Discovery and Mechanistic Investigation of Piperazinone Phenylalanine Derivatives with Terminal Indole or Benzene Ring as Novel HIV-1 Capsid Modulators
Abstract
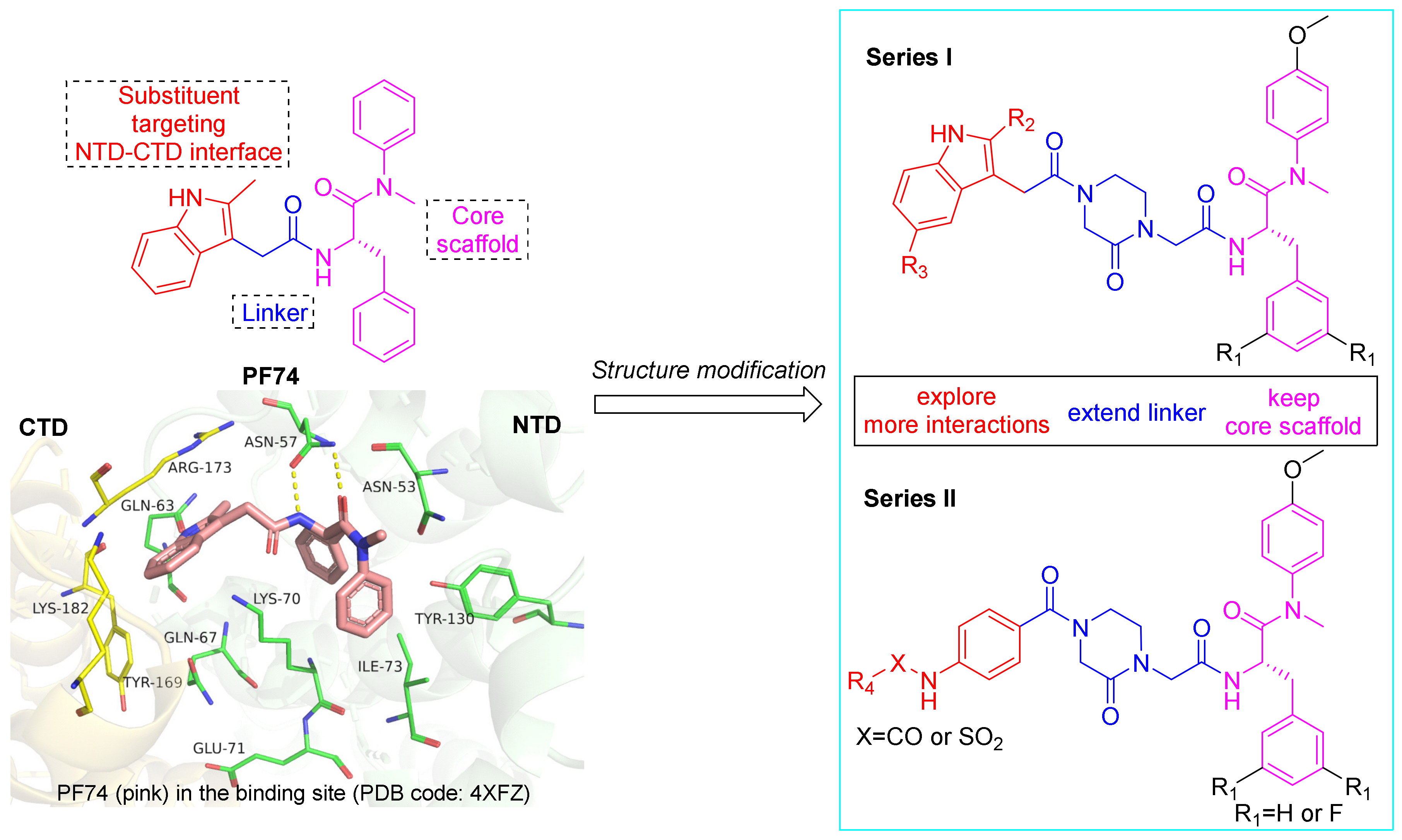
1. Introduction
2. Results and Discussion
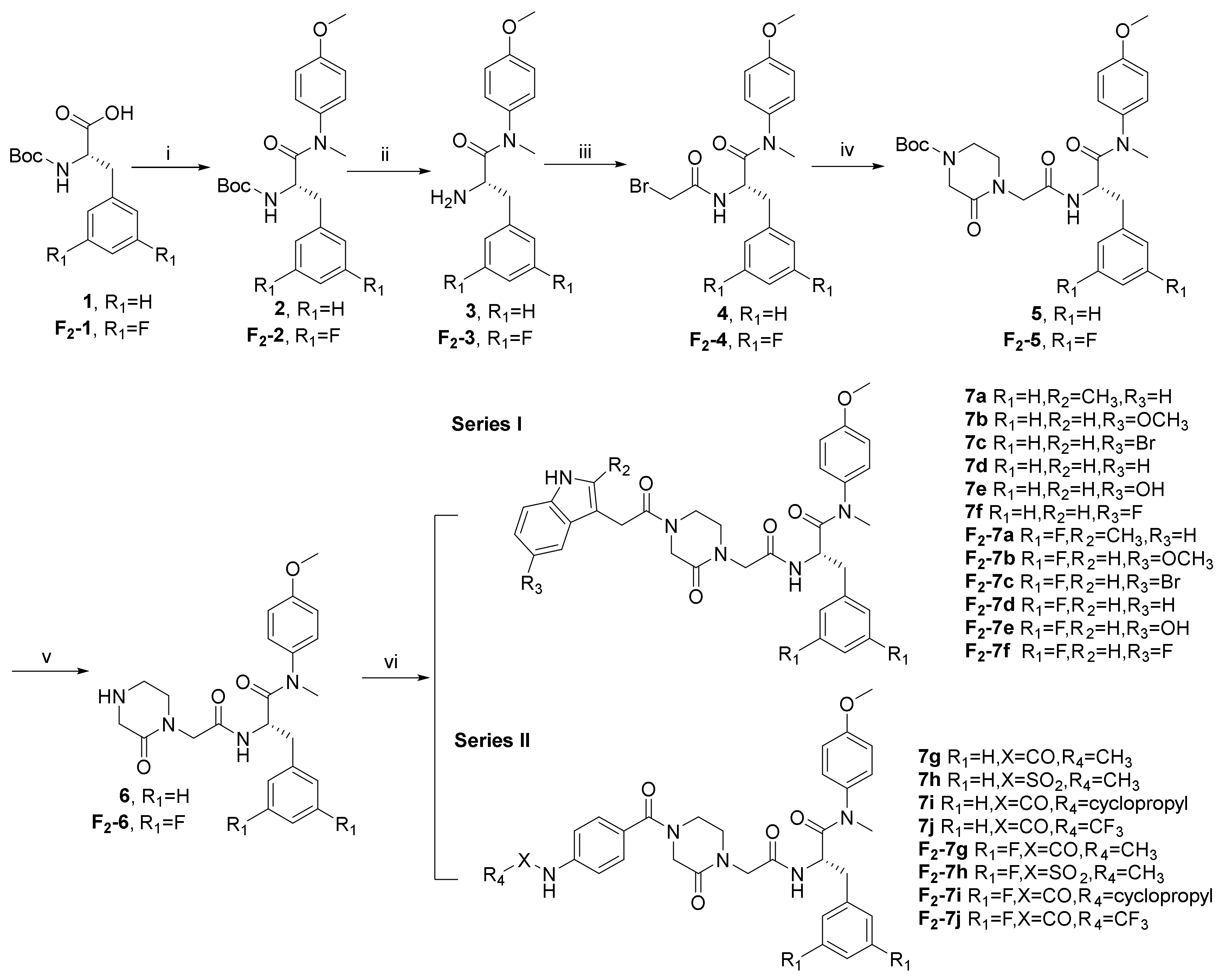
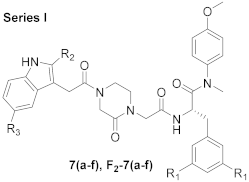
2.1. Chemistry
2.2. Antiviral Activity in MT-4 Cells against HIV-1 (IIIB) and HIV-2 (ROD) Replication
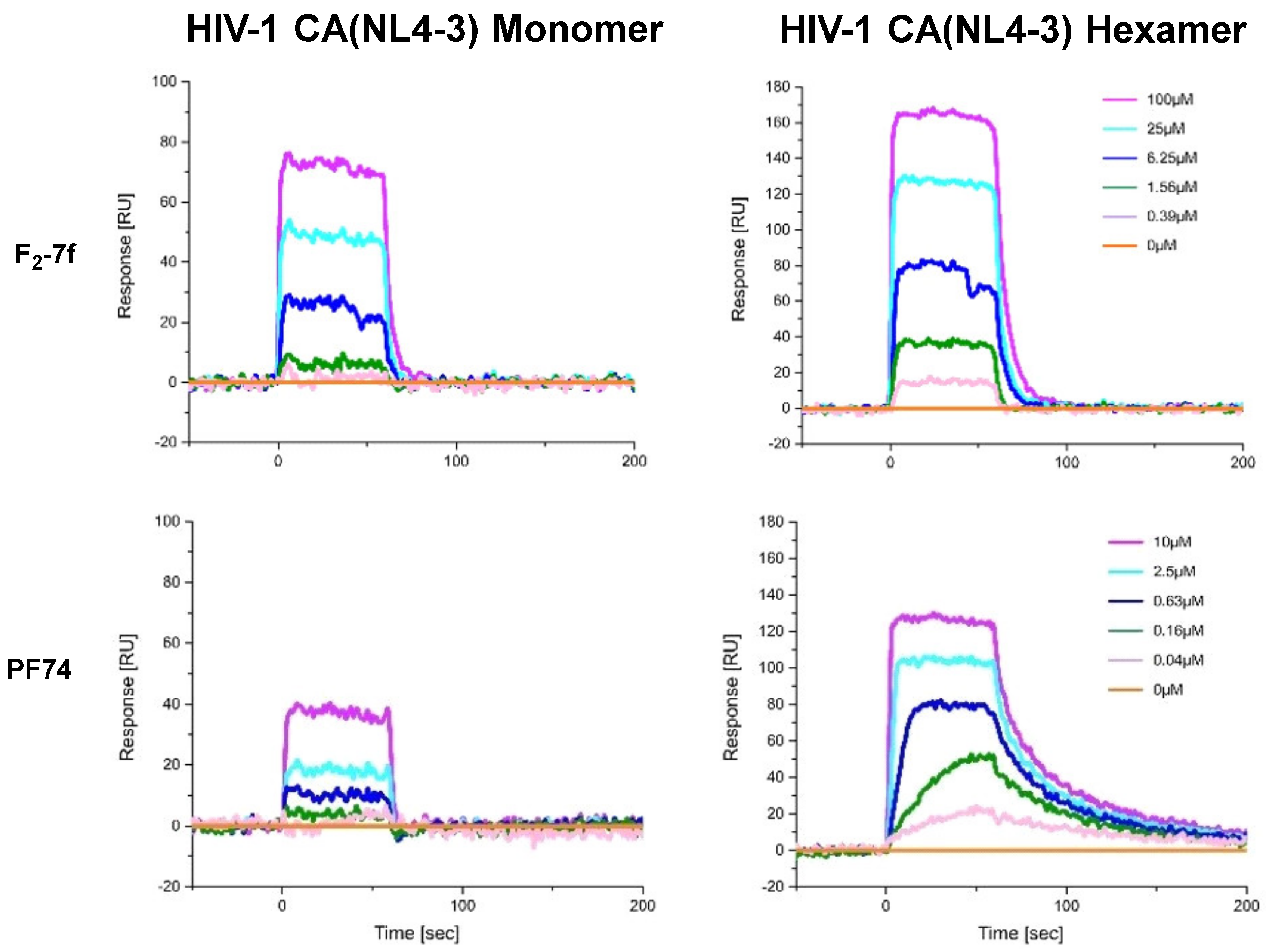
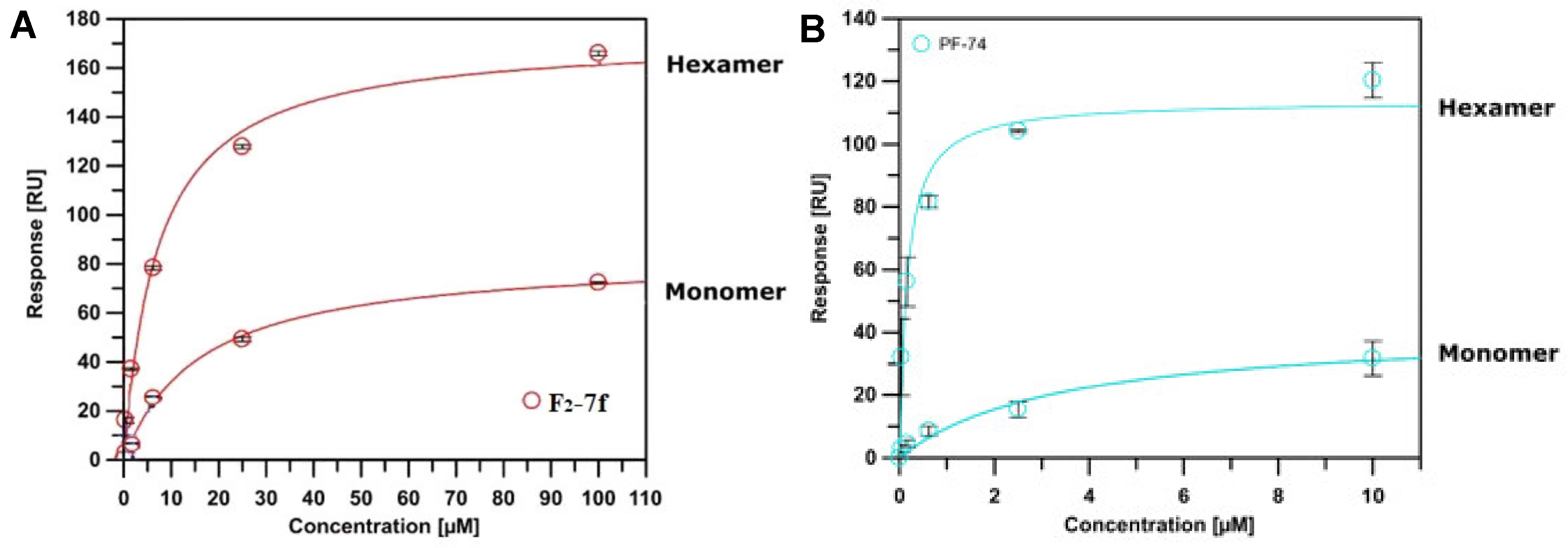
2.3. Surface Plasmon Resonance (SPR) Assay on CA Protein
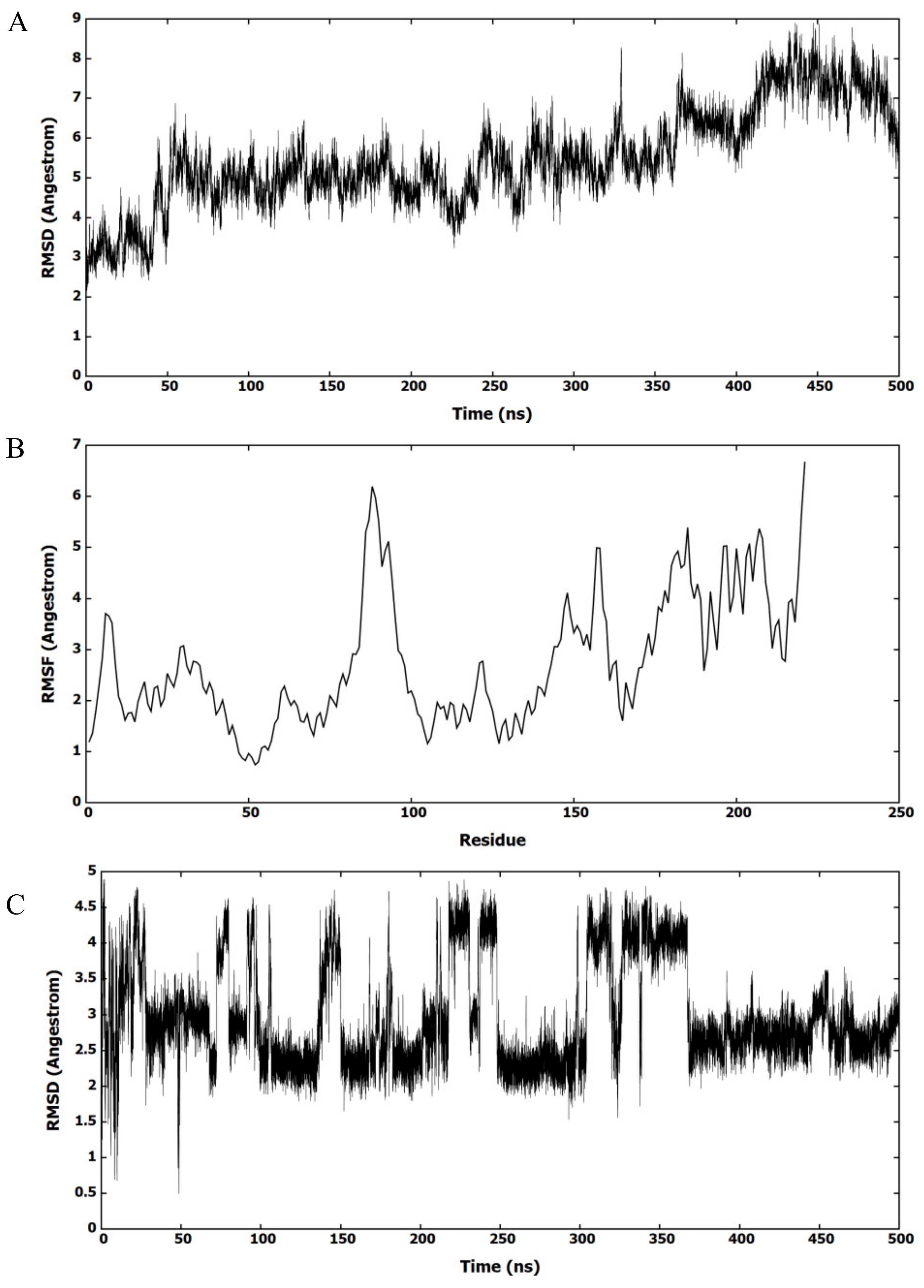
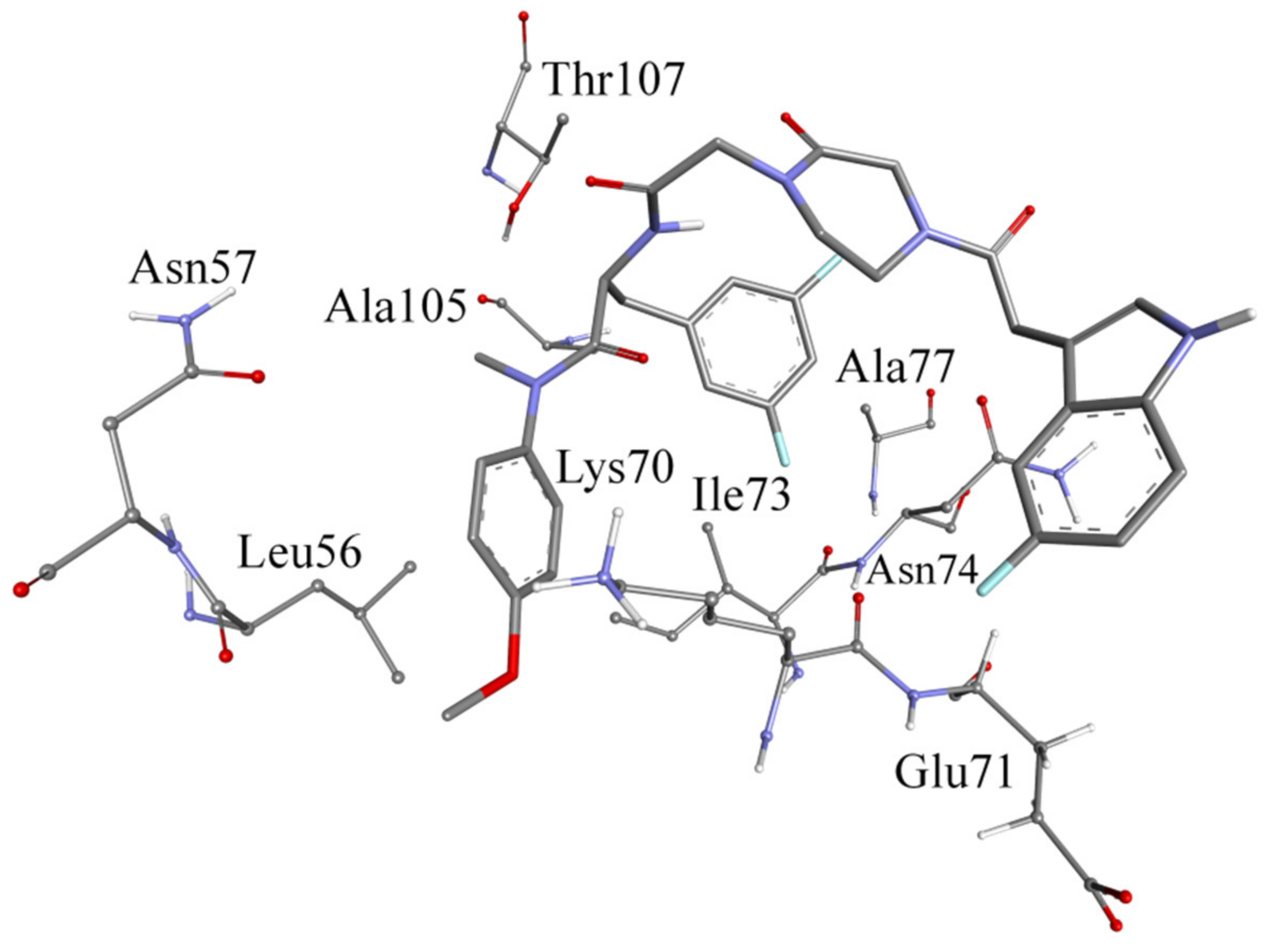
2.4. Molecular Dynamics (MD) Simulation with F2-7f Bound HIV-1 CA Hexamer
2.5. In Silico Predicted ADMET Properties of Representative Compounds
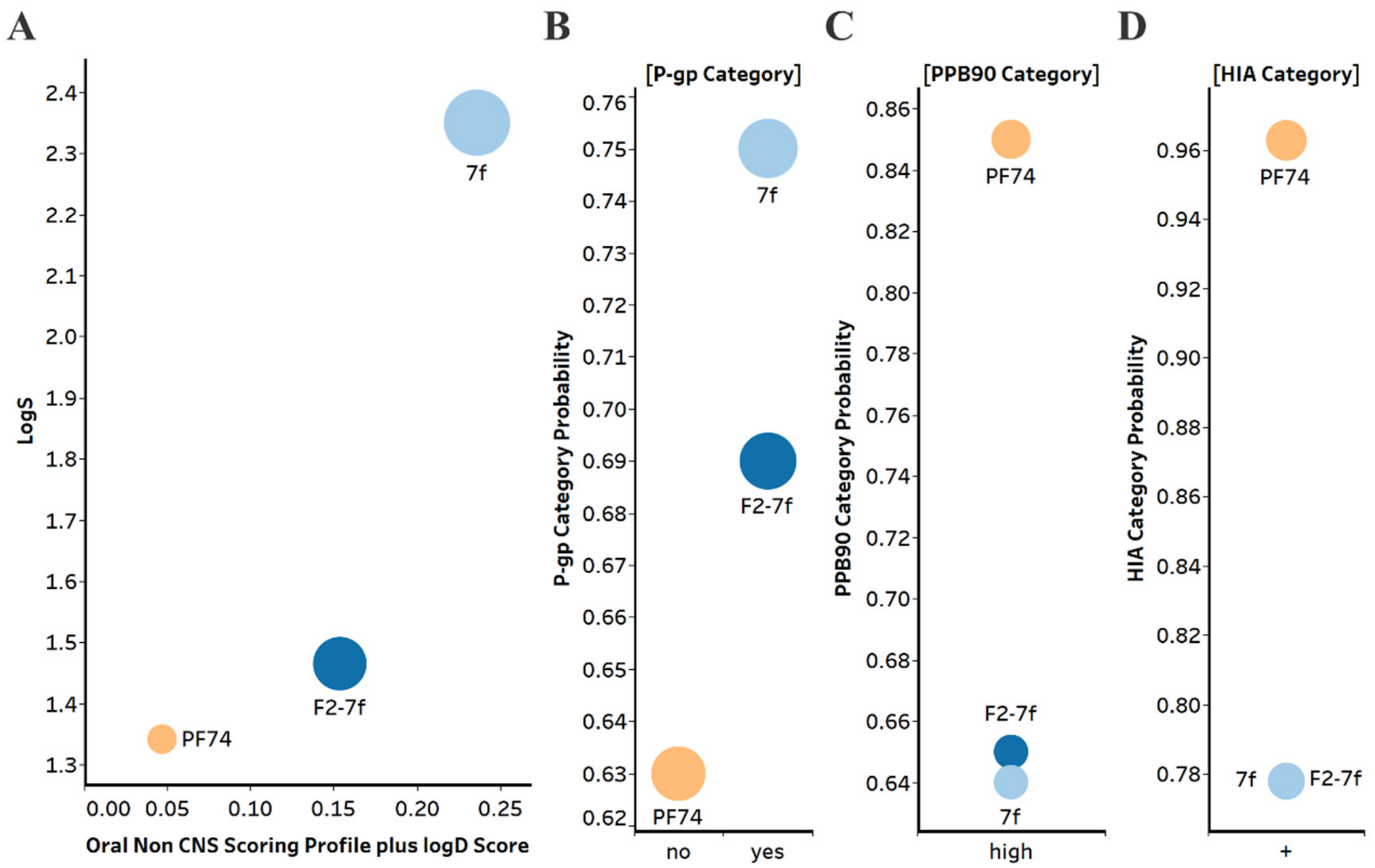
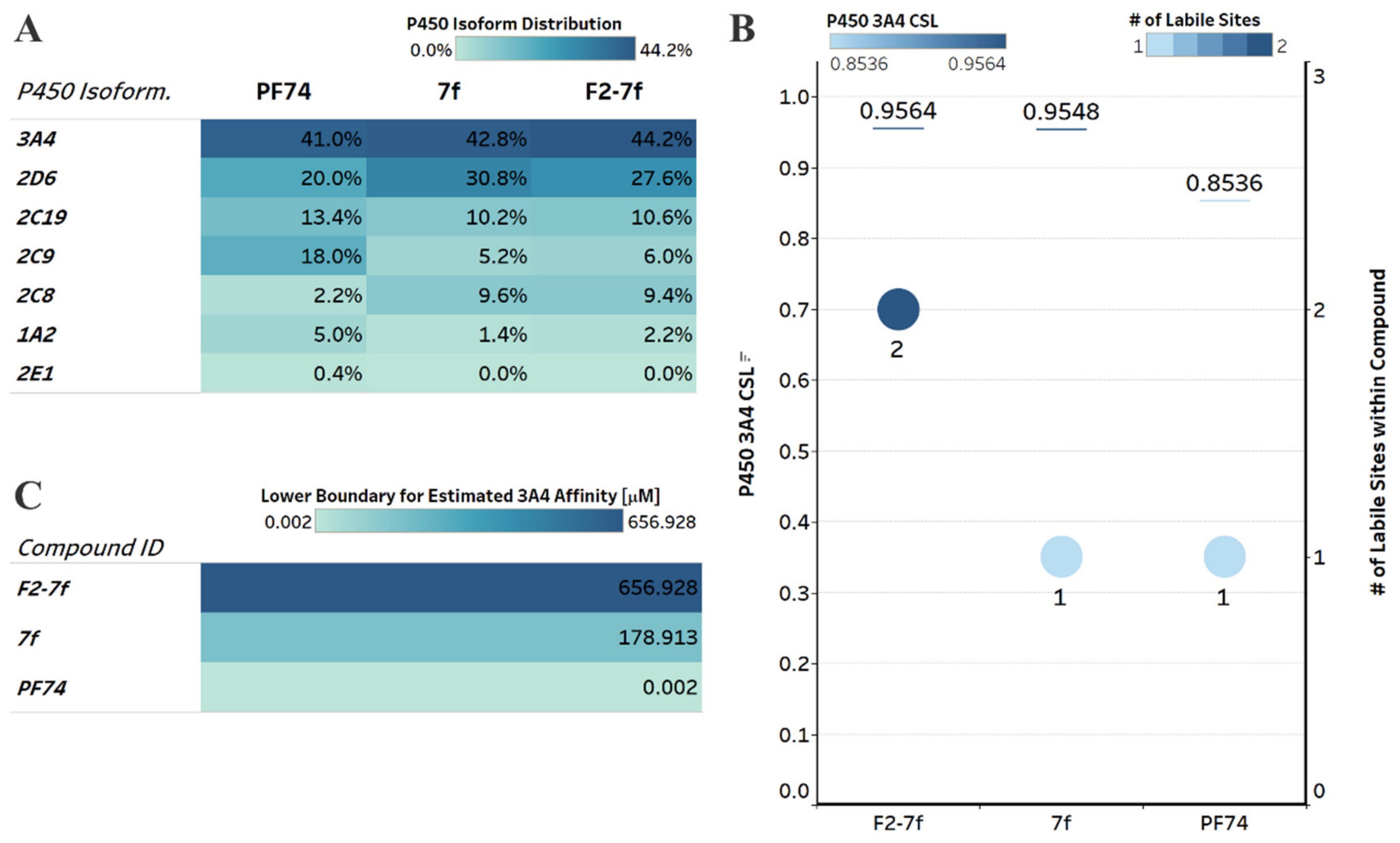
2.5.1. Drug-Like Properties and Metabolic Stability
2.5.2. Genotoxicity and Hepatotoxicity
