Well-Defined pH-Sensitive Self-Assembled Triblock Copolymer-Based Crosslinked Micelles for Efficient Cancer Chemotherapy
Abstract
1. Introduction
2. Result and Discussion
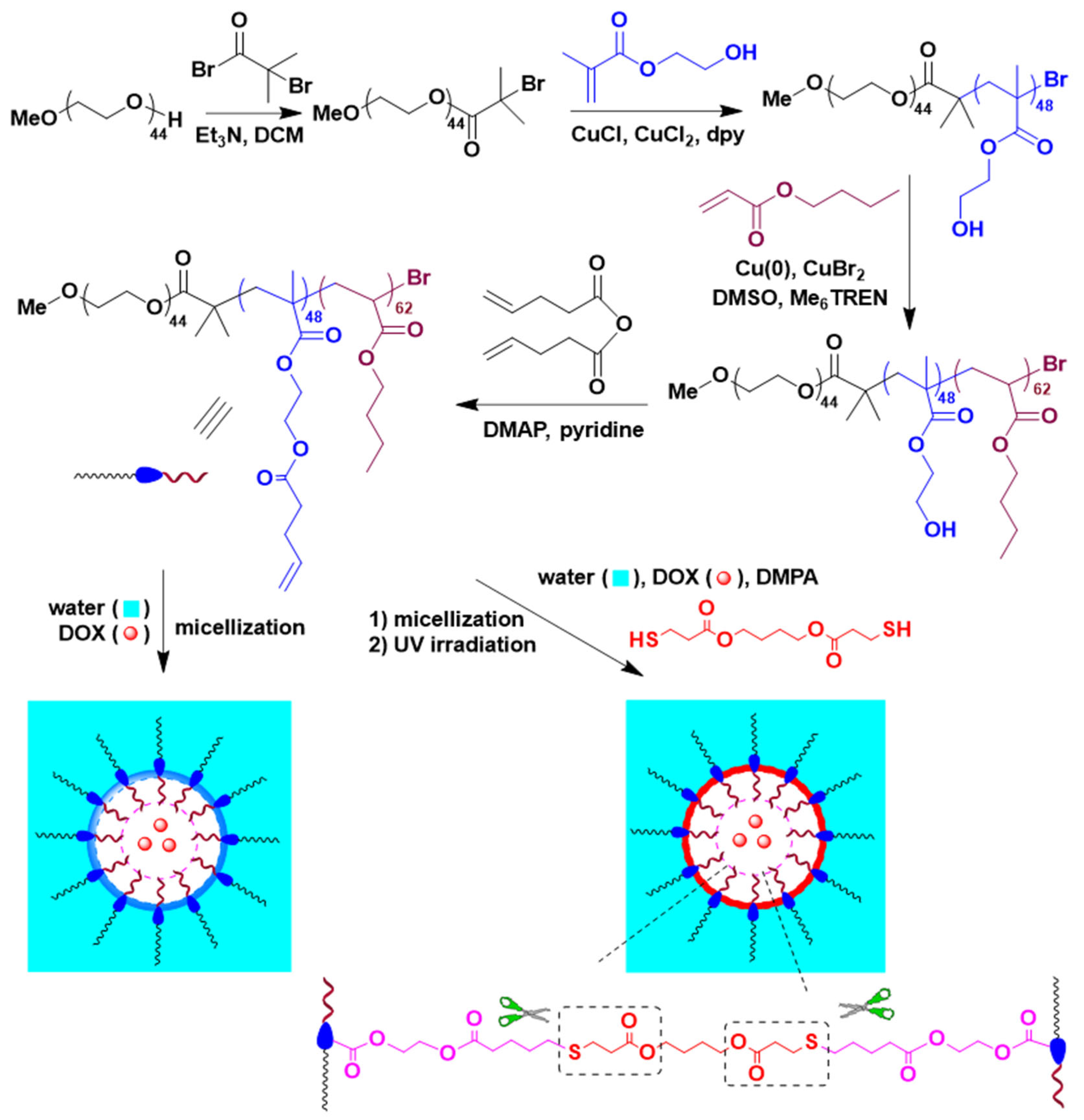
2.1. Synthesis and Characterization of PEG-b-P(HEMA-ene)-b-PBA
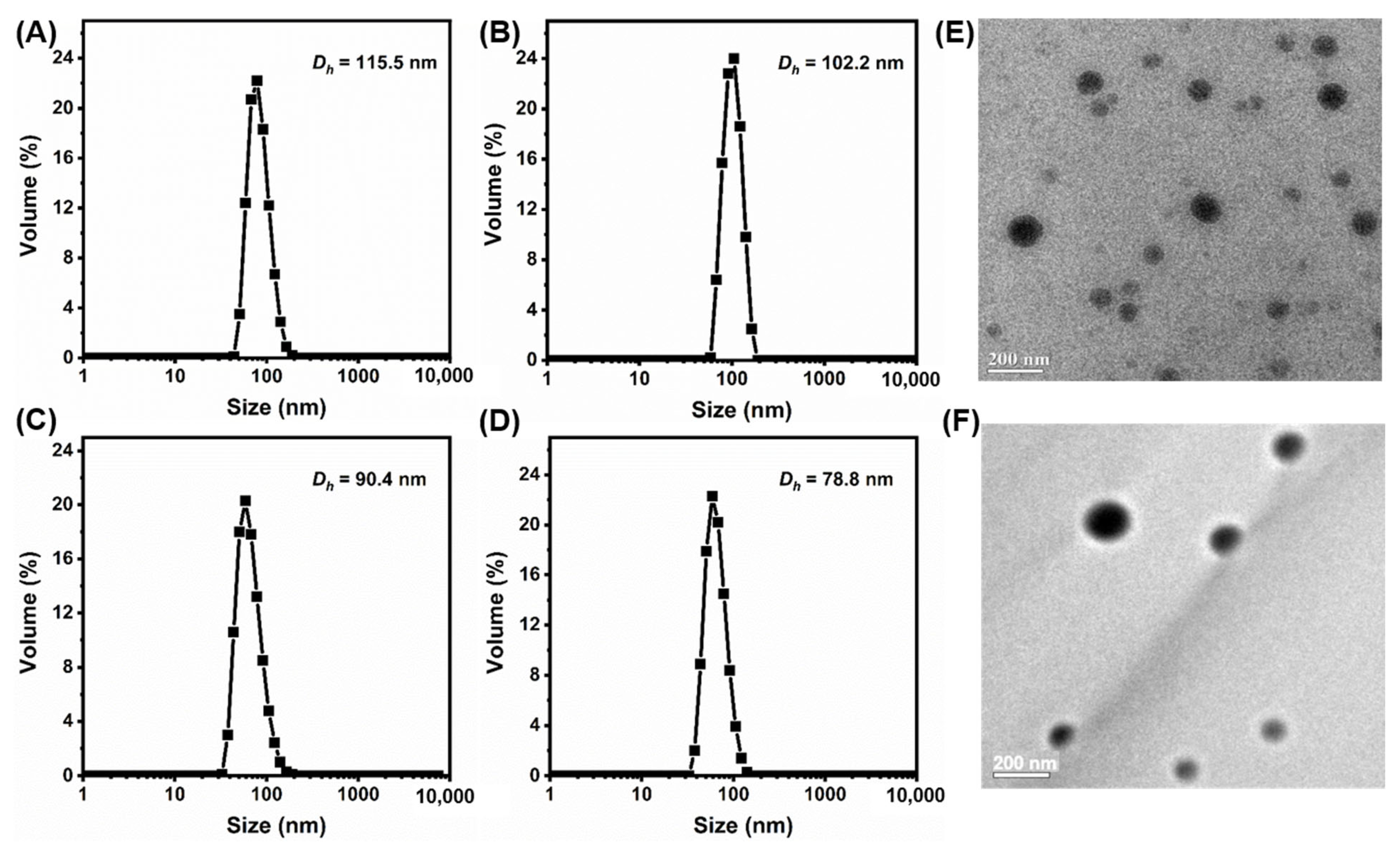
2.2. Preparation of PEG-b-P(HEMA-ene)-b-PBA NCMs and CMs
2.3. Preparation of DOX-Loaded PEG-b-P(HEMA-ene)-b-PBA NCMs and CMs
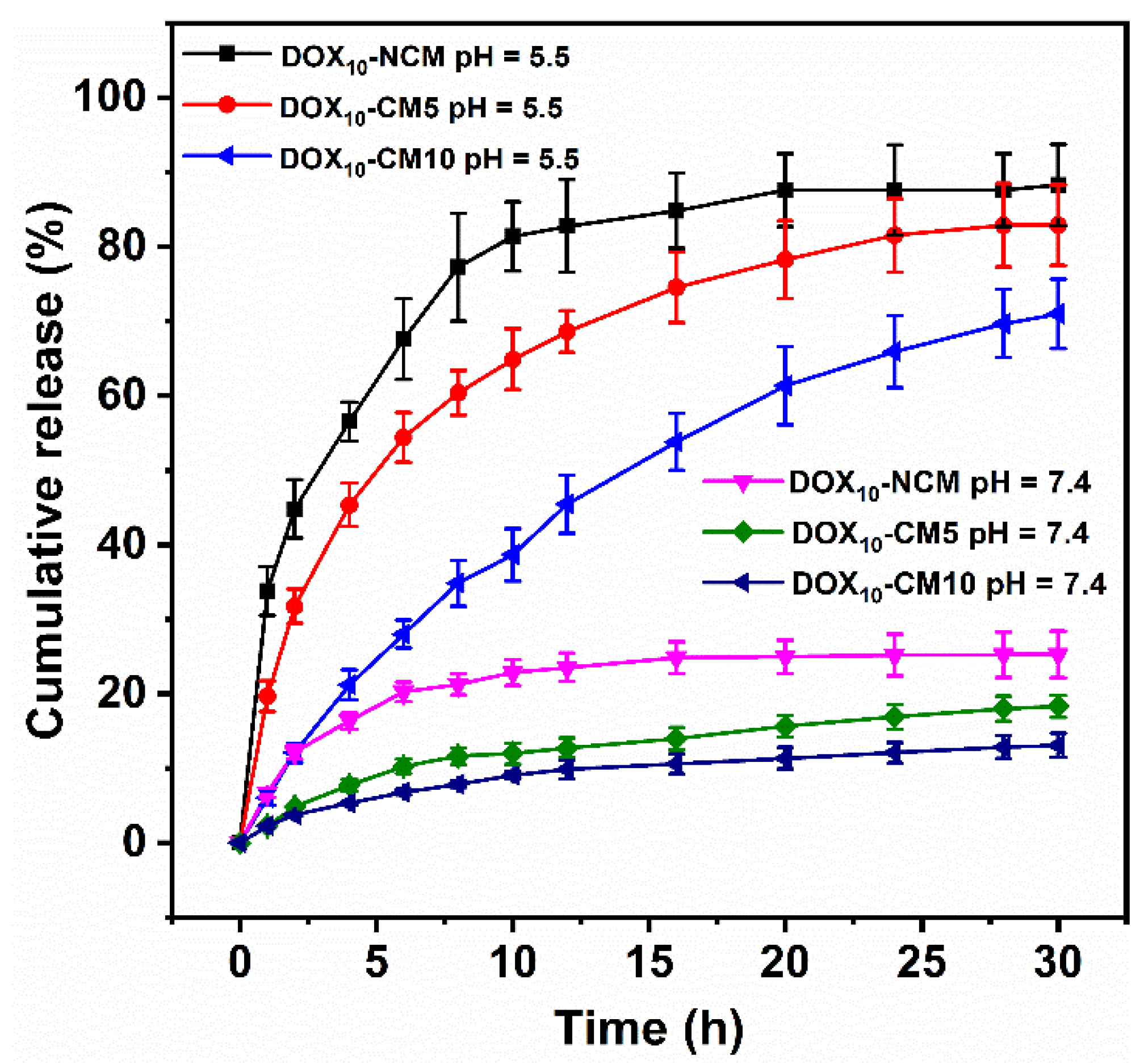
2.4. In Vitro pH-Responsive DOX Release
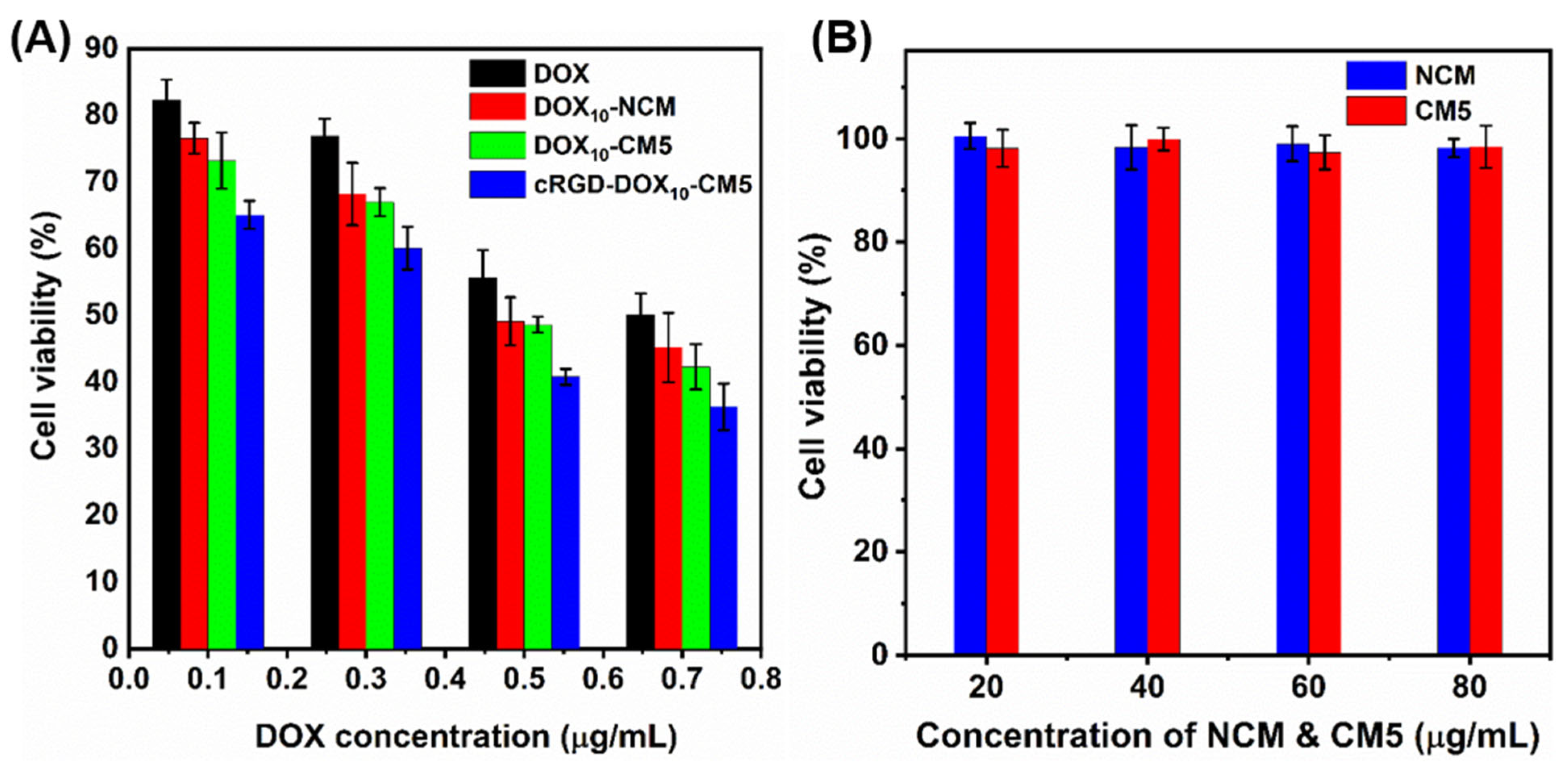
2.5. In Vitro Cytotoxicity
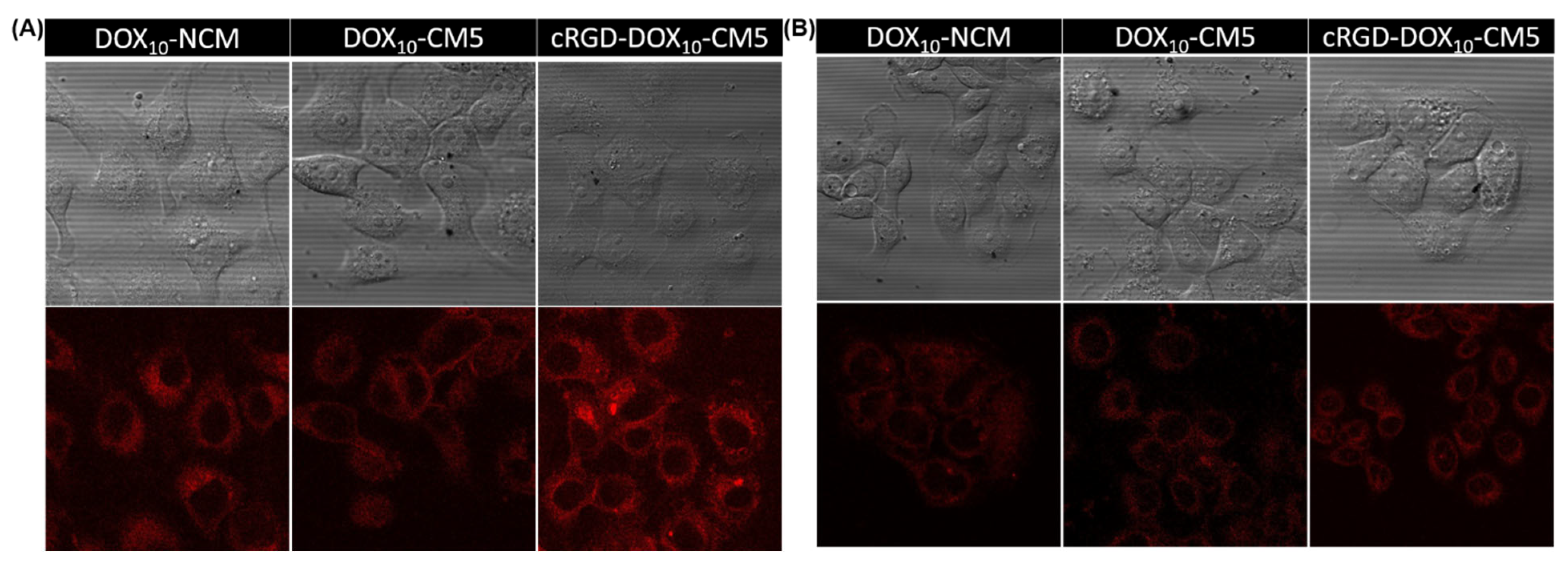
2.6. Cellular Uptake
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Measurements
4.3. Synthesis of MeO-PEG-Br Macroinitiator
4.4. Synthesis of MeO-PEG-b-PHEMA Diblock Copolymer
4.5. Synthesis of MeO-PEG-b-PHEMA-b-PBA Triblock Copolymer
4.6. Synthesis of MeO-PEG-b-P(HEMA-ene)-b-PBA
4.7. Determination of CMC of MeO-PEG-b-P(HEMA-ene)-b-PBA
4.8. Preparation of Blank and DOX-Loaded MeO-PEG-b-P(HEMA-ene)-b-PBA NCMs
4.9. Preparation of Blank and DOX-Loaded MeO-PEG-b-PHEMA(ene)-b-PBA CMs
4.10. Preparation of Targeted cRGD-DOX-CM5
4.11. In Vitro pH Sensitive Release of DOX
4.12. Cell Viability Studies
4.13. In Vitro Cellular Uptake and Imaging
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Serrano, M.; Blasco, M.A. The common biology of cancer and aging. Nature 2007, 448, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef]
- Mastria, E.M.; Cai, L.Y.; Kan, M.J.; Li, X.; Schaal, J.L.; Fiering, S.; Gunn, M.D.; Dewhirst, M.W.; Nair, S.K.; Chilkoti, A. Nanoparticle formulation improves doxorubicin efficacy by enhancing host antitumor immunity. J. Control. Release 2018, 269, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.; Allison, J. Cancer immunotherapy: Breaking the barriers to harvest the crop. Nat. Med. 2004, 10, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Forbes, D.C.; Creixell, M.; Frizzell, H.; Peppas, N.A. Polycationic nanoparticles synthesized using ARGET ATRP for drug delivery. Eur. J. Pharm. Biopharm. 2013, 84, 472–478. [Google Scholar] [CrossRef]
