Deep Eutectic Solvent-Mediated Electrocatalysts for Water Splitting
Abstract
1. Introduction
| Type | Composition | Terms | Example | Ref. |
|---|---|---|---|---|
| Type I | R1R2R3R4N+·X–+MClx | X is a Lewis base, generally a halide anion; M = Zn, Sn, Fe, Al, Ga, In, etc. | ChCl/ZnCl2 | [24] |
| Type II | R1R2R3R4N+·X–+MClx·yH2O | X is a Lewis base, generally a halide anion; M = Cr, Co, Cu, Ni, Fe, etc | ChCl/NiCl2·6H2O | [24] |
| Type III | R1R2R3R4N+·X–+HBD | HBDs include RCONH2, RCOOH, ROH, etc | ChCl/urea, ChCl/EG, ChCl/glycerol | [25] |
| Type IV | MClx·yH2O+HBD | M = Zn, Sn, Fe, Al, etc; HBDs include RCONH2, RCOOH, ROH, etc | [25] | |
| Type V | nonionic, molecular HBAs +HBDs | thymol/menthol thymol/lidocaine | [26] |
- (1)
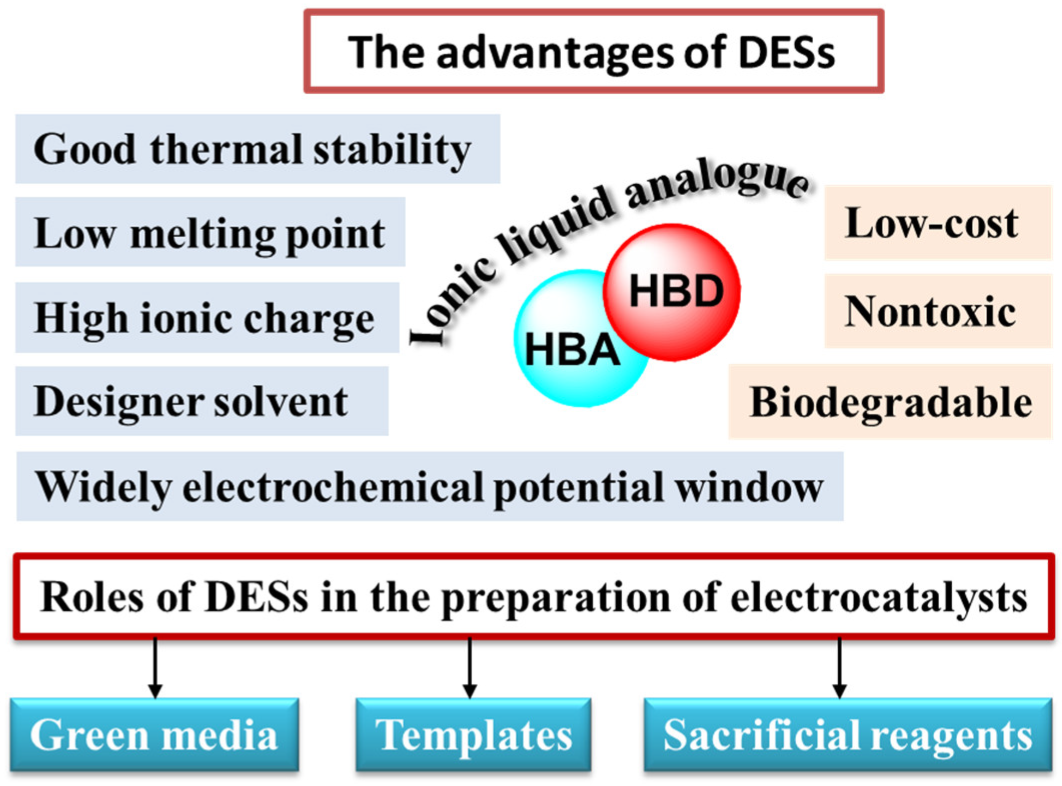
- DESs are considered emerging green solvents and promisingly soft templates [37]. This is because DESs have special properties. Firstly, they exhibit fine solubility for metal salts, so that they are beneficial in synthesizing catalysts. Secondly, the low vapor pressure and high thermal stability of DESs allow the progress of reactions at high temperature and ambient pressure, avoiding the danger of high pressure. Thirdly, owing to the hydrogen bond, highly ionic strength and the viscosity of DESs, the microenvironment is different from that in conventional solvents. The supramolecular nature of DESs can tune structures and sizes of micro/nanomaterials.
- (2)
- DESs can serve as active reactants to prepare catalysts. Their designability makes them P, S, N, C or metals sources (such as Fe, Co, Ni and so on) to form phosphides, sulfides, nitrides, carbides or metal-based catalysts [38,39]. Compared with traditional heteroatom sources, they are safe and green. Moreover, owing to the influence of the special liquid structure of DESs during the reaction process, the obtained products show different structures and performance from those obtained from the conventional reactants. In addition, the conversion of DESs into electrocatalysts can reduce waste emissions and simplify operation processes.
- (3)
- The unique physicochemical characteristics of DESs result in different nucleation and growth mechanisms from those in conventional solvents through charge neutralization, changes in reduction potential as well as chemical activity, and determination of growth along the preferred crystallographic directions. In addition, DESs are able to change the activity order of metals, leading to some displacement reactions that cannot occur in aqueous solutions being undertaken in DESs [40].
2. Deep Eutectic Solvents as Both Green Solvents and Structure-Directed Reagents Simultaneously for the Preparation of HER and OER Electrocatalysts
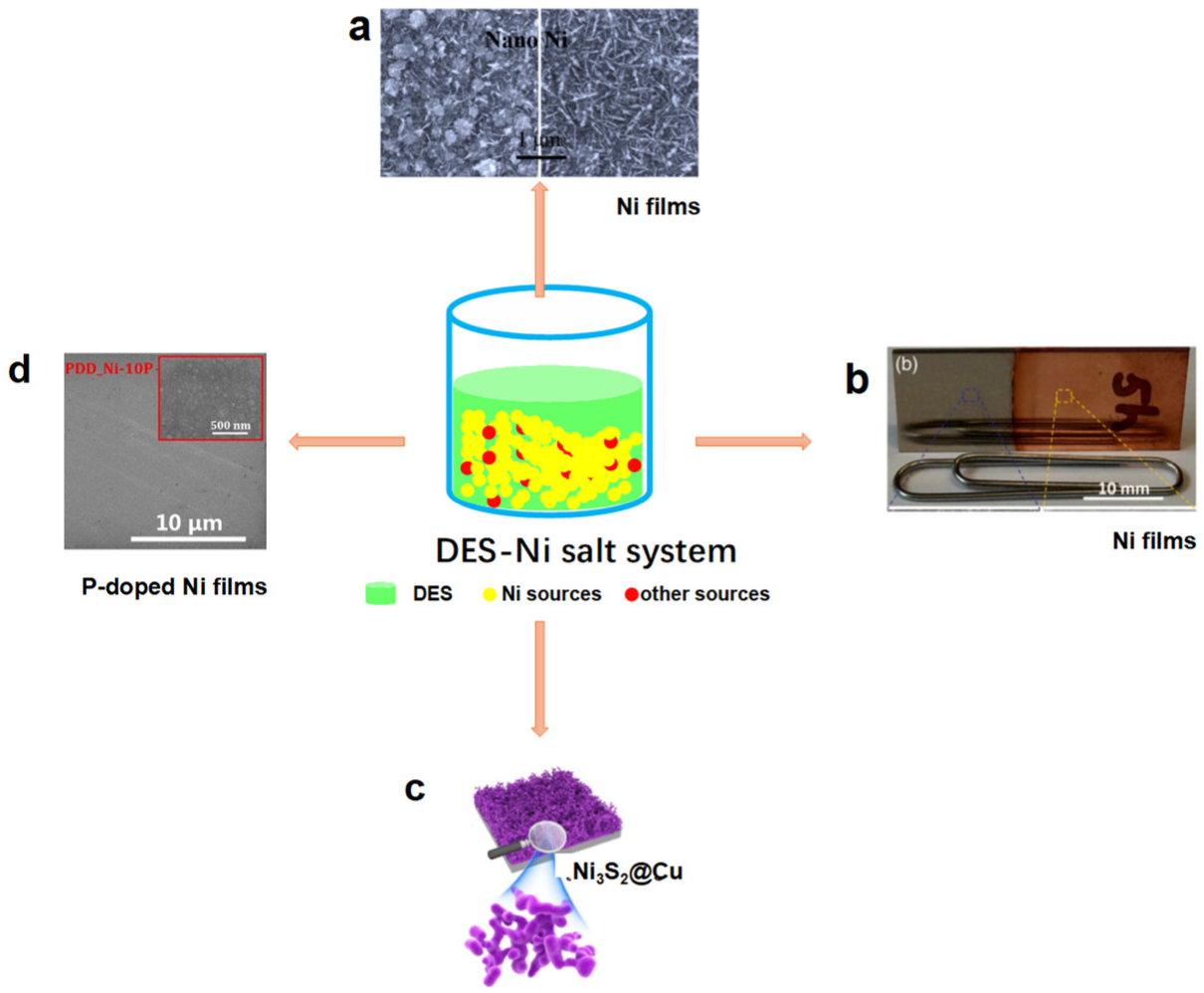
| Catalyst | Applied DES | Preparation Method | Catalytic Performance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HER | OER | Water Splitting | |||||||||
| Electrolyte | η (mV)@ Current Density (mA cm−2) | Tafel Slope (mV dec−1) | Electrolyte | η (mV)@ Current Density (mA cm−2) | Tafel Slope (mV dec−1) | Electrolyte | Potential (V)@ Current Density (mA cm−2) | Ref. | |||
| Ni | ChCl/Urea | Electrodeposition | 1 M KOH | 153@30 | 185 | –– | –– | –– | –– | –– | [57] |
| Ni/TiO2 | ChCl/EG | Electrodeposition | 1 M NaOH | –– | 122 | –– | –– | –– | –– | –– | [58] |
| Ni/Ni(OH)2 | ChCl/EG | Electrodeposition | 1 M KOH | 110@10 | 83.9 | 1 M KOH | 290@10 | 120.9 | –– | –– | [59] |
| NiSx | ChCl/EG | Electrodeposition | 1 M KOH | 54@10 | 54 | –– | –– | –– | –– | –– | [60] |
| Ni | ChCl/EG | GRR | 1 M KOH | 170@10 | 98.5 | –– | –– | –– | –– | –– | [61] |
| Ni3S2 | ChCl/EG | GRR | 1 M KOH | 60.8@10 | 67.5 | –– | –– | –– | –– | –– | [62] |
| Ni3S2 | ChCl/EG | GRR | 0.5 M H2SO4 | 63.5@10 | 91.6 | –– | –– | –– | –– | –– | [62] |
| NiPx | ChCl/EG | Electrodeposition | 1 M KOH | 105@10 | 44.7 | –– | –– | –– | –– | –– | [64] |
| Ni–P | ChCl/EG | Electrodeposition | 1 M KOH | 105@50 | 72.9 | –– | –– | –– | –– | –– | [65] |
| Ni–Mo | ChCl/EG | Electrodeposition | 1 M KOH | 63@20 | 49 | 1 M KOH | 335@20 | 108 | 1 M KOH | 1.59@10 | [66] |
| Ni–Mo–Cu | ChCl/Urea | Electrodeposition | 1 M KOH | 93@10 | 105 | –– | –– | –– | –– | –– | [67] |
| Ni–Cu | ChCl/EG | Electrodeposition | 1 M KOH | 128@10 | 57.2 | –– | –– | –– | –– | –– | [68] |
| Ni–Co–Sn | ChCl/EG | Electrodeposition | 1 M KOH | –– | 121 | –– | –– | –– | –– | –– | [69] |
| Ni–Fe | ChCl/EG | Electrodeposition | 0.1 M KOH | 316@10 | 62 | –– | –– | –– | –– | –– | [70] |
| Ni–Fe | ChCl/Urea | Electrodeposition | 0.5 M NaOH | 256@10 | 140.1 | 0.5 M NaOH | 406@10 | 84.4 | –– | –– | [71] |
| Cox–Ni(OH)2 | ChCl/EG | Electrodeposition | 1 M KOH | 106@10 | 98.2 | 1 M KOH | 330@100 | 126.7 | –– | –– | [72] |
| NiCo2O4 | ChCl/Glycerol | Calcining method | –– | –– | –– | 1 M KOH | 320@10 | 67 | –– | –– | [73] |
| (FeCoNiCuZn)(C2O4)· 2H2O | Polyethylene glycol (PEG)/Oxalic acid | Ionothermal method | 1 M KOH | 334@10 | 67.93 | [74] | |||||
| NiCoxSy | ChCl/EG | Electrodeposition | 1 M KOH | 65@10 | 62.5 | 1 M KOH | 300@20 | 109 | 1M KOH | 1.57@10 | [75] |
| S–NiFe2O4/Ni3Fe | ChCl/EG | Electrodeposition | –– | –– | –– | 1 M KOH | 260@10 | 35 | 1 M KOH | 1.52@10 | [76] |
| NiCoxSy | ChCl/EG | Electrodeposition | 1 M KOH | 65@20 | 54 | 1 M KOH | 270@20 | 35 | 1 M KOH | 1.57@10 | [77] |
| Co | ChCl/Malonic acid | Electrodeposition | –– | –– | –– | 1 M KOH | 350@10 | 76 | –– | –– | [78] |
| P–Co | ChCl–EG | Electrodeposition | 1 M KOH | 65@10 | 69.2 | 1 M KOH | 320@10 | 91.15 | 1 M KOH | 1.59@10 | [79] |
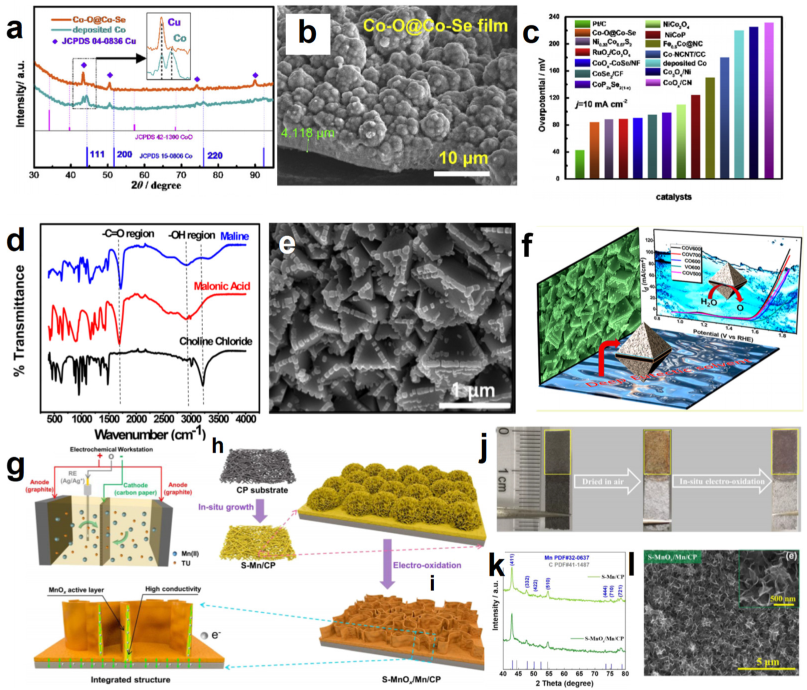
| Co–O/Co–Se | ChCl/Urea | Electrodeposition | 1 M KOH | 85@10 | 71.9 | 1 M KOH | 340@10 | 67.6 | 1 M KOH | 1.65@10 | [80] |
