Efficient Degradation of Congo Red in Water by UV-Vis Driven CoMoO4/PDS Photo-Fenton System
Abstract
1. Introduction
2. Experiment
2.1. Chemicals and Materials
2.2. Preparation of Samples
2.3. Characterization
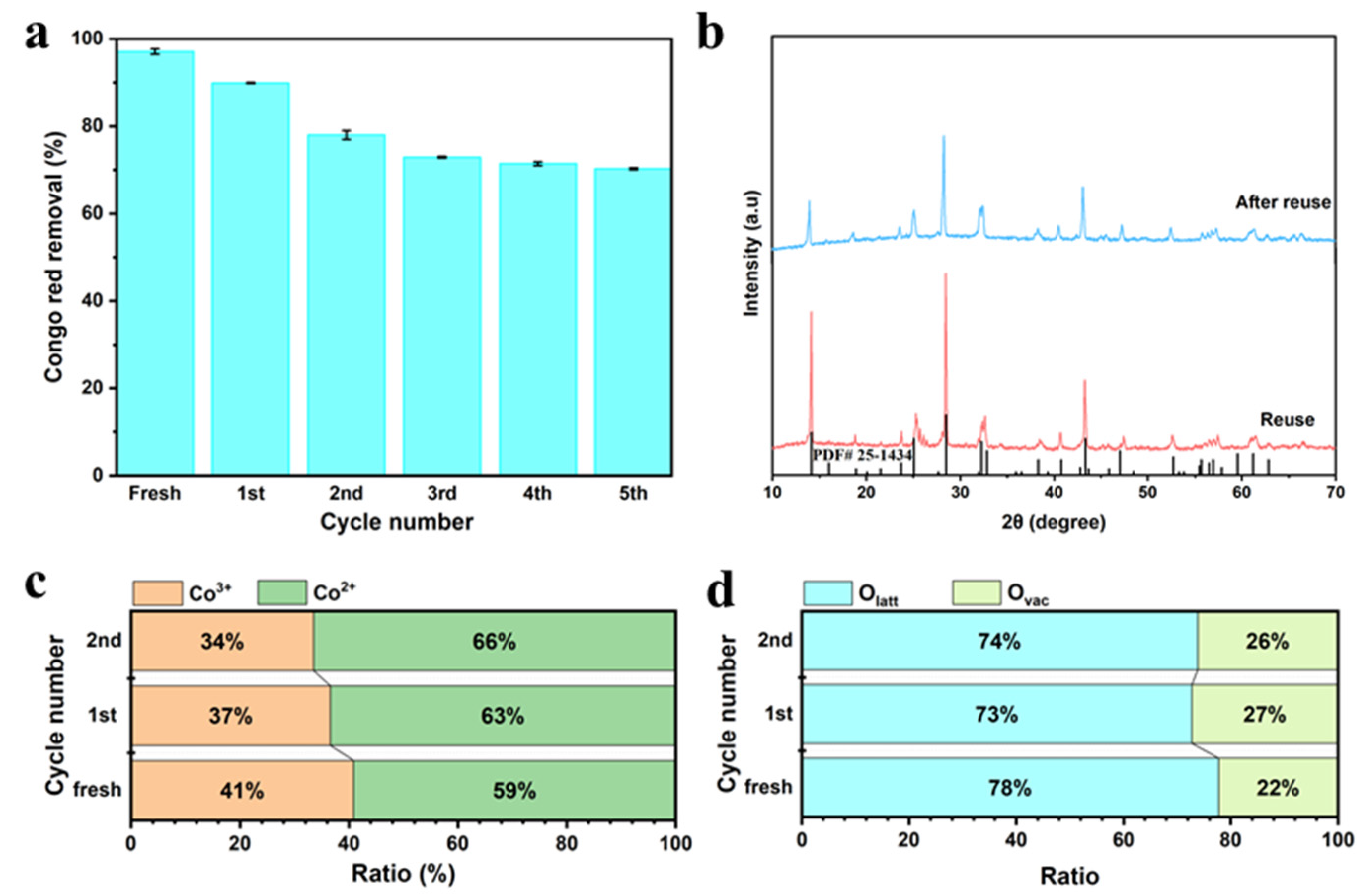
2.4. Catalytic Activation Experiments
3. Results and Discussion
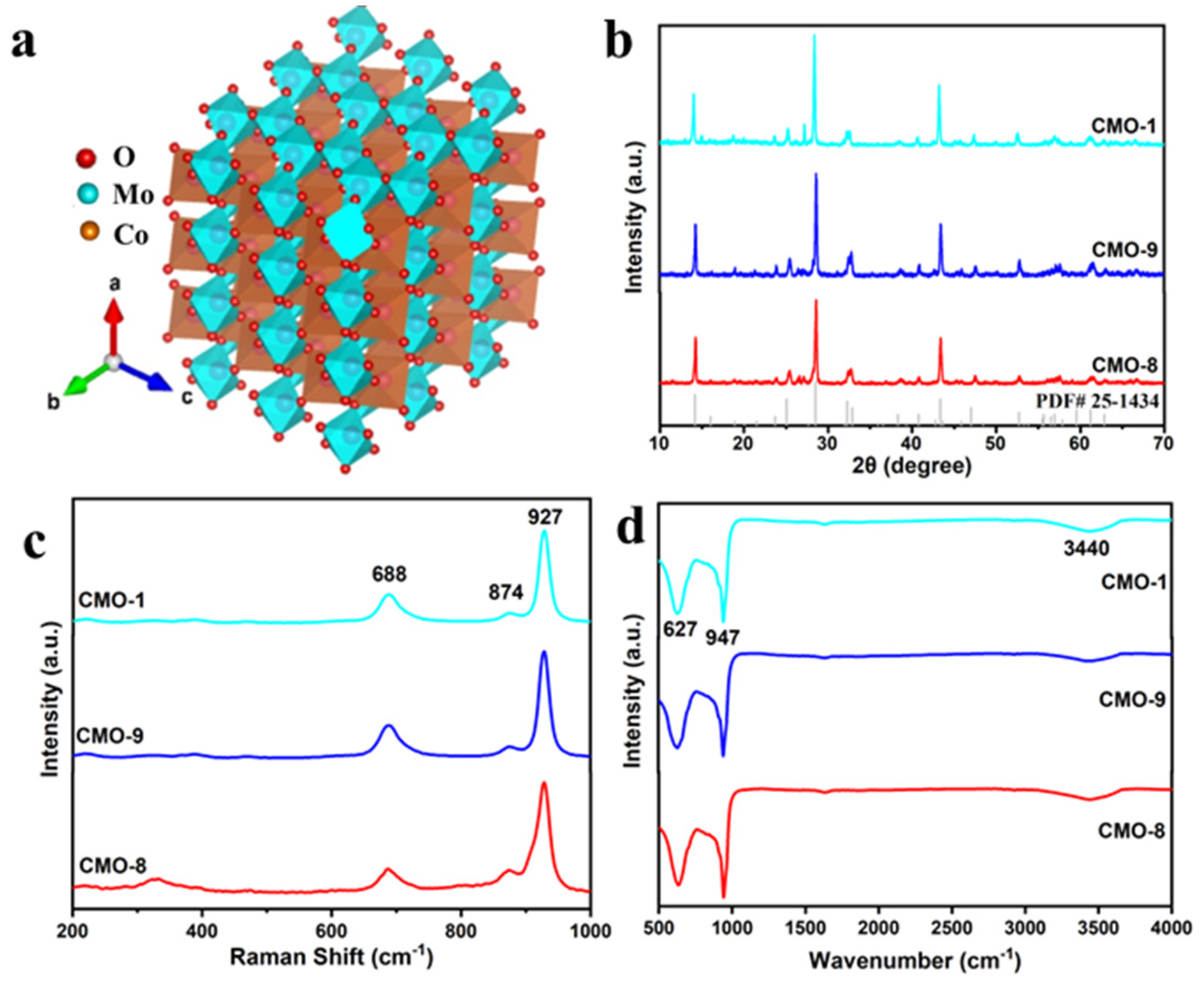
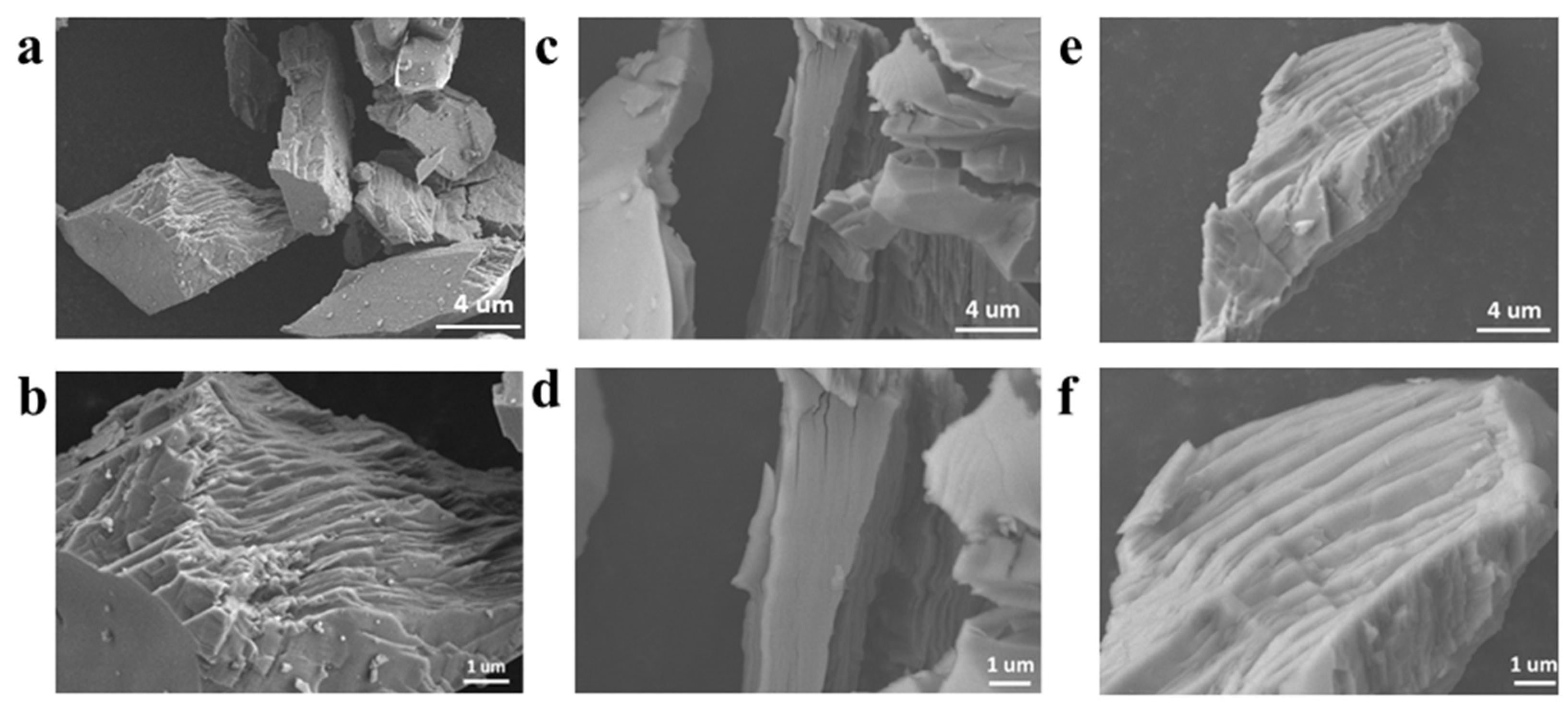
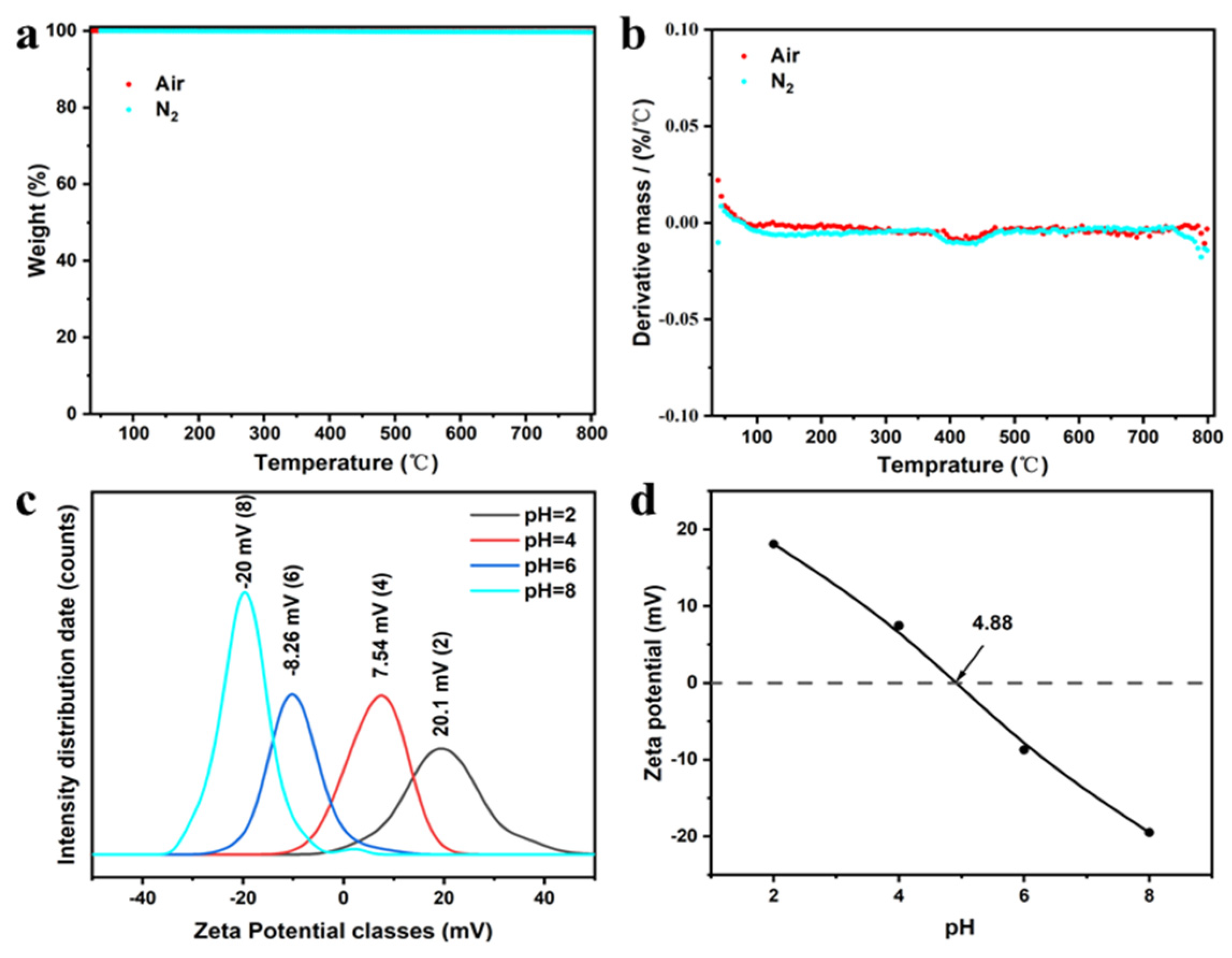
3.1. Characterization of CoMoO4
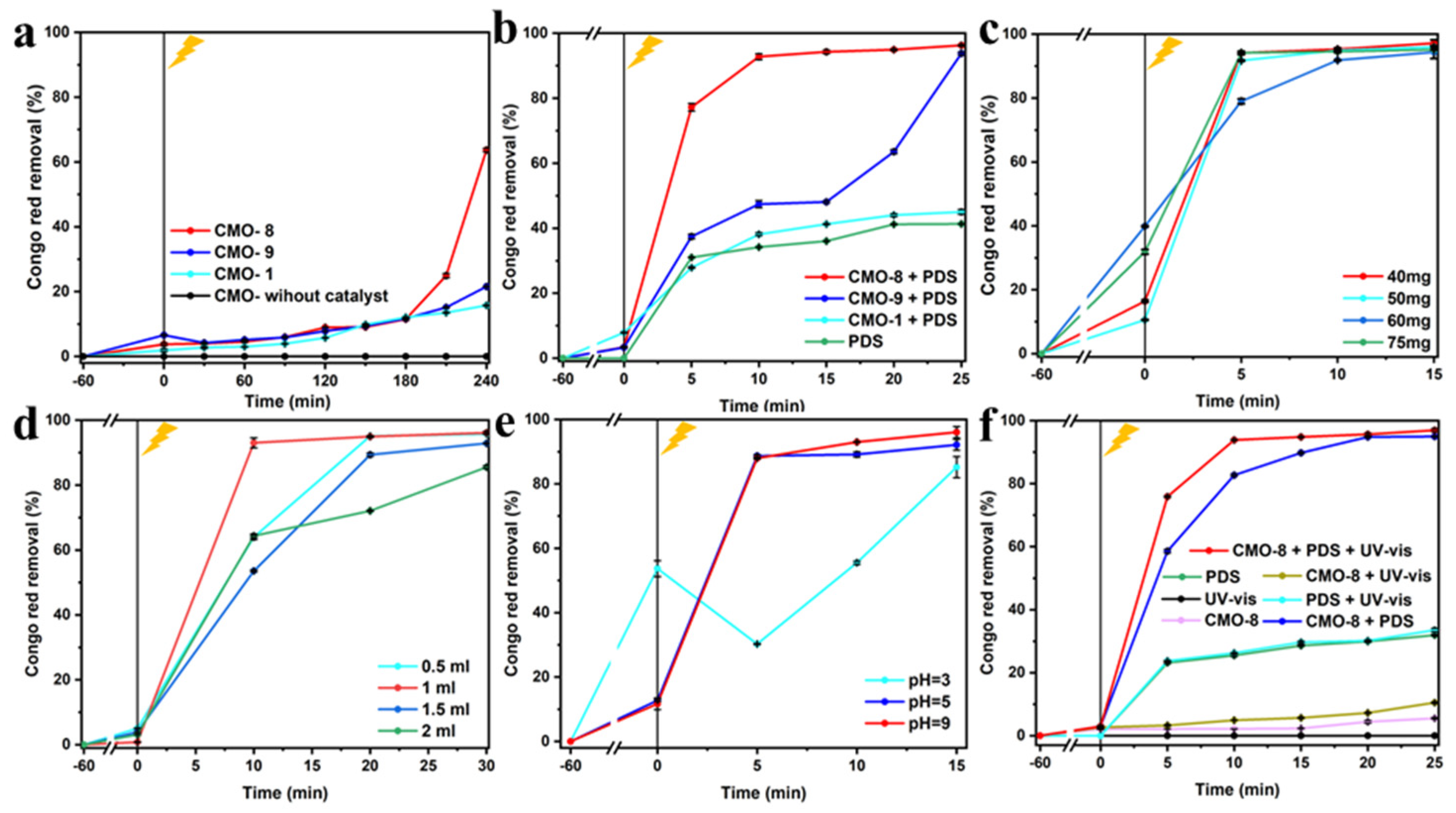
3.2. Degradation of CR in Different Condition
3.3. Degradation Mechanism by CMO under PDS Activation and Visible Light Irradiation
3.4. MS Analysis of Oxidation Intermediates from CR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Silva, R.A.; Carmona-Ribeiro, A.M.; Petri, D.F. Catalytic behavior of lipase immobilized onto congo red and PEG-decorated particles. Molecules 2014, 19, 8610–8628. [Google Scholar] [CrossRef] [PubMed]
- Haounati, R.; Alakhras, F.; Ouachtak, H.; Saleh, T.; Al-Mazaideh, G.; Alhajri, E.; Jada, A.; Hafid, N.; Addi, A. Synthesized of Zeolite@Ag2O Nanocomposite as Superb Stability Photocatalysis Toward Hazardous Rhodamine B Dye from Water. Arab. J. Sci. Eng. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Haounati, R.; El Guerdaoui, A.; Ouachtak, H.; El Haouti, R.; Bouddouch, A.; Hafid, N.; Bakiz, B.; Santos, D.M.F.; Taha, M.L.; Jada, A.; et al. Design of direct Z-scheme superb magnetic nanocomposite photocatalyst Fe3O4/Ag3PO4@Sep for hazardous dye degradation. Sep. Purif. Technol. 2021, 277, 119399. [Google Scholar]
- Das, T.; Ganguly, S.; Bhawal1, P.; Mondal1, S.; Das, N. A facile green synthesis of silver nanoparticle-decorated hydroxyapatite for efficient catalytic activity towards 4-nitrophenol reduction. Res. Chem. Intermed. 2018, 44, 1189–1208. [Google Scholar] [CrossRef]
- Das, T.; Remanan, S.; Ghosh, S.; Ghosh, S.; Das, N. Efficient synthesis of catalytic active silver nanoparticles illuminated cerium oxide nanotube: A mussel inspired approach. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100411. [Google Scholar] [CrossRef]
- Karci, A.; Arslan-Alaton, I.; Olmez-Hanci, T.; Bekbolet, M. Degradation and detoxification of industrially important phenol derivatives in water by direct UV-C photolysis and H2O2/UV-C process: A comparative study. Chem. Eng. J. 2013, 224, 4–9. [Google Scholar]
