DBU Promoted Polysubstituted Arene Formation via a Michael Addition/Cyclization/Elimination Cascade Reaction
Abstract
1. Introduction
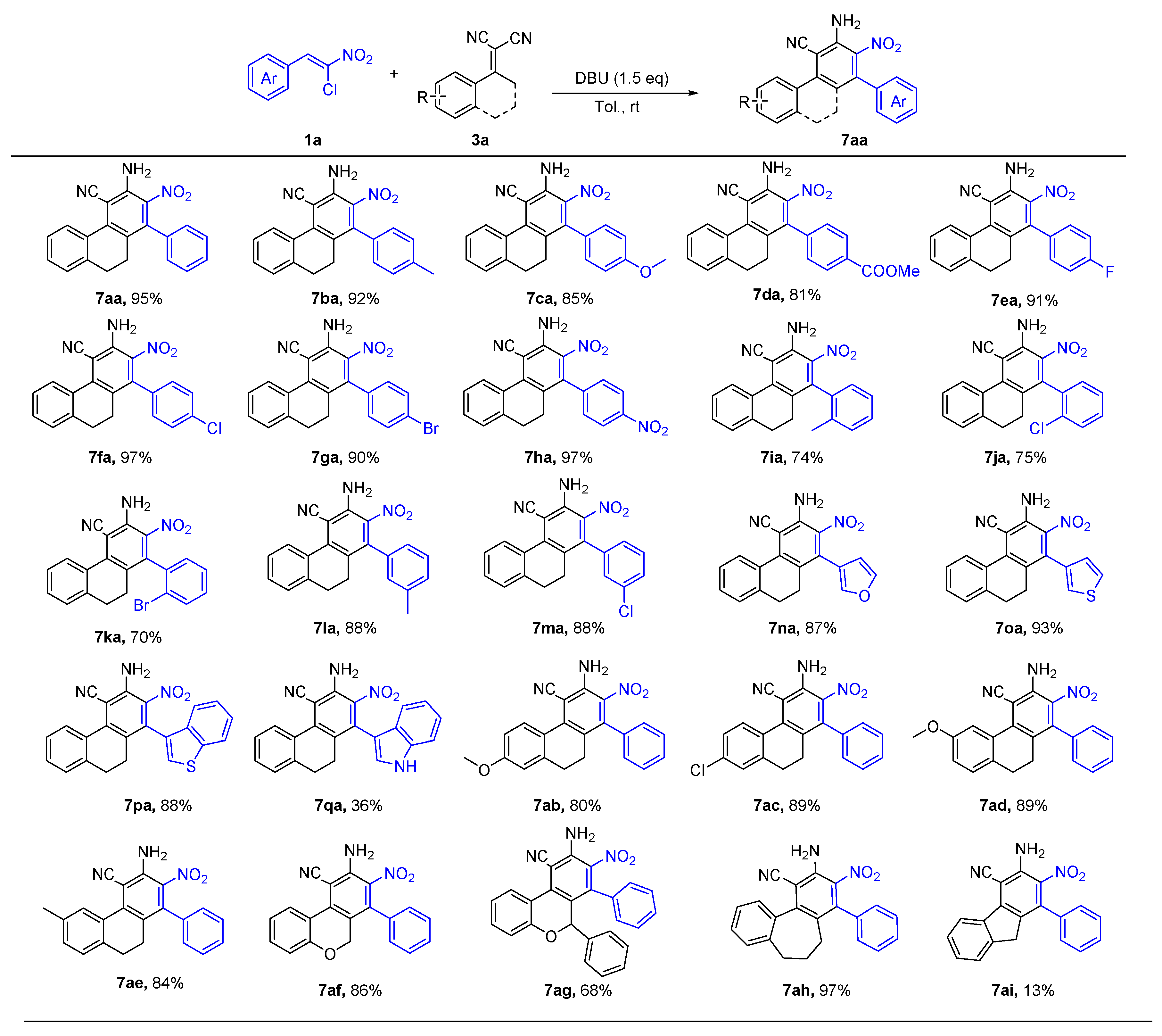
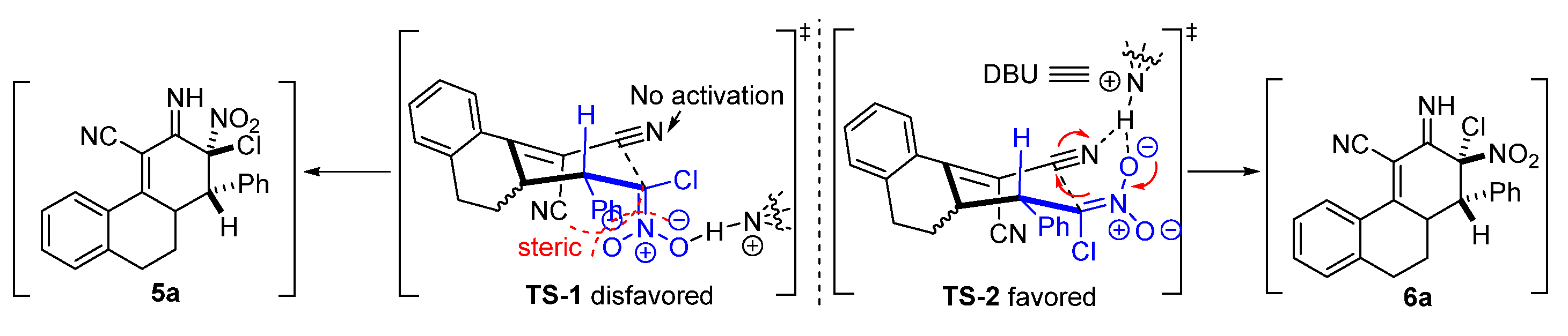
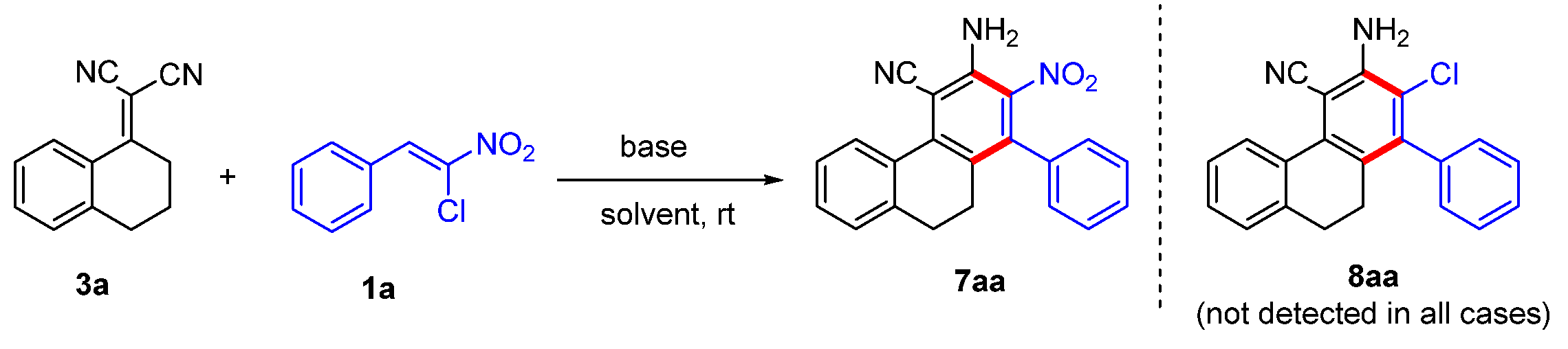
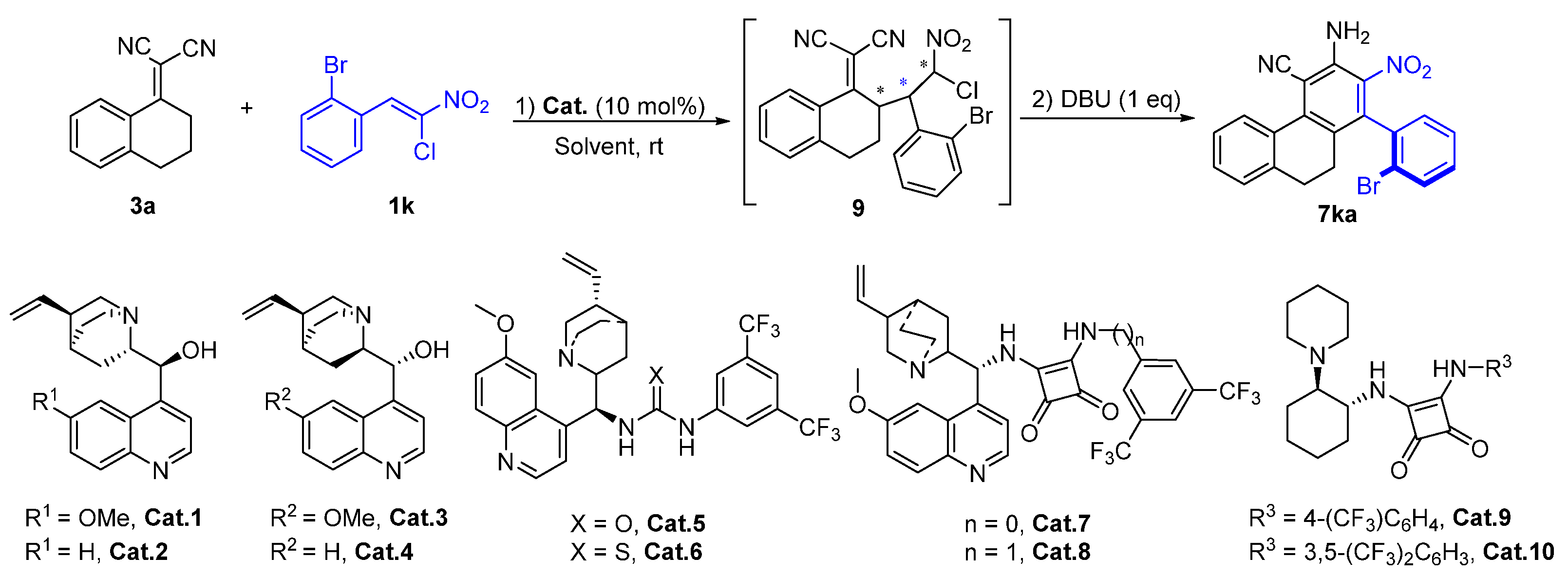
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer, E.A.; Castellano, R.K.; Diederich, F. Interactions with aromatic rings in chemical and biological recognition. Angew. Chem. Int. Ed. 2003, 42, 1210–1250. [Google Scholar] [CrossRef] [PubMed]
- Marson, C.M. New and unusual scaffolds in medicinal chemistry. Chem. Soc. Rev. 2011, 40, 5514–5533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peng, X.; Damu, G.L.V.; Geng, R.; Zhou, C. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef]
- Tsutsumi, L.S.; Gundisch, D.; Sun, D. Carbazole scaffold in medicinal chemistry and natural products: A review from 2010–2015. Curr. Top. Med. Chem. 2016, 16, 1290–1313. [Google Scholar] [CrossRef] [PubMed]
- Gromov, S.P.; Dmitrieva, S.N.; Vedernikov, A.I.; Churakova, M.V. Phenylaza- and benzoazacrown compounds with a nitrogen atom conjugated with a benzene ring. Russ. Chem. Rev. 2005, 74, 461–488. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sugawa, T.; Murahashi, T. Multinuclear coordination of fused benzene ring hydrocarbons. Coord. Chem. Rev. 2022, 466, 214575. [Google Scholar] [CrossRef]
- Subbaiah, M.A.M.; Meanwell, N.A. Bioisosteres of the phenyl ring: Recent strategic applications in lead optimization and drug design. J. Med. Chem. 2021, 64, 14046–14128. [Google Scholar] [CrossRef]
- Sharma, P.C.; Sinhmar, A.; Sharma, A.; Rajak, H.; Pathak, D.P. Medicinal significance of benzothiazole scaffold: An insight view. J. Enzyme. Inhib. Med. Chem. 2013, 28, 240–266. [Google Scholar] [CrossRef]
- Corbet, J.-P.; Mignani, G. Selected patented cross-coupling reaction technologies. Chem. Rev. 2006, 106, 2651–2710. [Google Scholar] [CrossRef]
- Ashenhurst, J.A. Intermolecular oxidative cross-coupling of arenes. Chem. Soc. Rev. 2010, 39, 540–548. [Google Scholar] [CrossRef]
- Kozlowski, M.C.; Morgana, B.J.; Lintona, E.C. Total synthesis of chiral biaryl natural products by asymmetric biaryl coupling. Chem. Soc. Rev. 2009, 38, 3193–3207. [Google Scholar] [CrossRef]
