Therapeutic Potential of 2-Methylquinazolin-4(3H)-one as an Antiviral Agent against Influenza A Virus-Induced Acute Lung Injury in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of EA Extract
2.3. Qualitative Analysis of Chemical Components in EA Extract
2.4. Animals and Virus
2.5. Determination of Antiviral Activity In Vitro
2.6. In Vivo Experiments of C1 on ALI Induced by H1N1 Influenza Virus
2.6.1. Organ Index Ratio Measurement and Lung Histopathology Analysis
2.6.2. Neuraminidase Activity Assay
2.6.3. Ionized Calcium Binding Adaptor Molecule-1 Assay Using Immunohistochemistry
2.6.4. Measurement of Cytokines in Lung Tissue Homogenates
2.7. Statistical Analysis
3. Results
3.1. Preparation and Physicochemical Characterization of C1
3.2. Antiviral Activity of C1 from EA Extract against IAV H1N1 In Vitro
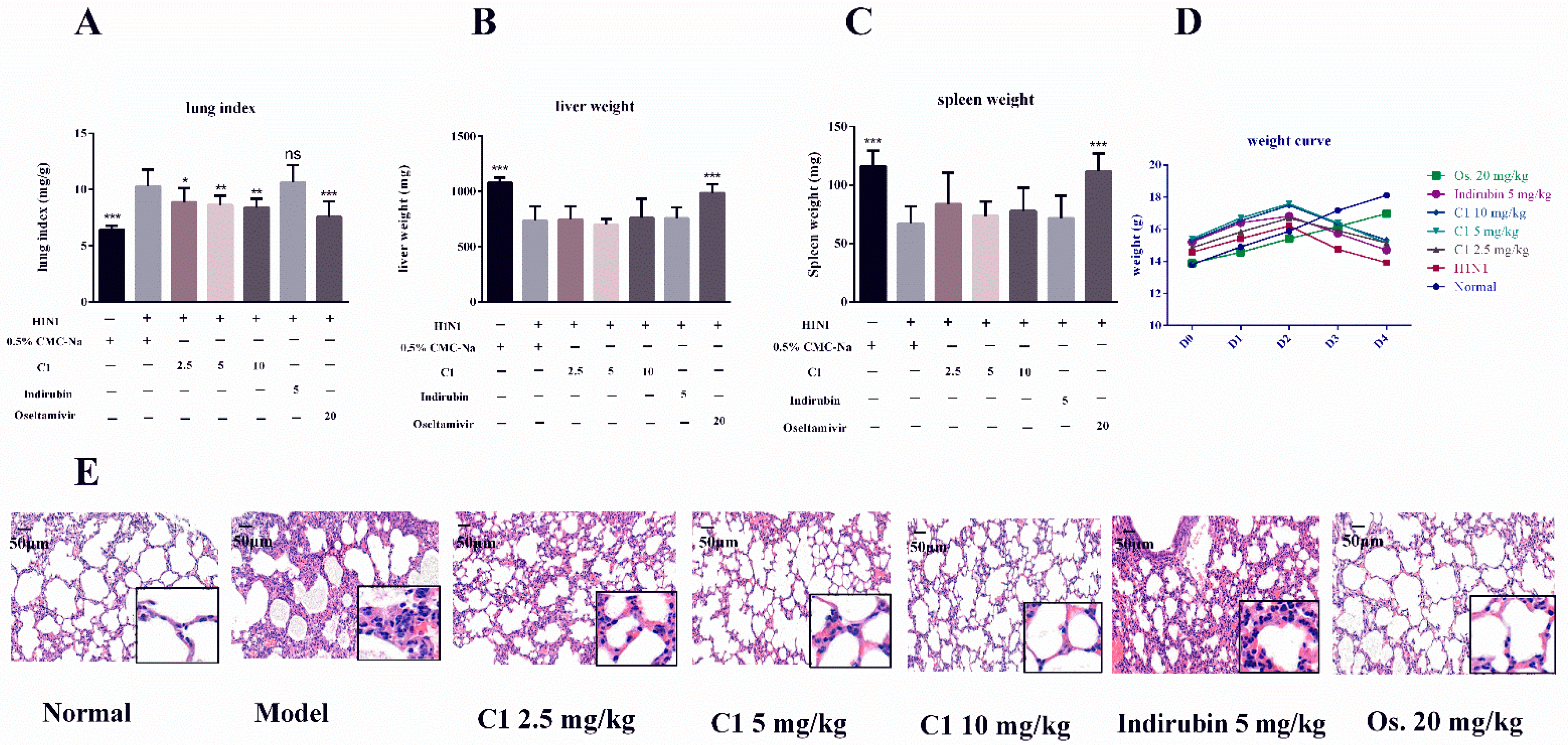
3.3. C1 Alleviates IAV-Induced Acute Lung Injury
3.4. Protective Effects of C1 on Liver and Spleen in Influenza A Virus-Infected Mice
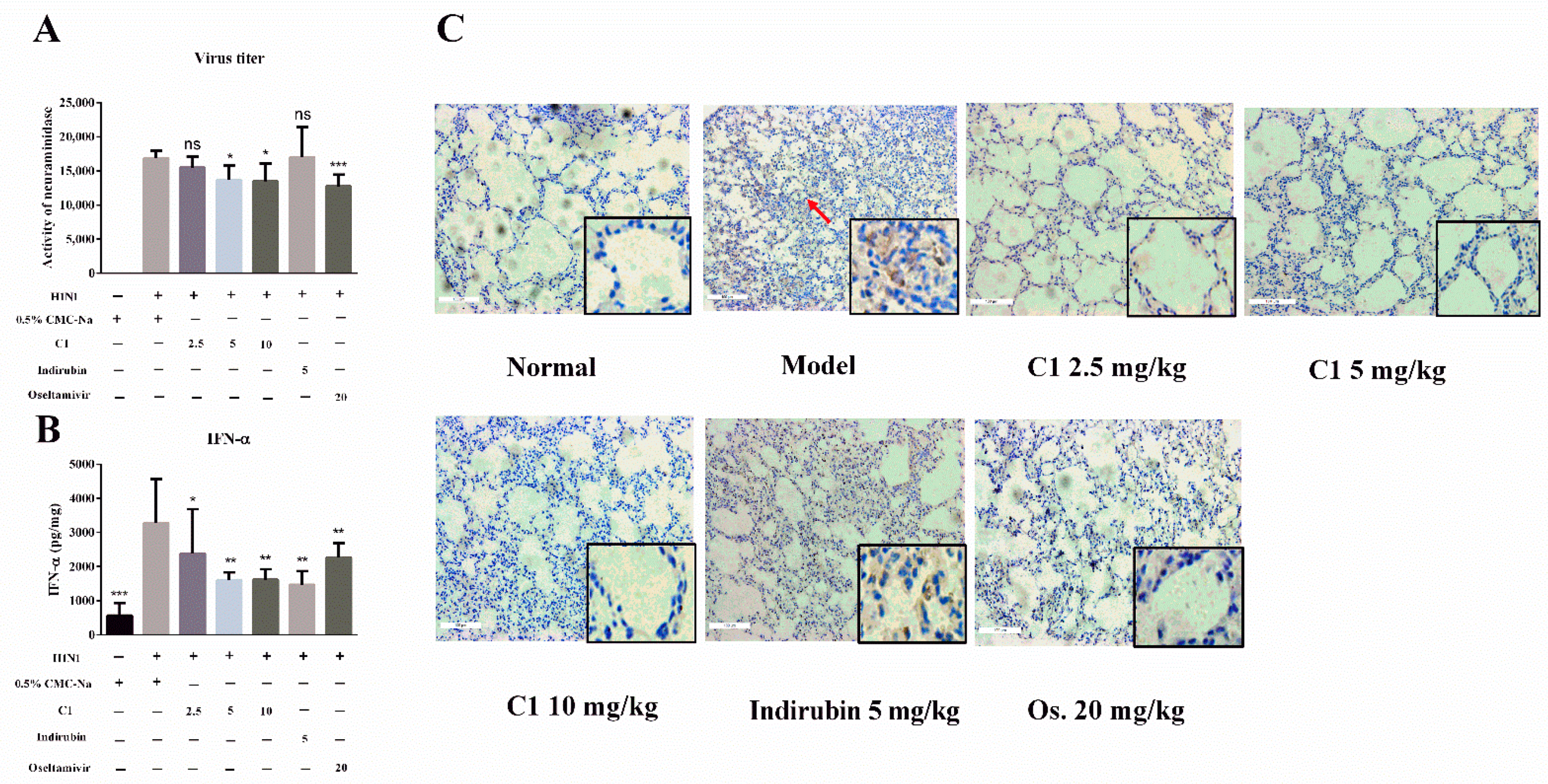
3.5. C1 Inhibits IAV Replication in Mice
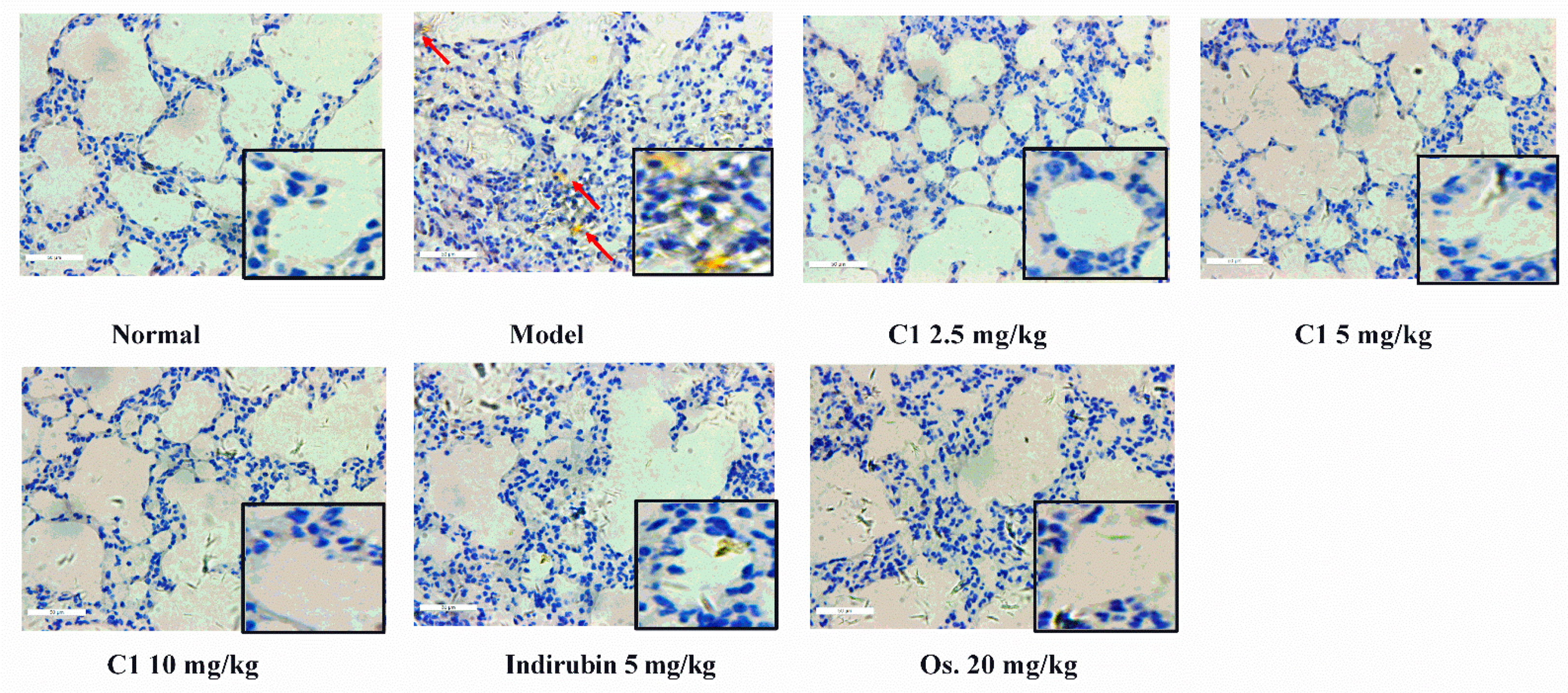
3.6. Effect of C1 on Cytokine Production and Recruitment of Macrophage in IAV-Infected Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maeda, N.; Uede, T. Swine-origin influenza-virus-induced acute lung injury: Novel or classical pathogenesis? World J. Biol. Chem. 2010, 1, 85–94. [Google Scholar] [CrossRef]
- Gao, W.; Mao, Q.; Feng, A.-W.; Sun, H.-M.; Sun, W.-K.; Lu, X.; Su, X.; Shi, Y. Inhibition of pre-B cell colony-enhancing factor attenuates inflammation and apoptosis induced by pandemic H1N1 2009 in lung endothelium. Respir. Physiol. Neurobiol. 2011, 178, 235–241. [Google Scholar] [CrossRef]