2.6. Metabolic Stability in the Presence of Human Liver Microsomes and Human Plasma
3. Experimental Section
3.1. Chemistry
3.1.1. Procedure for the Synthesis of Intermediates
3.1.2. General Procedure for the Synthesis of 7 (a-f) and F2-7 (a-f)
(S)-N-(4-methoxyphenyl)-N-methyl-2-(2-(4-(2-(2-methyl-1H-indol-3-yl)acetyl)-2-oxopiperazin-1-yl)acetamido)-3-phenylpropanamide (7a)
(S)-2-(2-(4-(2-(5-methoxy-1H-indol-3-yl)acetyl)-2-oxopiperazin-1-yl)acetamido)-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (7b)
(S)-2-(2-(4-(2-(5-bromo-1H-indol-3-yl)acetyl)-2-oxopiperazin-1-yl)acetamido)-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (7c)
(S)-2-(2-(4-(2-(1H-indol-3-yl)acetyl)-2-oxopiperazin-1-yl)acetamido)-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (7d)
(S)-2-(2-(4-(2-(5-hydroxy-1H-indol-3-yl)acetyl)-2-oxopiperazin-1-yl)acetamido)-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (7e)
(S)-2-(2-(4-(2-(5-fluoro-1H-indol-3-yl)acetyl)-2-oxopiperazin-1-yl)acetamido)-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (7f)
(S)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methyl-2-(2-(4-(2-(2-methyl-1H-indol-3-yl)acetyl)-2-oxopiperazin-1-yl)acetamido)propenamide (F2-7a)
(S)-3-(3,5-difluorophenyl)-2-(2-(4-(2-(5-methoxy-1H-indol-3-yl)acetyl)-2-oxopiperazin-1-yl)acetamido)-N-(4-methoxyphenyl)-N-methylpropanamide (F2-7b)
(S)-2-(2-(4-(2-(5-bromo-1H-indol-3-yl)acetyl)-2-oxopiperazin-1-yl)acetamido)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (F2-7c)
(S)-2-(2-(4-(2-(1H-indol-3-yl)acetyl)-2-oxopiperazin-1-yl)acetamido)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (F2-7d)
(S)-3-(3,5-difluorophenyl)-2-(2-(4-(2-(5-hydroxy-1H-indol-3-yl)acetyl)-2-oxopiperazin-1-yl)acetamido)-N-(4-methoxyphenyl)-N-methylpropanamide (F2-7e)
(S)-3-(3,5-difluorophenyl)-2-(2-(4-(2-(5-fluoro-1H-indol-3-yl)acetyl)-2-oxopiperazin-1-yl)acetamido)-N-(4-methoxyphenyl)-N-methylpropanamide (F2-7f)