- Gao, G.H.; Park, M.J.; Li, Y.; Im, G.H.; Kim, J.-H.; Kim, H.N.; Lee, J.W.; Jeon, P.; Bang, O.Y.; Lee, J.H.; et al. The use of pH-sensitive positively charged polymeric micelles for protein delivery. Biomaterials 2012, 33, 9157–9164. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Tong, R.; Mishra, A.; Xu, W.; Wong, G.C.L.; Cheng, J.; Lu, Y. Reversible Cell-Specific Drug Delivery with Aptamer-Functionalized Liposomes. Angew. Chem. Int. Ed. 2009, 48, 6494–6498. [Google Scholar] [CrossRef]
- Nochi, T.; Yuki, Y.; Takahashi, H.; Sawada, S.-I.; Mejima, M.; Kohda, T.; Harada, N.; Kong, I.G.; Sato, A.; Kataoka, N.; et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat. Mater. 2010, 9, 572–578. [Google Scholar] [CrossRef]
- Yan, M.; Du, J.; Gu, Z.; Liang, M.; Hu, Y.; Zhang, W.; Priceman, S.; Wu, L.; Zhou, Z.H.; Liu, Z.; et al. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nat. Nanotechnol. 2010, 5, 48–53. [Google Scholar] [CrossRef]
- Jafari, A.; Sun, H.; Sun, B.; Mohamed, M.A.; Cui, H.; Cheng, C. Layer-by-layer preparation of polyelectrolyte multilayer nanocapsules via crystallized miniemulsions. Chem. Commun. 2019, 55, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Erdman, I.W.; Yuan, Y.; Mohamed, M.A.; Xie, R.; Wang, Y.; Gong, S.; Cheng, C. Crosslinked polymer nanocapsules for therapeutic, diagnostic, and theranostic applications. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1653. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Trewyn, B.G.; Lin, V.S.Y. Mesoporous Silica Nanoparticles for Intracellular Delivery of Membrane-Impermeable Proteins. J. Am. Chem. Soc. 2007, 129, 8845–8849. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.