- Catanzaro, M.; Fagiani, F.; Racchi, M.; Corsini, E.; Govoni, S.; Lanni, C. Immune response in COVID-19: Addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct. Target. Ther. 2020, 5, 84. [Google Scholar] [CrossRef]
- Mauad, T.; Hajjar, L.A.; Callegari, G.D.; da Silva, L.F.F.; Schout, D.; Galas, F.R.B.G.; Alves, V.A.F.; Malheiros, D.M.A.C.; Auler, J.O.C., Jr.; Ferreira, A.F.; et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am. J. Respir. Crit. Care Med. 2010, 181, 72–79. [Google Scholar] [CrossRef]
- Murdaca, G.; Paladin, F.; Tonacci, A.; Isola, S.; Allegra, A.; Gangemi, S. The Potential Role of Cytokine Storm Pathway in the Clinical Course of Viral Respiratory Pandemic. Biomedicines 2021, 9, 1688. [Google Scholar] [CrossRef]
- Poux, C.; Dondalska, A.; Bergenstråhle, J.; Pålsson, S.; Contreras, V.; Arasa, C.; Järver, P.; Albert, J.; Busse, D.C.; LeGrand, R.; et al. A Single-Stranded Oligonucleotide Inhibits Toll-Like Receptor 3 Activation and Reduces Influenza A (H1N1) Infection. Front. Immunol. 2019, 10, 2161. [Google Scholar] [CrossRef]
- Uyeki, T.M. A Step Forward in the Treatment of Influenza. N. Engl. J. Med. 2018, 379, 975–977. [Google Scholar] [CrossRef]
- De Jong, M.D.; Thanh, T.T.; Khanh, T.H.; Hien, V.M.; Smith, G.J.; Chau, N.V.; Cam, B.V.; Qui, P.T.; Ha, D.Q.; Guan, Y.; et al. Oseltamivir Resistance during Treatment of Influenza A (H5N1) Infection. N. Engl. J. Med. 2005, 353, 2667–2672. [Google Scholar] [CrossRef]
- Shi, J.M. Shijinmo Variorum of Clinic Practices with Double-Herb Prescriptions, 1st ed.; People’s Medical Publishing House: Beijing, China, 2002; pp. 68–69. [Google Scholar]
