Abstract
The material with a high Curie temperature of cobalt-doped zinc oxide embedded with silver-nanoparticle thin films was studied by electron magnetic resonance. The nanoparticles were synthesized by the homogeneous nucleation technique. Thin films were produced with the pulsed laser deposition method. The main aim of this work was to investigate the effect of Ag nanoparticles on the magnetic properties of the films. Simultaneously, the coexisting Ag0 and Ag2+ centers in zinc oxide structures are shown. A discussion of the signal seen in the low field was conducted. To analyze the temperature dependence of the line parameters, the theory described by Becker was used. The implementation of silver nanoparticles causes a significant shift of the line, and the ferromagnetic properties occur in a wide temperature range with an estimated Curie temperature above 500 K.
1. Introduction
Zinc oxide (ZnO) is an inexpensive well-known semiconductor with potential in various applications [1,2,3], such as varistors [4] or sensors [5,6]. It is important that some of the zinc can be replaced by magnetic transition metal ions (TM) to create a metastable solid solution. Since both Zn2+ and Co2+ ions have nearly identical ion radii, doping ZnO with cobalt is most interesting [7]. Furthermore, parameters such as piezoelectricity and transparency in the visual region have attracted great interest from researchers in ZnO-based diluted magnetic semiconductors (DMS) [8,9] due to their possible technological applications in spintronics [10,11,12,13]. Using the modified Zener model, Dietl et al. suggested that p-type DMS based on ZnO could lead to a transition temperature greater than room temperature [14]. In this theory, p–d interactions are the cause of long-range magnetic coupling, but the studied ZnO samples are either insulating or conducting n-types. Some theoretical works using density functional theory (DFT) [15,16] show that n-type cobalt-doped ZnO shows ferromagnetism (FM) at room temperature (RTFM). Some research groups reported ferromagnetism in ZnO doped with transition metals with Curie temperatures (TC) from 30 to 550 K [17,18,19,20,21,22,23], and some found antiferromagnetic, spin-glass, or paramagnetic behavior [9,24,25]. The existence of a ferromagnetic order in Co-doped ZnO is suggested to be attributed to double exchange [8] or the Ruderman–Kittel–Kasuya–Yosida (RKKY) interaction between Co ions [26]. Theoretical calculations show that ground-state ZnO with Co ions is spin-glass because of the short-range interactions between TM atoms [27].
ZnO doped with gold (Au) or silver (Ag) increases the photocatalytic activity of the composite by reducing electron-hole recombination and improving separation [28]. Silver nanoparticles (NP) have been investigated by many scientists because of their significant role in applications of visible light absorption [29,30]. Many works have described the synthesis nanocomposites of heterogeneous ZnO/Ag via a variety of synthetic routes for various applications, such as disinfection and wastewater treatment [31,32,33,34,35,36,37,38,39]. In addition, research confirms that the existence of Ag NPs on the ZnO surface reduces the intensity of electron magnetic resonance (EMR) signals and may lead to improved photodegradation efficiency [40].
Obtaining a homogeneous thin layer with p-type ZnO is a challenging task. One of the proposed methods is the addition of silver ions [41,42,43]. The pulsed laser deposition (PLD) method has a wide range of particle energies, allowing mainly Zn ions to penetrate deeper into the substrate, forming a mixed structure with the desired conductive type [43]. The main aim of this work was to investigate the influence of silver nanoparticles on ZnO doped with cobalt-ion thin film and to determine the changes in magnetic properties compared to that of layers without silver NPs.
2. Results and Discussion
Measurements of EMR were taken on samples with a quartz and silicon substrate. Angular dependence measurements of the Zn0.8Co0.2O/Ag film on the quartz substrate were measured at room temperature; a summary is shown in Figure 1.

Figure 1.
EMR spectra as a function of the angular dependence of Zn0.8Co0.2O/Ag film.
The resulting angular dependence is characteristic of magnetic layers and the combination of magnetic and nonmagnetic layers. A strong anisotropy of the spectrum is observed, along with a change in the shape of the line, with the result that, at certain angles, the width of the line increases significantly as the intensity decreases, and the EMR line is no longer visible. However, a line visible all the time in the low field remains, the so-called low-field microwave absorption (LFMA), which is also called an indicator of FM properties for a large group of materials. The LFMA signal for ferrites and magnets is related to the beginning of the ordered phase and is a sensitive detector of magnetic ordering [44,45]. For soft magnetic materials, the signal is due to low-field processes of spin magnetization [46].