| Co–S | ChCl/EG | Electrodeposition | 1 M KOH | 59@10 | 65 | 1 M KOH | 307@50 | 66.4 | 1 M KOH | 1.69@50 | [81] |
| CoSx | Ethanedithiol/n–Butylamine | CO2–assited solution–processed method | –– | –– | –– | 1 M KOH | 302@10 | 64.8 | –– | –– | [82] |
| CoV2O6 | ChCl/Malonic acid | Calcining method | –– | –– | –– | 1 M KOH | 324@10 | –– | –– | –– | [83] |
| CoFe–LDH | ChCl/Urea | Water injection method | –– | –– | 0.5 M KOH | Onset overpotential 510 | –– | –– | –– | [88] | |
| FexCo3–x(PO4)2 | ChCl/Urea | Electrodeposition | 1 M KOH | 108.1@100 | 30.3 | 1 M KOH | 310@10 | 40.2 | 1 M KOH | 1.62@10 | [91] |
| FexCo3–x(PO4)2 | ChCl/Urea | Electrodeposition | 0.5 M H2SO4 | 128.8@100 | 42.4 | –– | –– | –– | –– | –– | [91] |
| FexCo3–x(PO4)2 | ChCl/Urea | Electrodeposition | 1 M Phosphate–buffered saline (PBS) | 291.5@100 | 117.6 | –– | –– | –– | –– | –– | [91] |
| FeSx | ChCl/EG | Electrodeposition | –– | –– | –– | 1 M KOH | 340@10 | –– | –– | –– | [92] |
| Fe7S8/Fe2O3 | ChCl/glycerol | Calcining method | –– | –– | –– | 1 M KOH | 229@10 | 49 | –– | –– | [93] |
| S–MnOx/Mn | ChCl/EG | Electrodeposition and in–situ electrochemical oxidation | –– | –– | –– | 1 M KOH | 435@10 | 89.97 | –– | –– | [94] |
| LaCoO3 | ChCl/Malonic acid | Calcining method | –– | –– | –– | 1 M NaOH | 390@10 | 55.8 | [95] | ||
| Pt–Pd@Ag | ChCl/EG | GRR and Electrodeposition | 0.5 M H2SO4 | 28.1@10 | 31.2 | –– | –– | –– | –– | –– | [97] |
| Pt–Pd@Ag | ChCl/EG | GRR and Electrodeposition | 1.0 M PBS | 34.8@10 | 32.2 | –– | –– | –– | –– | –– | [97] |
| Pt–Pd@Ag | ChCl/EG | GRR and Electrodeposition | 1 M KOH | 23.8@10 | 32.5 | –– | –– | –– | –– | –– | [97] |
| Ru | ChCl/urea | Electrodeposition | 0.5 M H2SO4 | 65.7@10 | 97 | –– | –– | –– | –– | –– | [99] |
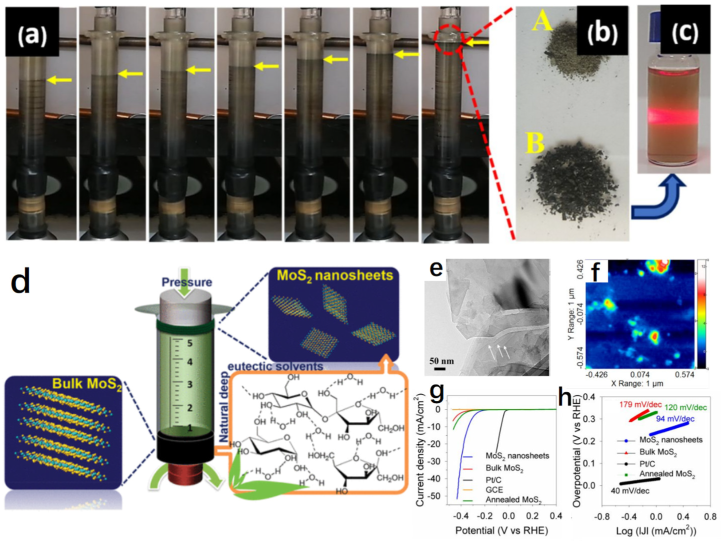
| MoS2 | A series of sugar–based natural DESs | Mechanical stirring | –– | –– | –– | 0.5 M H2SO4 | 339@10 | 94 | –– | –– | [105] |
3. Deep Eutectic Solvents as Reactive Reagents of Metal Electrocatalysts for HER and OER
3.1. Deep Eutectic Solvents as Heteroatom Sources to Prepare Metal Electrocatalysts
3.2. Hydrated Metal Chloride-Based Deep Eutectic Solvents as Metal Sources to Prepare Metal Electrocatalysts
4. Conclusions and Perspectives
4.1. Dialectically Understand the Greenness of DESs and Maximize Their Greenness through Reasonable Design and Effective Control Conditions
4.2. Requiring Further Understanding of the Structure-Composition-Performance Relationship
4.3. Exploring Research on the Preparation of Single-Atom Catalysts in DESs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moores, A.; Yan, N. Novel catalytic materials for energy and the environment. ACS Sustain. Chem. Eng. 2017, 5, 11124. [Google Scholar] [CrossRef]
- Nurul, S.; Kit, W.; Wei, L.; Ratchahat, S.; Rinklebe, J.; Loke, P. Recovery of microalgae biodiesel using liquid biphasic flotation system. Fuel 2022, 317, 123368. [Google Scholar]
- Tang, C.; Zhang, R.; Lu, W.; He, L.; Jiang, X.; Asiri, A.M.; Sun, X. Fe-doped CoP nanoarray: A monolithic multifunctional catalyst for highly efficient hydrogen generation. Adv. Mater. 2017, 29, 1602441. [Google Scholar] [CrossRef] [PubMed]
- Dibyendu, G.; Krishnendu, R.; Sarkar, K.; Devi, P.; Kumar, P. Surface plasmon-enhanced carbon dot-embellished multifaceted Si(111) nanoheterostructure for photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2020, 12, 28792–28800. [Google Scholar]
- Zhang, W.; Lai, W.; Cao, R. Energy-related small molecule activation reactions: Oxygen reduction and hydrogen and oxygen evolution reactions catalyzed by porphyrin- and corrole-based systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef]
- Ajeet, S.; Jyoti, R.; Samim, H.M.D.; Devaiah, C.; Pravin, P.; Pralay, K.; Dibyajyoti, G.; Sameer, S. MoSe2/SnS nanoheterostructures for water splitting. ACS Appl. Nano Mater. 2022, 5, 4293–4304. [Google Scholar]
- Callejas, J.; Read, C.; Roske, C.; Nathan, S.; Raymond, E. Synthesis, characterization, and properties of metal phosphide catalysts for the hydrogen evolution reaction. Chem. Mater. 2016, 28, 6017–6044. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, M.; Huang, Z.; Zhu, W.; Zheng, J.; Jiang, Q.; Wang, Z.; Lang, H. Surface reconstruction of water splitting electrocatalysts. Adv. Energy Mater. 2022, 12, 2201713. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Monica, D. Ionic liquids: Environment-friendly greener solvents for organic synthesis. Curr. Org. Synth. 2022, 19, 543–557. [Google Scholar] [CrossRef]
- Gebresilassie, E.G.; Armand, M.; Scrosati, B.; Passerini, S. Energy storage materials synthesized from ionic liquids. Angew. Chem. Int. Ed. 2014, 53, 13342–13359. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, L.Á.; Robles, R.; Miguel, D.; Cuerva, J.M. Reduction reactions in green solvents: Water, supercritical carbon dioxide, and ionic liquids. ChemSusChem 2011, 4, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liao, Y.; Zhang, Z. Toxicity of ionic liquids. Clean: Soil Air Water 2007, 35, 42–48. [Google Scholar] [CrossRef]
- Kudlak, B.; Owczarek, K.; Namieśnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—A review. Environ. Sci. Pollut. Res. 2015, 22, 11975–11992. [Google Scholar] [CrossRef]
- Tao, X.Y.; Li, Q.Z.; Wang, J.; Song, G.L.; Liu, H.M.; Cheng, H.; Yang, Z. Improved whole-cell biocatalysis by addition of deep eutectic solvents and natural deep eutectic solvents. ACS Sustain. Chem. Eng. 2017, 5, 5713–5722. [Google Scholar]
- Shi, R.; Yu, D.; Zhou, F.; Yu, J.; Mu, T. An emerging deep eutectic solvent based on halogen bonds. Chem. Commun. 2022, 58, 4607–4610. [Google Scholar] [CrossRef]