- Tu, P.; Tian, R.; Lu, Y.; Zhang, Y.; Zhu, H.; Ling, L.; Li, H.; Chen, D. Beneficial Effect of Indigo Naturalis on Acute Lung Injury Induced by Influenza a Virus. Chin. Med. 2020, 15, 128. [Google Scholar] [CrossRef]
- Yang, F.; Song, K.; Zhang, Z.; Chen, C.; Wang, G.; Yao, J.; Ling, F. Evaluation on the antiviral activity of ribavirin against Micropterus salmoides rhabdovirus (MSRV) in vitro and in vivo. Aquaculture 2021, 543, 736975. [Google Scholar] [CrossRef]
- Hu, J.M.; Hsiung, G.D. Evaluation of new antiviral agents: I. In vitro perspectives. Antivir. Res. 1989, 11, 217–232. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 70. [Google Scholar] [CrossRef]
- Iwata, A.; Iwata, N.M.; Saito, T.; Hamada, K.; Sokawa, Y.; Ueda, S. Cytopathic effect inhibition assay for canine interferon activity. J. Vet. Med. Sci. 1996, 58, 23–27. [Google Scholar] [CrossRef][Green Version]
- Watanabe, T.; Kiso, M.; Fukuyama, S.; Nakajima, N.; Imai, M.; Yamada, S.; Murakami, S.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Sakoda, Y.; et al. Characterization of H7N9 influenza A viruses isolated from humans. Nature 2013, 501, 551–555. [Google Scholar] [CrossRef]
- Ling, L.; Lu, Y.; Chen, D.F. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine 2020, 67, 153150. [Google Scholar] [CrossRef]
- Takahashi, E.; Indalao, I.L.; Sawabuchi, T.; Mizuno, K.; Sakai, S.; Kimoto, T.; Kim, H.; Kido, H. Clarithromycin suppresses induction of monocyte chemoattractant protein-1 and matrix metalloproteinase-l9 and improves pathological changes in the lungs and heart of mice infected with influenza A virus. Comp. Immunol. Microb. 2018, 56, 6–13. [Google Scholar] [CrossRef]