- Li, X.L.; Zhang, J.; Jiang, Y.C.; Hu, M.C.; Li, S.N.; Zhai, Q.G. Highly efficient biodecolorization/degradation of Congo red and Alizarin yellow R by chloroperoxidase from Caldariomyces fumago: Catalytic mechanism and degradation pathway. Ind. Eng. Chem. Res. 2013, 52, 13572–13579. [Google Scholar] [CrossRef]
- Mokhtari, P.; Ghaedi, M.; Dashtian, K.; Rahimi, M.R.; Purkait, M.K. Removal of methyl orange by copper sulfide nanoparticles loaded activated carbon: Kinetic and isotherm investigation. J. Mol. Liq. 2016, 219, 299–305. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Naushad, M.; Garcia-Penas, A.; Al-Muhtaseb, A.H.; Ghfar, A.A.; Sharma, V.; Ahamad, T.; Stadler, F.J. Fabrication and characterization of Gum arabic-cl-poly(acrylamide) nanohydrogel for effective adsorption of crystal violet dye. Carbohydr. Polym. 2018, 202, 444–453. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Ahamad, T.; Stadler, F.J. Fabrication and characterization of novel Fe0@Guar gum-crosslinked-soya lecithin nanocomposite hydrogel for photocatalytic degradation of methyl violet dye. Sep. Purif. Technol. 2019, 211, 895–908. [Google Scholar] [CrossRef]
- Sharma, G.; Dionysiou, D.D.; Sharma, S.; Kumar, A.; Al-Muhtaseb, A.H.; Naushad, M.; Stadler, F.J. Highly efficient Sr/Ce/activated carbon bimetallic nanocomposite for photoinduced degradation of rhodamine B. Catal. Today 2019, 335, 437–451. [Google Scholar]
- Paz, A.; Carballo, J.; Perez, M.J.; Dominguez, J.M. Biological treatment of model dyes and textile wastewaters. Chemosphere 2017, 181, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Kumar, A. Preparation of nanoporous composite carbon membrane for separation of rhodamine B dye. J. Membr. Sci. 2009, 329, 2–10. [Google Scholar]
- Fernandes, A.; Nunes, M.J.; Rodrigues, A.S.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Electro-Persulfate Processes for the Treatment of Complex Wastewater Matrices: Present and Future. Molecules 2021, 26, 4821. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Chen, H.; Peng, T.Y.; Ke, D.N. Photocatalytic degradation of methyl orange in aqueous suspension of mesoporous titania nanoparticles. Chemosphere 2007, 69, 1361–1367. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Hajati, S.; Rezaeinejad, M.; Goudarzi, A.; Purkait, M.K. Rapid removal of Auramine-O and Methylene blue by ZnS:Cu nanoparticles loaded on activated carbon: A response surface methodology approach. J. Taiwan. Inst. Chem. Eng. 2015, 53, 80–91. [Google Scholar]
