Treatment of Water Contaminated with Non-Steroidal Anti-Inflammatory Drugs Using Peroxymonosulfate Activated by Calcined Melamine@magnetite Nanoparticles Encapsulated into a Polymeric Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis Procedure
2.3. Experiments
2.4. Analysis
3. Results and Discussion
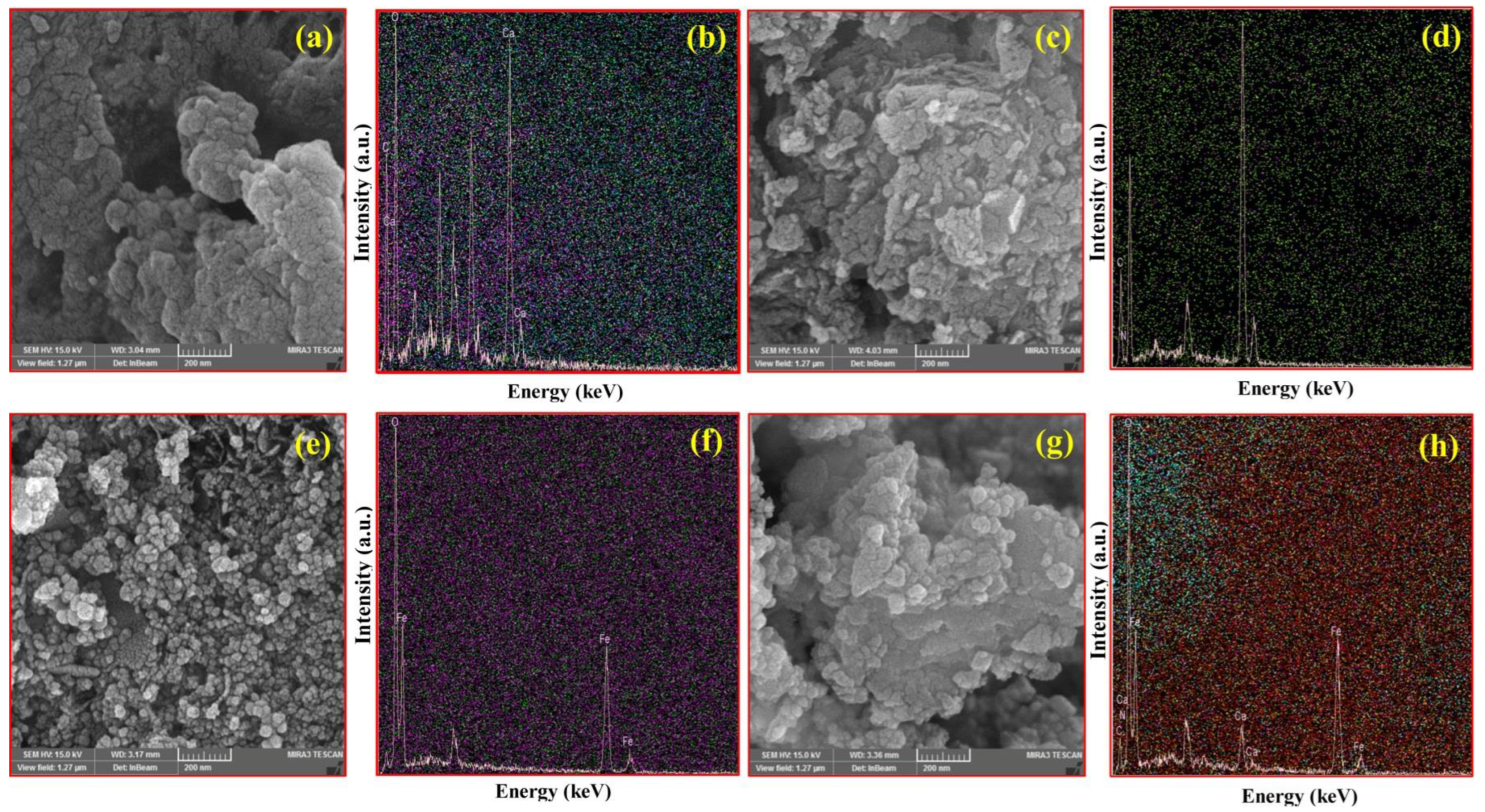
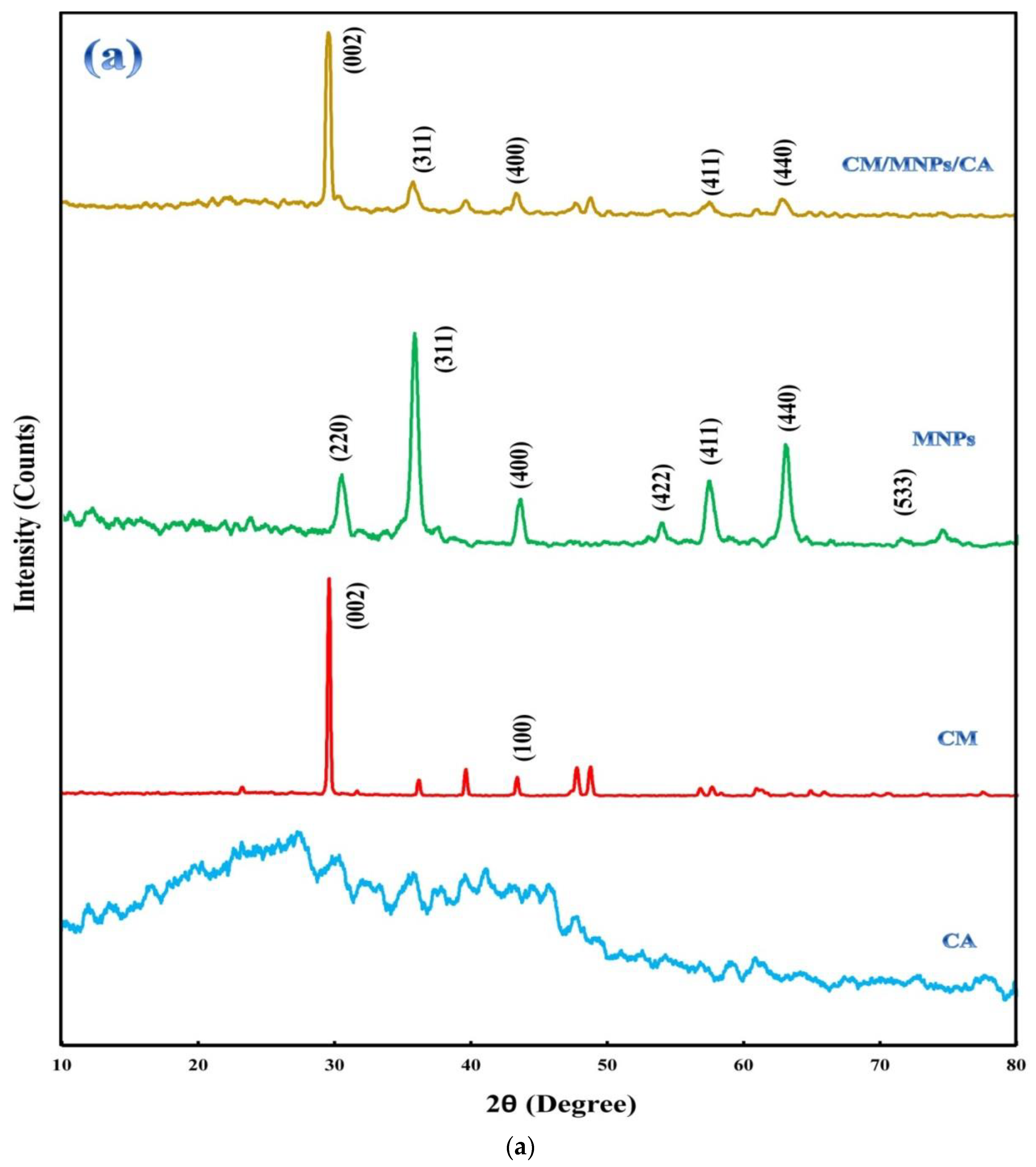
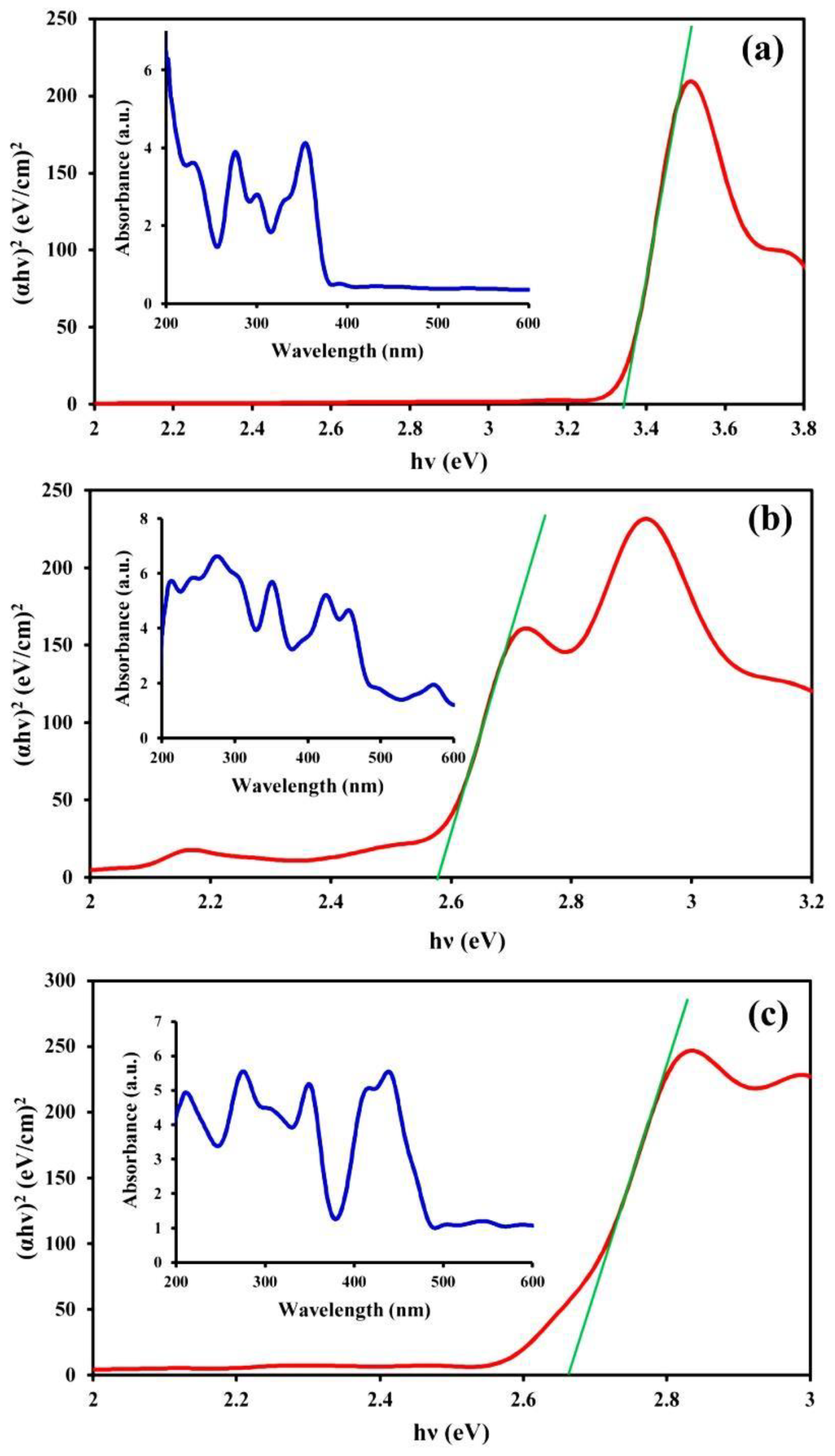
3.1. Characterization
3.2. Adsorption Role
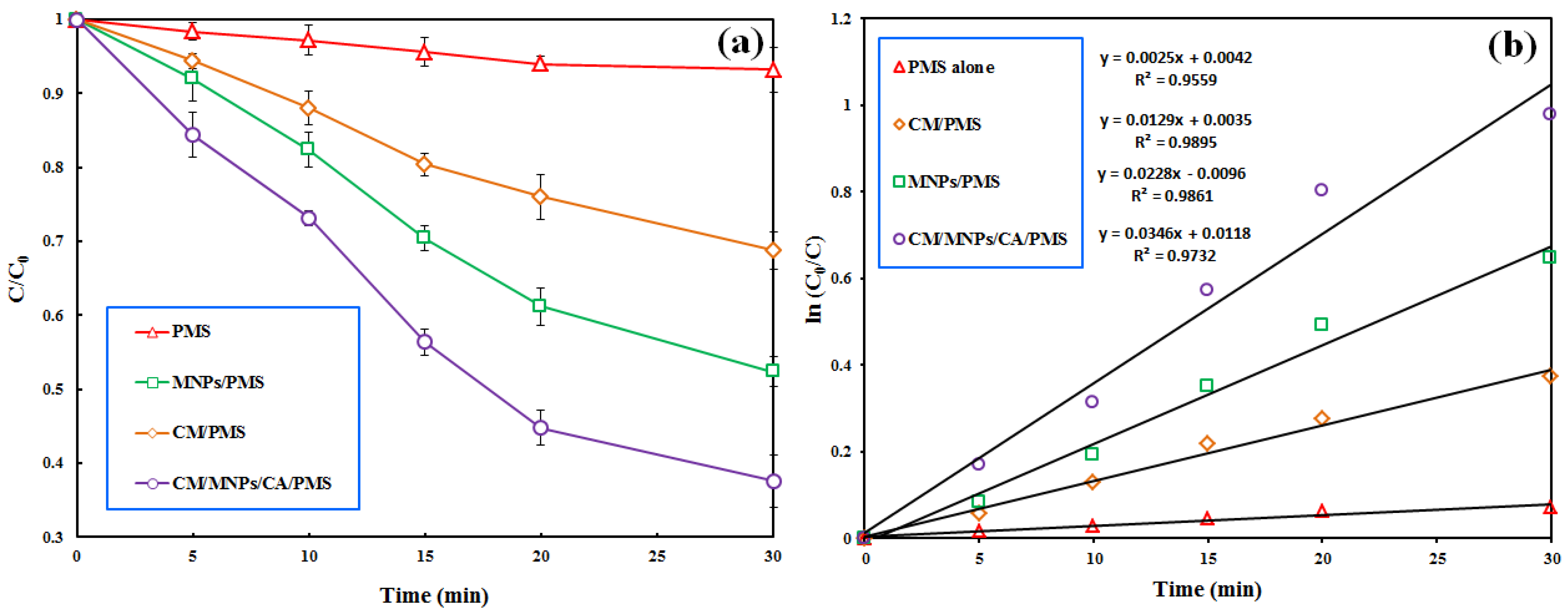
3.3. Comparison of PMS-Based Treatment Processes
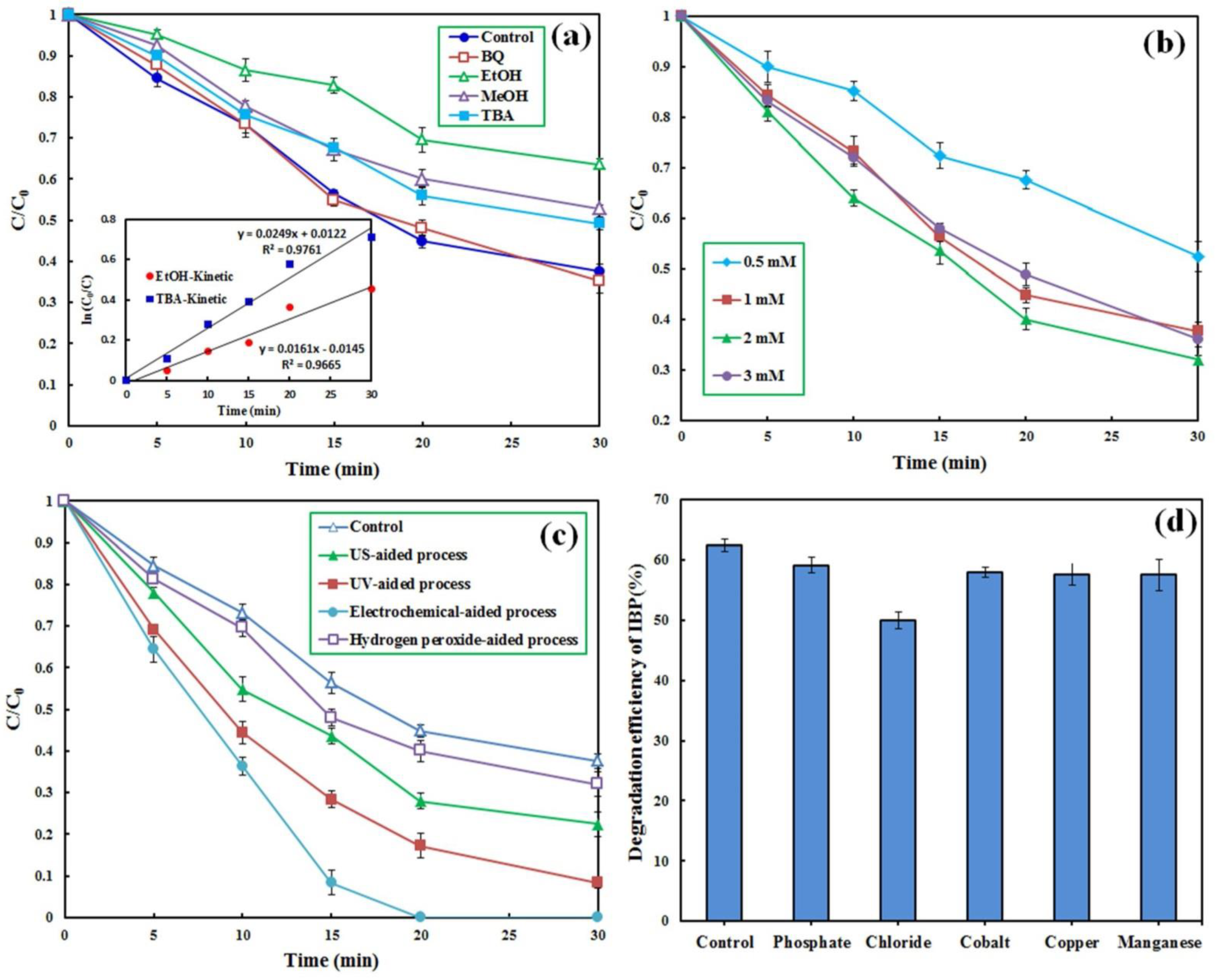
3.4. Role of the Radical Species
3.5. PMS Concentration Effect
3.6. Enhancing Strategies
3.6.1. Hydrogen Peroxide Addition
3.6.2. US, UV and Electrochemical Enhancement
3.7. Co-Existing Compounds Effect
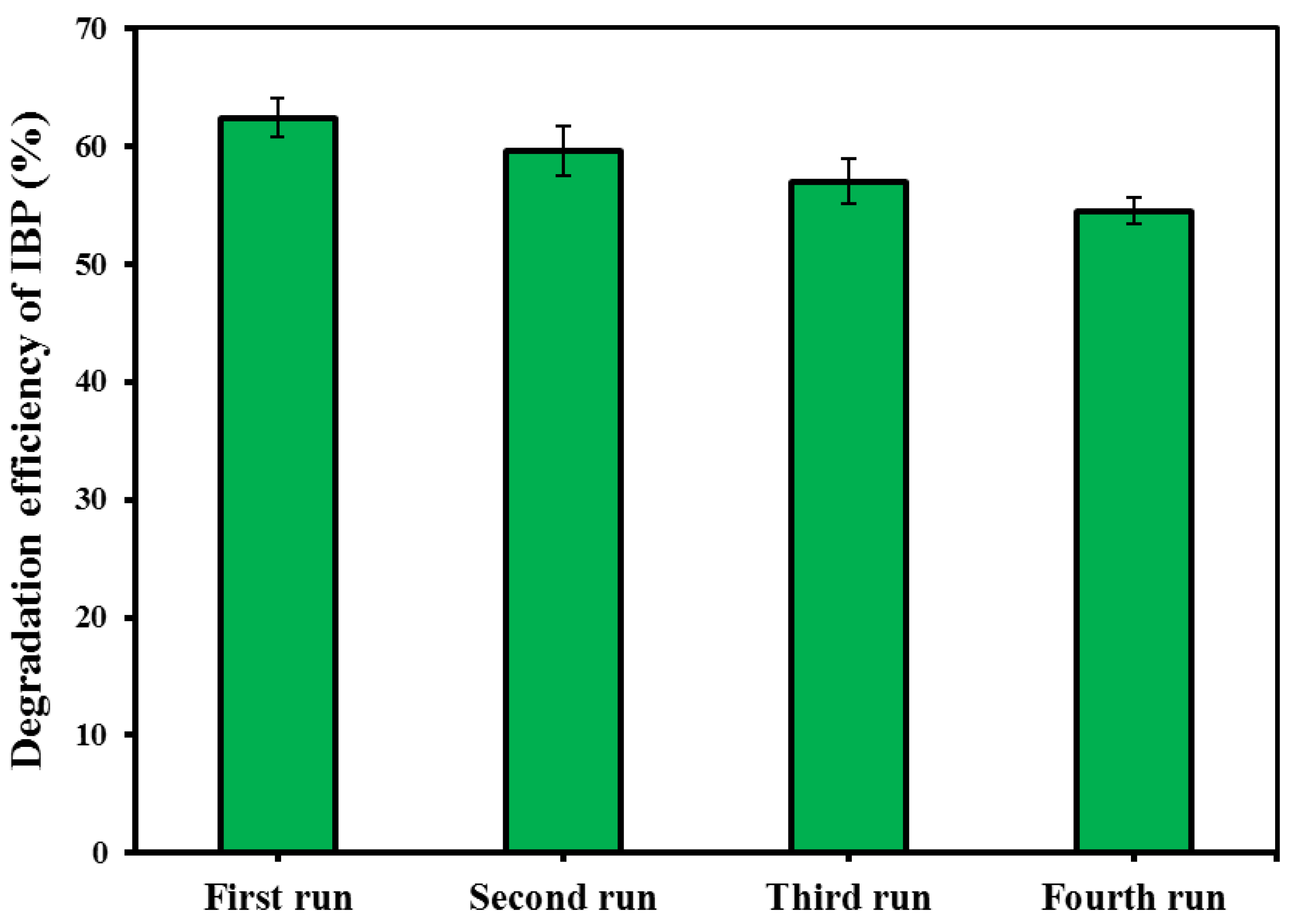
