Chemical Fractionations of Lead and Zinc in the Contaminated Soil Amended with the Blended Biochar/Apatite
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Amendment Preparation
2.2. Soil Incubation and Experiment Design
2.3. Analysis Method of Soil and Amendment
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of the Investigated Soil and Amendments
3.2. Analysis of the Amendments’ Characteristics
3.2.1. XRD Analysis
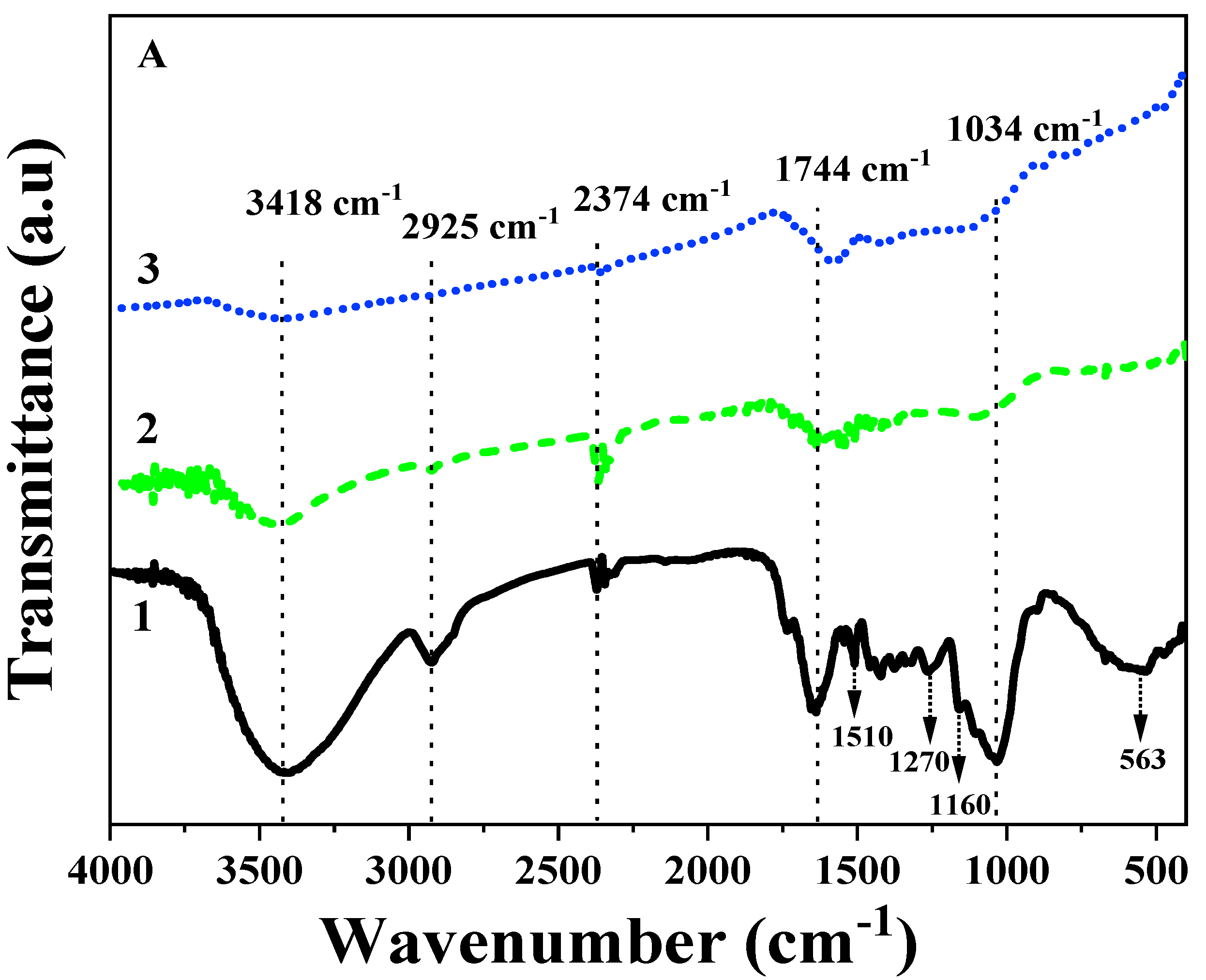
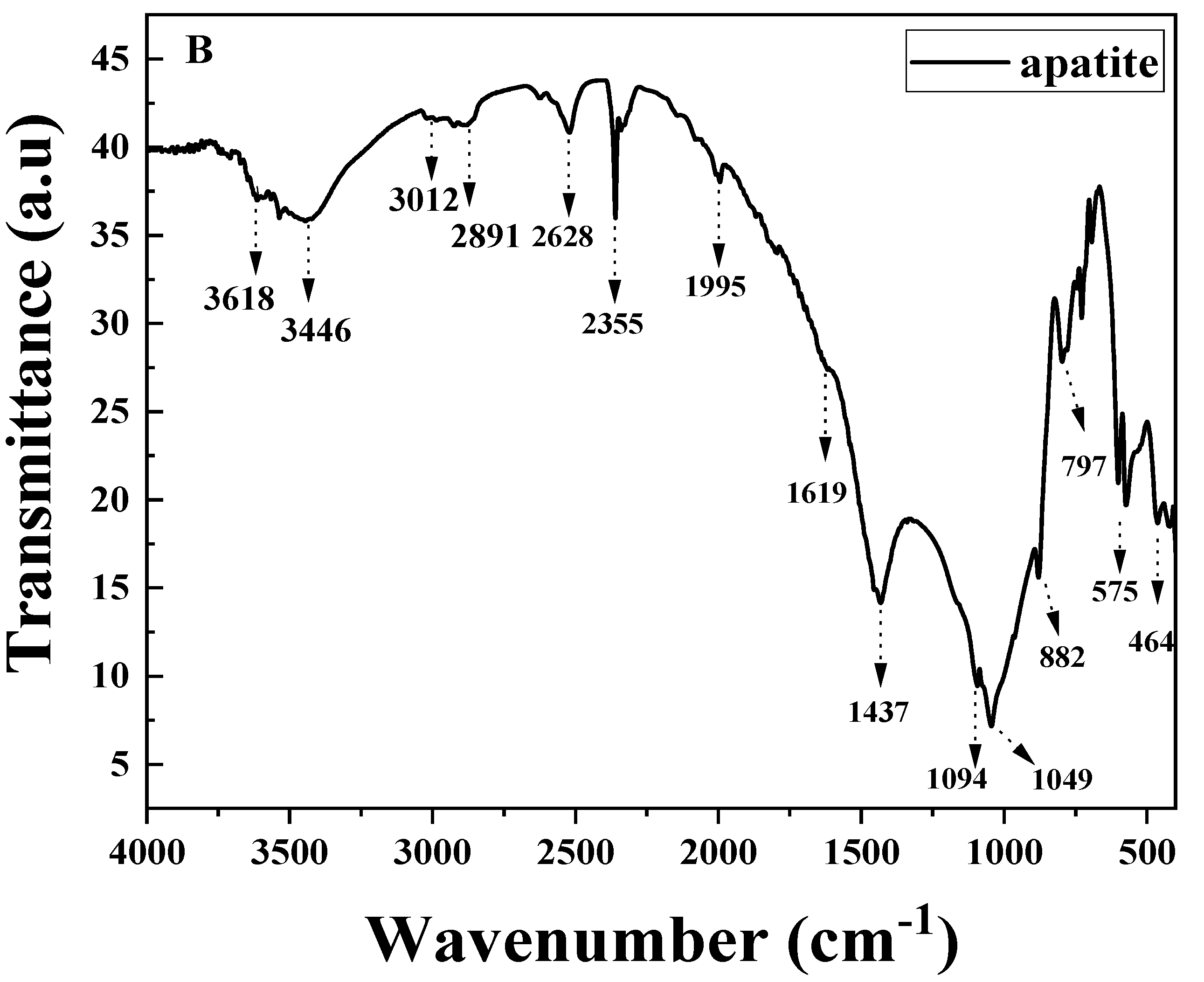
3.2.2. FT-IR Analysis of Amendments
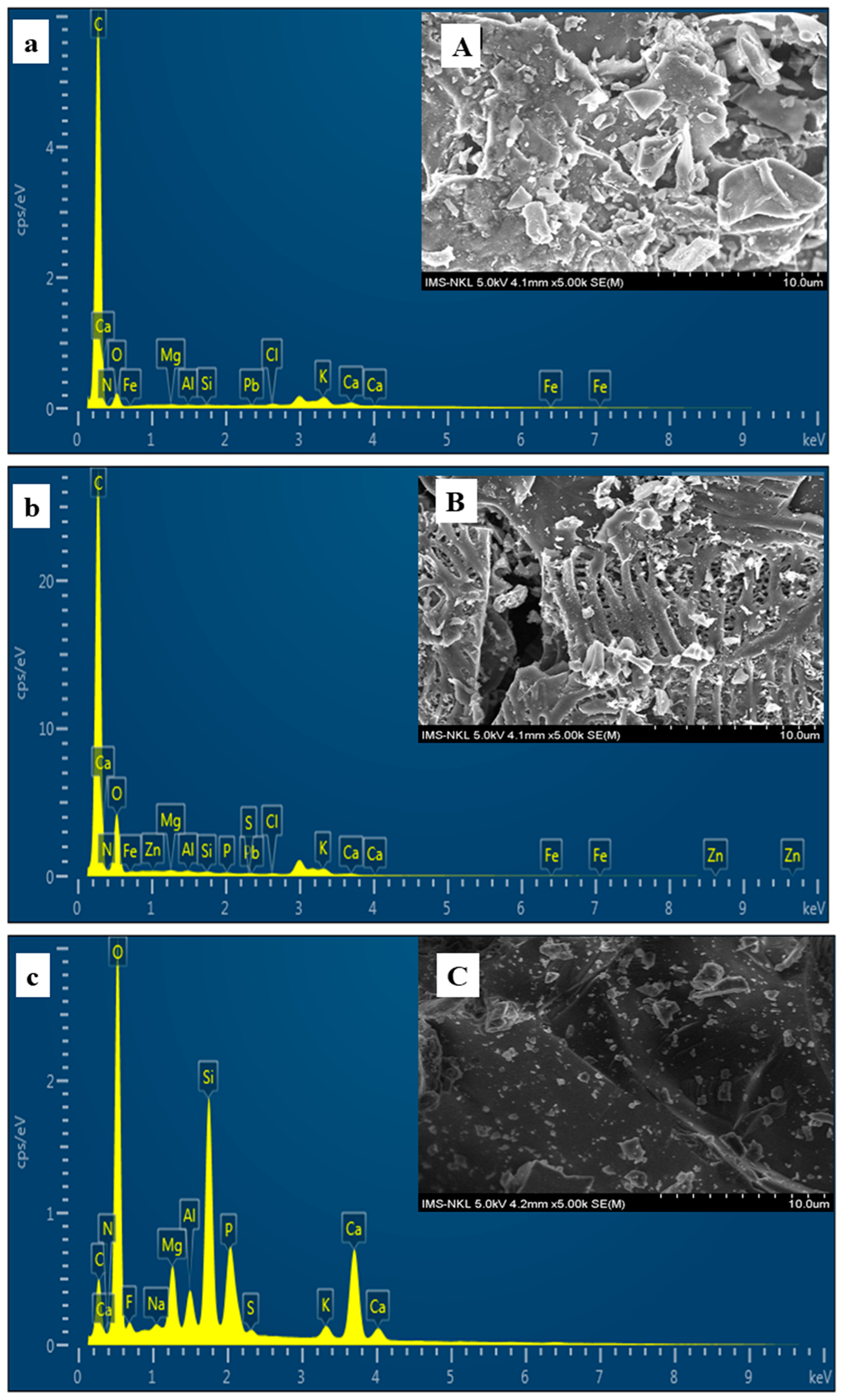
3.2.3. SEM and EDS Analysis of Amendments
3.3. Effects of Biochar and Apatite Amendment on OC, pH, and EC after a 30-Day Incubation
3.3.1. pH
3.3.2. Organic Carbon (OC)
3.3.3. Electrical Conductivity (EC)
3.4. Effects of Amendments on the Pb and Zn’s Chemical Fractionations in Soil Samples
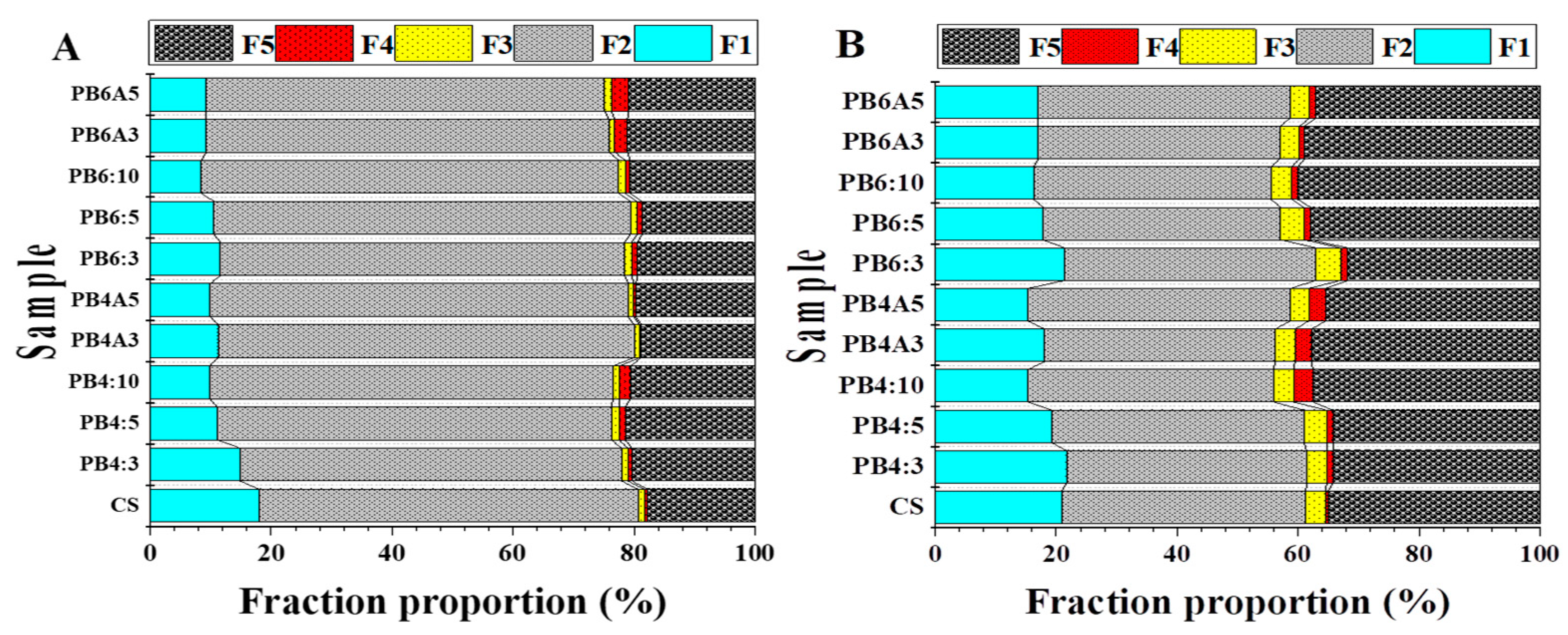
3.4.1. Chemical Fraction of Pb
3.4.2. Chemical Fraction of Zn
3.5. Correlation of the Exchangeable Fraction of Heavy Metals with pH, OC, and EC
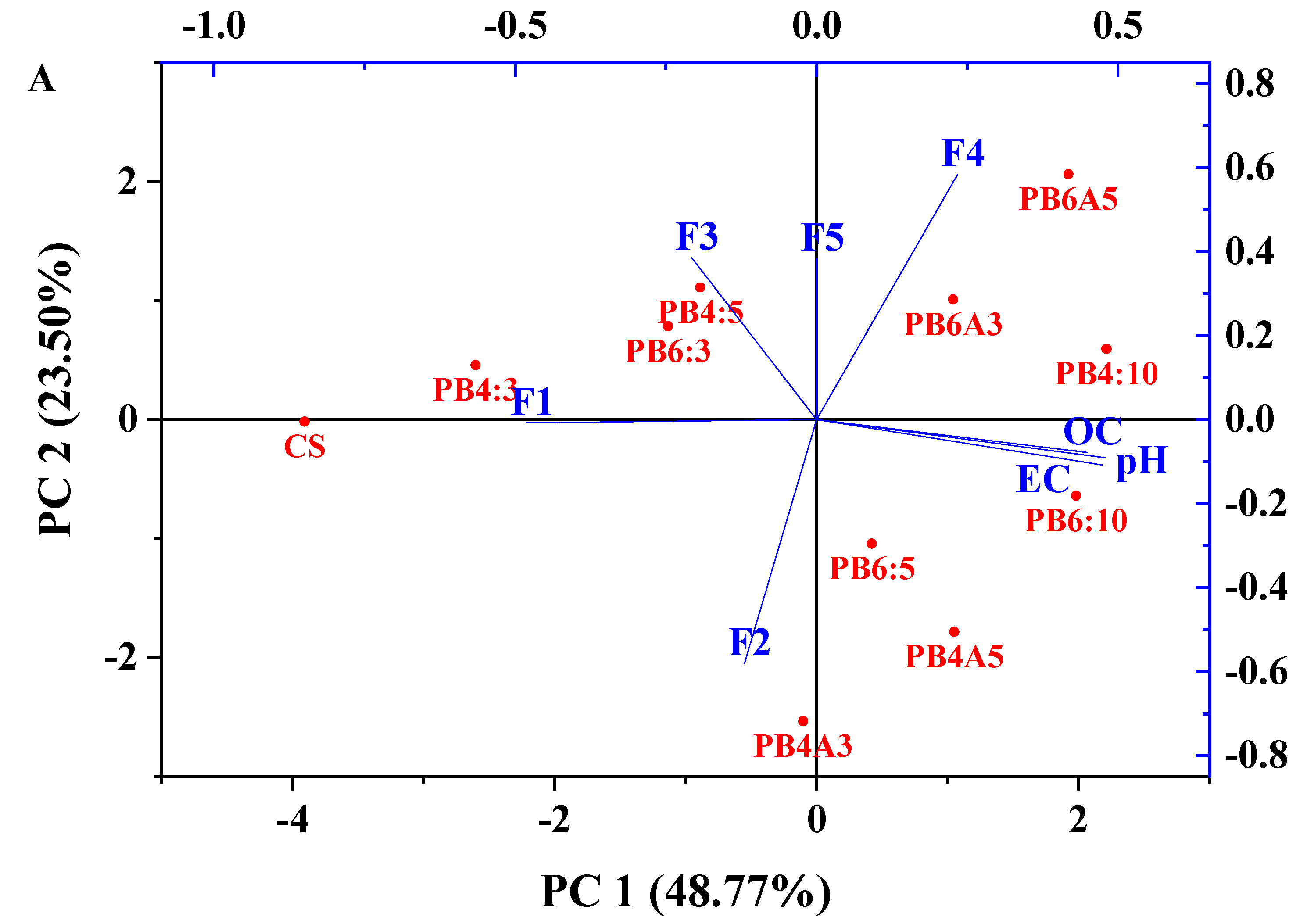
3.6. PCA Analysis of the Chemical Fractions of Zinc and Lead with pH, OC, and EC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviation
References
- Kheir, A.M.S.; Ali, E.F.; Ahmed, M.; Eissa, M.A.; Majrashi, A.; Ali, O.A.M. Biochar blended humate and vermicompost enhanced immobilization of heavy metals, improved wheat productivity, and minimized human health risks in different contaminated environments. J. Environ. Chem. Eng. 2021, 9, 105700. [Google Scholar] [CrossRef]
- Awad, M.; Liu, Z.; Skalicky, M.; Dessoky, E.S.; Brestic, M.; Mbarki, S.; Rastogi, A.; El Sabagh, A. Fractionation of heavy metals in multi-contaminated soil treated with biochar using the sequential extraction procedure. Biomolecules 2021, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Hans, N.; Kumar, S.; Rani, R. A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Elmayel, I.; Esbrí, J.M.; Efrén, G.O.; García-Noguero, E.M.; Elouear, Z.; Jalel, B.; Farieri, A.; Roqueñí, N.; Cienfuegos, P.; Higueras, P. Evolution of the speciation and mobility of Pb, Zn and Cd in relation to transport processes in a mining environment. Int. J. Environ. Res. Public Health. 2020, 17, 4912. [Google Scholar] [CrossRef]
- Ahn, Y.; Yun, H.S.; Pandi, K.; Park, S.; Ji, M.; Choi, J. Heavy metal speciation with prediction model for heavy metal mobility and risk assessment in mine-affected soils. Environ. Sci. Pollut. Res. 2020, 27, 3213–3223. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Tot. Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Hu, X. Removal of heavy metals from soil with biochar composite: A critical review of the mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Han, C.; Xie, W.; Chen, C.; Cheng, T. Health Risk Assessment of Heavy Metals in Soils before Rice Sowing and at Harvesting in Southern Jiangsu Province, China. J. Chem. 2020, 2020, 7391934. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Mazarji, M.; Bayero, M.T.