- Ribeiro, J.P.; Gomes, H.G.M.F.; Sarinho, L.; Marques, C.C.; Nunes, M.I. Synergies of metallic catalysts in the Fenton and photo-Fenton processes applied to the treatment of pulp bleaching wastewater. Chem. Eng. Process. Process Intensif. 2022, 181, 80–91. [Google Scholar]
- Liu, D.; Chen, D.; Hao, Z.; Tang, Y.; Jiang, L.; Li, T.; Tian, B.; Yan, C.; Luo, Y.; Jia, B. Efficient degradation of Rhodamine B in water by CoFe2O4/H2O2 and CoFe2O4/PMS systems: A comparative study. Chemosphere 2022, 307, 135935. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Duan, Z.; Yu, D.; Wang, Q.; Ji, D.; Liu, W. Zn/Co-ZIFs@MIL-101(Fe) metal–organic frameworks are effective photo-Fenton catalysts for RhB removal. Sep. Purif. Technol. 2022, 293, 121099. [Google Scholar]
- Parhi, P.K.; Misra, P.K. Environmental friendly approach for selective extraction and recovery of molybdenum (Mo) from a sulphate mediated spent Ni–Mo/Al2O3 catalyst baked leach liquor. J. Environ. Manag. 2022, 306, 114474. [Google Scholar]
- Xue, C.-M.; Li, S.-X.; Zhang, L.; Sha, J.-Q.; Zheng, T.-Y.; Zhang, Q.-N.; Li, L. Hydrothermal Synthesis, Characterization and Electrocatalytic/Photocatalytic Activities of New Polyoxometalate Based Hybrid Compound. J. Inorg. Organomet. Polym. Mater. 2013, 23, 1468–1476. [Google Scholar]
- Coué, V.; Dessapt, R.; Bujoli-Doeuff, M.; Evain, M.; Jobic, S. Hydrothermal Synthesis, Characterization and Electrocatalytic/Photocatalytic Activities of New Polyoxometalate Based Hybrid Compound. Inorg. Chem. 2007, 46, 2824–2835. [Google Scholar] [PubMed]
- Xu, Z.; Wang, Y.; Liu, M.; Sarwar, M.K.; Zhao, Y. Defects enriched cobalt molybdate induced by carbon dots for a high rate Li-ion battery anode. Nanotechnology 2021, 33, 1361–6528. [Google Scholar]
- Guo, J.; Chu, L.; Wang, L.; Liu, H.; Yang, M.; Wang, G. Self-templated fabrication of CoMoO4-Co3O4 hollow nanocages for efficient aerobic oxidative desulfurization. Appl. Surf. Sci. 2022, 579, 152251. [Google Scholar] [CrossRef]