EMR measurements as a function of temperature were performed in two temperature ranges, from 300 to 500 K and from 97 to 300 K. Figure 2 shows the EMR spectra of Zn0.8Co0.2O/Ag as a function of temperature in the range from 300 to 500 K. The measurement was performed at an angle when the EMR line was close to its extreme position, and it corresponds to an orientation of 115 degrees from the angular dependence in Figure 1.

Figure 2.
EMR spectra of Zn0.8Co0.2O/Ag as a function of temperature in the range from 300 to 500 K.
In contrast to the angular dependence of the EMR spectrum, the temperature dependence over the entire temperature range studied does not show large changes in the shape and position of the EMR line. We can see a broad line moving in the direction of the low magnetic field for Zn0.8Co0.2O/Ag compared to the sample without silver NPs (Figure 3) and to layers of Co-doped ZnO (in our previous papers [47,48,49]).
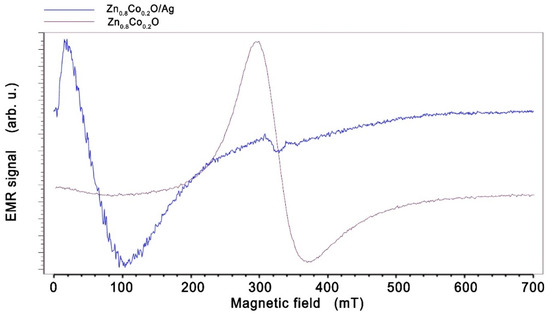
Figure 3.
EMR spectra of Zn0.8Co0.2O (purple) and Zn0.8Co0.2O embedded with Ag NPs (blue), obtained at temperature 160 K.
Such a large shift of the EMR line can be explained by the appearance of ferromagnetic properties in Zn0.8Co0.2O embedded with Ag NPs. A similar effect is observed in many works; for example, in spin-glasses or soft and hard magnetic layers [50,51,52,53]. The hard and soft layers of the spring magnets are coupled at the interfaces due to the strong exchange coupling between them. A high magnetic saturation is achieved by a soft magnet, whereas a high coercivity field is achieved by the magnetically hard material. The shift of the EMR line towards a low magnetic field for the layers of work [52] is related to the layer thickness and occurs when the thickness changes from 10 to 20 nm. In our sample, we observe conglomerates of nanoparticles with sizes in the order of 80 nm, as well as single nanoparticles, so, in addition to the EMR line, we also observe a spectrum from single silver nanoparticles. Low-intensity lines can also be seen in a field of about 340 mT. These were assigned to silver ions Ag0 (4d105s1) and/or Ag2+ and Ag0 (4d9) (Figure 4) (described in the literature [54]). This confirms that Ag0 and Ag2+ centers can coexist simultaneously in zinc oxide structures, with Ag+ being inactive in the EMR signal. Matching the EMR spectrum with the Dyson-type line results from a good fit to our sample Zn0.8Co0.2O (Figure 4), and hence the line parameters were obtained: the peak-to-peak linewidth (Hpp), the EMR intensity (I), and the resonance field (Hr). Xepr software was used to analyze and determine the parameters of the EMR line; this is the standard EPR spectrometer software used to control and analyze the spectrum.

Figure 4.
EMR spectra of Zn0.8Co0.2O embedded with Ag NPs film obtained at temperature 300 K with the simulated Dyson-type line. Small intensity lines from Ag2+ and Ag0 are described.
Figure 5 shows the intensities of the EMR line as a function of temperature in two temperature ranges.

Figure 5.
The EMR line intensities as a function of temperature in two temperature ranges, below (a), and close to TC (b).
The nature of the temperature dependence of the line parameters (Hpp, I, and Hr) suggests that the TC is higher than 500 K. The theory described by Becker [55] was used to analyze the temperature dependence (where T is the temperature of the measurement). Becker calculated the EMR resonance field shift and linewidth as a function of temperature and frequency near freezing temperature for spin-glass alloys, using RKKY exchange coupling and a smaller anisotropic interaction. To fit our line parameters’ behavior, we adopted Becker’s theory for the critical regime (T~TC) and spin-glass regime (T < TC). In Zn0.8Co0.2O/Ag, we can see an abnormal reduction in the linewidth resonance (Hpp) with a minimum at or near the critical temperature (Figure 6).