-B.; Falamarzian, A.; Garg, S.M.; Lavasanifar, A. Engineering of amphiphilic block copolymers for polymeric micellar drug and gene delivery. J. Control. Release 2011, 155, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, L.; Zhou, Z.; Sun, W.; Huang, Y. Dual-pH responsive micelle platform for co-delivery of axitinib and doxorubicin. Int. J. Pharm. 2016, 507, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control. Release 2012, 161, 175–187. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Huang, Y.; Yan, H.; Liu, K. Core-crosslinked polymeric micelles with high doxorubicin loading capacity and intracellular pH- and redox-triggered payload release. European Polymer Journal 2015, 68, 104–114. [Google Scholar] [CrossRef]
- Jafari, A.; Rajabian, N.; Zhang, G.; Mohamed, M.A.; Lei, P.; Andreadis, S.T.; Pfeifer, B.A.; Cheng, C. PEGylated Amine-Functionalized Poly(ε-caprolactone) for the Delivery of Plasmid DNA. Materials 2020, 13, 898. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, F.; Allen, C. In vivo fate of unimers and micelles of a poly(ethylene glycol)-block-poly(caprolactone) copolymer in mice following intravenous administration. Eur. J. Pharm. Biopharm. 2007, 65, 309–319. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Huang, S.-J.; Huang, X.-S.; Wu, Y.-T.; Chen, H.-Y.; Lo, Y.-L.; Wang, L.-F. The synthesis and comparison of poly(methacrylic acid)-poly(ε-caprolactone) block copolymers with and without symmetrical disulfide linkages in the center for enhanced cellular uptake. RSC Adv. 2016, 6, 75092–75103. [Google Scholar] [CrossRef]
- Savic, R.; Azzam, T.; Eisenberg, A.; Maysinger, D. Assessment of the Integrity of Poly(caprolactone)-b-poly(ethylene oxide) Micelles under Biological Conditions: A Fluorogenic-Based Approach. Langmuir 2006, 22, 3570–3578. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, R.; Yue, J.; Liu, S.; Xie, Z.; Jing, X. Targeting and anti-tumor effect of folic acid-labeled polymer-Doxorubicin conjugates with pH-sensitive hydrazone linker. J. Mater. Chem. 2012, 22, 13303–13310. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Lale, S.V.; Kumar, A.; Naz, F.; Bharti, A.C.; Koul, V. Multifunctional ATRP based pH responsive polymeric nanoparticles for improved doxorubicin chemotherapy in breast cancer by proton sponge effect/endo-lysosomal escape. Polym. Chem. 2015, 6, 2115–2132. [Google Scholar] [CrossRef]
- Zhong, Y.; Meng, F.; Deng, C.; Zhong, Z. Ligand-Directed Active Tumor-Targeting Polymeric Nanoparticles for Cancer Chemotherapy. Biomacromolecules 2014, 15, 1955–1969. [Google Scholar] [CrossRef]
- Zhang, L.; Nguyen, T.L.U.; Bernard, J.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Shell-Cross-Linked Micelles Containing Cationic Polymers Synthesized via the RAFT Process: Toward a More Biocompatible Gene Delivery System. Biomacromolecules 2007, 8, 2890–2901. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Meng, F.; Wang, Z.; Cheng, R.; Deng, C.; Liu, H.; Zhong, Z. Core-crosslinked pH-sensitive degradable micelles: A promising approach to resolve the extracellular stability versus intracellular drug release dilemma. J. Control. Release 2012, 164, 338–345. [Google Scholar] [CrossRef]
- Liu, S.; Weaver, J.V.M.; Save, M.; Armes, S.P. Synthesis of pH-Responsive Shell Cross-Linked Micelles and Their Use as Nanoreactors for the Preparation of Gold Nanoparticles. Langmuir 2002, 18, 8350–8357. [Google Scholar] [CrossRef]
- Nystrom, A.M.; Wooley, K.L. Thiol-functionalized shell crosslinked knedel-like (SCK) nanoparticles: A versatile entry for their conjugation with biomacromolecules. Tetrahedron 2008, 64, 8543–8552. [Google Scholar] [CrossRef][Green Version]
- Rios-Doria, J.; Carie, A.; Costich, T.; Burke, B.; Skaff, H.; Panicucci, R.; Sill, K. A versatile polymer micelle drug delivery system for encapsulation and in vivo stabilization of hydrophobic anticancer drugs. J. Drug Deliv. 2012, 2012, 951741. [Google Scholar] [CrossRef]