- Geng, X.; Ai, Y.; Shen, G. Facile construction of novel CoMoO4 microplates@CoMoO4 microprisms structures for well-stable supercapacitors. Prog. Nat. Sci. 2016, 26, 243–252. [Google Scholar]
- Konga, L.B.; Langa, J.W.; Liua, M.; Luo, Y.C.; Kang, L. Facile approach to prepare loose-packed cobalt hydroxide nano-flakes materials for electrochemical capacitors. J. Power Sources 2009, 194, 1194–1201. [Google Scholar] [CrossRef]
- Liu, M.-C.; Kong, L.-B.; Lu, C.; Li, X.-M.; Luo, Y.-C.; Kang, L. Facile fabrication of CoMoO4 nanorods as electrode material for electrochemical capacitors. Mater. Lett. 2013, 94, 197–200. [Google Scholar]
- Edrissi, M.; Samadanian-Isfahani, S.; Soleymani, M. Preparation of cobalt molybdate nanoparticles; Taguchi optimization and photocatalytic oxidation of Reactive Black 8 dye. Powder Technol. 2013, 249, 378–385. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, S.; Liu, L.; Lan, Y.; Chen, C. Three-dimensional blocky sponge based CoMoO4 catalyst activates peroxymonosulfate to degrade sulfadimethoxine: A remedy for ready-made catalysts to achieve convenient and continuous operation. Chem. Eng. J. 2023, 451, 138754. [Google Scholar] [CrossRef]
- Liu, Z.L.; Xu, J.; Xiang, C.J.; Liu, Y.; Ma, L.J.; Hu, L.Y. S-scheme heterojunction based on ZnS/CoMoO4 ball-and-rod composite. Appl. Surf. Sci. 2021, 569, 150973. [Google Scholar] [CrossRef]
- Adabavazeh, H.; Saljooqi, A.; Shamspur, T.; Mostafavi, A. Synthesis of polyaniline decorated with ZnO and CoMoO4 nanoparticles for enhanced photocatalytic degradation of imidacloprid pesticide under visible light. Polyhedron 2021, 198, 115058. [Google Scholar] [CrossRef]
- Umapathy, V.; Neeraja, P. Sol–Gel synthesis and characterizations of CoMoO4 nanoparticles: An efficient photocatalytic degradation of 4-chlorophenol. J. Nanosci. Nanotechnol. 2016, 16, 2960–2966. [Google Scholar] [PubMed]
- Fan, Y.A.; Ma, W.J.; He, J.L.; Du, Y.C. CoMoO4 as a novel heterogeneous catalyst of peroxymonosulfate activation for the degradation of organic dyes. RSC Adv. 2017, 7, 36193–36200. [Google Scholar]
- Sun, M.; Dang, Y.; Du, R.; Lu, Z.; Wang, Y.; Wang, X.; Song, C.; Li, T.; Wang, G.; Ye, J.; et al. Experiment and theoretical insights into CuNi/CoMoO4 multi-functional catalyst with laccase-like: Catalysis mechanism, smartphone biosensing and organic pollutant efficient degradation. Chem. Eng. J. 2021, 425, 130586. [Google Scholar]