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Tereshchenko, A.; Timofeeva, A.; Bauer, T.; Burachevskaya, M.; Kızılkaya, R.; et al. Realizing united nations sustainable development goals for greener remediation of heavy metals-contaminated soils by biochar: Emerging trends and future directions. Sustainability 2021, 13, 13825. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Ju, M.; Liu, L. Preparation and modification of biochar materials and their application in soil remediation. Appl. Sci. 2019, 9, 1365. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Wang, J.; Zhang, C. Stabilization of heavy metal-contaminated soils by biochar: Challenges and recommendations. Sci. Total Environ. 2020, 729, 139060. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Wu, J.; Wang, T.; Zhang, Y.; Pan, W.P. The distribution of Pb(II)/Cd(II) adsorption mechanisms on biochars from aqueous solution: Considering the increased oxygen functional groups by HCl treatment. Bioresour Technol. 2019, 291, 121859. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619, 815–826. [Google Scholar] [CrossRef]
- Wu, P.; Ata-Ul-Karim, S.T.; Singh, B.P.; Wang, H.; Wu, T.; Liu, C.; Fang, G.; Zhou, D.; Wang, Y.; Chen, W. A scientometric review of biochar research in the past 20 years (1998–2018). Biochar 2019, 1, 23–43. [Google Scholar] [CrossRef]
- Wu, P.; Wang, Z.; Wang, H.; Bolan, N.S.; Wang, Y.; Chen, W. Visualizing the emerging trends of biochar research and applications in 2019: A scientometric analysis and review. Biochar 2020, 2, 135–150. [Google Scholar] [CrossRef]
- Puga, A.P.; Melo, L.C.A.; de Abreu, C.A.; Coscione, A.R.; Paz-Ferreiro, J. Leaching and fractionation of heavy metals in mining soils amended with biochar. Soil Tillage Res. 2016, 164, 25–33. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.S.; Tang, C.S.; Gu, K.; Shi, B. Remediation of heavy-metal-contaminated soils by biochar: A review. Environ. Geotech. 2019, 9, 135–148. [Google Scholar] [CrossRef]
- Wang, J.; Shi, L.; Zhai, L.; Zhang, H.; Wang, S.; Zou, J. Analysis of the long-term effectiveness of biochar immobilization remediation on heavy metal contaminated soil and the potential environmental factors weakening the remediation effect: A review. Ecotoxicol. Environ. Saf. 2021, 207, 111261. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.-H. Benefits and limitations of biochar amendment in agricultural soils: A review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wu, J.; Luo, L.; Yang, G.; Zhang, X.; Peng, H.; Xu, X.; Wang, L. The factors affecting biochar application in restoring heavy metal-polluted soil and its potential applications. Chem. Ecol. 2018, 34, 177–197. [Google Scholar] [CrossRef]
- Zheng, R.L.; Cai, C.; Liang, J.H.; Huang, Q.; Chen, Z.; Huang, Y.Z.; Sun, G.; Arp, H.P.H. The effects of biochars from rice residue on the formation of iron plaque and the accumulation of Cd, Zn, Pb, As in rice (Oryza sativa L.) seedlings. Chemosphere 2012, 89, 856–862. [Google Scholar] [CrossRef]