3.8. Reusability, Stability, Mineralization and Bio-Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vieno, N.M.; Tuhkanen, T.; Kronberg, L. Seasonal Variation in the Occurrence of Pharmaceuticals in Effluents from a Sewage Treatment Plant and in the Recipient Water. Environ. Sci. Technol. 2005, 39, 8220–8226. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Mashayekhi, M.; Jorfi, S.; Khataee, A.; Ghanadzadeh, M.-J.; Sillanpää, M. Implementation of martite nanoparticles prepared through planetary ball milling as a heterogeneous activator of oxone for degradation of tetracycline antibiotic: Ultrasound and peroxy-enhancement. Chemosphere 2018, 210, 699–708. [Google Scholar] [CrossRef]
- Liu, J.; Lu, G.; Xie, Z.; Zhang, Z.; Li, S.; Yan, Z. Occurrence, bioaccumulation and risk assessment of lipophilic pharmaceutically active compounds in the downstream rivers of sewage treatment plants. Sci. Total Environ. 2015, 511, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Kumar, D. Ibuprofen as an emerging organic contaminant in environment, distribution and remediation. Heliyon 2020, 6, e04087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ding, Y.; Nie, W.; Tang, H. Efficient degradation of drug ibuprofen through catalytic activation of peroxymonosulfate by Fe3C embedded on carbon. J. Environ. Sci. 2019, 78, 1–12. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Y.; Fu, J. Urchin-like Co3O4 anchored on reduced graphene oxide with enhanced performance for peroxymonosulfate activation in ibuprofen degradation. J. Environ. Manag. 2022, 307, 114572. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Ngo, H.H.; Guo, W.; Huo, J.; Du, Q.; Zhang, Y.; Li, C.; Yang, F. Sorptive removal of ibuprofen from water by natural porous biochar derived from recyclable plane tree leaf waste. J. Water Process Eng. 2022, 46, 102627. [Google Scholar] [CrossRef]