- McKimm-Breschkin, J.L. Influenza neuraminidase inhibitors: Antiviral action and mechanisms of resistance. Influenza Other Resp. 2013, 7, 25–36. [Google Scholar] [CrossRef]
- Gamblin, S.J.; Skehel, J.J. Influenza Hemagglutinin and Neuraminidase Membrane Glycoproteins. J. Biol. Chem. 2010, 285, 28403–28409. [Google Scholar]
- Fu, Z.; Xia, L.; De, J.; Zhu, M.; Li, H.; Lu, Y.; Chen, D. Beneficial effects on H1N1-induced acute lung injury and structure characterization of anti-complementary acidic polysaccharides from Juniperus pingii var. wilsonii. Int. J. Biol. Macromol. 2019, 129, 246–253. [Google Scholar] [CrossRef]
- Cauldwell, A.V.; Long, J.S.; Moncorge, O.; Barclay, W.S. Viral determinants of influenza A virus host range. J. Gen. Virol. 2014, 95, 1193–1210. [Google Scholar] [CrossRef]
- Manz, B.; Schwemmle, M.; Brunotte, L. Adaptation of Avian Influenza A Virus Polymerase in Mammals to Overcome the Host Species Barrier. J. Virol. 2013, 87, 7200–7209. [Google Scholar] [CrossRef]
- García-Sastre, A. Induction and evasion of type I interferon responses by influenza viruses. Virus Res. 2011, 162, 12–18. [Google Scholar] [CrossRef]
- Seo, S.H.; Webby, R.; Webster, R.G. No apoptotic deaths and different levels of inductions of inflammatory cytokines in alveolar macrophages infected with influenza viruses. Virology 2004, 329, 270–279. [Google Scholar] [CrossRef]
- Ji, K.-A.; Yang, M.-S.; Jeong, H.-K.; Min, K.-J.; Kang, S.-H.; Jou, I.; Joe, E.-H. Resident microglia die and infiltrated neutrophils and monocytes become major inflammatory cells in lipopolysaccharide-injected brain. Glia 2007, 55, 1577–1588. [Google Scholar] [CrossRef]
