinene | 39029-41-9 | C15H24 | 1507 | 1508 | 0.05 ± 0.004 | 0.04 ± 0.02 | ||||||||||
| (Z)-calamenene | 72937-55-4 | C15H22 | 1510 | 1510 | 0.08 ± 0.03 | 0.01 ± 0.001 | 0.01 ± 0.002 | 0.01 ± 0.001 | 0.19 ± 0.02 | 0.17 ± 0.05 | 0.11 ± 0.02 | |||||
| δ-cadinene | 483-76-1 | C15H24 | 1515 | 1513 | 0.04 ± 0.02 | <0.01 | 0.01 ± 0.002 | 0.01 ± 0.001 | 0.04 ± 0.004 | 0.08 ± 0.01 | 0.06 ± 0.01 | |||||
| cadina-1,4-diene | 16728-99-7 | C15H24 | 1525 | 1524 | <0.01 | <0.01 | 0.04 ± 0.001 | 0.04 ± 0.01 | 0.04 ± 0.01 | |||||||
| Compound | CAS Number | Molecular Formula | RI | RI | Relative Content (%) | |||||||||||
| HS-SPME | Essential Oil HS-SPME | Essential Oil Direct Injection | ||||||||||||||
| Leaf | Stem | Rhizome | Whole | Leaf | Stem | Rhizome | Whole | Leaf | Stem | Rhizome | Whole | |||||
| Terpene Alcohols | ||||||||||||||||
| linalool | 78-70-6 | C10H18O | 1086 | 1074 | 0.10 ± 0.02 | 0.04 ± 0.003 | 0.11 ± 0.03 | 0.14 ± 0.02 | 0.08 ± 0.01 | 0.06 ± 0.01 | 0.23 ± 0.03 | 0.37 ± 0.02 | 0.11 ± 0.001 | 0.18 ± 0.01 | ||
| borneol | 507-70-0 | C10H18O | 1152 | 1144 | 0.01 ± 0.01 | 0.02 ± 0.004 | ||||||||||
| terpinen-4-ol | 562-74-3 | C10H18O | 1163.5 | 1162 | 0.03 ± 0.01 | 0.05 ± 0.01 | <0.01 | 0.04 ± 0.01 | 0.24 ± 0.05 | 0.13 ± 0.02 | 0.43 ± 0.05 | 0.40 ± 0.03 | ||||
| α-terpineol | 98-55-5 | C10H18O | 1175 | 1171 | 0.01 ± 0.003 | 0.08 ± 0.02 | 0.15 ± 0.03 | 0.13 ± 0.03 | 0.42 ± 0.12 | 0.38 ± 0.04 | 0.51 ± 0.09 | |||||
| (E)-geraniol | 106-24-1 | C10H18O | 1237 | 1231 | <0.01 | <0.01 | <0.01 | <0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | <0.01 | 0.02 ± 0.002 | ||||
| perilla alcohol | 536-59-4 | C10H18O | 1281 | 1273 | 0.10 ± 0.03 | <0.01 | ||||||||||
| nerolidol | 40716-66-3 | C15H26O | 1549 | 1540 | 0.15 ± 0.01 | 0.04 ± 0.001 | <0.01 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.32 ± 0.01 | 1.22 ± 0.48 | 0.08 ± 0.01 | 0.26 ± 0.04 | |||
| spathulenol | 6750-60-3 | C15H24O | 1568 | 1569 | <0.01 | 0.35 ± 0.02 | 0.66 ± 0.45 | 0.29 ± 0.02 | ||||||||
| globulol | 51371-47-2 | C15H26O | 1579 | 1580 | 0.09 ± 0.01 | 0.19 ± 0.07 | 0.11 ± 0.02 | |||||||||
| veridiflorol | 552-02-3 | C15H26O | 1580 | 1588 | 0.13 ± 0.01 | 0.18 ± 0.04 | 0.09 ± 0.02 | |||||||||
| isospathulenol | 88395-46-4 | C15H24O | 1626 | 1625 | 0.08 ± 0.01 | 0.13 ± 0.05 | 0.05 ± 0.01 | |||||||||
| α-cadinol | 481-34-5 | C15H26O | 1641 | 1642 | 0.09 ± 0.01 | 0.07 ± 0.03 | 0.05 ± 0.01 | |||||||||
| (2E,6E)-farnesol | 106-28-5 | C15H26O | 1709 | 1700 | 0.04 ± 0.01 | 0.05 ± 0.02 | 0.03 ± 0.01 | |||||||||