- Sui, F.; Wang, J.; Zuo, J.; Joseph, S.; Munroe, P.; Drosos, M.; Li, L.; Pan, G. Effect of amendment of biochar supplemented with Si on Cd mobility and rice uptake over three rice growing seasons in an acidic Cd-tainted paddy from central South China. Sci. Total Environ. 2020, 709, 136101. [Google Scholar] [CrossRef]
- Sui, F.; Zuo, J.; Chen, D.; Li, L.; Pan, G.; Crowley, D.E. Biochar effects on uptake of cadmium and lead by wheat in relation to annual precipitation: A 3-year field study. Environ. Sci. Pollut. Res. 2018, 25, 3368–3377. [Google Scholar] [CrossRef]
- Cui, L.; Pan, G.; Li, L.; Yan, J.; Zhang, A.; Bian, R.; Chang, A. The reduction of wheat cd uptake in contaminated soil via biochar amendment: A two-year field experiment. Bioresources 2012, 7, 5666–5676. [Google Scholar] [CrossRef]
- Bian, R.; Joseph, S.; Cui, L.; Pan, G.; Li, L.; Liu, X.; Zhang, A.; Rutlidge, H.; Wong, S.; Chia, C.; et al. A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. J. Hazard. Mater. 2014, 272, 121–138. [Google Scholar] [CrossRef]
- Ali, A.; Guo, D.; Zhang, Y.; Sun, X.; Jiang, S.; Guo, Z.; Huang, H.; Liang, W.; Li, R.; Zhang, Z. Using bamboo biochar with compost for the stabilization and phytotoxicity reduction of heavy metals in mine-contaminated soils of China. Sci. Rep. 2017, 7, 2690. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Hou, S.; Li, Y.; Khan, M.A.; Luo, W.; Chen, Z.; Li, Y.; Wu, X.; Ye, Z.; Liu, D. Bioavailability and Speciation of Heavy Metals in Polluted Soil as Alleviated by Different Types of Biochars. Bull. Environ. Contam. Toxicol. 2020, 104, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Lin, H.; He, Y.; Zhang, Y. The influence of corncob-based biochar on remediation of arsenic and cadmium in yellow soil and cinnamon soil. Sci. Total Environ. 2020, 717, 137014. [Google Scholar] [CrossRef] [PubMed]
- Deenik, J.L.; Cooney, M.J. The potential benefits and limitations of corn cob and sewage sludge biochars in an infertile Oxisol. Sustainability 2016, 8, 131. [Google Scholar] [CrossRef]
- Haddad, S.A.; Lemanowicz, J. Benefits of corn-cob biochar to the microbial and enzymatic activity of soybean plants grown in soils contaminated with heavy metals. Energies 2021, 14, 5763. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, G.; Mao, R. Effects of particle sizes of rock phosphate on immobilizing heavy metals in lead zinc mine soils. J. Soil Sci. Plant Nutr. 2014, 14, 258–266. [Google Scholar] [CrossRef]
- Cao, X. Immobilization of heavy metals in contaminated soils amended by phosphate-, carbonate-, and silicate-based materials: From lab to field. In Twenty Years of Research and Development on Soil Pollution and Remediation in China; Springer: Singapore, 2018; pp. 535–543. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Tang, L.; Su, M.; Tian, D.; Zhang, L.; Li, Z.; Hu, S. Enhanced Pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar] [CrossRef]
- Oustriere, N.; Marchand, L.; Rosette, G.; Friesl-Hanl, W.; Mench, M. Wood-derived-biochar combined with compost or iron grit for in situ stabilization of Cd, Pb, and Zn in a contaminated soil. Environ. Sci. Pollut. Res. 2017, 24, 7468–7481. [Google Scholar] [CrossRef]