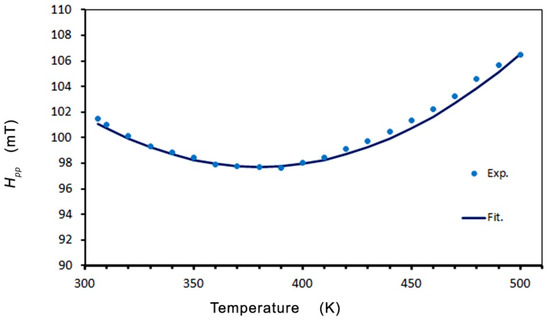
Figure 6.
Temperature dependence of the resonance linewidth Hpp.
To fit the linewidth about the minimum, we used the function [50,51,55]:
where a0 is the residual linewidth; b′ is the thermal broadening constant (independent of the orientation of the static field); n is the exponent of the expression for the length of the correlation associated with the distributed magnetization of Huber’s theory of linewidth in isolated ferromagnets and antiferromagnets in the region near the critical temperature [56,57]; and Tmin represents the temperature of the minimum linewidth. The large value of the exponent n = 3 v/2 that we obtain is consistent with the presence of a strong perturbation, which is expected to increase the rate at which the correlation length decreases as the temperature moves away from the critical temperature. In the theory of the mean-field 3D Heisenberg model v = 0.71, and the value that we obtained was v = 1.33 [58]. The best fit of Equation (1) is shown in Figure 6 as the black line, where a0 = 97.7 mT, b′ = 89 mT, Tmin = 380 K, and n = 2.0. This agrees with experimental data in the temperature range of 300 to 500 K, and the value of a0 = 97.7 mT implies that the effects of the crystal field and demagnetization in Zn0.8Co0.2O/Ag are high. The origin of the residual linewidth component in the spin-glass alloys has been assigned to local moment imperfections and crystal-field effects via a mechanism of demagnetization.
In the low-temperature regime (T < TC), we can see a linewidth broadening and a shift in the resonance field. This is similar to the dependence in spin-glass alloys (for example, AgMnxSby) and in molecule-based magnets [50,55]. In these alloys, the excess linewidth was assigned to an exchange-narrowed anisotropic interaction. With the decreasing temperature, the slowing down of the spin fluctuations reduces the effectiveness of the exchange-narrowing. Moreover, these alloys also show a related shift in the resonance field, which is neither a frequency-independent internal field nor a pure g-shift. Becker calculated the EMR linewidth and line shift effects for spin-glasses with anisotropy [55]. For systems with no remnant magnetization, Becker has shown that the resonance field and linewidth are given by formulas [50,51,55]:
and
where , , ; and is the resonance frequency. Here K is the constant of anisotropy, is the static susceptibility of transverse, and M2 is associated with spin relaxation.
The best fits to the experimental data (shown in Figure 7 and Figure 8) are A = 39.62 mT, B = 44.49 K, and H0 = 19.12 mT. Very good agreement between the experimental data and the fitting (Equations (2) and (3)) is found. The linewidth and resonance field shift are compatible with a disordered ferrimagnet near and below critical temperature. Additional confirmation of the observed ferromagnetic properties is provided by the LFMA line. The absorption of microwave power centered at zero magnetic field has been reported in ferromagnetic materials and in various other materials, such as ferrites, high-temperature superconductors, and soft magnetic materials [44,46,59]. For soft magnetic materials, the LFMA signal is induced by low-field processes of spin magnetization [46]. The appearance of LMFA lines is an indicator of the ferromagnetic properties of the material. Figure 9 shows the magnetic hysteresis that is very often peeled off in the literature for LFMA lines.
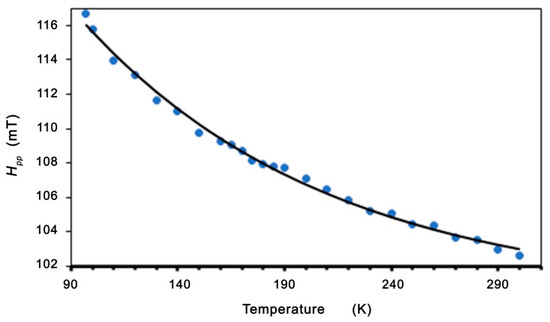
Figure 7.
Temperature dependence of the peak-to-peak linewidth for Zn0.8Co0.2O/Ag. Theoretical line fitted based on Equation (2).