- Song, X.; Ni, J.; Liu, D.; Shi, W.; Yuan, Y.; Cui, F.; Tian, J.; Wang, W. Molybdenum disulfide as excellent Co-catalyst boosting catalytic degradation of sulfamethoxazole by nZVI/PDS process. Sep. Purif. Technol. 2022, 285, 120398. [Google Scholar]
- Saleem, S.S. Infrared and Raman Spectroscopic Studies of the Polymorphic forms of Nickel, Cobalt and Ferric Molybdates. Infkred Phys. 1987, 27, 309–315. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Chaturvedi, S.; Hanson, J.C. Reaction of H2 and H2S with CoMoO4 and NiMoO4: TPR, XANES, time-resolved XRD, and molecular-orbital studies. J. Phy. Chem. 1999, 103, 770–781. [Google Scholar] [CrossRef]
- Baskar, S.; Meyrick, D.; Ramakrishnan, K.S.; Minakshi, M.J.C.E.J. Facile and large scale combustion synthesis of α-CoMoO4: Mimics the redox behavior of a battery in aqueous hybrid device. Chem. Eng. J. 2014, 253, 502–507. [Google Scholar] [CrossRef]
- Ette, P.M.; Chithambararaj, A.; Prakash, A.; Ramesha, K.J.A. Interfaces, MoS2 nanoflower-derived interconnected CoMoO4 nanoarchitectures as a stable and high rate performing anode for lithium-ion battery applications. ACS Appl. Mater. Interfaces 2020, 12, 11511–11521. [Google Scholar] [CrossRef]
- Mandal, M.; Ghosh, D.; Giri, S.; Shakir, I.; Das, C.K. Polyaniline-wrapped 1D CoMoO4·0.75H2O nanorods as electrode materials for supercapacitor energy storage applications. RSC. Adv. 2014, 4, 30832–30839. [Google Scholar]
- Rahulan, K.M.; Flower, N.A.L.; Sujatha, R.A.; Padmanathan, N.; Gopalakrishnan, C. Nonlinear optical absorption studies of CoMoO4 hybrid nanostructures. J. Mater. Sci. Mater. Electron. 2017, 29, 1504–1509. [Google Scholar] [CrossRef]
- Dang, Y.; Wang, G.; Su, G.; Lu, Z.; Wang, Y.; Liu, T.; Pu, X.; Wang, X.; Wu, C.; Song, C.; et al. Rational construction of a Ni/CoMoO4 heterostructure with strong Ni-O-Co bonds for improving multifunctional nanozyme activity. ACS Nano 2022, 16, 4536–4550. [Google Scholar] [PubMed]
- Rosić, M.; Zarubica, A.; Šaponjić, A.; Babić, B.; Zagorac, J.; Jordanov, D.; Matović, B. Structural and photocatalytic examination of CoMoO4 nanopowders synthesized by GNP method. Mater. Res. Bull. 2018, 98, 111–120. [Google Scholar] [CrossRef]