- Dang, V.M.; Joseph, S.; Van, H.T.; Mai, T.L.A.; Duong, T.M.H.; Weldon, S.; Munroe, P.; Mitchell, D.; Taherymoosavi, S. Immobilization of heavy metals in contaminated soil after mining activity by using biochar and other industrial by-products: The significant role of minerals on the biochar surfaces. Environ. Technol. 2019, 40, 3200–3215. [Google Scholar] [CrossRef]
- Chu, T.T.H. Survey on heavy metals contaminated soils in Thai Nguyen and Hung Yen provinces in Northern Vietnam. J. Vietnam. Environ. 2011, 1, 34–39. [Google Scholar] [CrossRef]
- Dang, V.M.; Van, H.T.; Duong, H.T.M.; Nguyen, D.H.; Chao, H.P.; Nguyen, L.H.; Lin, C.C. Evaluation of fly ash, apatite and rice straw derived-biochar in varying combinations for in situ remediation of soils contaminated with multiple heavy metals. Soil Sci. Plant Nutr. 2020, 66, 379–388. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sun, J.K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Han, X.; Chu, L.; Liu, S.; Chen, T.; Ding, C.; Yan, J.; Cui, L.; Quan, G. Removal of methylene blue from aqueous solution using porous biochar obtained by KOH activation of peanut shell biochar. Bioresources 2015, 10, 2836–2849. [Google Scholar] [CrossRef]
- Jung, K.W.; Hwang, M.J.; Ahn, K.H.; Ok, Y.S. Kinetic study on phosphate removal from aqueous solution by biochar derived from peanut shell as renewable adsorptive media. Int. J. Environ. Sci. Technol. 2015, 12, 3363–3372. [Google Scholar] [CrossRef]
- Chao, X.; Qian, X.; Han-hua, Z.; Shuai, W.; Qi-hong, Z.; Dao-you, H.; Yang-zhu, Z. Effect of biochar from peanut shell on speciation and availability of lead and zinc in an acidic paddy soi. Ecotoxicol. Environ. Saf. 2018, 164, 554–561. [Google Scholar] [CrossRef]
- An, Q.; Jiang, Y.Q.; Nan, H.Y.; Yu, Y.; Jiang, J.N. Unraveling sorption of nickel from aqueous solution by KMnO4 and KOH-modified peanut shell biochar: Implicit mechanism. Chemosphere 2019, 214, 846–854. [Google Scholar] [CrossRef]
- Yang, J.; Ji, G.; Gao, Y.; Fu, W.; Irfan, M.; Mu, L.; Zhang, Y.; Li, A. High-yield and high-performance porous biochar produced from pyrolysis of peanut shell with low-dose ammonium polyphosphate for chloramphenicol adsorption. J. Clean. Prod. 2020, 264, 121516. [Google Scholar] [CrossRef]
- Awad, M.; El-Sayed, M.M.; Li, X.; Liu, Z.; Mustafa, S.K.; Ditta, A.; Hessini, K. Diminishing heavy metal hazards of contaminated soil via biochar supplementation. Sustainability 2021, 13, 12742. [Google Scholar] [CrossRef]
- Miller, W.P.; Miller, D.M. A micro-pipette method for soil mechanical analysis. Commun. Soil. Sci. Plant Anal. 1987, 18, 1–15. [Google Scholar] [CrossRef]
- Gelman, F.; Binstock, R.; Halicz, L. Application of the Walkley-Black titration for the organic carbon quantification in organic rich sedimentary rocks. Fuel 2012, 96, 608–610. [Google Scholar] [CrossRef]