- Ma, Q.H.; Ren, M.Y.; Luo, J.B. San Wu Huangqin decoction regulates inflammation and immune dysfunction induced by influenza virus by regulating the NF-κB signaling pathway in H1N1-infected mice. J. Ethnopharmacol. 2020, 264, 112800. [Google Scholar] [CrossRef]
- Zhi, H.-J.; Zhu, H.-Y.; Zhang, Y.-Y.; Lu, Y.; Li, H.; Chen, D.-F. In vivo effect of quantified flavonoids-enriched extract of Scutellaria baicalensis root on acute lung injury induced by influenza A virus. Phytomedicine 2019, 57, 105–116. [Google Scholar] [CrossRef]
- Gao, L.; Gou, N.; Yuan, E.; Ren, J.Y. Bioactivity-Oriented Purification of Polyphenols from Cinnamomum cassia Presl. with Anti-Proliferation Effects on Colorectal Cancer Cells. Plant Food Hum. Nutr. 2020, 75, 561–568. [Google Scholar]
- Wang, R.; Xiao, H.; Guo, R.; Li, Y.; Shen, B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg. Microbes Infect. 2015, 4, e28. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Zhang, T.; He, Y.N.; Huang, S.-J.; Deng, X.; Han, L.; Xie, C.-G. From natural dye to herbal medicine: A systematic review of chemical constituents, pharmacological effects and clinical applications of indigo naturalis. Chin. Med. 2020, 15, 127. [Google Scholar]
- Baz, M.; Carbonneau, J.; Rhéaume, C.; Cavanagh, M.-H.; Boivin, G. Combination Therapy with Oseltamivir and Favipiravir Delays Mortality but Does Not Prevent Oseltamivir Resistance in Immunodeficient Mice Infected with Pandemic A(H1N1) Influenza Virus. Virus 2018, 10, 610. [Google Scholar] [CrossRef]
- Chen, S.-F.; Li, S.-H.; Fei, X.; Wu, Z.-L. Phospholipase A2-Induced Lung Edema And Its Mechanism In Isolated Perfused Guinea-Pig Lung. Inflammation 1990, 14, 267–273. [Google Scholar] [CrossRef]