3.1.3. General Procedure for the Synthesis of 7 (g-j) and F2-7 (g-j)
(S)-2-(2-(4-(4-acetamidobenzoyl)-2-oxopiperazin-1-yl)acetamido)-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (7g)
(S)-N-(4-methoxyphenyl)-N-methyl-2-(2-(4-(4-(methylsulfonamido)benzoyl)-2-oxopiperazin-1-yl)acetamido)-3-phenylpropanamide (7h)
(S)-N-(4-(4-(2-((1-((4-methoxyphenyl)(methyl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-2-oxoethyl)-3-oxopiperazine-1-carbonyl)phenyl)cyclopropanecarboxamide (7i)
(S)-N-(4-methoxyphenyl)-N-methyl-2-(2-(2-oxo-4-(4-(2,2,2-trifluoroacetamido)benzoyl)piperazin-1-yl)acetamido)-3-phenylpropanamide (7j)
(S)-2-(2-(4-(4-acetamidobenzoyl)-2-oxopiperazin-1-yl)acetamido)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (F2-7g)
(S)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methyl-2-(2-(4-(4-(methylsulfonamido)benzoyl)-2-oxopiperazin-1-yl)acetamido)propenamide (F2-7h)
(S)-N-(4-(4-(2-((3-(3,5-difluorophenyl)-1-((4-methoxyphenyl)(methyl)amino)-1-oxopropan-2-yl)amino)-2-oxoethyl)-3-oxopiperazine-1-carbonyl)phenyl)cyclopropanecarboxamide (F2-7i)
(S)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methyl-2-(2-(2-oxo-4-(4-(2,2,2-trifluoroacetamido)benzoyl)piperazin-1-yl)acetamido)propenamide (F2-7j)
3.2. In Vitro Anti-HIV Assay with MT-4 Cells
3.3. Analysis of Binding to HIV-1 CA Proteins via Surface Plasmon Resonance
3.4. Molecular Dynamics Simulation
3.5. In Silico ADMET Analysis
3.6. Metabolic Stability in Human Liver Microsomes
3.7. Metabolic Stability in Human Plasma
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhan, P.; Pannecouque, C.; De Clercq, E.; Liu, X. Anti-HIV Drug Discovery and Development: Current Innovations and Future Trends. J. Med. Chem. 2016, 59, 2849–2878. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/health-topics/hiv-aids#tab=tab_1 (accessed on 12 October 2022).