| Terpene Aldehydes | ||||||||||||||||
| β-cyclocitral | 432-25-7 | C10H16O | 1196 | 1194 | 0.01 ± 0.001 | 0.01 ± 0.004 | 0.01 ± 0.004 | 0.01 ± 0.002 | 0.02 ± 0.004 | 0.04 ± 0.004 | 0.05 ± 0.003 | 0.03 ± 0.003 | ||||
| geranial | 141-27-5 | C10H16O | 1247 | 1242 | <0.01 | 0.11 ± 0.02 | ||||||||||
| Terpene Esters | ||||||||||||||||
| fenchyl acetate | 13851-11-1 | C12H20O2 | 1214 | 1205 | 0.01 ± 0.004 | 0.02 ± 0.01 | 0.01 ± 0.002 | 0.05 ± 0.004 | 0.05 ± 0.01 | |||||||
| linalyl acetate | 115-95-7 | C12H20O2 | 1241 | 1235 | 0.08 ± 0.04 | |||||||||||
| bornyl acetate | 76-49-3 | C12H20O2 | 1270 | 1271 | 0.60 ± 0.08 | 3.12 ± 0.05 | 3.37 ± 0.43 | 1.91 ± 0.39 | 0.49 ± 0.20 | 3.29 ± 0.96 | 2.62 ± 0.62 | 1.36 ± 0.29 | 1.79 ± 0.18 | 14.55 ± 1.61 | 5.56 ± 0.10 | 4.15 ± 0.18 |
| α-terpinyl acetate | 80-26-2 | C12H20O2 | 1333 | 1330 | 0.31 ± 0.03 | 0.07 ± 0.02 | ||||||||||
| neryl acetate | 141-12-8 | C12H20O2 | 1343 | 1333 | 0.13 ± 0.01 | 0.03 ± 0.01 | 0.09 ± 0.01 | 0.30 ± 0.04 | 0.15 ± 0.004 | 0.12 ± 0.01 | ||||||
| geranyl acetate | 105-87-3 | C12H20O2 | 1361 | 1348 | 0.33 ± 0.05 | 0.46 ± 0.03 | 0.48 ± 0.03 | 0.27 ± 0.03 | 0.08 ± 0.02 | 0.18 ± 0.03 | 0.10 ± 0.03 | 0.18 ± 0.03 | 3.11 ± 0.19 | 0.96 ± 0.52 | ||
| Compound | CAS Number | Molecular Formula | RI | RI | Relative Content (%) | |||||||||||
| HS-SPME | Essential Oil HS-SPME | Essential Oil Direct Injection | ||||||||||||||
| Leaf | Stem | Rhizome | Whole | Leaf | Stem | Rhizome | Whole | Leaf | Stem | Rhizome | Whole | |||||
| (2E,6E)-farnesyl acetate | 4128-17-0 | C17H28O2 | 1816 | 1803 | 0.01 ± 0.01 | |||||||||||
| Terpene Ketones | ||||||||||||||||
| pinocarvone | 30460-92-5 | C10H14O | 1140 | 1137 | <0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.004 | 0.03 ± 0.001 | |||||||
| carvone | 99-49-0 | C10H14O | 1218 | 1215 | <0.01 | 0.03 ± 0.003 | ||||||||||
| piperitone | 89-81-6 | C10H16O | 1232.5 | 1228 | <0.01 | <0.01 | 0.06 ± 0.03 | 0.02 ± 0.002 | ||||||||
| β-damascenone | 23726-93-4 | C13H18O | 1361 | 1361 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.09 ± 0.02 | 0.64 ± 0.23 | ||||||||
| Terpene Oxides | ||||||||||||||||
| (E)-limonene oxide | 4959-35-7 | C10H16O | 1124 | 1115 | <0.01 | 0.01 ± 0.001 | ||||||||||
| caryophyllene oxide | 1139-30-6 | C15H24O | 1573 | 1576 | 0.11 ± 0.005 | 0.03 ± 0.005 | <0.01 | 0.27 ± 0.04 | 0.66 ± 0.42 | 0.27 ± 0.05 | 0.25 ± 0.02 | |||||