- Rezaee, A.; Masoumbeigi, H.; Soltani, R.D.C.; Khataee, A.R.; Hashemiyan, S. Photocatalytic decolorization of methylene blue using immo bilized ZnO nanoparticles prepared by solution combustion method. Desalination Water Treat. 2012, 44, 174–179. [Google Scholar] [CrossRef]
- Kwon, S.C.; Kim, J.Y.; Yoon, S.M.; Bae, W.; Kang, K.S.; Rhee, Y.W. Treatment characteristic of 1,4-dioxane by ozone-based advanced oxidation processes. J. Ind. Eng. Chem. 2012, 18, 1951–1955. [Google Scholar] [CrossRef]
- Tizhoosh, N.Y.; Khataee, A.; Hassandoost, R.; Soltani, R.D.C.; Doustkhah, E. Ultrasound-engineered synthesis of WS2@CeO2 heterostructure for sonocatalytic degradation of tylosin. Ultrason. Sonochem. 2020, 67, 105114. [Google Scholar] [CrossRef]
- Adityosulindro, S.; Barthe, L.; González-Labrada, K.; Haza, U.J.J.; Delmas, H.; Julcour, C. Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste) water. Ultrason. Sonochem. 2017, 39, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, L.; Janani, B.; Khan, S.S. Ibuprofen removal from aqueous solution via light-harvesting photocatalysis by nano-heterojunctions: A review. Sep. Purif. Technol. 2021, 279, 119709. [Google Scholar] [CrossRef]