- Bringmann, G.; Gulder, T.; Gulder, T.A.M.; Breuning, M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 2011, 111, 563–639. [Google Scholar] [CrossRef]
- Bauvois, B.; Puiffe, M.-L.; Bongui, J.-B.; Paillat, S.; Monneret, C.; Dauzonne, D. Synthesis and biological evaluation of novel flavone-8-acetic acid derivatives as reversible inhibitors of aminopeptidase N/CD13. J. Med. Chem. 2003, 46, 3900–3913. [Google Scholar] [CrossRef]
- Ni, Q.; Wang, X.; Zeng, D.; Wu, Q.; Song, X. Organocatalytic asymmetric synthesis of aza-spirooxindoles via michael/friedel-crafts cascade reaction of 1,3-nitroenynes and 3-pyrrolyloxindoles. Org. Lett. 2021, 23, 2273–2278. [Google Scholar] [CrossRef]
- Raut, V.S.; Jean, M.; Vanthuyne, N.; Roussel, C.; Constantieux, T.; Bressy, C.; Bugaut, X.; Bonne, D.; Rodriguez, J. Enantioselective syntheses of furan atropisomers by an oxidative central-to-axial chirality conversion strategy. J. Am. Chem. Soc. 2017, 139, 2140–2143. [Google Scholar] [CrossRef]
- Mane, V.; Sivanandan, S.T.; Santana, R.G.; Beatriz, A.; Junior, E.N.S.; Namboothiri, I.N.N. Synthesis of densely substituted sulfonylfurans and dihydrofurans via cascade reactions of α-functionalized nitroalkenes with β-ketosulfones. J. Org. Chem. 2020, 85, 8825–8843. [Google Scholar] [CrossRef]
- Feng, J.; Wang, S.; Feng, J.; Li, Q.; Yue, J.; Yue, G.; Zou, P.; Wang, G. Mild and efficient synthesis of trans-3-aryl-2-nitro-2,3-dihydrobenzofurans on water. New J. Chem. 2020, 44, 11937–11940. [Google Scholar] [CrossRef]
- Raimondi, W.; Dauzonne, D.; Constantieux, T.; Bonne, D.; Rodriguez, J. Expeditious, metal-free, domino, regioselective synthesis of highly substituted 2-carbonyl- and 2-phosphorylfurans by formal [3+2] cycloaddition. Eur. J. Org. Chem. 2012, 2012, 6119–6123. [Google Scholar] [CrossRef]
- Xie, J.-W.; Wang, Z.; Yang, W.-J.; Kong, L.-C.; Xu, D.-C. Efficient method for the synthesis of functionalized pyrazoles by catalyst-free one-pot tandem reaction of nitroalkenes with ethyl diazoacetate. Org. Biomol. Chem. 2009, 7, 4352–4354. [Google Scholar] [CrossRef]