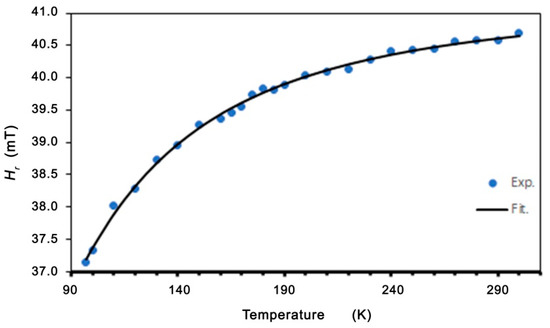
Figure 8.
Temperature dependence of the resonance field for Zn0.8Co0.2O/Ag. Theoretical line fitted based on Equation (3).
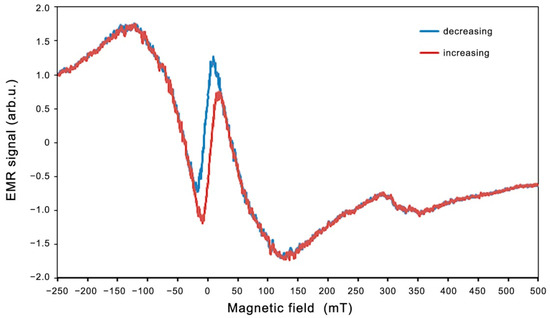
Figure 9.
EMR spectrum recorded for an increasing (red) and decreasing (blue) magnetic field at 300 K for Zn0.8Co0.2O/Ag on a quartz substrate.
A similar hysteresis of the LFMA line was observed for Zn0.8Co0.2O/Ag on a silicon substrate, shown in Figure 10.

Figure 10.
EMR spectrum recorded for an increasing (red) and a decreasing (blue) magnetic field at 140 K for Zn0.8Co0.2O/Ag on a silicon substrate.
For the Zn0.8Co0.2O/Ag film on a silicon substrate, we observe a lower intensity of the EMR lines and LFMA lines, but the nature of the observed magnetic properties is similar. The addition of gold produces a similar effect, although with a weaker intensity. The same layer deposited on a silicon substrate produces an analogous effect, including a hysteresis loop, although with a weaker intensity. The observed changes in the EMR spectrum obtained in both directions of registration show hysteresis only near the zero magnetic field; meanwhile in the rest of the range, the spectrum has an identical form and we do not observe changes in the shape of the EMR spectrum as presented in the paper [60].
3. Materials and Methods
Electron magnetic resonance measurements in the continuous wave X-band were taken on the Bruker FT-EPR ELEXSYS E580 spectrometer (Bruker Analytische Messtechnik, Rheinstetten, Germany). To control the temperature, Bruker liquid nitrogen cryostats were used with the 41131 VT digital controller, and the angular dependences of the EPR spectra were performed using a one-degree programmable goniometer E218-1001 (Bruker Analytische Messtechnik, Rheinstetten, Germany).
Samples of ZnO doped with cobalt thin films (Zn1−xCoxO, x = 0.2) embedded with Ag NPs were obtained using a combination of PLD and homogeneous nucleation techniques. A homogeneous nucleation technique was chosen for the synthesis of silver nanoparticles (more details on the creation of noble metal NPs are given in the work [61]). A droplet of aqueous Ag NPs was then deposited on the surface of the substrate with further drying under surrounding conditions. For the PLD method, a silicon and quartz substrate was chosen. The KGd(WO4)2 laser was used—radiation characteristics: λ = 1067 nm, beam energy density 6–8 J/cm2, repetition rate 10–0.3 Hz, pulse duration t = 20 ns. The technology module used the Q-switch to irradiate in modulated goodness factor mode. The deposition temperature (substrate temperature) was about 200 °C. The thickness of the resulting layer was about 300 nm. Therefore, a planar nanocomposite consisting of a Zn0.8Co0.2O thin film deposited on silver nanoparticles (Zn0.8Co0.2O/Ag) was formed. The specificity of the PLD method is the wide particle energies spread, so we expect a deeper penetration of the applied particles into the substrate and the formation of a complex structure at the contact zone of the resulting layer and Ag NPs.
The basic parameters of the obtained layers were controlled, and the results are included in the Supplementary Materials.