- Yang, B.; Lv, Y.; Zhu, J.-Y.; Han, Y.-T.; Jia, H.-Z.; Chen, W.-H.; Feng, J.; Zhang, X.-Z.; Zhuo, R.-X. A pH-responsive drug nanovehicle constructed by reversible attachment of cholesterol to PEGylated poly(L-lysine) via catechol-boronic acid ester formation. Acta Biomater. 2014, 10, 3686–3695. [Google Scholar] [CrossRef] [PubMed]
- Du, J.-Z.; Chen, D.-P.; Wang, Y.-C.; Xiao, C.-S.; Lu, Y.-J.; Wang, J.; Zhang, G.-Z. Synthesis and Micellization of Amphiphilic Brush-Coil Block Copolymer Based on Poly(ε-caprolactone) and PEGylated Polyphosphoester. Biomacromolecules 2006, 7, 1898–1903. [Google Scholar] [CrossRef]
- Lale, S.V.; Girija, A.R.; Aravind, A.; Kumar, D.S.; Koul, V. AS1411 Aptamer and Folic Acid Functionalized pH-Responsive ATRP Fabricated pPEGMA-PCL-pPEGMA Polymeric Nanoparticles for Targeted Drug Delivery in Cancer Therapy. Biomacromolecules 2014, 15, 1737–1752. [Google Scholar] [CrossRef]
- Zhao, H.; Sterner, E.S.; Coughlin, E.B.; Theato, P. O-Nitrobenzyl Alcohol Derivatives: Opportunities in Polymer and Materials Science. Macromolecules 2012, 45, 1723–1736. [Google Scholar] [CrossRef]
- Jafari, A.; Yan, L.; Mohamed, M.A.; Wu, Y.; Cheng, C. Well-Defined Diblock Poly(ethylene glycol)-b-Poly(ε-caprolactone)-Based Polymer-Drug Conjugate Micelles for pH-Responsive Delivery of Doxorubicin. Materials 2020, 13, 1510. [Google Scholar] [CrossRef]
- Yang, X.; Grailer, J.J.; Pilla, S.; Steeber, D.A.; Gong, S. Tumor-Targeting, pH-Responsive, and Stable Unimolecular Micelles as Drug Nanocarriers for Targeted Cancer Therapy. Bioconjug. Chem. 2010, 21, 496–504. [Google Scholar] [CrossRef]