- Paiva, A.; Matias, A.A.; Duarte, A.R.C. How do we drive deep eutectic systems towards an industrial reality? Curr. Opin. Green Sustain. Chem. 2018, 11, 81–85. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Raymond, K.R.; Vasuki, T. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Cooper, E.R.; Andrews, C.D.; Wheatley, P.S.; Webb, P.B.; Wormald, P.; Morris, R.E. Ionic liquids and eutectic mixtures as solvent and template in the synthesis of zeolite analogues. Nature 2004, 430, 1012–1016. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.D.; Wang, X.; Tu, J. Deep eutectic solvents (DESs)-derived advanced functional materials for energy and environmental applications: Challenges, opportunities, and future vision. J. Mater. Chem. A 2017, 5, 8209–8229. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Synthesis, properties, and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Catarina, F.; Filipa, L.; Bernardo, D.R.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar]
- Patrycja, J.; Massoud, K.; Justyna, P.; Gębicki, J. Supramolecular deep eutectic solvents and their applications. Green Chem. 2022, 24, 5035–5045. [Google Scholar]
- McDonald, T.R.; Mills, L.R.; West, M.S.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar]
- Abbott, A.P.; Barron, J.C.; Ryder, K.S.; Wilson, D. Eutectic-based ionic liquids with metal-containing anions and cations. Chem. Eur. J. 2007, 13, 6495–6501. [Google Scholar] [CrossRef] [PubMed]
- Abranches, D.O.; Martins, M.A.; Silva, L.P.; Schaeffer, N.; Pinho, S.P.; Coutinho, J.A.P. Phenolic hydrogen bond donors in the formation of nonionic deep eutectic solvents: The quest for type V DES. Chem. Commun. 2019, 55, 10253–10256. [Google Scholar] [CrossRef] [PubMed]
- Rachiero, G.P.; Berton, P.; Shamshina, J. Deep eutectic solvents: Alternative solvents for biomass-based waste valorization. Molecules 2022, 27, 6606. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acid: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Yu, D.K.; Xue, Z.M.; Mu, T.C. Eutectics: Formation, properties, and applications. Chem. Soc. Rev. 2021, 50, 8596–8638. [Google Scholar] [CrossRef]
- Saif, R.; Rafiq, S.; Muhammad, N. Surface tuning of silica by deep eutectic solvent to synthesize biomass derived based membranes for gas separation to enhance the circular bioeconomy. Fuel 2022, 310, 122355. [Google Scholar] [CrossRef]
- Macfarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.J.H.; Watanabe, M.; Simon, P.; Angell, A.C. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef]
- Yu, D.; Xue, Z.; Mu, T. Deep eutectic solvents as a green toolbox for synthesis. Cell Rep. Phys. Sci. 2022, 3, 100809. [Google Scholar] [CrossRef]
- Hammond, O.S.; Edler, K.J.; Bowron, D.T.; Laura, T.M. Deep eutectic solvothermal synthesis of nanostructured ceria. Nat. Commun. 2017, 8, 14150. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.S.; Lv, X.W.; Zhao, Y.M.; Ren, T.Z.; Yuan, Z.Y. Engineering of cobalt oxide/phosphate-carbon nanohybrids for high-efficiency electrochemical water oxidation and reduction. J. Energy Chem. 2021, 52, 139–146. [Google Scholar] [CrossRef]
- Deng, J.; Fang, S.; Fang, Y.; Hao, Q.; Wang, L.; Hu, Y.H. Multiple roles of graphene in electrocatalysts for metal-air batteries. Catal. Today 2022, in press. [Google Scholar] [CrossRef]
- Zhang, C.; Bai, T.; Sun, Y.; Xin, B.; Zhang, S. Ionic Liquid/Deep Eutectic Solvent-Mediated Ni-Based Catalysts and Their Application in Water Splitting Electrocatalysis. Catalysts 2022, 12, 928. [Google Scholar] [CrossRef]
- Chen, C.H.; Wu, D.; Li, Z.; Zhang, R.; Kuai, C.G.; Zhao, X.R.; Dong, C.K.; Qiao, S.Z.; Liu, H.; Du, X.W. Ruthenium-based single-atom alloy with high electrocatalytic activity for hydrogen evolution. Adv. Energy Mater. 2019, 9, 1803913. [Google Scholar] [CrossRef]
- Fechler, N.; Zussblatt, N.P.; Rothe, R.; Schlögl, R.; Willinger, M.G.; Chmelka, B.F.; Antonietti, M. Eutectic syntheses of graphitic carbon with high pyrazinic nitrogen content. Adv. Mater. 2016, 28, 1287–1294. [Google Scholar] [CrossRef]
- Shishov, A.; Chromá, R.; Vakh, C.; Kuchár, J.; Simon, A.; Andruch, V.; Bulatov, A. In situ decomposition of deep eutectic solvent as a novel approach in liquid-liquid microextraction. Anal. Chim. Acta 2019, 1065, 49–55. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Cecilia, O.Z.; Javier, G.S.; Javier, H.B. Deep Eutectic solvents application in food analysis. Molecules 2021, 26, 6846. [Google Scholar]
- Wang, J.; Teng, C.; Yan, L. Applications of deep eutectic solvents in the extraction, dissolution, and functional materials of chitin: Research progress and prospects. Green Chem. 2022, 24, 552–564. [Google Scholar] [CrossRef]
- Carolin, R.; Burkhard, K. Low melting mixtures in organic synthesis—An alternative to ionic liquids? Green Chem. 2012, 14, 2969–2982. [Google Scholar]
- Durgesh, V.W.; Zhao, H.; Gary, A.B. Deep eutectic solvents: Sustainable media for nanoscale and functional materials. Acc. Chem. Res. 2014, 47, 2299–2308. [Google Scholar]
- Abbott, A.P. Deep eutectic solvents and their application in electrochemistry. Curr. Opin. Green Sustain. Chem. 2022, 36, 100649. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, B.; Lin, T.; Faaij, A.P.C. Economic optimization for a dual-feedstock lignocellulosic-based sustainable biofuel supply chain considering greenhouse gas emissions and soil carbon stock. Biofuels Bioprod. Biorefin. 2022, 16, 653–670. [Google Scholar]
- Rangaswamy, P.; Chanchal, M.; Dibyendu, M.; Ghosh, D. An account on deep eutectic solvent-based electrolytes for rechargeable batteries and supercapacitors. Sustain. Mater. Technol. 2022, 33, 477. [Google Scholar]