- Li, J.; Xia, C.; Cheng, R.; Lan, J.; Chen, F.; Li, X. Passivation of multiple heavy metals in lead–zinc tailings facilitated by straw biochar-loaded N-doped carbon aerogel nanoparticles: Mechanisms and microbial community evolution. Sci. Total Environ. 2022, 803, 149866. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.A.M.; Irshad, S.; Yousaf, B.; Ali, M.U.; Dan, C.; Abbas, Q. Interactive assessment of lignite and bamboo-biochar for geochemical speciation, modulation and uptake of Cu and other heavy metals in the copper mine tailing. Sci. Total Environ. 2021, 779, 146536. [Google Scholar] [CrossRef] [PubMed]
- Vuong, X.T.; Vu, L.D.; Duong, A.T.T.; Duong, H.T.; Hoang, T.H.T.; Luu, M.N.T.; Nguyen, T.N.; Nguyen, V.D.; Nguyen, T.T.T.; Van, T.H.; et al. Speciation and environmental risk assessment of heavy metals in soil from a lead/zinc mining site in Vietnam. Int. J. Environ. Sci. Technol. 2022, 1–16. [Google Scholar] [CrossRef]
- Chaoua, S.; Boussaa, S.; Gharmali, A.E.l.; Boumezzough, A. Impact of irrigation with wastewater on accumulation of heavy metals in soil and crops in the region of Marrakech in Morocco. J. Saudi Soc. Agric. Sci. 2019, 18, 429–436. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M. Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Anal. Appl. Pyrolysis 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Zhao, S.X.; Ta, N.; Wang, X.D. Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Picard, M.; Thakur, S.; Misra, M.; Mielewski, D.F.; Mohanty, A.K. Biocarbon from peanut hulls and their green composites with biobased poly(trimethylene terephthalate) (PTT). Sci. Rep. 2020, 10, 3310. [Google Scholar] [CrossRef]
- Major, I.; Pin, J.M.; Behazin, E.; Rodriguez-Uribe, A.; Misra, M.; Mohanty, A. Graphitization of Miscanthus grass biocarbon enhanced by in situ generated FeCo nanoparticles. Green Chem. 2018, 20, 2269–2278. [Google Scholar] [CrossRef]
- Liu, M.; Bai, J.; Kong, L.; Bai, Z.; He, C.; Li, W. The correlation between coal char structure and reactivity at rapid heating condition in TGA and heating stage microscope. Fuel 2020, 260, 116318. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Hoekman, S.K.; Liu, T.; Gai, C.; Peng, N. Homogeneously Dispersed Zerovalent Iron Nanoparticles Supported on Hydrochar-Derived Porous Carbon: Simple, in Situ Synthesis and Use for Dechlorination of PCBs. ACS. Sustain. Chem. Eng. 2016, 4, 3261–3277. [Google Scholar] [CrossRef]
- Tun, Z.M.; Myat, Z.M.; Win, T.T.; Maung, Y.M. Characterization of activated carbons from coconut and peanut shells biomass. J. Myanmar. Acad. Arts Sci. 2020, XVIII, 65–74. [Google Scholar]
- Nguyen, P.T.; Nguyen, X.T.; Nguyen TVan Nguyen, T.T.; Vu, T.Q.; Nguyen, H.T. Treatment of Cd2+ and Cu2+ Ions Using Modified Apatite Ore. J. Chem. 2020, 2020, 6527197. [Google Scholar] [CrossRef]