- Du, Y.; Chen, W.; Zheng, M.; Meng, F.; Zhong, Z. pH-sensitive degradable chimaeric polymersomes for the intracellular release of doxorubicin hydrochloride. Biomaterials 2012, 33, 7291–7299. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, X.; Liu, Y.; Liu, C.; Jiang, B.; Jiang, Y. Tumor penetrability and anti-angiogenesis using iRGD-mediated delivery of doxorubicin-polymer conjugates. Biomaterials 2014, 35, 8735–8747. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Li, Y.-M.; Lv, Y.; Cheng, Y.-J.; He, F.; Zhuo, R.-X. Amphiphilic polycarbonate conjugates of doxorubicin with pH-sensitive hydrazone linker for controlled release. Colloids Surf. B 2013, 111, 542–548. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. Stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.K.; Lee, S.C.; Han, B.; Park, K. Analysis on the current status of targeted drug delivery to tumors. J. Control. Release 2012, 164, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; van Nostrum, C.F.; Hennink, W.E. Interfacially hydrazone cross-linked thermosensitive polymeric micelles for acid-triggered release of paclitaxel. ACS Biomater. Sci. Eng. 2015, 1, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Guo, W.; Yuan, W. Supramolecular hydrogels from inclusion complexation of α-cyclodextrin with densely grafted chains in micelles for controlled drug and protein release. J. Mater. Chem. B 2013, 1, 6235–6244. [Google Scholar] [CrossRef]
- Hu, X.; Li, H.; Luo, S.; Liu, T.; Jiang, Y.; Liu, S. Thiol and pH dual-responsive dynamic covalent shell cross-linked micelles for triggered release of chemotherapeutic drugs. Polym. Chem. 2013, 4, 695–706. [Google Scholar] [CrossRef]
- Kawamura, W.; Miura, Y.; Kokuryo, D.; Toh, K.; Yamada, N.; Nomoto, T.; Matsumoto, Y.; Sueyoshi, D.; Liu, X.; Aoki, I.; et al. Density-tunable conjugation of cyclic RGD ligands with polyion complex vesicles for the neovascular imaging of orthotopic glioblastomas. Sci. Technol. Adv. Mater. 2015, 16, 035004. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Wang, T.-H.; Hu, S.-H.; Hsu, T.-C.; Yow, J.-L.; Tzang, B.-S.; Chiang, W.