- Kristina, R.; Jelena, Ž.; Marina, C.B. Comparative in vitro study of cholinium-based ionic liquids and deep eutectic solvents on a fish cell line. Ecotox. Environ. Saf. 2016, 131, 30–36. [Google Scholar]
- Cheng, Q.B.; Zhang, L.W. Highly efficient enzymatic preparation of daidzein in deep eutectic solvents. Molecules 2017, 22, 186. [Google Scholar] [CrossRef]
- Nakhle, L.; Kfoury, M.; Mallard, I.; Mallard, I.; Landy, D. Microextraction of bioactive compounds using deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3747–3759. [Google Scholar] [CrossRef]
- Potka-Wasylka, J.; Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ballantyne, A.; Harris, R.C.; Juma, J.A.; Ryder, K.S.; Forrest, G. A comparative study of nickel electrodeposition using deep eutectic solvents and aqueous solutions. Electrochim. Acta 2015, 176, 718–726. [Google Scholar] [CrossRef]
- Cruz, H.; Jordão, N.; Branco, L.C. Deep eutectic solvents (DESs) as low-cost and green electrolytes for electrochromic devices. Green Chem. 2017, 19, 1653. [Google Scholar] [CrossRef]
- Sebastian, P.; Giannotti, M.I.; Gómez, E.; Feliu, J.M. Surface sensitive nickel electrodeposition in deep eutectic solvent. ACS Appl. Energy Mater. 2018, 1, 1016–1028. [Google Scholar] [CrossRef]
- Elsharkawya, S.; Hammad, S.; El-hallaga, I. Electrodeposition of Ni nanoparticles from deep eutectic solvent and aqueous solution promotes high stability electrocatalyst for hydrogen and oxygen evolution reactions. J. Solid State Electrochem. 2022, 26, 1501–1517. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Bobrova, L.S.; Korniy, S.A.; Danilov, F.I. Electrochemical synthesis and characterization of electrocatalytic materials for hydrogen production using Cr(III) baths based on a deep eutectic solvent. Mater. Lett. 2022, 313, 131800. [Google Scholar] [CrossRef]
- Wang, S.; Zou, X.; Lu, Y.; Rao, S.; Xie, X.; Pang, Z.; Lu, X.; Xu, Q.; Zhou, Z. Electrodeposition of nanonickel in deep eutectic solvents for hydrogen evolution reaction in alkaline solution. Int. J. Hydrogen Energy 2018, 43, 15673–15686. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Bogdanov, D.A.; Korniy, S.A.; Kityk, A.A.; Baskevich, A.S.; Danilov, F.I. Application of a deep eutectic solvent to prepare nanocrystalline Ni and Ni/TiO2 coatings as electrocatalysts for the hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 24604–24616. [Google Scholar] [CrossRef]
- Gao, M.Y.; Sun, C.; Lei, H.; Zeng, J.R.; Zhang, Q.B. Nitrate-induced and in situ electrochemical activation synthesis of oxygen deficiency-rich nickel/nickel (oxy)hydroxide hybrid films for enhanced electrocatalytic water splitting. Nanoscale 2018, 10, 17546–17551. [Google Scholar] [CrossRef]
- Zeng, J.; Gao, M.Y.; Zhang, Q.; Yang, C.; Li, X.T.; Yang, W.Q.; Hua, Y.X.; Xu, C.Y.; Li, Y. Facile electrodeposition of cauliflower-like S-doped nickel microsphere films as highly active catalysts for electrochemical hydrogen evolution. J. Mater. Chem. A 2017, 5, 15056–15064. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Q.; Abbott, A.P. Facile fabrication of nickel nanostructures on a copper-based template via a galvanic replacement reaction in a deep eutectic solvent. Electrochem. Commun. 2016, 70, 60–64. [Google Scholar] [CrossRef]
- Yang, C.; Gao, M.Y.; Zhang, Q.; Zeng, J.R.; Li, X.T.; Abbott, A.P. In-situ activation of self-supported 3D hierarchically porous Ni3S2 films grown on nanoporous copper as excellent pH-universal electrocatalysts for hydrogen evolution reaction. Nano Energy 2017, 36, 85–94. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Feng, H.; Shen, J. Synthesis of nickel phosphide nanoparticles in a eutectic mixture for hydrotreating reactions. J. Mater. Chem. 2011, 21, 8137–8145. [Google Scholar] [CrossRef]
- Sun, C.; Zeng, J.; Lei, H.; Yang, W.; Zhang, Q. Direct electrodeposition of phosphorus-doped nickel superstructures from choline chloride-ethylene glycol deep eutectic solvent for enhanced hydrogen evolution catalysis. ACS Sustain. Chem. Eng. 2019, 7, 1529–1537. [Google Scholar] [CrossRef]
- Li, L.; Sheng, S.; Wang, H.; Qu, T.; Hou, D.; Wang, D.; Sheng, M. Electrodeposition of Ni–P alloy in deep eutectic solvent and its electrocatalytic activity toward hydrogen evolution reaction. Can. J. Chem. Eng. 2022, 100, 3381–3394. [Google Scholar] [CrossRef]
- Gao, M.Y.; Yang, C.; Zhang, Q.; Zeng, J.R.; Li, X.T.; Hua, Y.X.; Xu, C.Y.; Dong, P. Facile electrochemical preparation of self-supported porous Ni–Mo alloy microsphere films as efficient bifunctional electrocatalysts for water splitting. J. Mater. Chem. A 2017, 5, 5797–5805. [Google Scholar] [CrossRef]
- Lu, Y.; Geng, S.; Wang, S.; Rao, S.; Huang, Y.; Zou, X.; Zhang, Y.; Xu, Q.; Lu, X. Electrodeposition of NiMoCu coatings on roasted nickel matte in deep eutectic solvent for hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 5704–5716. [Google Scholar] [CrossRef]
- Gao, M.Y.; Yang, C.; Zhang, Q.; Yu, Y.W.; Hua, Y.X.; Li, Y.; Dong, P. Electrochemical fabrication of porous Ni–Cu alloy nanosheets with high catalytic activity for hydrogen evolution. Electrochim. Acta 2016, 215, 609–616. [Google Scholar] [CrossRef]
- Vijayakumar, J.; Mohan, S.; Kumar, S.A.; Suseendiran, S.R.; Pavithra, S. Electrodeposition of Ni–Co–Sn alloy in choline chloride-based deep eutectic solvent and characterization as cathode for hydrogen evolution in alkaline solution. Int. J. Hydrogen Energy 2013, 38, 10208–10214. [Google Scholar] [CrossRef]
- Vo, T.; Hidalgo, S.D.S.; Chiang, C. Controllable electrodeposition of binary metal films from deep eutectic solvent as an efficient and durable catalyst for the oxygen evolution reaction. Dalton Trans. 2019, 48, 14748–14757. [Google Scholar] [CrossRef]