- Chi, K.; Zhang, Z.; Lv, Q.; Xie, C.; Xiao, J.; Xiao, F.; Wang, S. Well-Ordered Oxygen-Deficient CoMoO4 and Fe2O3 Nanoplate Arrays on 3D Graphene Foam: Toward Flexible Asymmetric Supercapacitors with Enhanced Capacitive Properties. ACS Appl. Mater. Interfaces 2017, 9, 6044–6053. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, J.; Zhan, C.; Yu, J.; Cao, Y.; Tu, J.; Shi, C. Phosphide-oxide honeycomb-like heterostructure CoP@CoMoO4/CC for enhanced hydrogen evolution reaction in alkaline solution. J. Mater. Sci. Technol. 2020, 46, 177–184. [Google Scholar]
- Sun, M.M.; Huang, S.; Su, G.H.; Wang, X.X.; Lu, Z.W.; Wamg, Y.Y.; Liu, T.; Jiang, Y.Y.; Song, C.; Rao, H.B. Synthesis of pH-switchable Pt/Co3O4 nanoflowers: Catalytic mechanism, four-enzyme activity and smartphone biosensing applications. Chem. Eng. J. 2022, 437, 134414. [Google Scholar]
- Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar]
- Huerta-Aguilar, C.; Gutiérrez, Y.; Thangarasu, P. Crystal plane directed interaction of TiO2 [1 0 1] with AgNPs [1 1 1] silver nanoparticles enhancing solar light induced photo-catalytic oxidation of ciprofloxacin: Experimental and theoretical studies. Chem. Eng. J 2020, 394, 124286. [Google Scholar]
- Huerta-Aguilar, C.; Ramírez-Alejandre, A.; Thangarasu, P.; Arenas-Alatorre, J.; Reyes-Dominguez, I.; Corea, M. Crystal phase induced band gap energy enhancing the photo-catalytic properties of Zn–Fe2O4/Au NPs: Experimental and theoretical studies. Catal. Sci. Technol. 2019, 9, 3066–3080. [Google Scholar] [CrossRef]
- Huerta-Aguilar, C.; Diaz-Puerto, Z.; Tecuapa-Flores, E.; Thangarasu, P. Crystal Plane Impact of ZnFe2O4−Ag Nanoparticles Influencing Photocatalytical and Antibacterial Properties: Experimental and Theoretical Studies. ACS Omega 2022, 7, 33985–34001. [Google Scholar] [CrossRef]
- Ortega-Granados, J.; Thangarasu, P. Photo-deposition of Ag/AuNPs on TiO2 accelerating the oxidation of tetracycline under visible light. Mater. Lett. 2022, 321, 132455. [Google Scholar] [CrossRef]
- Ko, S.; Kim, E.S.; Park, S.; Daigle, H.; Milner, T.E.; Huh, C.; Bennetzen, M.V.; Geremia, G.A. Amine functionalized magnetic nanoparticles for removal of oil droplets from produced water and accelerated magnetic separation. J. Nanoparticle Res. 2017, 19, 132. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, J.; Mao, M.; Li, L.J.; Li, X.H. NiCo2S4@Zn0.5Cd0.5S with direct Z-scheme heterojunction constructed by band structure adjustment of ZnxCd1-xS for efficient photocatalytic H2 evolution. Appl. Surf. Sci. 2020, 528, 147016. [Google Scholar] [CrossRef]