- Deng, X.; Liang, J.T.; Mani, N.S. Regioselective synthesis of 4-nitro- or 4-chloro-tetrasubstituted pyrazoles from hydrazones and β-halo-β-nitrostyrenes. Eur. J. Org. Chem. 2014, 2014, 410–417. [Google Scholar] [CrossRef]
- Pleshchev, M.I.; Gupta, N.V.D.; Kuznetsov, V.V.; Fedyanin, I.V.; Kachala, V.V.; Makhova, N.N. CAN-mediated new, regioselective one-pot access to bicyclic cationic structures with 2,3-dihydro-1H-pyrazolo[1,2-a]pyrazol-4-ium core. Tetrahedron 2015, 71, 9012–9021. [Google Scholar] [CrossRef]
- Gopi, E.; Kumar, T.; Barreto, R.F.S.; Valenca, W.O.; da Junior, E.N.S.; Namboothiri, I.N.N. Imidazoles from nitroallylic acetates and α-bromonitroalkenes with amidines: Synthesis and trypanocidal activity studies. Org. Biomol. Chem. 2015, 13, 9862–9871. [Google Scholar] [CrossRef]
- Sheremet, E.A.; Tomanov, R.I.; Trukhin, E.V.; Berestovitskaya, V.M. Synthesis of 4-Aryl-5-nitro-1,2,3-triazoles. Russ. J. Org. Chem. 2004, 40, 594–595. [Google Scholar] [CrossRef]
- Jana, S.; Adhikari, S.; Cox, M.R.; Roy, S. Regioselective synthesis of 4-fluoro-1,5-disubstituted-1,2,3- triazoles from synthetic surrogates of α-fluoroalkynes. Chem. Commun. 2020, 56, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Motornov, V.A.; Tabolin, A.A.; Novikov, R.A.; Nelyubina, Y.V.; Ioffe, S.L.; Smolyar, I.V.; Nenajdenko, V.G. Synthesis and regioselective n-2 functionalization of 4-fluoro-5-aryl-1,2,3-nh-triazoles. Eur. J. Org. Chem. 2017, 2017, 6851–6860. [Google Scholar] [CrossRef]
- Kumar, V.; Awasthi, A.; Salam, A.; Khan, T. Scalable total syntheses of some natural and unnatural lamellarins: Application of a one-pot domino process for regioselective access to the central 1,2,4-trisubstituted pyrrole core. J. Org. Chem. 2019, 84, 11596–11603. [Google Scholar] [CrossRef]
- Rao, M.P.; Gunaga, S.S.; Zuegg, J.; Pamarthi, R.; Ganesh, M. Highly regio- and diastereoselective [3+2]-cycloadditions involving indolediones and α,β-disubstituted nitroethylenes. Org. Biomol. Chem. 2019, 17, 9390–9402. [Google Scholar] [CrossRef]
- Santos, C.M.; Barrera, C.J.; Parra, A.; Esteban, F.; Ranninger, C.N.; Aleman, J. Modular three-component organocatalytic synthesis of 3,4-disubstituted pyrroles by a one-pot domino reaction. ChemCatChem 2012, 4, 976–979. [Google Scholar] [CrossRef]