- Francisco, G.S.; Oliveira, L.P.M.; Santos, R.B.; Silva, R.B.D.; Correa, M.A.; Bohn, F.; Correia, A.N.; Vieira, L.; Vasconcelos, I.F.; Lima-Neto, P. FexNi(1–x) coatings electrodeposited from choline chloride-urea mixture: Magnetic and electrocatalytic properties for water electrolysis. Mater. Chem. Phys. 2022, 279, 125738. [Google Scholar]
- Sun, C.B.; Guo, M.W.; Siwal, S.S.; Zhang, Q.B. Efficient hydrogen production via urea electrolysis of cobalt-doped nickel hydroxide-riched hybrid films: Cobalt doping effect and mechanism aspects. J. Catal. 2020, 381, 454–461. [Google Scholar] [CrossRef]
- Zhang, C.; Xin, B.; Chen, T.; Ying, H.; Li, Z.; Hao, J. Deep eutectic solvent strategy enables an octahedral Ni–Co precursor for creating high-performance NiCo2O4 catalyst with oxygen evolution reaction. Green Energy Environ. 2022, 7, 1217–1227. [Google Scholar] [CrossRef]
- Yang, H.Y.; Cheng, Z.F.; Wu, P.C.; Wei, Y.; Jiang, J.; Xu, Q. Deep eutectic solvent regulation synthesis of multimetal oxalate for electrocatalytic oxygen evolution reaction and supercapacitor applications. Electrochim. Acta 2022, 427, 140879. [Google Scholar] [CrossRef]
- Zhang, Y.; Ru, J.; Hua, Y.; Huang, P.; Xu, C. One-step preparation, and adsorption property of mesoporous nickel sulfides in deep eutectic solvent. Ceram. Int. 2022, 48, 20341–20350. [Google Scholar] [CrossRef]
- Gao, M.; Zeng, J.; Zhang, Q.; Yang, C.; Li, X.T.; Hua, Y.X.; Xu, C.Y. Scalable one-step electrochemical deposition of nanoporous amorphous S-doped NiFe2O4/Ni3Fe composite films as highly efficient electrocatalysts for oxygen evolution with ultrahigh stability. J. Mater. Chem. A 2018, 6, 1551–1560. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, J.; Siwal, S.S.; Yang, W.; Fu, X.; Zhang, Q. Morphological and electronic modification of 3D porous nickel microsphere arrays by cobalt and sulfur dual synergistic modulation for overall water splitting electrolysis and supercapacitors. Appl. Surf. Sci. 2019, 491, 570–578. [Google Scholar] [CrossRef]
- Renjith, A.; Lakshminarayanan, V. Single-step electrochemical synthesis of cobalt nanoclusters embedded in dense graphite sheets for electrocatalysis of the oxygen evolution reaction. ACS Appl. Nano Mater. 2020, 3, 2705–2712. [Google Scholar] [CrossRef]
- Li, K.; Ren, T.; Yuan, Z.; Bandosz, T.J. Electrodeposited P–Co nanoparticles in deep eutectic solvents and their performance in water splitting. Int. J. Hydrogen Energy 2018, 43, 10448–10457. [Google Scholar] [CrossRef]
- Yang, W.; Hua, Y.X.; Zhang, Q.; Lei, H.; Xu, C.Y. Electrochemical fabrication of 3D quasi-amorphous pompon-ike Co–O and Co–Se hybrid films from choline chloride/urea deep eutectic solvent for efficient overall water splitting. Electrochim. Acta 2018, 273, 71–79. [Google Scholar] [CrossRef]
- Yang, W.; Zeng, J.; Hua, Y.; Xu, C.; Siwal, S.S.; Zhang, Q. Defect engineering of cobalt microspheres by S doping and electrochemical oxidation as efficient, bifunctional, and durable electrocatalysts for water splitting at high current densities. J. Power Sources 2019, 436, 226887. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, J.; Xue, Z.; Yan, C.; Mu, T. An ambient temperature, CO2-assisted solution processing of amorphous cobalt sulfide in a thiol/amine based quasi-ionic liquid for oxygen evolution catalysis. Chem. Commun. 2017, 53, 9418–9421. [Google Scholar] [CrossRef] [PubMed]
- Thorat, G.M.; Jadhav, H.S.; Roy, A.; Chung, W.; Seo, J.G. Dual role of deep eutectic solvent as a solvent and template for the synthesis of octahedral cobalt vanadate by an oxygen evolution reaction. ACS Sustain. Chem. Eng. 2018, 6, 16255–16266. [Google Scholar] [CrossRef]
- Liu, J.; Ji, Y.; Nai, J.; Niu, X.; Luo, Y.; Guo, L.; Yang, S. Ultrathin amorphous cobalt-vanadium hydr(oxy)oxide catalysts for the oxygen evolution reaction. Energy Environ. Sci. 2018, 11, 1736–1741. [Google Scholar] [CrossRef]
- Liardet, L.; Hu, X. Amorphous Cobalt vanadium oxide as a highly active electrocatalyst for oxygen evolution. ACS Catal. 2018, 8, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, D.A. The catalysis of the oxygen evolution reaction by iron impurities in thin film nickel oxide electrodes. J. Electrochem. Soc. 1987, 134, 377–384. [Google Scholar] [CrossRef]
- Burke, M.S.; Kast, M.G.; Trotochaud, L.; Smith, A.M.; Boettcher, S.W. Cobalt-iron (oxy)hydroxide oxygen evolution electrocatalysts: The role of structure and composition on activity, stability, and mechanism. J. Am. Chem. Soc. 2015, 137, 3638–3648. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.D.; Wang, X.L.; Tu, J.P. Ionothermal synthesis of cobalt iron layered double hydroxides (LDHs) with expanded interlayer spacing as advanced electrochemical materials. J. Mater. Chem. A 2014, 2, 17066–17076. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.D.; Lu, Y.; Wang, X.L.; Tu, J.P. A versatile protocol for the ionothermal synthesis of nanostructured nickel compounds as energy storage materials from a choline chloride-based ionic liquid. J. Mater. Chem. A 2013, 1, 13454–13461. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.D.; Wang, X.L.; Tu, J.P. Correlation between microstructure and electrochemical behavior of the mesoporous Co3O4 sheet and its ionothermal synthesized hydrotalcite-like α–Co(OH)2 precursor. J. Phys. Chem. C 2014, 118, 911–923. [Google Scholar] [CrossRef]
- Yang, C.; He, T.; Zhou, W.; Deng, R.; Zhang, Q. Iron-tuned 3D cobalt-phosphate catalysts for efficient hydrogen and oxygen evolution reactions over a wide pH range. ACS Sustain. Chem. Eng. 2020, 8, 13793–13804. [Google Scholar] [CrossRef]