- Yang, S.P.; Yang, J.; Zhang, X.Q.; Tang, J.; Li, J.L.; Zhang, A.P. Degradation of refractory organic matter in MBR effluent from treating landfill leachate by UV/PMS and UV/H2O2: A comparative study. Environ. Technol. 2022, 2022, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, P.; Liu, J.; Chen, M.; Ahmed, Z.; Zhu, N. Efficient removal of bisphenol A by superoxide radical and singlet oxygen generated from peroxymonosulfate activated with Fe0-montmorillonite. Chem. Eng. J. 2018, 350, 484–495. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Mafa, P.J.; Mamba, B.B.; Kuvarega, A.T. Construction of hierarchical BiPW12O40/BiOI p–n heterojunction with enhanced visible light activity for degradation of endocrine disrupting Bisphenol A. Sep. Purif. Technol. 2020, 253, 117349. [Google Scholar] [CrossRef]
- Guo, J.; Shen, C.-H.; Sun, J.; Xu, X.-J.; Li, X.-Y.; Fei, Z.-H.; Liu, Z.-T.; Wen, X.J. Highly efficient activation of peroxymonosulfate by Co3O4/Bi2MoO6 pn heterostructure composites for the degradation of norfloxacin under visible light irradiation. Sep. Purif. Technol. 2021, 259, 118109. [Google Scholar] [CrossRef]
- Shakeel, M.; Arif, M.; Yasin, G.; Li, B.; Khan, H.D. Layered by layered Ni-Mn-LDH/g-C3N4 nanohybrid for multi-purpose photo/electrocatalysis: Morphology controlled strategy for effective charge carriers separation. Appl. Catal. B Environ. 2019, 242, 485–498. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Activation of peroxymonosulfate by chemically modified sludge biochar for the removal of organic pollutants: Understanding the role of active sites and mechanism. Chem. Eng. J. 2020, 392, 123681. [Google Scholar] [CrossRef]
- Aguilar, C.; Pandiyan, T.; Arenas-Alatorre, J.; Singh, N. Oxidation of phenols by TiO2-Fe3O4-M (M = Ag or Au) hybrid composites under visible light. Sep. Purif. Technol. 2015, 149, 265–278. [Google Scholar] [CrossRef]
- Shaha, N.; Khanb, J.; Sayedb, M.; Khana, Z.; Iqbalc, J.; Arshada, S.; Junaida, M.; Khan, H. Synergistic effects of H2O2 and S2O82− in the gamma radiation induced degradation of congo-red dye: Kinetics and toxicities evaluation. Sep. Purif. Technol. 2020, 233, 115966. [Google Scholar] [CrossRef]