- Motornov, V.A.; Tabolin, A.A.; Nelyubina, Y.V.; Nenajdenko, V.G.; Ioffe, S.L. Copper-catalyzed [3+2]-cycloaddition of α-halonitroalkenes with azomethine ylides: Facile synthesis of multisubstituted pyrrolidines and pyrroles. Org. Biomol. Chem. 2021, 19, 3413–3427. [Google Scholar] [CrossRef]
- Chen, F.-Y.; Xiang, L.; Zhan, G.; Liu, H.; Kang, B.; Zhang, S.-C.; Peng, C.; Han, B. Highly stereoselective organocatalytic synthesis of pyrrolidinyl spirooxindoles containing halogenated contiguous quaternary carbon stereocenters. Tetrahedron Lett. 2020, 61, 151806. [Google Scholar] [CrossRef]
- Mosse, S.S.; Alexakis, A. Chiral amines as organocatalysts for asymmetric conjugate addition to nitroolefins and vinyl sulfones via enamine activation. Chem. Commun. 2007, 30, 3123–3135. [Google Scholar] [CrossRef]
- Cui, D.-X.; Li, Y.-D.; Zhu, J.-C.; Jia, Y.-Y.; Wen, A.-D.; Wang, P.-A. Highly efficient michael reactions of nitroolefins by grinding means. Curr. Org. Synth. 2019, 16, 449–457. [Google Scholar] [CrossRef]
- Uraguchi, D.; Oyaizu, K.; Ooi, T. Nitroolefins as a nucleophilic component for highly stereoselective aza Henry reaction under the catalysis of chiral ammonium betaines. Chem. Eur. J. 2012, 18, 8306–8309. [Google Scholar] [CrossRef]
- Bao, X.; Rodriguez, J.; Bonne, D. Bidirectional enantioselective synthesis of bis-benzofuran atropisomeric oligoarenes featuring two distal C-C stereogenic axes. Chem. Sci. 2020, 11, 403–408. [Google Scholar] [CrossRef]
- Liu, P.; Bao, X.; Naubron, J.-V.; Chentouf, S.; Humbel, S.; Vanthuyne, N.; Jean, M.; Giordano, L.; Rodriguez, J.; Bonne, D. Simultaneous control of central and helical chiralities: Expedient helicoselective synthesis of dioxa[6]helicenes. J. Am. Chem. Soc. 2020, 142, 16199–16204. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Zhou, L. Construction of axially chiral compounds via central-to-axial chirality conversion. Chem. Asian. J. 2020, 15, 2939–2951. [Google Scholar] [CrossRef]
- Min, X.-L.; Zhang, X.-L.; Shen, R.; Zhang, Q.; He, Y. Recent advances in the catalytic asymmetric construction of atropisomers by central-to-axial chirality transfer. Org. Chem. Front. 2022, 9, 2280–2292. [Google Scholar] [CrossRef]