4. Conclusions
We have performed X-band EMR studies of the Zn0.8Co0.2O embedded with Ag NPs. A line in a broad asymmetric Dyson shape associated with magnetic interactions was observed. The results of a temperature dependence analysis for the EMR linewidth and resonant field based on Becker’s model were developed. From the critical regime (T~TC), the temperature Tmin = 380 K was determined for the minimum width of the EMR linewidth. The value of a0 = 97.7 mT suggests that demagnetization and the effects of the crystal field in Zn0.8Co0.2O/Ag are high. For the range of the low-temperature regime (T < TC), constants A and B related to the magnetic properties were determined. A = 39.62 mT, B = 44.49 K, and H0 = 19.12 mT. Very good agreement between the experimental data and the fitting was found. The linewidth and resonance field shift are in accordance with a disordered ferrimagnet near and below the critical temperature. In addition, small, intense lines are assigned to the silver ions Ag2+ and Ag0 [54]. There is a shift of the line towards the low-field direction for samples with silver nanoparticles.
The aim of this study was to investigate the effect of silver nanoparticles on the magnetic properties of Zn0.8Co0.2O. It was shown that the implementation of silver nanoparticles causes a significant shift of the ferromagnetic resonance (FMR) line (Figure 3) and that the ferromagnetic properties occur in a wide temperature range with an estimated Curie temperature above 500 K; the description is consistent with the adopted model, which can be considered as a major achievement of this work. Based on the results obtained, it can be claimed that a material with desirable magnetic properties at room temperature has been obtained, with potential applications that are important in spintronics.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules27238500/s1, Figure S1: SEM images of the Zn0.8Co0.2O/Ag film with clusters of Ag NPs; Figure S2: XRD diffractogram of Zn0.8Co0.2O/Ag film on a silicon substrate; Figure S3: EDS analysis of the Zn0.8Co0.2O/Ag composition; Table S1: The average crystallite size of Ag NPs; Table S2: EDS analysis of Zn0.8Co0.2O/Ag composites
Author Contributions
Conceptualization, B.C. and I.S.; methodology, B.C. and I.S.; investigation, B.C., I.V. and R.V.G.; data curation, B.C., I.S. and I.R.; writing—original draft preparation, B.C. and I.S.; writing—review and editing, B.C., I.S. and I.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the Zn0.8Co0.2O/Ag compounds are available from the authors.
References
- Pearton, S. GaN and ZnO-Based Materials and Devices; Pearton, S., Ed.; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2012; Volume 156, ISBN 978-3-642-23520-7. [Google Scholar]
- Weng, J.; Zhang, Y.; Han, G.; Zhang, Y.; Xu, L.; Xu, J.; Huang, X.; Chen, K. Electrochemical deposition and characterization of wide band semiconductor ZnO thin film. Thin Solid Films 2005, 478, 25–29. [Google Scholar] [CrossRef]
- Wilkinson, J.; Ucer, K.B.; Williams, R.T. Picosecond excitonic luminescence in ZnO and other wide-gap semiconductors. Radiat. Meas. 2004, 38, 501–505. [Google Scholar] [CrossRef]
- Greuter, F. ZnO Varistors: From Grain Boundaries to Power Applications. In Oxide Electronics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 157–234. [Google Scholar]
- Tsai, Y.T.; Chang, S.J.; Ji, L.W.; Hsiao, Y.J.; Tang, I.T.; Lu, H.Y.; Chu, Y.L. High Sensitivity of NO Gas Sensors Based on Novel Ag-Doped ZnO Nanoflowers Enhanced with a UV Light-Emitting Diode. ACS Omega 2018, 3, 13798–13807. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Kolesnik, S.; Dabrowski, B.; Mais, J. Structural and magnetic properties of transition metal substituted ZnO. J. Appl. Phys. 2004, 95, 2582–2586. [Google Scholar] [CrossRef]