- Huerta-Aguilar, C.; Palos-Barba, V.; Thangarasu, P.; Koodali, R. Visible light driven photo-degradation of Congo red by TiO2ZnO/Ag:DFT approach on synergetic effect on band gap energy. Chemosphere 2018, 213, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Kim, E.; Park, S.; Daigle, H.; Milner, T.; Huh, C. Oil Droplet Removal from Produced Water Using Nanoparticles and Their Magnetic Separation. In Proceedings of the SPE Annual Technical Conference and Exhibition 2016, Dubai, United Arab Emirates, 26–28 September 2016. [Google Scholar]








| Photocatalyst | Light Source | Dye (mg/L) | Degradation (%) | Time (min) | Ref |
|---|---|---|---|---|---|
| CoMoO4@CMS | UV-vis | SDM 30 mg/L | 98 | 10 min | [29] |
| CoMoO4-Co3O4 | UV-vis | DBT 2000 ppm | 100 | 180 min | [31] |
| CoMoO4 | UV-vis | 4-CP 50 mg/L | 88 | 275 min | [32] |
| CoMoO4 | UV-vis | MB 100 mg/L | 100 | 40 min | [33] |
| CuNi/CoMoO4 | UV-vis | AF 15 mg/L | 99.45 | 40 min | [34] |
| CoMoO4 | UV-vis | CR 100 mg | 96.975 | 35 min | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Qiu, Y.; Yang, C.; Zang, J.; Song, Z.; Yang, T.; Li, J.; Fan, Y.; Dang, F.; Wang, W. Efficient Degradation of Congo Red in Water by UV-Vis Driven CoMoO4/PDS Photo-Fenton System. Molecules 2022, 27, 8642. https://doi.org/10.3390/molecules27248642
Zhou H, Qiu Y, Yang C, Zang J, Song Z, Yang T, Li J, Fan Y, Dang F, Wang W. Efficient Degradation of Congo Red in Water by UV-Vis Driven CoMoO4/PDS Photo-Fenton System. Molecules. 2022; 27(24):8642. https://doi.org/10.3390/molecules27248642
Chicago/Turabian StyleZhou, Huimin, Yang Qiu, Chuanxi Yang, Jinqiu Zang, Zihan Song, Tingzheng Yang, Jinzhi Li, Yuqi Fan, Feng Dang, and Weiliang Wang. 2022. "Efficient Degradation of Congo Red in Water by UV-Vis Driven CoMoO4/PDS Photo-Fenton System" Molecules 27, no. 24: 8642. https://doi.org/10.3390/molecules27248642
APA StyleZhou, H., Qiu, Y., Yang, C., Zang, J., Song, Z., Yang, T., Li, J., Fan, Y., Dang, F., & Wang, W. (2022). Efficient Degradation of Congo Red in Water by UV-Vis Driven CoMoO4/PDS Photo-Fenton System. Molecules, 27(24), 8642. https://doi.org/10.3390/molecules27248642