- Pei, S.; Xia, S.; Yang, F.; Chen, J.; Wang, M.; Sun, W.; Li, Z.; Yuan, K.; Chen, J. Design, synthesis and in vitro biological evaluation of isoxazol-4-carboxa piperidyl derivatives as new anti-influenza A agents targeting virus nucleoprotein. RSC Adv. 2020, 10, 4446–4454. [Google Scholar] [CrossRef]
- Gao, X.; Cai, X.; Yan, K.; Song, B.; Gao, L.; Chen, Z. Synthesis and antiviral bioactivities of 2-aryl- or 2-methyl-3-(substituted-benzalamino)-4(3H)-quinazolinone derivatives. Molecules 2007, 12, 2621–2642. [Google Scholar] [CrossRef]
- Akgun, H.; Us Yilmaz, D.; Cetin Atalay, R.; Gozen, D. A Series of 2,4(1H,3H)-Quinazolinedione Derivatives: Synthesis and Biological Evaluation as Potential Anticancer Agents. Lett. Drug Des. Discov. 2016, 13, 64–76. [Google Scholar] [CrossRef]
- Smith, K.; El-Hiti, G.A.; Abdel-Megeed, M.F. Unexpected Products from Carbonylation of Lithiated Quinazolin-4(3H)-one Derivatives. Russ. J. Org. Chem. 2003, 34, 430–435. [Google Scholar] [CrossRef]
- Van Dycke, A.; Verstraete, A.; Pil, K.; Raedt, R.; Vonck, K.; Boison, D.; Boon, P. Quantitative analysis of adenosine using liquid chromatography/atmospheric pressure chemical ionization-tandem mass spectrometry (LC/APCI-MS/MS). J Chromatogr. B 2010, 878, 1493–1498. [Google Scholar] [CrossRef][Green Version]
- Chung, C.-Y.; Liu, C.-H.; Burnouf, T.; Wang, G.-H.; Chang, S.-P.; Jassey, A.; Tai, C.-J.; Tai, C.-J.; Huang, C.-J.; Richardson, C.D.; et al. Activity-based and fraction-guided analysis of Phyllanthus urinaria identifies loliolide as a potent inhibitor of hepatitis C virus entry. Antivir Res. 2016, 130, 58–68. [Google Scholar] [CrossRef]