- Samarth, N.; Furdyna, J.K. Electron paramagnetic resonance in Cd1−xMnxS, Cd1−xMnxSe, and Cd1−xMnxTe. Phys. Rev. B 1988, 37, 9227–9239. [Google Scholar] [CrossRef]
- Volbers, N.; Zhou, H.; Knies, C.; Pfisterer, D.; Sann, J.; Hofmann, D.M.; Meyer, B.K. Synthesis and characterization of ZnO:Co2+ nanoparticles. Appl. Phys. A Mater. Sci. Process. 2007, 88, 153–155. [Google Scholar] [CrossRef]
- Awschalon, D.D.; Loss, D. Semiconductors Spintronics and Quantum Computation; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 9783642075773. [Google Scholar]
- Pearton, S.J.; Norton, D.P.; Heo, Y.W.; Tien, L.C.; Ivill, M.P.; Li, Y.; Kang, B.S.; Ren, F.; Kelly, J.; Hebard, A.F. ZnO spintronics and nanowire devices. J. Electron. Mater. 2006, 35, 862–868. [Google Scholar] [CrossRef]
- Pan, F.; Song, C.; Liu, X.J.; Yang, Y.C.; Zeng, F. Ferromagnetism and possible application in spintronics of transition-metal-doped ZnO films. Mater. Sci. Eng. R Rep. 2008, 62, 1–35. [Google Scholar] [CrossRef]
- Kisan, B.; Kumar, J.; Alagarsamy, P. Experimental and first-principles study of defect-induced electronic and magnetic properties of ZnO nanocrystals. J. Phys. Chem. Solids 2020, 146, 109580. [Google Scholar] [CrossRef]
- Dietl, T.; Ohno, H.; Matsukura, F.; Cibert, J.; Ferrand, D. Zener Model Description of Ferromagnetism in Zinc-Blende Magnetic Semiconductors. Science 2000, 287, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Katayama-Yoshida, H. Material design for transparent ferromagnets with ZnO-based magnetic semiconductors. Jpn. J. Appl. Phys. Part 2 Lett. 2000, 39, L555–L558. [Google Scholar] [CrossRef]
- Sato, K.; Katayama-Yoshida, H. Stabilization of ferromagnetic states by electron doping in Fe-, Co- or Ni-doped ZnO. Jpn. J. Appl. Phys. Part 2 Lett. 2001, 40, L334–L336. [Google Scholar] [CrossRef]
- Jung, S.W.; An, S.-J.; Yi, G.-C.; Jung, C.U.; Lee, S.-I.; Cho, S. Ferromagnetic properties of Zn1−xMnxO epitaxial thin films. Appl. Phys. Lett. 2002, 80, 4561–4563. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Norberg, N.S.; Nguyen, Q.P.; Parker, J.M.; Gamelin, D.R. Magnetic Quantum Dots: Synthesis, Spectroscopy, and Magnetism of Co2+- and Ni2+-Doped ZnO Nanocrystals. J. Am. Chem. Soc. 2003, 125, 13205–13218. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Jeong, S.-Y.; Cho, C.R.; Park, C.H. Study of diluted magnetic semiconductor: Co-doped ZnO. Appl. Phys. Lett. 2002, 81, 4020–4022. [Google Scholar] [CrossRef]
- Cho, Y.M.; Choo, W.K.; Kim, H.; Kim, D.; Ihm, Y. Effects of rapid thermal annealing on the ferromagnetic properties of sputtered Zn1−x(Co0.5Fe0.5)xO thin films. Appl. Phys. Lett. 2002, 80, 3358–3360. [Google Scholar] [CrossRef]
- Sharma, P.; Gupta, A.; Rao, K.V.; Owens, F.J.; Sharma, R.; Ahuja, R.; Osorio-Guillen, J.M.; Johansson, B.; Gehring, G.A. Ferromagnetism above room temperature in bulk and transparent thin films of Mn-doped ZnO. Nat. Mater. 2003, 2, 673–677. [Google Scholar] [CrossRef]
- Wakano, T.; Fujimura, N.; Morinaga, Y.; Abe, N.; Ashida, A.; Ito, T. Magnetic and magneto-transport properties of ZnO:Ni films. Phys. E 2001, 10, 260–264. [Google Scholar] [CrossRef]
- Ueda, K.; Tabata, H.; Kawai, T. Magnetic and electric properties of transition-metal-doped ZnO films. Appl. Phys. Lett. 2001, 79, 988–990. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.; Kim, D.; Ihm, Y.E.; Choo, W.K. Magnetic properties of epitaxially grown semiconducting Zn 1−xCoxO thin films by pulsed laser deposition. J. Appl. Phys. 2002, 92, 6066–6071. [Google Scholar] [CrossRef]