- Wang, W.; Xu, R.; Yu, B.; Wang, X.; Feng, S. Electrochemical fabrication of FeSx films with high catalytic activity for oxygen evolution. RSC Adv. 2019, 9, 31979–31987. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, T.; Ying, H.; Hao, J. Deep eutectic solvent-mediated construction of oxygen vacancy-rich Fe-based electrocatalysts for efficient oxygen evolution reaction. Adv. Sustain. Syst. 2020, 4, 2000038. [Google Scholar] [CrossRef]
- Guo, M.; Wei, Z.; Zhang, Q. Electrochemical construction of S-doped MnO/Mn integrated film on carbon paper in a choline deep eutectic solvent for enhanced electrochemical water oxidation. Int. J. Hydrogen Energy 2022, 47, 6029–6043. [Google Scholar] [CrossRef]
- Hong, S.; Díez, A.M.; Adedoyin, N.A.; Sousa, J.P.S.; Salonen, L.M.; Lebedev, O.I.; Kolen’ko, Y.V.; Zaikina, J.V. Deep eutectic solvent synthesis of perovskite electrocatalysts for water oxidation. ACS Appl. Mater. Interfaces 2022, 14, 23277–23284. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Prokhorov, E.; Arias de Fuentes, O.; Ramírez, M.; Bogdanchikova, N.; Sanchez, I.C.; Mota-Morales, J.D.; Luna-Bárcenas, G. Temperature-induced Au nanostructure synthesis in a nonaqueous deep-eutectic solvent for high performance electrocatalysis. J. Mater. Chem. A 2015, 3, 15869–15875. [Google Scholar] [CrossRef]
- Yang, C.; Lei, H.; Zhou, W.Z.; Zeng, J.R.; Zhang, Q.B.; Hua, Y.X.; Xu, C.Y. Engineering nanoporous Ag/Pd core/shell interfaces with ultrathin Pt doping for efficient hydrogen evolution reaction over a wide pH range. J. Mater. Chem. A 2018, 6, 14281–14290. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Edison, T.N.J.I.; Atchudan, R.; Karthik, N.; Chandrasekaran, S.; Perumal, S.; Raja, P.B.; Perumal, V.; Lee, Y.R. Deep eutectic solvent assisted electrosynthesis of ruthenium nanoparticles on stainless steel mesh for electrocatalytic hydrogen evolution reaction. Fuel 2021, 297, 120786. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, S.; Yu, C.; Zhang, J.; Pan, X.; Li, G. Ionic liquid-assisted one-step preparation of ultrafine amorphous metallic hydroxide nanoparticles for the highly efficient oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 15767–15773. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Chen, P. Galvanic displacement of electrodeposited tangled Zn nanowire sacrificial template for preparing porous and hollow Ni electrodes in ionic liquid. J. Mol. Liq. 2020, 298, 112050. [Google Scholar] [CrossRef]
- Abdelkader, A.M.; Patten, H.V.; Li, Z.; Chen, Y.; Kinloch, I.A. Electrochemical exfoliation of graphite in quaternary ammonium-based deep eutectic solvents: A route for the mass production of graphane. Nanoscale 2015, 7, 11386–11392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Zhu, J.; Li, L.; Du, X.; Sun, X. Electrochemically exfoliated high-yield graphene in ambient temperature molten salts and its application for flexible solid-state supercapacitors. Carbon 2018, 127, 392–403. [Google Scholar] [CrossRef]
- Abdelkader, A.M.; Kinloch, I.A. Mechanochemical exfoliation of 2D crystals in deep eutectic solvents. ACS Sustain. Chem. Eng. 2016, 4, 4465. [Google Scholar] [CrossRef]
- Mohammadpour, Z.; Abdollahi, S.H.; Safavi, A. Sugar-based natural deep eutectic mixtures as green intercalating solvents for high-yield preparation of stable MoS2 nanosheets: Application to electrocatalysis of hydrogen evolution reaction. ACS Appl. Energy Mater. 2018, 1, 5896–5906. [Google Scholar] [CrossRef]
- Mohammadpour, Z.; Abdollahi, S.H.; Omidvar, A.; Mohajeri, A.; Safavi, A. Aqueous solutions of carbohydrates are new choices of green solvents for highly efficient exfoliation of two-dimensional nanomaterials. J. Mol. Liq. 2020, 309, 113087. [Google Scholar] [CrossRef]
- Zhang, T.; Doert, T.; Wang, H.; Zhang, S.; Ruck, M. Inorganic synthesis based on reactions of ionic liquids and deep eutectic solvents. Angew. Chem. Int. Ed. 2021, 60, 22148–22165. [Google Scholar] [CrossRef]
- Pariiska, O.; Mazur, D.; Cherchenko, K.; Zhang, S.; Ruck, M. Efficient Co–NC electrocatalysts for oxygen reduction derived from deep eutectic solvents. Electrochim. Acta 2022, 413, 140132. [Google Scholar] [CrossRef]
- Titirici, M.M.; White, R.J.; Brun, N.; Budarin, V.L.; Sheng, S.D.; Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef]
- Brandão, A.T.; Costa, R.; Silva, A.F.; Pereira, C.M. Sustainable preparation of nanoporous carbons via dry ball milling: Electrochemical studies using nanocarbon composite electrodes and a deep eutectic solvent as electrolyte. Nanomaterials 2021, 11, 3258. [Google Scholar] [CrossRef]
- Zhang, J.J.; Sun, Y.; Guo, L.K. Using urea to improve the ORR performance of N–, P–, and S–ternary-doped porous carbon derived from biomass. Mod. Phys. Lett. B 2022, 36, 2242018. [Google Scholar] [CrossRef]
- Zhu, C.; Li, H.; Fu, S.; Du, D.; Lin, Y. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 2016, 45, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhang, Q.; Wang, Y. Catalytic transformation of cellulose and cellulose-derived carbohydrates into organic acids. Catal. Today 2014, 234, 31–41. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Rubio, F.; Monte, F.D. Resorcinol-formaldehyde polycondensation in deep eutectic solvents for the preparation of carbons and carbon−carbon nanotube composites. Chem. Mater. 2010, 22, 2711. [Google Scholar] [CrossRef]
- Rong, K.; Wei, J.; Huang, L.; Fang, Y.; Dong, S. Synthesis of low-dimensional hierarchical transition metal oxides via a direct deep eutectic solvent calcining method for enhanced oxygen evolution catalysis. Nanoscale 2020, 12, 20719–20725. [Google Scholar] [CrossRef]