- Kali, A.; Amar, A.; Loulidi, I.; Jabri, M.; Hadey, C.; Lgaz, H. Characterization and adsorption capacity of four low-cost adsorbents based on coconut, almond, walnut, and peanut shells for copper removal. Biomass Convers. Biorefin. 2022, 3, 1–12. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, P.; Yang, X.; Li, H.; Liu, Y.; Pan, B. An integrated study on the pyrolysis mecanism of peanut shell based on the kinetic analysis and solid/gas characterization. Bioresour. Technol. 2021, 329, 124860. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, A.K.; Srinivasan, P.; Smernik, R.J.; Manley-Harris, M.; Antal, M.J.; Downie, A. Retention capacity of biochar-amended New Zealand dairy farm soil for an estrogenic steroid hormone and its primary metabolite. Soil. Res. 2010, 48, 648–658. [Google Scholar] [CrossRef]
- Elnour, A.Y.; Alghyamah, A.A.; Shaikh, H.M.; Poulose, A.M.; Al-Zahrani, S.M.; Anis, A.; Al-Wabel, M.I. Effect of pyrolysis temperature on biochar microstructural evolution, physicochemical characteristics, and its influence on biochar/polypropylene composites. Appl. Sci. 2019, 9, 1149. [Google Scholar] [CrossRef]
- Tatzber, M.; Stemmer, M.; Spiegel, H.; Katzlberger, C.; Haberhauer, G.; Mentler, A. FTIR-spectroscopic characterization of humic acids and humin fractions obtained by advanced NaOH, Na4P2O7, and Na 2CO3 extraction procedures. J. Plant Nutri. Soil Sci. 2007, 170, 522–529. [Google Scholar] [CrossRef]
- Behazin, E.; Ogunsona, E.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M.; Anyia, A.O. Mechanical, chemical, and physical properties of wood and perennial grass biochars for possible composite application. BioResources 2016, 11, 1334–1348. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Jiang, Z.; Zhao, J.; Wang, Z.; Pan, B.; Xing, B. Formation and Physicochemical Characteristics of Nano Biochar: Insight into Chemical and Colloidal Stability. Environ. Sci. Technol. 2018, 52, 10369–10379. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Jiang, Z.; Wang, Z. Effects of biochar input on the properties of soil nanoparticles and dispersion/sedimentation of natural mineral nanoparticles in aqueous phase. Sci. Total Environ. 2018, 634, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tang, J.; Gao, K.; He, R.; Zhao, H.; Werner, D. Characterization of KOH modified biochars from different pyrolysis temperatures and enhanced adsorption of antibiotics. RSC Adv. 2017, 7, 14640–14648. [Google Scholar] [CrossRef]
- Nikčević, I.; Jokanović, V.; Mitrić, M.; Nedić, Z.; Makovec, D.; Uskoković, D. Mechanochemical synthesis of nanostructured fluorapatite/ fluorhydroxyapatite and carbonated fluorapatite/fluorhydroxyapatite. J. Solid State Chem. 2004, 177, 2565–2574. [Google Scholar] [CrossRef]
- Nunes, A.P.L.; Peres, A.E.C.; Chaves, A.P.; Ferreira, W.R. Effect of alkyl chain length of amines on fluorapatite and aluminium phosphates floatabilities. J. Mater. Res. Technol. 2019, 8, 3623–3634. [Google Scholar] [CrossRef]
- Wang, M.; Qian, R.; Bao, M.; Gu, C.; Zhu, P. Raman, FT-IR and XRD study of bovine bone mineral and carbonated apatites with different carbonate levels. Mater. Lett. 2018, 210, 203–216. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Y.; Ma, Q.; Zhou, H.; Luo, X.; Liu, X.; Wang, S. Evolution of the chemical composition, functional group, pore structure and crystallographic structure of bio-char from palm kernel shell pyrolysis under different temperatures. J. Anal. Appl. Pyrolysis 2017, 127, 350–359. [Google Scholar] [CrossRef]