- Ankiewicz, A.O.; Carmo, M.C.; Sobolev, N.A.; Gehlhoff, W.; Kaidashev, E.M.; Rahm, A.; Lorenz, M.; Grundmann, M. Electron paramagnetic resonance in transition metal-doped ZnO nanowires. J. Appl. Phys. 2007, 101, 024324. [Google Scholar] [CrossRef]
- Jalbout, A.F.; Chen, H.; Whittenburg, S.L. Monte Carlo simulation on the indirect exchange interactions of Co-doped ZnO film. Appl. Phys. Lett. 2002, 81, 2217–2219. [Google Scholar] [CrossRef]
- Lee, E.C.; Chang, K.J.J. Ferromagnetic versus antiferromagnetic interaction in Co-doped ZnO. Phys. Rev. B-Condens. Matter Mater. Phys. 2004, 69, 085205. [Google Scholar] [CrossRef]
- Merga, G.; Cass, L.C.; Chipman, D.M.; Meisel, D. Probing silver nanoparticles during catalytic H2 evolution. J. Am. Chem. Soc. 2008, 130, 7067–7076. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Jun, Y.W.; Yeon, S.I.; Kim, H.C.; Shin, J.S.; Cheon, J. Biocompatible heterostructured nanoparticles for multimodal biological detection. J. Am. Chem. Soc. 2006, 128, 15982–15983. [Google Scholar] [CrossRef]
- Jeong, S.H.; Park, B.N.; Lee, S.B.; Boo, J.-H. Structural and optical properties of silver-doped zinc oxide sputtered films. Surf. Coat. Technol. 2005, 193, 340–344. [Google Scholar] [CrossRef]
- Gouvêa, C.A.K.; Wypych, F.; Moraes, S.G.; Durán, N.; Peralta-Zamora, P. Semiconductor-assisted photodegradation of lignin, dye, and kraft effluent by Ag-doped ZnO. Chemosphere 2000, 40, 427–432. [Google Scholar] [CrossRef]
- Georgekutty, R.; Seery, M.K.; Pillai, S.C. A highly efficient Ag-ZnO photocatalyst: Synthesis, properties, and mechanism. J. Phys. Chem. C 2008, 112, 13563–13570. [Google Scholar] [CrossRef]
- Yin, X.; Que, W.; Fei, D.; Shen, F.; Guo, Q. Ag nanoparticle/ZnO nanorods nanocomposites derived by a seed-mediated method and their photocatalytic properties. J. Alloys Compd. 2012, 524, 13–21. [Google Scholar] [CrossRef]
- Wang, J.; Fan, X.M.; Tian, K.; Zhou, Z.W.; Wang, Y. Largely improved photocatalytic properties of Ag/tetrapod-like ZnO nanocompounds prepared with different PEG contents. Appl. Surf. Sci. 2011, 257, 7763–7770. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, L.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K. Ag/ZnO heterostructure nanocrystals: Synthesis, characterization, and photocatalysis. Inorg. Chem. 2007, 46, 6980–6986. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wu, H.; Zhang, R.; Pan, W. Enhanced photocatalysis of electrospun Ag-ZnO heterostructured nanofibers. Chem. Mater. 2009, 21, 3479–3484. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, X.; Wang, X. Growth and photocatalytic activity of ZnO nanosheets stabilized by Ag nanoparticles. J. Alloys Compd. 2011, 509, 4972–4977. [Google Scholar] [CrossRef]
- Hu, H.; Wang, Z.; Wang, S.; Zhang, F.; Zhao, S.; Zhu, S. ZnO/Ag heterogeneous structure nanoarrays: Photocatalytic synthesis and used as substrate for surface-enhanced Raman scattering detection. J. Alloys Compd. 2011, 509, 2016–2020. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Rodríguez, H.B.; San Román, E.; Feldhoff, A.; Grela, M.A. Ag@ZnO Core–Shell Nanoparticles Formed by the Timely Reduction of Ag+ Ions and Zinc Acetate Hydrolysis in N,N-Dimethylformamide: Mechanism of Growth and Photocatalytic Properties. J. Phys. Chem. C 2011, 115, 24967–24974. [Google Scholar] [CrossRef]
- Wang, C.C.; Shieu, F.S.; Shih, H.C. Ag-nanoparticle enhanced photodegradation of ZnO nanostructures: Investigation using photoluminescence and ESR studies. J. Environ. Chem. Eng. 2021, 9, 104707. [Google Scholar] [CrossRef]
- Yan, Y.; Al-Jassim, M.M.; Wei, S.H. Doping of ZnO by group-IB elements. Appl. Phys. Lett. 2006, 89, 181912. [Google Scholar] [CrossRef]
- Ahn, B.D.; Kang, H.S.; Kim, J.H.; Kim, G.H.; Chang, H.W.; Lee, S.Y. Synthesis and analysis of Ag-doped ZnO. J. Appl. Phys. 2006, 100, 093701. [Google Scholar] [CrossRef]