- Li, D.; Huang, Y.; Li, Z.; Zhong, L.; Liu, C.; Peng, X. Deep eutectic solvent derived carbon-based efficient electrocatalyst for boosting H2 production coupled with glucose oxidation. Chem. Eng. J. 2022, 430, 132783. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, B.; Li, Z.; Hao, J. Deep eutectic solvent-mediated hierarchically structured Fe-based organic-inorganic hybrid catalyst for oxygen evolution reaction. ACS Appl. Energy Mater. 2019, 2, 3343–3351. [Google Scholar] [CrossRef]
- Song, S.; Zhou, J.; Su, X.; Wang, Y.; Li, J.; Zhang, L.; Xiao, G.; Guan, C.; Liu, R.; Chen, S.; et al. Operando X-ray spectroscopic tracking of self-reconstruction for anchored nanoparticles as high-performance electrocatalysts towards oxygen evolution. Energy Environ. Sci. 2018, 11, 2945–2953. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, Y.; Liang, Y.; Du, Y.; Zhang, B. In situ electrochemical conversion of an ultrathin tannin-nickel-iron complex film as an efficient oxygen evolution reaction electrocatalyst. Angew. Chem. 2019, 58, 3769–3773. [Google Scholar] [CrossRef]
- Niu, S.; Jiang, W.; Wei, Z.; Tang, T.; Ma, J.; Hu, J.S.; Wan, L.J. Se-doping activates FeOOH for cost-effective and efficient electrochemical water oxidation. J. Am. Chem. Soc. 2019, 141, 7005–7013. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhang, L. Highly conductive NiCo₂S₄ urchin-like nanostructures for high-rate pseudocapacitors. Nanoscale 2013, 5, 8879–8883. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Zhou, M.; Li, W.; Li, C.; Xue, Y.; Jiang, X.; Zeng, X.; Bando, Y.; Golberg, D. Engineering sulfur vacancies and impurities in NiCo2S4 nanostructures with optimal supercapacitive performance. Nano Energy 2016, 26, 313–323. [Google Scholar] [CrossRef]
- Jiang, J.; Yan, C.; Zhao, X.; Luo, H.; Xue, Z.; Mu, T. A PEGylated deep eutectic solvent for controllable solvothermal synthesis of porous NiCo2S4 for efficient oxygen evolution reaction. Green Chem. 2017, 19, 3023–3031. [Google Scholar] [CrossRef]
- Zhao, X.; Lan, X.; Yu, D.; Fu, H.; Liu, Z.; Mu, T. Deep eutectic solvothermal synthesis of nanostructured Fe3S4 by electrochemical N2 fixation under ambient conditions. Chem. Commun. 2018, 54, 13010–13013. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, H. A green and cost-effective rechargeable battery with high energy density based on a deep eutectic catholyte. Energy Environ. Sci. 2016, 9, 2267–2272. [Google Scholar] [CrossRef]
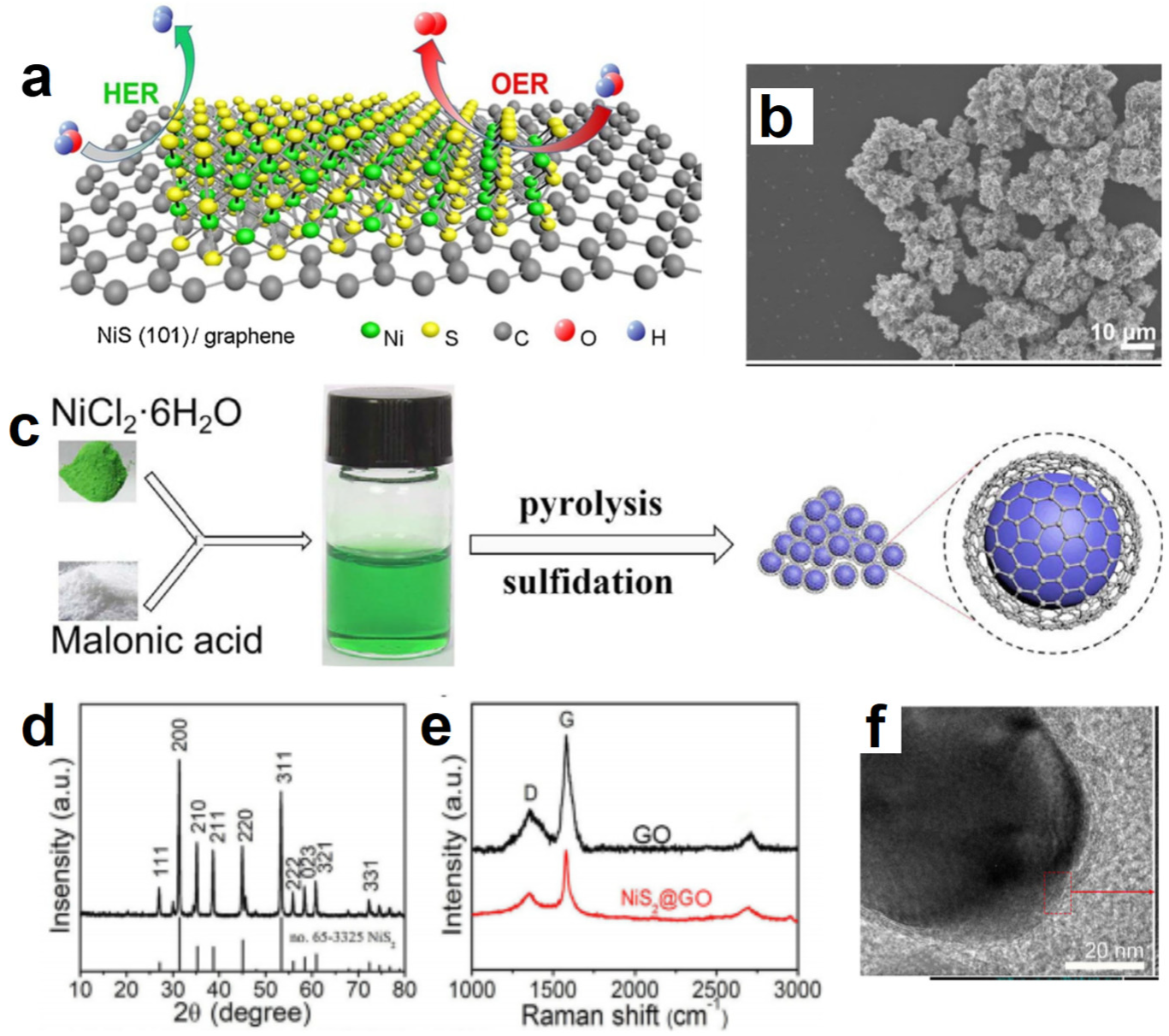
- Zhang, D.; Mou, H.; Lu, F.; Song, C.; Wang, D. A novel strategy for 2D/2D NiS/graphene heterostructures as efficient bifunctional electrocatalysts for overall water splitting. Appl. Catal. B 2019, 254, 471–478. [Google Scholar] [CrossRef]
- Zhang, D.; Mou, H.; Chen, L.; Wang, D.; Song, C. Design and in-situ synthesis of a unique catalyst via embedding graphene oxide shell membrane in NiS2 for efficient hydrogen evolution. Appl. Surf. Sci. 2020, 510, 145483. [Google Scholar] [CrossRef]
- Mou, H.; Wang, J.F.; Yu, D.; Zhang, D.; Lu, F.; Chen, L.; Wang, D.; Mu, T. A facile and controllable, deep eutectic solvent aided strategy for synthesis graphene encapsulated metal phosphides for enhanced electrocatalytic overall water splitting. J. Mater. Chem. A 2019, 7, 13455–13459. [Google Scholar] [CrossRef]
- Zhang, D.; Mou, H.; Chen, L.; Xing, G.; Wang, D.; Song, C. Surface/interface engineering N-doped carbon/NiS2 nanosheets for efficient electrocatalytic H2O splitting. Nanoscale 2020, 12, 3370–3376. [Google Scholar] [CrossRef]
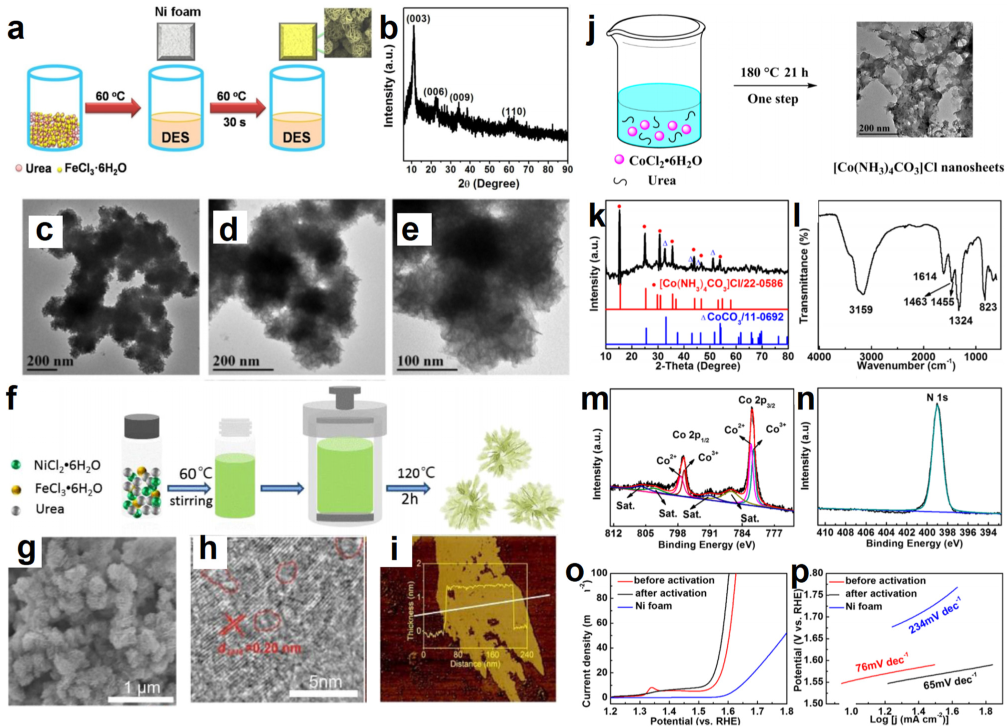
- Zhang, C.; Chen, T.; Zhang, H.; Li, Z.; Hao, J. Hydrated metal halide-based deep eutectic solvent-mediated NiFe layered double hydroxide: An excellent electrocatalyst for urea electrolysis and water splitting. Chem. Asian J. 2019, 14, 2995–3002. [Google Scholar] [CrossRef]
- Gao, Z.; Xie, S.; Zhang, B.; Qiu, X.; Chen, F. Ultrathin Mg–Al layered double hydroxide prepared by ionothermal synthesis in a deep eutectic solvent for highly effective boron removal. Chem. Eng. J. 2017, 319, 108–118. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.; Sun, C.; Song, C.; Wang, D. One-step ionothermal accompanied thermolysis strategy for N-doped carbon quantum dot hybridized NiFe LDH ultrathin nanosheets for electrocatalytic water oxidation. Electrochim. Acta 2021, 391, 138932. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, D.; Chen, W.; Li, F.; Mu, T. Water absorption by deep eutectic solvents. Phys. Chem. Chem. Phys. 2019, 21, 2601–2610. [Google Scholar] [CrossRef]
- Kivelä, H.; Salomäki, M.; Vainikka, P.; Mäkilä, E.; Poletti, F.; Ruggeri, S.; Terzi, F.; Lukkari, J. Effect of water on a hydrophobic deep eutectic solvent. J. Phys. Chem. B 2022, 126, 513–527. [Google Scholar] [CrossRef]
- Tracy, E.A.; Sophie, F.; Hélène, G.G. Deep eutectic solvents: An overview on their interactions with water and biochemical compounds. J. Mol. Liq. 2019, 288, 111028. [Google Scholar]
- Liu, S.; Zhang, C.; Zhang, B.; Li, Z.; Hao, J. All-in-one deep eutectic solvent with cobalt-based electrocatalyst for oxygen evolution reaction. ACS Sustain. Chem. Eng. 2019, 7, 8964–8971. [Google Scholar] [CrossRef]
- Mou, H.; Xue, Z.; Zhang, B.; Xue, L.; Mu, T. A deep eutectic solvent strategy to form defect-rich N, S, and O tridoped carbon/Co9S8 hybrid materials by a pH-universal hydrogen evolution reaction. J. Mater. Chem. A 2021, 9, 2099–2103. [Google Scholar] [CrossRef]
- Li, X.; Duan, F.; Deng, M.; Zheng, W.; Lin, Y.; Dan, Y.; Cheng, X.; Chen, L. Effect of Fe doping on Co–S/carbon cloth as bifunctional electrocatalyst for enhanced water splitting. J. Electroanal. Chem. 2022, 922, 116723. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Sun, Q.; Cheng, Y.; Wang, L. Self-assembly of hierarchical Ni–Mo–polydopamine microflowers and their conversion to a Ni–Mo2C/C composite for water splitting. Chem. Eur. J. 2017, 23, 4644–4650. [Google Scholar] [CrossRef]