- Wang, Z.; Cherukupalli, S.; Xie, M.; Wang, W.; Jiang, X.; Jia, R.; Pannecouque, C.; De Clercq, E.; Kang, D.; Zhan, P.; et al. Contemporary Medicinal Chemistry Strategies for the Discovery and Development of Novel HIV-1 Non-nucleoside Reverse Transcriptase Inhibitors. J. Med. Chem. 2022, 65, 3729–3757. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, D.N.; Vieira, N.; Hønge, B.L.; da Silva Té, D.; Jespersen, S.; Bjerregaard-Andersen, M.; Oliveira, I.; Furtado, A.; Gomes, M.A.; Sodemann, M.; et al. HIV-1 and HIV-2 Prevalence, Risk Factors and Birth Outcomes Among Pregnant Women in Bissau, Guinea-Bissau: A Retrospective Cross-sectional Hospital Study. Sci. Rep. 2020, 10, 12174. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Gupta, K.K.; Singh, S.K. A Review on Pharmacokinetics Properties of Antiretroviral Drugs to Treat HIV-1 Infections. Curr. Comput. Aid. Drug Des. 2021, 17, 850–864. [Google Scholar] [CrossRef]
- Menéndez-Arias, L.; Delgado, R. Update and Latest Advances in Antiretroviral Therapy. Trends Pharmacol. Sci. 2022, 43, 16–29. [Google Scholar] [CrossRef]
- Du, J.; Guo, J.; Kang, D.; Li, Z.; Wang, G.; Wu, J.; Zhang, Z.; Fang, H.; Hou, X.; Huang, Z.; et al. New Techniques and Strategies in Drug Discovery. Chin. Chem. Lett. 2020, 31, 695–1708. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Xu, S.; Huang, T.; Song, S.; Cherukupalli, S.; Zhan, P.; Liu, X. An Insight on Medicinal Aspects of Novel HIV-1 Capsid Protein Inhibitors. Eur. J. Med. Chem. 2021, 217, 113380. [Google Scholar] [CrossRef]
- Rossi, E.; Meuser, M.E.; Cunanan, C.J.; Cocklin, S. Structure, Function, and Interactions of the HIV-1 Capsid Protein. Life 2021, 11, 100. [Google Scholar] [CrossRef]
- Aiken, C.; Rousso, I. The HIV-1 Capsid and Reverse Transcription. Retrovirology 2021, 18, 29. [Google Scholar] [CrossRef]
- Novikova, M.; Zhang, Y.; Freed, E.O.; Peng, K. Multiple Roles of HIV-1 Capsid during the Virus Replication Cycle. Virol. Sin. 2019, 34, 119–134. [Google Scholar] [CrossRef]
- Wang, L.; Casey, M.C.; Vernekar, S.K.V.; Sahani, R.L.; Kirby, K.A.; Du, H.; Zhang, H.; Tedbury, P.R.; Xie, J.; Sarafianos, S.G.; et al. Novel PF74-like Small Molecules Targeting the HIV-1 Capsid Protein: Balance of Potency and Metabolic Stability. Acta. Pharm. Sin. B 2021, 11, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.M.; Hope, T.J. HIV-1 Capsid: The Multifaceted Key Player in HIV-1 Infection. Nat. Rev. Microbiol. 2015, 13, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Mattei, S.; Glass, B.; Hagen, W.J.; Kräusslich, H.G.; Briggs, J.A. The Structure and Flexibility of Conical HIV-1 Capsids Determined within Intact Virions. Science 2016, 354, 1434–1437. [Google Scholar] [CrossRef]
- Domenech, R.; Neira, J.L. The HIV-1 Capsid Protein as a Drug Target: Recent Advances and Future Prospects. Curr. Prot. Pept. Sci. 2013, 14, 658–668. [Google Scholar] [CrossRef]
- Yufenyuy, E.L.; Aiken, C. The NTD-CTD Intersubunit Interface Plays a Critical Role in Assembly and Stabilization of the HIV-1 Capsid. Retrovirology 2013, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Achuthan, V.; Perreira, J.M.; Ahn, J.J.; Brass, A.L.; Engelman, A.N. Capsid-CPSF6 Interaction: Master Regulator of Nuclear HIV-1 Positioning and Integration. J. Life Sci. 2019, 1, 39–45. [Google Scholar] [CrossRef]
- Li, S.; Patel, J.S.; Yang, J.; Crabtree, A.M.; Rubenstein, B.M.; Lund-Andersen, P.K.; Ytreberg, F.M.; Rowley, P.A. Defining the HIV Capsid Binding Site of Nucleoporin 153. mSphere 2022, 7, e0031022. [Google Scholar] [CrossRef]
- Rebensburg, S.V.; Wei, G.; Larue, R.C.; Lindenberger, J.; Francis, A.C.; Annamalai, A.S.; Morrison, J.; Shkriabai, N.; Huang, S.W.; KewalRamani, V.; et al. Sec24C is an HIV-1 Host Dependency Factor Crucial for Virus Replication. Nat. Microbiol. 2021, 6, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, B. HIV Capsid Assembly, Mechanism, and Structure. Biochemistry 2016, 55, 2539–2552. [Google Scholar] [CrossRef]
- Xu, S.; Sun, L.; Dick, A.; Zalloum, W.A.; Huang, T.; Meuser, M.E.; Zhang, X.; Tao, Y.; Cherukupalli, S.; Ding, D.; et al. Design, Synthesis, and Mechanistic Investigations of Phenylalanine Derivatives Containing a Benzothiazole Moiety as HIV-1 Capsid Inhibitors with Improved Metabolic Stability. Eur. J. Med. Chem. 2022, 227, 113903. [Google Scholar] [CrossRef]
- Blair, W.S.; Pickford, C.; Irving, S.L.; Brown, D.G.; Anderson, M.; Bazin, R.; Cao, J.; Ciaramella, G.; Isaacson, J.; Jackson, L.; et al. HIV Capsid is a Tractable Target for Small Molecule Therapeutic Intervention. PLoS Pathog. 2010, 6, e1001220. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, L.; Meuser, M.E.; Zalloum, W.A.; Xu, S.; Huang, T.; Cherukupalli, S.; Jiang, X.; Ding, X.; Tao, Y.; et al. Design, Synthesis, and Mechanism Study of Dimerized Phenylalanine Derivatives as Novel HIV-1 Capsid Inhibitors. Eur. J. Med. Chem. 2021, 226, 113848. [Google Scholar] [CrossRef]
- Gres, A.T.; Kirby, K.A.; KewalRamani, V.N.; Tanner, J.J.; Pornillos, O.; Sarafianos, S.G. STRUCTURAL VIROLOGY. X-ray Crystal Structures of Native HIV-1 Capsid Protein Reveal Conformational Variability. Science 2015, 349, 99–103. [Google Scholar] [CrossRef]
- Sun, L.; Huang, T.; Dick, A.; Meuser, M.E.; Zalloum, W.A.; Chen, C.H.; Ding, X.; Gao, P.; Cocklin, S.; Lee, K.H.; et al. Design, Synthesis and Structure-activity Relationships of 4-phenyl-1H-1,2,3-triazole Phenylalanine Derivatives as Novel HIV-1 Capsid Inhibitors with Promising Antiviral Activities. Eur. J. Med. Chem. 2020, 190, 112085. [Google Scholar] [CrossRef]