- Farhat, J.; Alzyoud, L.; Alwahsh, M.; Omari, B. Structure-activity relationship of benzofuran derivatives with potential anticancer activity. Cancers 2022, 14, 2196. [Google Scholar] [CrossRef]
- Ostrowski, T. Bioactive furanyl- or thienyl-substituted nucleobases, nucleosides and their analogues. Mini. Rev. Med. Chem. 2022, 2, 1–18. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, N.; Mishra, I.; Sachan, N. A review on anticancer activities of thiophene and its analogs. Mini. Rev. Med. Chem. 2020, 20, 1944–1965. [Google Scholar] [CrossRef]
- Archna, P.S.; Chawla, P.A. Thiophene-based derivatives as anticancer agents: An overview on decade’s work. Bioorg. Chem. 2020, 101, 104026. [Google Scholar] [CrossRef] [PubMed]
- Duvauchelle, V.; Meffre, P.; Benfodda, Z. Recent contribution of medicinally active 2-aminothiophenes: A privileged scaffold for drug discovery. Eur. J. Med. Chem. 2022, 238, 114502. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Chand, K.; Budagumpi, S.; Somappa, S.B.; Patil, S.A.; Nagaraja, B.M. An overview of benzo[b]thiophene-based medicinal chemistry. Eur. J. Med. Chem. 2017, 138, 1002–1033. [Google Scholar] [CrossRef] [PubMed]
- George, N.; Akhtar, M.J.; Balushi, K.A.A.; Khan, S.A. Rational drug design strategies for the development of promising multi-target directe d indole hybrids as anti-alzheimer agents. Bioorg. Chem. 2022, 127, 105941. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhu, Y.-Y.; He, Q.; Gu, S.-X. Indole derivatives as tubulin polymerization inhibitors for the development of promising anticancer agents. Bioorg. Med. Chem. 2021, 55, 116597. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Kaur, A.; Goyal, B. Benzofuran and indole: Promising scaffolds for drug development in alzheimer’s disease. ChemMedChem 2018, 13, 1275–1299. [Google Scholar] [CrossRef]
- Lunagariya, J.; Bhadja, P.; Zhong, S.; Vekariya, R.; Xu, S. Marine natural product bis-indole alkaloid caulerpin: Chemistry and biology. Mini. Rev. Med. Chem. 2019, 19, 751–761. [Google Scholar] [CrossRef]
- Bringmann, G.; Mortimer, A.J.P.; Keller, P.A.; Gresser, M.J.; Garner, J.; Breuning, M. Atroposelective synthesis of axially chiral biaryl compounds. Angew. Chem. Int. Ed. 2005, 44, 5384–5427. [Google Scholar] [CrossRef]