| Compound | Relative Content (%) a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HS-SPME | Essential Oil HS-SPME | Essential Oil Direct Injection | ||||||||||
| Leaf | Stem | Rhizome | Whole b | Leaf | Stem | Rhizome | Whole | Leaf | Stem | Rhizome | Whole | |
| Aliphatic alcohols | 5.14 | 0.70 | 0.75 | 0.10 | 0.09 | 0.30 | 0.12 | 0.16 | 0.44 | 0.28 | ||
| Aliphatic aldehydes | 3.65 | 3.30 | 7.24 | 4.14 | 1.47 | 0.13 | 0.09 | 0.39 | 0.78 | 0.34 | 1.68 | 0.81 |
| Aliphatic ester | 0.19 | 0.05 | ||||||||||
| Aliphatic ketones | 0.23 | 0.12 | 5.60 | 2.10 | 5.58 | 4.25 | 24.55 | 13.05 | ||||
| Aromatic compounds | 0.03 | 0.07 | 0.11 | 0.13 | 0.03 | 0.14 | 0.17 | 0.14 | 0.04 | 0.16 | 0.06 | 0.14 |
| Hydrocarbon | <0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |||||
| Monoterpenes | 79.91 | 68.47 | 80.66 | 85.33 | 94.57 | 93.30 | 89.11 | 92.69 | 74.10 | 50.19 | 55.67 | 68.50 |
| Sesquiterpenes | 4.27 | 4.69 | 2.79 | 3.00 | 0.32 | 0.78 | 0.59 | 0.59 | 3.09 | 4.65 | 3.10 | 2.95 |
| Terpene alcohols | 0.35 | 0.03 | 0.13 | 0.12 | 0.28 | 0.47 | 0.33 | 1.37 | 3.34 | 1.00 | 1.99 | |
| Terpene aldehydes | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.04 | 0.17 | 0.03 | ||||
| Terpene esters | 1.01 | 3.58 | 4.29 | 2.18 | 0.57 | 3.48 | 2.84 | 1.55 | 1.88 | 17.96 | 6.73 | 4.32 |
| Terpene ketones | 0.01 | 0.02 | 0.01 | 0.09 | 0.09 | 0.08 | 0.67 | |||||
| Terpene oxides | 0.11 | 0.03 | <0.01 | 0.27 | 0.66 | 0.28 | 0.25 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Chao, L.K.; Lin, L.-Y.; Wu, C.-S.; Chu, L.-P.; Huang, C.-H.; Chen, H.-C. Analysis of Volatile Compounds from Different Parts of Houttuynia cordata Thunb. Molecules 2022, 27, 8893. https://doi.org/10.3390/molecules27248893
Lin C-H, Chao LK, Lin L-Y, Wu C-S, Chu L-P, Huang C-H, Chen H-C. Analysis of Volatile Compounds from Different Parts of Houttuynia cordata Thunb. Molecules. 2022; 27(24):8893. https://doi.org/10.3390/molecules27248893
Chicago/Turabian StyleLin, Chen-Hsiang, Louis Kuoping Chao, Li-Yun Lin, Chin-Sheng Wu, Lee-Ping Chu, Chien-Hsueh Huang, and Hsin-Chun Chen. 2022. "Analysis of Volatile Compounds from Different Parts of Houttuynia cordata Thunb." Molecules 27, no. 24: 8893. https://doi.org/10.3390/molecules27248893
APA StyleLin, C.-H., Chao, L. K., Lin, L.-Y., Wu, C.-S., Chu, L.-P., Huang, C.-H., & Chen, H.-C. (2022). Analysis of Volatile Compounds from Different Parts of Houttuynia cordata Thunb. Molecules, 27(24), 8893. https://doi.org/10.3390/molecules27248893