- Xu, S.; Sun, L.; Ding, D.; Zhang, X.; Liu, X.; Zhan, P. Metabolite Identification of HIV-1 Capsid Modulators PF74 and 11L in Human Liver Microsomes. Metabolites 2022, 12, 752. [Google Scholar] [CrossRef]
- Link, J.O.; Rhee, M.S.; Tse, W.C.; Zheng, J.; Somoza, J.R.; Rowe, W.; Begley, R.; Chiu, A.; Mulato, A.; Hansen, D.; et al. Clinical Targeting of HIV Capsid Protein with a Long-acting Small Molecule. Nature 2020, 584, 614–661. [Google Scholar] [CrossRef]
- Margot, N.A.; Naik, V.; Vander, V.L.; Anoshchenko, O.; Singh, R.; Dvory-Sobol, H.; Rhee, M.S.; Callebaut, C. Resistance Analyses in Highly Treatment-Experienced People with HIV Treated with the Novel Capsid HIV Inhibitor Lenacapavir. J. Infect. Dis. 2022. ahead of print. [Google Scholar] [CrossRef]
- Sun, L.; Dick, A.; Meuser, M.E.; Huang, T.; Zalloum, W.A.; Chen, C.H.; Cherukupalli, S.; Xu, S.; Ding, X.; Gao, P.; et al. Design, Synthesis, and Mechanism Study of Benzenesulfonamide-Containing Phenylalanine Derivatives as Novel HIV-1 Capsid Inhibitors with Improved Antiviral Activities. J. Med. Chem. 2020, 63, 4790–4810. [Google Scholar] [CrossRef]
- Xu, S.; Sun, L.; Zalloum, W.A.; Zhang, X.; Huang, T.; Ding, D.; Tao, Y.; Zhao, F.; Gao, S.; Kang, D.; et al. From Design to Biological Mechanism Evaluation of Phenylalanine-bearing HIV-1 Capsid Inhibitors Targeting a Vital Assembly Interface. Chin. Chem. Lett. 2022, in press. [Google Scholar] [CrossRef]
- Xu, J.P.; Francis, A.C.; Meuser, M.E.; Mankowski, M.; Ptak, R.G.; Rashad, A.A.; Melikyan, G.B.; Cocklin, S. Exploring Modifications of an HIV-1 Capsid Inhibitor: Design, Synthesis, and Mechanism of Action. J. Drug Des. Res. 2018, 5, 1070. [Google Scholar]
- Segall, M.; Champness, E.; Obrezanova, O.; Leeding, C. Beyond Profiling: Using ADMET Models to Guide Decisions. Chem. Biodivers. 2009, 6, 2144–2151. [Google Scholar] [CrossRef]
- Tuyishime, M.; Danish, M.; Princiotto, A.; Mankowski, M.K.; Lawrence, R.; Lombart, H.G.; Esikov, K.; Berniac, J.; Liang, K.; Ji, J.; et al. Discovery and Optimization of Novel Small-molecule HIV-1 Entry Inhibitors Using Field-based Virtual Screening and Bioisosteric Replacement. Bioorg. Med. Chem. Lett. 2014, 24, 5439–5445. [Google Scholar] [CrossRef]
- Karadsheh, R.; Meuser, M.E.; Cocklin, S. Composition and Orientation of the Core Region of Novel HIV-1 Entry Inhibitors Influences Metabolic Stability. Molecules 2020, 25, 1430. [Google Scholar] [CrossRef]
- Meuser, M.E.; Reddy, P.A.N.; Dick, A.; Maurancy, J.M.; Salvino, J.M.; Cocklin, S. Rapid Optimization of the Metabolic Stability of a Human Immunodeficiency Virus Type-1 Capsid Inhibitor Using a Multistep Computational Workflow. J. Med. Chem. 2021, 64, 3747–3766. [Google Scholar] [CrossRef]
- Tyzack, J.D.; Hunt, P.A.; Segall, M.D. Predicting Regioselectivity and Lability of Cytochrome P450 Metabolism Using Quantum Mechanical Simulations. J. Chem. Inf. Model 2016, 56, 2180–2193. [Google Scholar] [CrossRef]
- Hunt, P.A.; Segall, M.D.; Tyzack, J.D. WhichP450: A Multi-class Categorical Model to Predict the Major Metabolising CYP450 Isoform for a Compound. J. Comput. Aid. Mol. Des. 2018, 32, 537–546. [Google Scholar] [CrossRef]
- Reulecke, I.; Lange, G.; Albrecht, J.; Klein, R.; Rarey, M. Towards an Integrated Description of Hydrogen Bonding and Dehydration: Decreasing False Positives in Virtual Screening with the HYDE Scoring Function. ChemMedChem 2008, 3, 885–897. [Google Scholar] [CrossRef]
- Kaur, P.; Chamberlin, A.R.; Poulos, T.L.; Sevrioukova, I.F. Structure-Based Inhibitor Design for Evaluation of a CYP3A4 Pharmacophore Model. J. Med. Chem. 2016, 59, 4210–4220. [Google Scholar] [CrossRef]
- Sega, G.A. A Review of The Genetic Effects of Ethyl Methanesulfonate. Mutat. Res. 1984, 134, 113–142. [Google Scholar] [CrossRef]
- Pillans, P.I.; Ghiculescu, R.A.; Lampe, G.; Wilson, R.; Wong, R.; Macdonald, G.A. Severe Acute Liver Injury Associated with Lumiracoxib. J. Gastroenterol. Hepatol. 2012, 27, 1102–1105. [Google Scholar] [CrossRef]
- Kortagere, S.; Madani, N.; Mankowski, M.K.; Schon, A.; Zentner, I.; Swaminathan, G.; Princiotto, A.; Anthony, K.; Oza, A.; Sierra, L.J.; et al. Inhibiting Early-stage Events in HIV-1 Replication by Small-molecule Targeting of the HIV-1 Capsid. J. Virol. 2012, 86, 8472–8481. [Google Scholar] [CrossRef]
- Studier, F.W. Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef]
- Jacques, D.A.; McEwan, W.A.; Hilditch, L.; Price, A.J.; Towers, G.J.; James, L.C. HIV-1 Uses Dynamic Capsid Pores to Import Nucleotides and Fuel Encapsidated DNA Synthesis. Nature 2016, 536, 349. [Google Scholar] [CrossRef]
- Ester, M.; Kriegel, H.P.; Sander, J.; Xu, X. A Density-Based Algorithm for Discovering Clusters in Large Spatial Databases with Noise. In Proceedings of the 2nd International Conference on Knowledge Discovery and Data Mining; Simoudis, E., Han, J., Fayyad, U., Eds.; AAAI Press: Menlo Park, CA, USA, 1996; pp. 226–231. [Google Scholar]
- Hawkins, P.C.D.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. OMEGA, Version 3.0.0.1; OpenEye Scientific: Santa Fe, NM, USA, 2010. [Google Scholar]
- Hawkins, P.C.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. Conformer Generation with OMEGA: Algorithm and Validation Using High Quality Structures from the Protein Databank and the Cambridge Structural Database. J. Chem. Inf. Model. 2010, 50, 572–584. [Google Scholar] [CrossRef]
- OEDOCKING 3.0.1: OpenEye Scientific Software, Santa Fe, NM. Available online: http://www.eyesopen.com (accessed on 12 October 2022).