- Delord, J.W.; Panossian, A.; Leroux, F.R.; Colobert, F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 2015, 44, 3418–3430. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, L.; Liu, X.; Zhang, L.; Yu, Z.; Wu, G. Recent progress toward developing axial chirality bioactive compounds. Eur. J. Med. Chem. 2022, 243, 114700. [Google Scholar] [CrossRef]
- Huettel, W.; Mueller, M. Regio- and stereoselective intermolecular phenol coupling enzymes in secondary metabolite biosynthesis. Nat. Prod. Rep. 2021, 38, 1011–1043. [Google Scholar] [CrossRef]
- Carlsson, A.-C.C.; Karlsson, S.; Munday, R.H.; Tatton, M.R. Approaches to Synthesis and Isolation of Enantiomerically Pure Biologically Active Atropisomers. Acc. Chem. Res. 2022, 55, 2938–2948. [Google Scholar] [CrossRef]
- Li, Y.-M.; Kwong, F.-Y.; Yu, W.-Y.; Chan, A.S.C. Recent advances in developing new axially chiral phosphine ligands for asymmetric catalysis. Coord. Chem. Rev. 2007, 257, 2119–2144. [Google Scholar] [CrossRef]
- Mancinelli, M.; Bencivenni, G.; Pecorari, D.; Mazzanti, A. Stereochemistry and recent applications of axially chiral organic molecules. Eur. J. Org. Chem. 2020, 2020, 4070–4086. [Google Scholar] [CrossRef]
- Shirakawa, S.; Liu, S.; Kaneko, S. Organocatalyzed asymmetric synthesis of axially, planar, and helical chiral compounds. Chem. Asian J. 2016, 11, 330–341. [Google Scholar] [CrossRef]
- Bonne, D.; Rodriguez, J. Enantioselective syntheses of atropisomers featuring a five-membered ring. Chem. Commun. 2017, 53, 12385–12393. [Google Scholar] [CrossRef]
- Bai, X.-F.; Cui, Y.-M.; Cao, J.; Xu, L.-W. Atropisomers with axial and point chirality: Synthesis and applications. Acc. Chem. Res. 2022, 55, 2545–2561. [Google Scholar] [CrossRef]
- Pan, H.; Han, M.-Y.; Li, P.; Wang, L. “On Water” Direct Catalytic Vinylogous Aldol Reaction of Silyl Glyoxylates. J. Org. Chem. 2019, 8421, 14281–14290. [Google Scholar] [CrossRef]
- Yang, W.; Du, D.-M. Highly enantioselective Michael addition of nitroalkanes to chalcones using chiral squaramides as hydrogen bonding organocatalysts. Org. Lett. 2010, 12, 5450–5453. [Google Scholar] [CrossRef]