-H. Tumor site-specific PEG detachment and active tumor homing of therapeutic PEGylated chitosan/folate-decorated polydopamine nanoparticles to augment antitumor efficacy of photothermal/chemo combination therapy. Chem. Eng. J. 2022, 446, 137243. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Yan, L.; Shahini, A.; Rajabian, N.; Jafari, A.; Andreadis, S.T.; Wu, Y.; Cheng, C. Well-Defined pH-Responsive Self-Assembled Block Copolymers for the Effective Codelivery of Doxorubicin and Antisense Oligonucleotide to Breast Cancer Cells. ACS Appl. Bio Mater. 2022, 5, 4779–4792. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Shahini, A.; Rajabian, N.; Caserto, J.; El-Sokkary, A.M.A.; Akl, M.A.; Andreadis, S.T.; Cheng, C. Fast photocurable thiol-ene elastomers with tunable biodegradability, mechanical and surface properties enhance myoblast differentiation and contractile function. Bioact. Mater. 2021, 6, 2120–2133. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Radiolabeled Multimeric Cyclic RGD Peptides as Integrin αvβ3 Targeted Radiotracers for Tumor Imaging. Mol. Pharm. 2006, 3, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Radiolabeled Cyclic RGD Peptides as Integrin αvβ3-Targeted Radiotracers: Maximizing Binding Affinity via Bivalency. Bioconjug. Chem. 2009, 20, 2199–2213. [Google Scholar] [CrossRef]
- Beers, K.L.; Boo, S.; Gaynor, S.G.; Matyjaszewski, K. Atom Transfer Radical Polymerization of 2-Hydroxyethyl Methacrylate. Macromolecules 1999, 32, 5772–5776. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Percec, V. Acid Dissolution of Copper Oxides as a Method for the Activation of Cu (0) Wire Catalyst for SET-LRP. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4241–4252. [Google Scholar] [CrossRef]
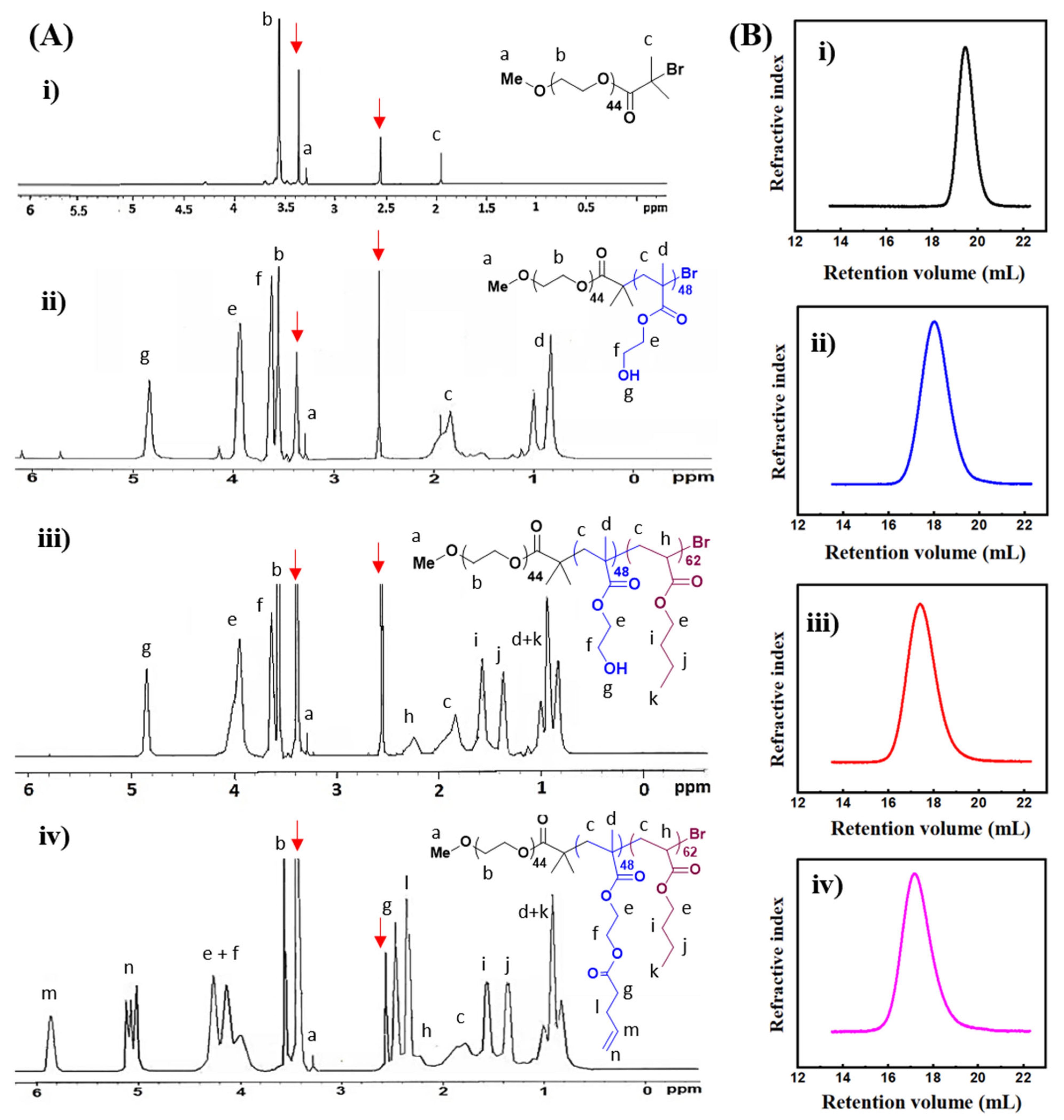
| DPn for Blocks a | Mn,NMRa (kDa) | Mn,GPCb (kDa) | Ðb | |||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | ||||
| MeO-PEG-Br | 44 | - | - | 2.3 | 2.2 | 1.01 |