- Kelley, B.P.; Brown, S.P.; Warren, G.L.; Muchmore, S.W. POSIT: Flexible Shape-Guided Docking for Pose Prediction. J. Chem. Inf. Model. 2015, 55, 1771–1780. [Google Scholar] [CrossRef]
- McGann, M. FRED Pose Prediction and Virtual Screening Accuracy. J. Chem. Inf. Model. 2011, 51, 578–596. [Google Scholar] [CrossRef]
- McGann, M. FRED and HYBRID Docking Performance on Standardized Datasets. J. Comput. Aided Mol. Des. 2012, 26, 897–906. [Google Scholar] [CrossRef]
- Case, D.A.; Betz, R.M.; Botello-Smith, W.; Cerutti, D.S.; Cheatham, T.E.; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; et al. Amber 2016; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Shao, J.; Tanner, S.W.; Thompson, N.; Cheatham, T.E. Clustering molecular Dynamics Trajectories: 1. Characterizing the Performance of Different Clustering Algorithms. J. Chem. Theory Comput. 2007, 3, 2312–2334. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.; Olson, A.J.; Goodsell, D.S. A Semiempirical Free Energy Force Field with Charge-based Desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for Ligand-receptor Docking. Curr. Protoc. Bioinform. 2008, 24, 8–14. [Google Scholar] [CrossRef]
- Bikadi, Z.; Hazai, E. Application of the PM6 Semi-empirical Method to Modeling Proteins Enhances Docking Accuracy of AutoDock. J. Cheminform. 2009, 1, 15. [Google Scholar] [CrossRef]

 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound ID | R1 | R2 | R3 | EC50 a (μM) | CC50 b (μM) | SI c | ||
| IIIB | ROD | IIIB | ROD | |||||
| 7a | H | CH3 | H | 14.28 ± 0.76 | 7.74 ± 0.79 | 107.56 ± 3.26 | 7.73 | 13.90 |
| 7b | H | H | OCH3 | 14.58 ± 0.39 | 16.95 ± 3.58 | 104.21 ± 3.37 | 7.15 | 6.15 |
| 7c | H | H | Br | >16.37 | >16.37 | 16.37 ± 3.44 | <1 | <1 |
| 7d | H | H | H | 14.97 ± 0.24 | 4.89 ± 1.39 | 112.41 ± 3.22 | 7.51 | 22.99 |
| 7e | H | H | OH | 198.10 ± 9.74 | 186.48 ± 4.42 | >209.29 | >1.06 | >1.12 |
| 7f | H | H | F | 21.81 ± 4.81 | 4.52 ± 0.87 | 90.06 ± 15.72 | 4.13 | 19.92 |
| F2-7a | F | CH3 | H | 20.83 ± 5.09 | 19.66 ± 6.59 | 76.85 ± 19.98 | 3.69 | 3.91 |
| F2-7b | F | H | OCH3 | >15.94 | >15.94 | 15.94 ± 3.83 | <1 | <1 |
| F2-7c | F | H | Br | >3.15 | >3.15 | 3.15 ± 0.40 | <1 | <1 |
| F2-7d | F | H | H | 29.37 ± 7.03 | 17.61 ± 9.14 | 57.84 ± 21.81 | 1.97 | 3.28 |
| F2-7e | F | H | OH | >131.51 | ≥49.55 | 131.51 ± 32.96 | <1 | ≤2.65 |
| F2-7f | F | H | F | 5.89 ± 2.03 | 5.07 ± 0.63 | 16.36 ± 3.38 | 2.78 | 3.23 |
| PF74 | 0.75 ± 0.33 | 4.16 ± 2.02 | 32.27 ± 2.94 | 43.03 | 7.76 | |||
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound ID | R1 | X | R4 | EC50 a (µM) | CC50 b (µM) | SI c | ||
| IIIB | ROD | IIIB | ROD | |||||
| 7g | H | CO | CH3 | 203.14 ± 10.98 | 184.07 ± 19.33 | >213.43 | >1.05 | >1.16 |
| 7h | H | SO2 | CH3 | 192.39 ± 3.17 | >201.06 | >201.06 | >1.05 | ND |
| 7i | H | CO | cyclopropyl | >167.50 | 15.02 ± 2.27 | 167.50 ± 19.81 | <1 | 11.15 |
| 7j | H | CO | CF3 | 157.50 ± 6.77 | 14.95 ± 4.83 | >195.42 | >1.24 | >13.08 |
| F2-7g | F | CO | CH3 | 177.40 ± 16.55 | 192.14 ± 4.57 | 196.03 ± 6.80 | 1.11 | 1.02 |
| F2-7h | F | SO2 | CH3 | 160.53 ± 15.43 | 183.40 ± 2.77 | >190.06 | >1.18 | >1.04 |
| F2-7i | F | CO | cyclopropyl | 14.36 ± 1.31 | 15.10 ± 0.68 | 88.56 ± 11.27 | 6.17 | 5.87 |
| F2-7j | F | CO | CF3 | 13.74 ± 0.70 | 16.55 ± 6.13 | 150.38 ± 25.46 | 10.95 | 9.09 |
| PF74 | 0.75 ± 0.33 | 4.16 ± 2.02 | 32.27 ± 2.94 | 43.03 | 7.76 | |||
| Compound ID | KD a (μM) | Ratio b | koff c (s−1) | |
|---|---|---|---|---|
| Monomer | Hexamer | Hexamer | ||
| F2-7f | 16.063 ± 1.316 | 7.203 ± 1.101 | 2.230 | 0.151 ± 0.028 |
| PF74 | 3.410 ± 1.310 | 0.159 ± 0.041 | 21.446 | 0.0177 ± 0.0004 |
| Sample | HLM (Final Concentration of 0.5 mg Protein/mL) | |||||
|---|---|---|---|---|---|---|
| R2 a | T1/2 b (min) | CLint(mic) c (μL/min/mg) | CLint(liver) d (mL/min/kg) | Remaining (T = 60 min) | Remaining (NCF e = 60 min) | |
| F2-7f | 1.0000 | 0.5 | 2759.1 | 2483.2 | 0.0% | 103.5% |
| PF74 | 1.0000 | 0.5 | 2862.5 | 2576.2 | 0.0% | 112.6% |
| Testosterone | 0.9982 | 16.7 | 82.8 | 74.5 | 7.9% | 90.7% |
| Diclofenac | 0.9947 | 3.7 | 372.0 | 334.8 | 0.0% | 96.7% |
| Propafenone | 0.9350 | 5.0 | 279.5 | 251.5 | 0.0% | 93.6% |
| Compound ID | Batch | Time Point (min) | % Remaining a | T1/2 (min) |
|---|---|---|---|---|
| Human | Human | |||
| F2-7f | / | 0 | 100.0 | >289.1 |
| 10 | 98.0 | |||
| 30 | 97.8 | |||
| 60 | 90.9 | |||
| 120 | 86.9 | |||
| PF74 | / | 0 | 100.0 | >289.1 |
| 10 | 84.7 | |||
| 30 | 81.7 | |||
| 60 | 80.7 | |||
| 120 | 85.2 | |||
| Propantheline bromide | ODR-4547 | 0 | 100.0 | 11.2 |
| 10 | 56.5 | |||
| 30 | 19.4 | |||
| 60 | 1.9 | |||
| 120 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Sun, L.; Zalloum, W.A.; Huang, T.; Zhang, X.; Ding, D.; Shao, X.; Jiang, X.; Zhao, F.; Cocklin, S.; et al. Discovery and Mechanistic Investigation of Piperazinone Phenylalanine Derivatives with Terminal Indole or Benzene Ring as Novel HIV-1 Capsid Modulators. Molecules 2022, 27, 8415. https://doi.org/10.3390/molecules27238415
Xu S, Sun L, Zalloum WA, Huang T, Zhang X, Ding D, Shao X, Jiang X, Zhao F, Cocklin S, et al. Discovery and Mechanistic Investigation of Piperazinone Phenylalanine Derivatives with Terminal Indole or Benzene Ring as Novel HIV-1 Capsid Modulators. Molecules. 2022; 27(23):8415. https://doi.org/10.3390/molecules27238415
Chicago/Turabian StyleXu, Shujing, Lin Sun, Waleed A. Zalloum, Tianguang Huang, Xujie Zhang, Dang Ding, Xiaoyu Shao, Xiangyi Jiang, Fabao Zhao, Simon Cocklin, and et al. 2022. "Discovery and Mechanistic Investigation of Piperazinone Phenylalanine Derivatives with Terminal Indole or Benzene Ring as Novel HIV-1 Capsid Modulators" Molecules 27, no. 23: 8415. https://doi.org/10.3390/molecules27238415
APA StyleXu, S., Sun, L., Zalloum, W. A., Huang, T., Zhang, X., Ding, D., Shao, X., Jiang, X., Zhao, F., Cocklin, S., De Clercq, E., Pannecouque, C., Dick, A., Liu, X., & Zhan, P. (2022). Discovery and Mechanistic Investigation of Piperazinone Phenylalanine Derivatives with Terminal Indole or Benzene Ring as Novel HIV-1 Capsid Modulators. Molecules, 27(23), 8415. https://doi.org/10.3390/molecules27238415