- Rimpa, K.; Madhupriya, S.; Shrabani, G.; Sankar, D.N.; Chattopadhyay, K.K. Co incorporated Ni3S2 hierarchical nano/micro cactus for electrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 21315–21323. [Google Scholar]
- Jiang, J.; Chang, L.; Zhao, W.; Tian, Q.; Xu, Q. An advanced FeCoNi nitro-sulfide hierarchical structure in deep eutectic solvents for enhanced oxygen evolution reaction. Chem. Commun. 2019, 55, 10174–10177. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, Z.; Chen, W.; Wang, Y.; Mu, T. Eutectic synthesis of high-entropy metal phosphides for electrocatalytic water splitting. ChemSusChem 2020, 13, 2038–2042. [Google Scholar] [CrossRef]
- Samori, C.; Mazzei, L.; Ciurli, S.; Cravotto, G.; Grillo, G.; Guidi, E.; Pasteris, A.; Tabasso, S.; Galletti, P. Urease inhibitory potential and soil ecotoxicity of novel “polyphenols-deep eutectic solvents” formulations. ACS Sustain. Chem. Eng. 2019, 7, 15558–15567. [Google Scholar] [CrossRef]
- de Morais, P.; Goncalves, F.; Coutinho, J.A.P.; Ventura, S.P.M. Ecotoxicity of cholinium-based deep eutectic solvents. ACS Sustain. Chem. Eng. 2015, 3, 3398–3404. [Google Scholar] [CrossRef]
- Ahmadi, R.; Hemmateenejad, B.; Safavi, A.; Shojaeifard, Z.; Mohabbati, M.; Firuzi, O. Assessment of cytotoxicity of choline chloride-based natural deep eutectic solvents against human HEK–293 cells: A QSAR analysis. Chemosphere 2018, 209, 831–838. [Google Scholar] [CrossRef]
- Macario, I.P.E.; Oliveira, H.; Menezes, A.C.; Ventura, S.P.M.; Pereira, J.L.; Goncalves, A.M.M.; Coutinho, J.A.P.; Goncalves, F.J.M. Cytotoxicity profifiling of deep eutectic solvents to human skin cells. Sci. Rep. 2019, 9, 3932. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T.C. Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem. Eng. 2021, 2, 174–186. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Jung, W.; Zhou, W.; Shao, Z. Electrochemical water splitting: Bridging the gaps between fundamental research and industrial applications. Energy Environ. Mater 2022, e12441. [Google Scholar] [CrossRef]
| Catalyst | Applied DES | Preparation Method | Catalytic Performance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HER | OER | Water Splitting | |||||||||
| Electrolyte | η (mV)@ Current Density (mA cm−2) | Tafel Slope (mV dec−1) | Electrolyte | η (mV)@ Current Density (mA cm−2) | Tafe l Slope (mV dec−1) | Electrolyte | Potential (V)@ Current Density (mA cm−2) | Ref. | |||
| La0.5Sr0.5Co0.8Fe0.2O3 | Glucose/Urea | Calcining method | –– | –– | –– | 1.0 M KOH | 304@10 | 62.9 | –– | –– | [115] |
| Co@NPC | ChCl/Urea/Gluconic acid ternary | Calcining method | 1 M KOH | 215@10 | 70 | 1 M KOH | 430@10 | 87 | 1 M KOH | 1.74@10 | [116] |
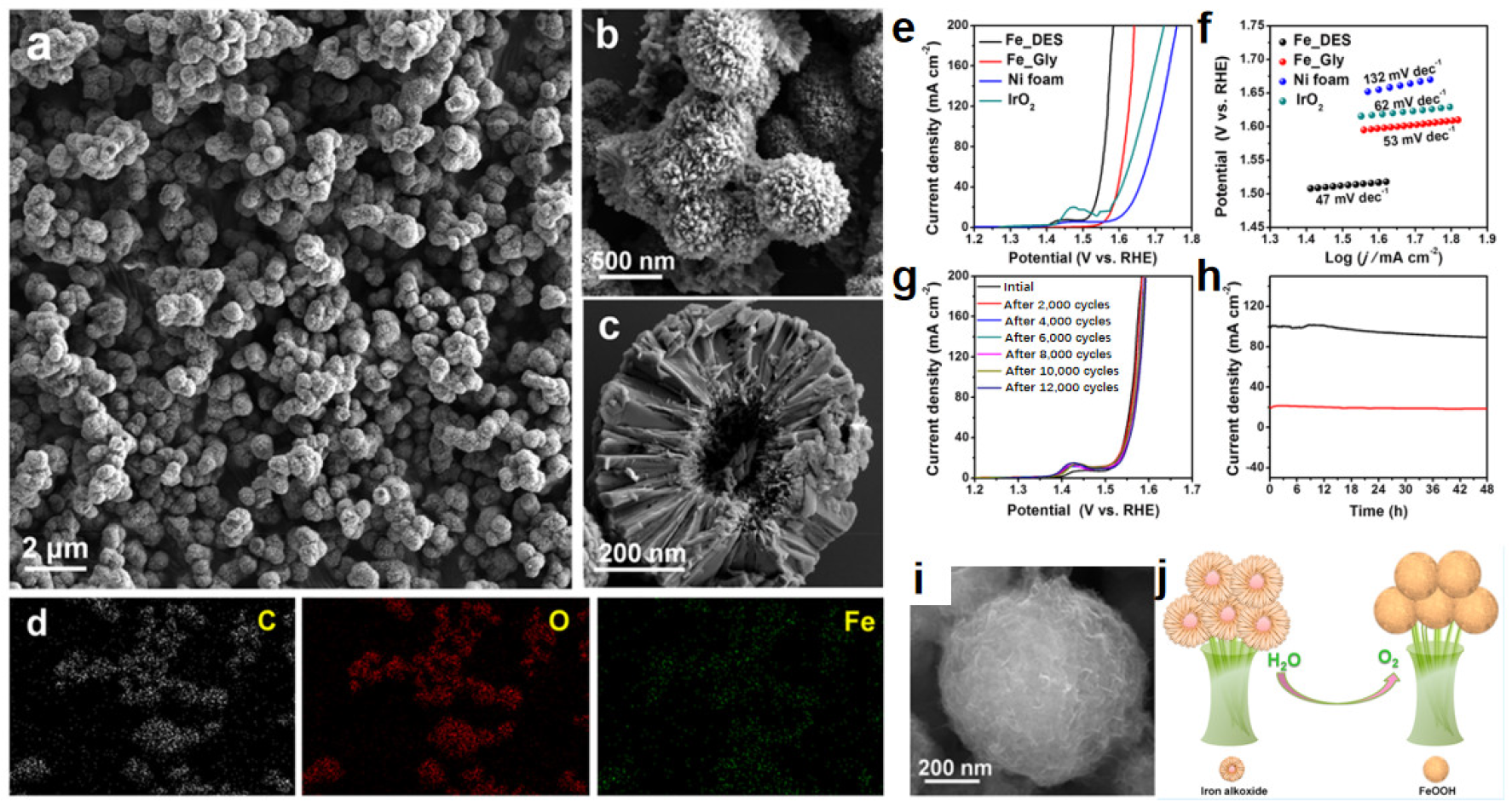
| Iron alkoxide | ChCl/Glycerol | Ionothermal method | –– | –– | –– | 1 M KOH | 280@10 | 47 | –– | –– | [117] |
| NiCo2S4 | PEG 200/Thiourea | Ionothermal method | –– | –– | –– | 1 M KOH | 337@10 | 64 | –– | –– | [123] |
| NiS/Graphene | NiCl2·6H2O/PEG 200 | Calcining method | 1 M KOH | 70@10 | 50.1 | 1 M KOH | 300@10 | 55.8 | 1 M KOH | 1.54@10 | [126] |
| NiS2/Graphene | NiCl2·6H2O/Malonic acid | Calcining method | 1 M KOH | 57@10 | 47 | 1 M KOH | 294@10 | 54 | 1 M KOH | 1.52@10 | [127] |
| Ni2P/Graphene | NiCl2·6H2O/Malonic acid | Calcining method | 1 M KOH | 103@10 | 56.5 | 1 M KOH | 275@20 | 56.2 | 1 M KOH | 1.51@10 | [128] |
| N–C/NiS2 | NiCl2·6H2O/Urea | Calcining method | 1 M KOH | 78@10 | 63.4 | 1 M KOH | 264@10 | 51.3 | 1 M KOH | 1.53@10 | [129] |
| NiFe–LDH | FeCl3·6H2O/Urea | Dipping–redox method | 1 M KOH | 160@10 | 42 | 1 M KOH | –– | –– | 1 M KOH | 1.61@10 | [130] |
| NiFe–LDH/N–C | NiCl2·6H2O/FeCl3· 6H2O/Urea/Water | Ionothermal method | –– | –– | –– | 0.1 M KOH | 363@500 | 49.8 | –– | –– | [132] |
| [Co(NH3)4CO3]Cl | CoCl2·6H2O/Urea | Calcining method | –– | –– | –– | 1 M KOH | 291@10 | 65 | –– | –– | [136] |
| N,S,O–C/Co9S8 | CoCl2·6H2O/Thiourea | Calcining method | 1.0 M KOH | 53@10 | 31 | –– | –– | –– | –– | –– | [137] |
| N,S,O–C/Co9S8 | CoCl2·6H2O/Thiourea | Calcining method | 1.0 M PBS | 103@10 | 91.2 | –– | –– | –– | –– | –– | [137] |
| N,S,O–C/Co9S8 | CoCl2·6H2O/Thiourea | Calcining method | 0.5 M H2SO4 | 68@10 | 45.3 | –– | –– | –– | –– | –– | [137] |
| FeCoNi–NS | FeCl3·6H2O/CoCl26H2O/NiCl2·6H2O/L–cysteine | Calcining method | –– | –– | –– | 1 M KOH | 251@10 | 58 | –– | –– | [141] |
| High–entropy metal phosphides | [P4444]Cl/Ethylene glycol/Five equimolar hydrated metal chlorides | Eutectic solvent method | 1 M KOH | 136@10 | 85.5 | 1 M KOH | 320@10 | 60.8 | 1 M KOH | 1.78@100 | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Fu, Y.; Gao, W.; Bai, T.; Cao, T.; Jin, J.; Xin, B. Deep Eutectic Solvent-Mediated Electrocatalysts for Water Splitting. Molecules 2022, 27, 8098. https://doi.org/10.3390/molecules27228098
Zhang C, Fu Y, Gao W, Bai T, Cao T, Jin J, Xin B. Deep Eutectic Solvent-Mediated Electrocatalysts for Water Splitting. Molecules. 2022; 27(22):8098. https://doi.org/10.3390/molecules27228098
Chicago/Turabian StyleZhang, Chenyun, Yongqi Fu, Wei Gao, Te Bai, Tianyi Cao, Jianjiao Jin, and Bingwei Xin. 2022. "Deep Eutectic Solvent-Mediated Electrocatalysts for Water Splitting" Molecules 27, no. 22: 8098. https://doi.org/10.3390/molecules27228098
APA StyleZhang, C., Fu, Y., Gao, W., Bai, T., Cao, T., Jin, J., & Xin, B. (2022). Deep Eutectic Solvent-Mediated Electrocatalysts for Water Splitting. Molecules, 27(22), 8098. https://doi.org/10.3390/molecules27228098