- Bastami, T.R.; Ahmadpour, A.; Hekmatikar, F.A. Synthesis of Fe3O4/Bi2WO6 nanohybrid for the photocatalytic degradation of pharmaceutical ibuprofen under solar light. J. Ind. Eng. Chem. 2017, 51, 244–254. [Google Scholar] [CrossRef]
- Liu, N.; Dai, W.; Fei, F.; Xu, H.; Lei, J.; Quan, G.; Zheng, Y.; Zhang, X.; Tang, L. Insights into the photocatalytic activation persulfate by visible light over ReS2/MIL-88B (Fe) for highly efficient degradation of ibuprofen: Combination of experimental and theoretical study. Sep. Purif. Technol. 2022, 297, 121545. [Google Scholar] [CrossRef]
- Jothinathan, L.; Hu, J. Kinetic evaluation of graphene oxide based heterogenous catalytic ozonation for the removal of ibuprofen. Water Res. 2018, 134, 63–73. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Cui, M.; Ren, Y.; Park, B.; Ma, J.; Han, Z.; Khim, J. Activation of peroxodisulfate and peroxymonosulfate by ultrasound with different frequencies: Impact on ibuprofen removal efficient, cost estimation and energy analysis. Chem. Eng. J. 2020, 413, 127487. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, J.; Zhang, Y.; Zhou, P.; Wang, J.; Liu, Y. Heterogeneous catalytic oxidation degradation of BPAF by peroxymonosulfate active with manganic manganous oxide: Mineralization, mechanism and degradation pathways. Chemosphere 2020, 263, 127950. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, H.; Murugananthan, M.; Sun, P.; Dionysiou, D.; Zhang, K.; Khan, A.; Zhang, Y. Glucose and melamine derived nitrogen-doped carbonaceous catalyst for nonradical peroxymonosulfate activation. Carbon 2020, 156, 399–409. [Google Scholar] [CrossRef]