- Yang, H.; Huan, B.; Chen, Y.; Gao, Y.; Li, J.; Chen, H. Biomass-Based Pyrolytic Polygeneration System for Bamboo Industry Waste: Evolution of the Char Structure and the Pyrolysis Mechanism. Energy Fuels 2016, 30, 6430–6439. [Google Scholar] [CrossRef]
- Zhang, R.H.; Li, Z.G.; Liu, X.D.; Wang, B.C.; Zhou, G.L.; Huang, X.X.; Lin, C.; Wang, A.; Brooks, M. Immobilization and bioavailability of heavy metals in greenhouse soils amended with rice straw-derived biochar. Ecol. Eng. 2017, 98, 183–198. [Google Scholar] [CrossRef]
- Dai, S.; Li, H.; Yang, Z.; Dai, M.; Dong, X.; Ge, X.; Sun, M.; Shi, L. Effects of biochar amendments on speciation and bioavailability of heavy metals in coal-mine-contaminated soil. Hum. Ecol. Risk Assess. 2018, 24, 1887–1900. [Google Scholar] [CrossRef]
- Elouear, Z.; Bouzid, J.; Boujelben, N.; Feki, M.; Jamoussi, F.; Montiel, A. Heavy metal removal from aqueous solutions by activated phosphate rock. J. Hazard. Mater. 2008, 156, 412–420. [Google Scholar] [CrossRef]
- Nzihou, A.; Sharrock, P. Role of phosphate in the remediation and reuse of heavy metal polluted wastes and sites. Waste Biomass Valorization 2010, 1, 163–174. [Google Scholar] [CrossRef]
- Chen, X.; Wright, J.V.; Conca, J.L.; Peurrung, L.M. Evaluation of heavy metal remediation using mineral apatite. Water Air Soil Pollut. 1997, 98, 57–78. [Google Scholar] [CrossRef]
- Shao, Y.; Yan, T.; Wang, K.; Huang, S.; Yuan, W.; Qin, F.G.F. Soil heavy metal lead pollution and its stabilization remediation technology. Energy Rep. 2020, 6, 122–127. [Google Scholar] [CrossRef]
- Chen, X.; Wright, J.V.; Conca, J.L.; Peurrung, L.M. Effects of pH on heavy metal sorption on mineral apatite. Environ. Sci. Technol. 1997, 31, 624–631. [Google Scholar] [CrossRef]




| Sample | Sample Code | Biochar Weight (g) | Apatite Weight (g) | Soil Weight (g) | Ratio (%) |
|---|---|---|---|---|---|
| CS | CS | 0 | 0 | 100 | 0 |
| CS + 3% PSB400 | BC4:3 | 3 | 0 | 97 | 3 |
| CS + 5% PSB400 | BC4:5 | 5 | 0 | 95 | 5 |
| CS +10% PSB400 | BC4:10 | 10 | 0 | 90 | 10 |
| CS + 3% PSB400 + 3% APA | B4A3 | 3 | 3 | 94 | 3:3 |
| CS + 5% PSB400 + 5% APA | B4A5 | 5 | 5 | 90 | 5:5 |
| CS + 3% PSB600 | BC6:3 | 3 | 0 | 97 | 3 |
| CS + 5% PSB600 | BC6:5 | 5 | 0 | 95 | 5 |
| CS +10% PSB600 | BC6:10 | 10 | 0 | 90 | 10 |
| CS + 3% PSB600 + 3% APA | B6A3 | 3 | 3 | 94 | 3:3 |
| CS + 5% PSB600 + 5% APA | B6A5 | 5 | 5 | 90 | 5:5 |
| Properties | Unit | Soil | PSB400 | PSB600 | APA |
|---|---|---|---|---|---|
| Sand | % | 69.78 ± 0.72 | na | na | na |
| Silt | % | 5.48 ± 0.32 | na | na | na |
| Clay | % | 24.74 ± 0.43 | na | na | na |
| pH | 6.47 ± 0.02 | 10.90 ± 0.01 | 11.13 ± 0.02 | 9.36 ± 0.02 | |
| OC | % | 2.49 ± 0.12 | 80.79 ± 8.34 | 73.34 ± 0.21 | 3.34 ± 0.21 |
| EC | µS cm−1 | 118.50 ± 0.50 | >1999 | 1104.51 ± 1.50 | 1104.53 ± 1.51 |
| Pb | mg kg−1 | 2447.80 ± 98.60 | <LOD | <LOD | <LOD |
| Zn | mg kg−1 | 2034.30 ± 35.40 | 0.70 ± 0.03 | 9.43 ± 0.03 | 9.43 ± 0.03 |