| PEG-b-PHEMA | 44 | 62 | - | 10.2 | 10.4 | 1.15 |
| PEG-b-PHEMA-b-PBA | 44 | 62 | 48 | 16.4 | 16.7 | 1.21 |
| PEG-b-P(HEMA-ene)-b-PBA | 44 | 62 | 48 | 21.7 | 22.0 | 1.27 |
| Entry | [ene]0/[SH]0/[DMPA]0 | PDI b | |
|---|---|---|---|
| NCM | 1/0/0 | 115.5 ± 4.4 | 0.207 |
| CM5 | 1/0.05/0.02 a | 102.2 ± 2.4 | 0.265 |
| CM10 | 1/0.10/0.02 a | 90.4 ± 2.3 | 0.132 |
| CM20 | 1/0.20/0.02 a | 78.8 ± 1.6 | 0.196 |
| Entry | [ene]0/[SH]0/[DMPA]0 a | DOX (wt %) | DLC (wt %) | DLE (%) | PDI b | |
|---|---|---|---|---|---|---|
| DOX7-NCM | - | 7.0 | 6.2 | 89.6 | 122.6 ± 4.8 | 0.283 |
| DOX10-NCM | - | 10.0 | 9.1 | 91.7 | 128.3 ± 3.4 | 0.245 |
| DOX10-CM5 | 1/0.05/0.02 | 10.0 | 9.7 | 97.3 | 106.9 ± 1.1 | 0.112 |
| DOX10-CM10 | 1/0.10/0.02 | 10.0 | 9.5 | 95.5 | 95.1 ± 2.4 | 0.105 |
| cRGD-DOX10-CM5 | 1/0.10/0.02 | 10.0 | 9.4 | 94.8 | 135.5 ± 4.4 | 0.248 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, M.A.; Singh, A.; Prasad, P.N.; Cheng, C. Well-Defined pH-Sensitive Self-Assembled Triblock Copolymer-Based Crosslinked Micelles for Efficient Cancer Chemotherapy. Molecules 2022, 27, 8153. https://doi.org/10.3390/molecules27238153
Mohamed MA, Singh A, Prasad PN, Cheng C. Well-Defined pH-Sensitive Self-Assembled Triblock Copolymer-Based Crosslinked Micelles for Efficient Cancer Chemotherapy. Molecules. 2022; 27(23):8153. https://doi.org/10.3390/molecules27238153
Chicago/Turabian StyleMohamed, Mohamed Alaa, Ajay Singh, Paras N. Prasad, and Chong Cheng. 2022. "Well-Defined pH-Sensitive Self-Assembled Triblock Copolymer-Based Crosslinked Micelles for Efficient Cancer Chemotherapy" Molecules 27, no. 23: 8153. https://doi.org/10.3390/molecules27238153
APA StyleMohamed, M. A., Singh, A., Prasad, P. N., & Cheng, C. (2022). Well-Defined pH-Sensitive Self-Assembled Triblock Copolymer-Based Crosslinked Micelles for Efficient Cancer Chemotherapy. Molecules, 27(23), 8153. https://doi.org/10.3390/molecules27238153