- Requena, J.V.A.; Lopez, E.M.; Herrera, R.P. One-pot synthesis of unsymmetrical squaramides. RSC Adv. 2015, 5, 33450–33462. [Google Scholar] [CrossRef]

| Entry a | Solvent | Base | Reaction Time (h) | Yield b (%) |
|---|---|---|---|---|
| 1 c | DCM | Et3N | 12 | trace |
| 2 | DCM | DABCO | 2 | 29 |
| 3 | DCM | DBU | 0.5 | 80 |
| 4 | DCM | (CH3)3 COLi | 12 | trace |
| 5 | DCM | NaOH | 12 | trace |
| 6 | DCM | K2CO3 | 12 | trace |
| 7 | DCM | CsCO3 | 12 | 16 |
| 8 | DCM | K3PO3 | 12 | trace |
| 9 d | EA | DBU | 0.5 | 81 |
| 10 | CH3CN | DBU | 0.25 | 80 |
| 11 e | DCE | DBU | 0.25 | 82 |
| 12 | Toluene | DBU | 0.25 | 85 |
| 13 f | Toluene | DBU | 0.25 | 95 |
| Entry a | Cat. | Solvent | Yield (%) | Ee b (%) |
|---|---|---|---|---|
| 1 | Cat. 1 | DCM | 70 | 17 |
| 2 | Cat. 2 | DCM | 63 | −14 |
| 3 | Cat. 3 | DCM | 71 | 23 |
| 4 | Cat. 4 | DCM | 61 | 14 |
| 5 | Cat. 5 | DCM | 65 | 31 |
| 6 | Cat. 6 | DCM | 56 | 33 |
| 7 | Cat. 7 | DCM | 40 | 40 |
| 8 | Cat. 8 | DCM | 48 | 13 |
| 9 | Cat. 9 | DCM | 48 | −31 |
| 10 | Cat. 10 | DCM | 67 | −14 |
| 11 | Cat. 7 | DCE | 38 | 37 |
| 12 | Cat. 7 | CHCl3 | 35 | 39 |
| 13 | Cat. 7 | Toluene | 59 | 27 |
| 14 | Cat. 7 | EA | 63 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, G.; Yang, Y.; Wang, X.; Wu, J.; Wang, H.; Ye, X.; Bao, X. DBU Promoted Polysubstituted Arene Formation via a Michael Addition/Cyclization/Elimination Cascade Reaction. Molecules 2022, 27, 8167. https://doi.org/10.3390/molecules27238167
Bai G, Yang Y, Wang X, Wu J, Wang H, Ye X, Bao X. DBU Promoted Polysubstituted Arene Formation via a Michael Addition/Cyclization/Elimination Cascade Reaction. Molecules. 2022; 27(23):8167. https://doi.org/10.3390/molecules27238167
Chicago/Turabian StyleBai, Guishun, Yang Yang, Xingyue Wang, Jiamin Wu, Hong Wang, Xinyi Ye, and Xiaoze Bao. 2022. "DBU Promoted Polysubstituted Arene Formation via a Michael Addition/Cyclization/Elimination Cascade Reaction" Molecules 27, no. 23: 8167. https://doi.org/10.3390/molecules27238167
APA StyleBai, G., Yang, Y., Wang, X., Wu, J., Wang, H., Ye, X., & Bao, X. (2022). DBU Promoted Polysubstituted Arene Formation via a Michael Addition/Cyclization/Elimination Cascade Reaction. Molecules, 27(23), 8167. https://doi.org/10.3390/molecules27238167