- Brauer, G.; Kuriplach, J.; Ling, C.C.; Djurišić, A.B. Activities towards p-type doping of ZnO. J. Phys. Conf. Ser. 2011, 265, 012002. [Google Scholar] [CrossRef]
- Srinivasu, V.V.; Lofland, S.E.; Bhagat, S.M.; Ghosh, K.; Tyagi, S.D. Temperature and field dependence of microwave losses in manganite powders. J. Appl. Phys. 1999, 86, 1067–1072. [Google Scholar] [CrossRef]
- Alvarez, G.; Zamorano, R. Characteristics of the magnetosensitive non-resonant power absorption of microwave by magnetic materials. J. Alloys Compd. 2004, 369, 231–234. [Google Scholar] [CrossRef]
- Montiel, H.; Alvarez, G.; Betancourt, I.; Zamorano, R.; Valenzuela, R. Correlations between low-field microwave absorption and magnetoimpedance in Co-based amorphous ribbons. Appl. Phys. Lett. 2005, 86, 072503. [Google Scholar] [CrossRef]
- Stefaniuk, I.; Cieniek, B.; Virt, I.S. Magnetic Properties of Zinc-Oxide Composite Doped with Transition Metal Ions (Mn, Co, Cr). Curr. Top. Biophys. 2010, 33, 221–226. [Google Scholar]
- Cieniek, B.; Stefaniuk, I.; Virt, I.S. EPR study of ZnO:Co thin films grown by the PLD method. Nukleonika 2013, 58, 359–363. [Google Scholar]
- Cieniek, B.; Stefaniuk, I.; Virt, I.S. EMR spectra thin films doped with high concentration of Co and Cr on quartz and sapphire substrates. Acta Phys. Pol. A 2017, 132, 30–33. [Google Scholar] [CrossRef]
- Mozurkewich, G.; Elliott, J.H.; Hardiman, M.; Orbach, R. Exchange-narrowed anisotropy contribution to the EPR width and shift in the Ag-Mn spin-glass. Phys. Rev. B 1984, 29, 278–287. [Google Scholar] [CrossRef]
- Long, S.M.; Zhou, P.; Miller, J.S.; Epstein, A.J. Electron Spin Resonance Study of The Disorder in The V(TCNE)x.y(MeCN) High-Tc Molecule-Based Magnet. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1995, 272, 207–215. [Google Scholar] [CrossRef]
- Yildiz, F.; Yalçm, O.; Özdemir, M.; Aktaş, B.; Köseoǧlu, Y.; Jiang, J.S. Magnetic properties of Sm-Co/Fe exchange spring magnets. J. Magn. Magn. Mater. 2004, 272, E1941–E1942. [Google Scholar] [CrossRef]
- Yaln, O. Ferromagnetic Resonance. In Ferromagnetic Resonance-Theory and Applications; IntechOpen: London, UK, 2013; ISBN 978-953-51-1186-3. [Google Scholar]
- Mandal, A.R.; Mandal, S.K. Electron spin resonance in silver-doped PbS nanorods. J. Exp. Nanosci. 2010, 5, 189–198. [Google Scholar] [CrossRef]
- Becker, K.W. Theory of electron-spin-resonance linewidth and line-shift effects in spin-glasses with anisotropy and zero remanent magnetization. Phys. Rev. B 1982, 26, 2409–2413. [Google Scholar] [CrossRef]
- Huber, D.L. EPR linewidths in RbMnF3 and MnF2. Phys. Lett. A 1971, 37, 283–284. [Google Scholar] [CrossRef]
- Zomack, M.; Baberschke, K.; Barnes, S.E. Magnetic resonance in the spin-glass (LaGd)Al2. Phys. Rev. B 1983, 27, 4135–4148. [Google Scholar] [CrossRef]
- Yeomans, J.M. Statistical Mechanics of Phase Transitions; Clarendon Press: Oxford, UK, 1992; Volume 19, ISBN 9780198517306. [Google Scholar]
- Bhat, S.V.; Ramakrishnan, T.V.; Ganguly, P.; Rao, C.N.R. Absorption of electromagnetic radiation by superconducting YBa2Cu3O7: An oxygen-induced phenomenon. J. Phys. C Solid State Phys. 1987, 20, L559–L563. [Google Scholar] [CrossRef]
- Gafurov, M.R.; Kurkin, I.N.; Kurzin, S.P. Inhomogeneity of the intrinsic magnetic field in superconducting YBa2Cu3OX compounds as revealed by a rare-earth EPR probe. Supercond. Sci. Technol. 2005, 18, 1183–1189. [Google Scholar] [CrossRef][Green Version]
- Savchuk, V.V.; Gamernyk, R.V.; Virt, I.S.; Malynych, S.Z.; Pinchuk, A.O. Plasmon-exciton coupling in nanostructured metal-semiconductor composite films. AIP Adv. 2019, 9, 045021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).