- Zhao, Y.Y. The Study of Chemical Constituents and Bioactive Constituents of Polyporus umbellatus. Ph.D. Thesis, Northwest University, Xi’an, China, 2010. [Google Scholar]
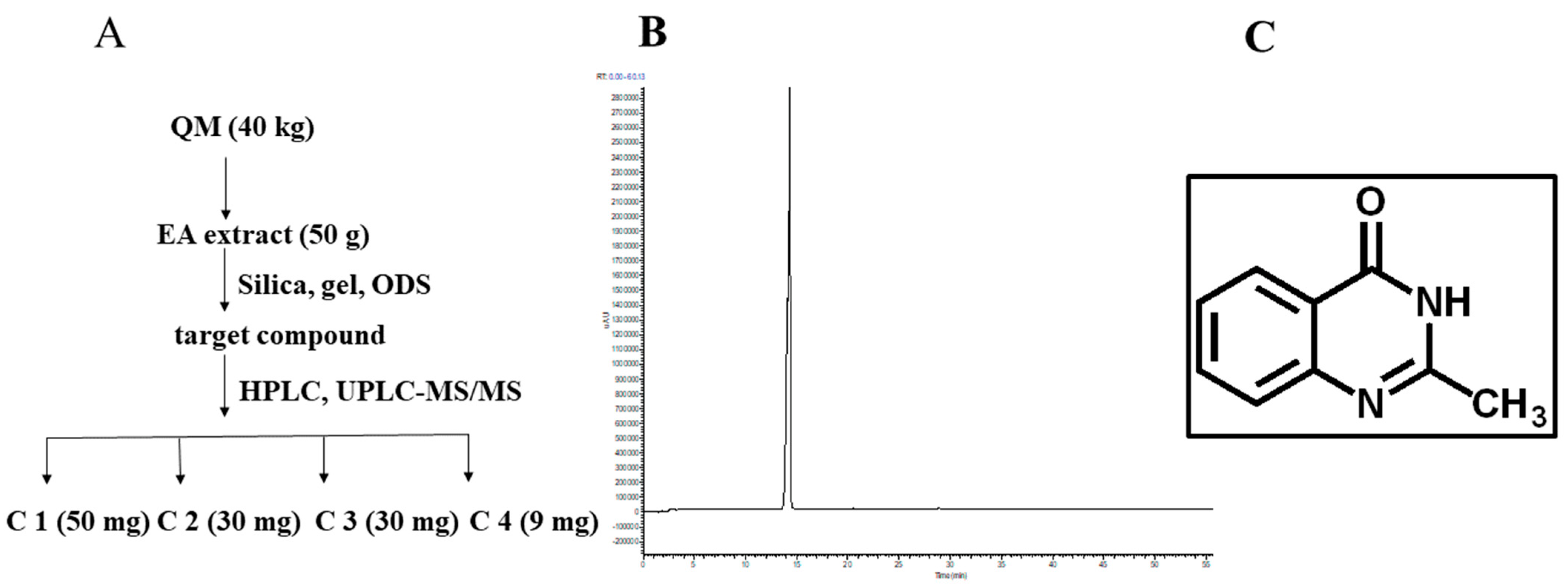


| Compound | TC50 (μg/mL) | IC50 (μg/mL) |
|---|---|---|
| C1 | 79.7 | 23.8 |
| C2 | 26.7 | / |
| C3 | 106.8 | 100 |
| C4 | 167.7 | / |
| Ribavrin | / | 37.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, R.; Zhu, H.; Lu, Y.; Shi, X.; Tu, P.; Li, H.; Huang, H.; Chen, D. Therapeutic Potential of 2-Methylquinazolin-4(3H)-one as an Antiviral Agent against Influenza A Virus-Induced Acute Lung Injury in Mice. Molecules 2022, 27, 7857. https://doi.org/10.3390/molecules27227857
Tian R, Zhu H, Lu Y, Shi X, Tu P, Li H, Huang H, Chen D. Therapeutic Potential of 2-Methylquinazolin-4(3H)-one as an Antiviral Agent against Influenza A Virus-Induced Acute Lung Injury in Mice. Molecules. 2022; 27(22):7857. https://doi.org/10.3390/molecules27227857
Chicago/Turabian StyleTian, Rong, Haiyan Zhu, Yan Lu, Xunlong Shi, Peng Tu, Hong Li, Hai Huang, and Daofeng Chen. 2022. "Therapeutic Potential of 2-Methylquinazolin-4(3H)-one as an Antiviral Agent against Influenza A Virus-Induced Acute Lung Injury in Mice" Molecules 27, no. 22: 7857. https://doi.org/10.3390/molecules27227857
APA StyleTian, R., Zhu, H., Lu, Y., Shi, X., Tu, P., Li, H., Huang, H., & Chen, D. (2022). Therapeutic Potential of 2-Methylquinazolin-4(3H)-one as an Antiviral Agent against Influenza A Virus-Induced Acute Lung Injury in Mice. Molecules, 27(22), 7857. https://doi.org/10.3390/molecules27227857