| Cd | mg kg−1 | 14.10 ± 0.90 | <LOD | < LOD | <LOD |
| S(BET) | m2 g−1 | 1.48 | 79.62 | 0.45 | 0.45 |
| Sample | F1_Pb (mg kg−1) | F1_Zn (mg kg−1) | pH | OC (g kg−1) | EC (µS cm−1) |
|---|---|---|---|---|---|
| CS | 495.77 ± 5.20 a | 424.82 ± 4.69 a | 6.57 ± 0.01 h | 19.46 ± 2.11 g | 136.4 ± 2.5 h |
| PB4:3 | 455.73 ± 15.24 b | 416.97 ± 6.93 ab | 6.79 ± 0.01 f | 26.37 ± 1.21 f | 154.8 ± 1.5 g |
| PB4:5 | 328.46 ± 7.32 c | 348.97 ± 34.08 cd | 6.82 ± 0.01 e | 42.12 ± 0.65 c | 185.3 ± 1.6 d |
| PB4:10 | 275.15 ± 31.29 d | 277.69 ± 16.52 e | 7.24 ± 0.01 a | 86.46 ± 0.80 a | 211.3 ± 2.3 b |
| PB4A3 | 324.88 ± 16.60 c | 326.84 ± 23.90 d | 7.02 ± 0.01 cd | 25.93 ± 1.23 f | 190.4 ± 3.2 c |
| PB4A5 | 275.37 ± 9.01 d | 279.72 ± 32.17 de | 7.10 ± 0.01 b | 37.73 ± 3.21 d | 210.6 ± 5.1 b |
| PB6:3 | 319.82 ± 39.99 cd | 388.75 ± 27.62 bc | 6.67 ± 0.01 g | 31.61 ± 1.45 e | 175.6 ± 2.5 e |
| PB6:5 | 295.26 ± 12.05 d | 321.33 ± 22.01 d | 6.99 ± 0.01 d | 38.63 ± 2.02 d | 195.6 ± 1.3 c |
| PB6:10 | 234.55 ± 18.27 e | 302.89 ± 22.68 de | 7.23 ± 0.01 a | 77.85 ± 1.04 b | 231.3 ± 2.1 a |
| PB6A3 | 256.83 ± 28.50 de | 308.82 ± 20.76 de | 7.05 ± 0.02 c | 31.74 ± 1.23 e | 183.9 ± 2.4 e |
| PB6A5 | 252.83 ± 17.81 e | 311.78 ± 11.83 d | 7.11 ± 0.01 b | 34.65 ± 1.65 d | 213.5 ± 3.5 b |
| Metal | Element | Component 1 | Component 2 |
|---|---|---|---|
| Pb | F1 | −0.48 | 0.00 |
| F2 | −0.12 | −0.58 | |
| F3 | −0.21 | 0.39 | |
| F4 | 0.23 | 0.58 | |
| F5 | 0.23 | 0.58 | |
| pH | 0.47 | −0.11 | |
| OC | 0.45 | −0.08 | |
| EC | 0.48 | −0.09 | |
| Eigenvalue | 3.90 | 1.88 | |
| Cumulative variances (%) | 48.77 | 72.27 | |
| Zn | F1 | −0.43 | 0.00 |
| F2 | −0.23 | −0.57 | |
| F3 | −0.27 | 0.66 | |
| F4 | 0.29 | −0.17 | |
| F5 | 0.27 | −0.28 | |
| pH | 0.45 | −0.06 | |
| OC | 0.40 | 0.32 | |
| EC | 0.41 | 0.17 | |
| Eigenvalue | 4.81 | 1.29 | |
| Cumulative variances (%) | 60.10 | 76.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuong, T.X.; Stephen, J.; Minh, T.B.; Nguyen, T.T.T.; Duong, T.H.; Pham, D.T.N. Chemical Fractionations of Lead and Zinc in the Contaminated Soil Amended with the Blended Biochar/Apatite. Molecules 2022, 27, 8044. https://doi.org/10.3390/molecules27228044
Vuong TX, Stephen J, Minh TB, Nguyen TTT, Duong TH, Pham DTN. Chemical Fractionations of Lead and Zinc in the Contaminated Soil Amended with the Blended Biochar/Apatite. Molecules. 2022; 27(22):8044. https://doi.org/10.3390/molecules27228044
Chicago/Turabian StyleVuong, Truong Xuan, Joseph Stephen, Tu Binh Minh, Thu Thuy Thi Nguyen, Tuan Hung Duong, and Dung Thuy Nguyen Pham. 2022. "Chemical Fractionations of Lead and Zinc in the Contaminated Soil Amended with the Blended Biochar/Apatite" Molecules 27, no. 22: 8044. https://doi.org/10.3390/molecules27228044
APA StyleVuong, T. X., Stephen, J., Minh, T. B., Nguyen, T. T. T., Duong, T. H., & Pham, D. T. N. (2022). Chemical Fractionations of Lead and Zinc in the Contaminated Soil Amended with the Blended Biochar/Apatite. Molecules, 27(22), 8044. https://doi.org/10.3390/molecules27228044