- Liu, N.; Wu, J.; Fei, F.; Lei, J.; Shi, W.; Quan, G.; Zeng, S.; Zhang, X.; Tang, L. Ibuprofen degradation by a synergism of facet-controlled MIL-88B(Fe) and persulfate under simulated visible light. J. Colloid Interface Sci. 2022, 612, 1–12. [Google Scholar] [CrossRef]
- Su, L.; Ou, L.; Wen, Y.; Wang, Y.; Zhao, W.; Zhou, Z.; Zhong, M.-E.; Zhu, Y.; Zhou, N. High-efficiency degradation of quinclorac via peroxymonosulfate activated by N-doped CoFe2O4/Fe0@CEDTA hybrid catalyst. J. Ind. Eng. Chem. 2021, 102, 177–185. [Google Scholar] [CrossRef]
- Bicalho, H.A.; Rios, R.D.; Binatti, I.; Ardisson, J.D.; Howarth, A.J.; Lago, R.M.; Teixeira, A.P.C. Efficient activation of peroxymonosulfate by composites containing iron mining waste and graphitic carbon nitride for the degradation of acetaminophen. J. Hazard. Mater. 2020, 400, 123310. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; He, X.; Zhang, J.; Ma, J. Efficient degradation of refractory organic contaminants by zero-valent copper/hydroxylamine/peroxymonosulfate process. Chemosphere 2019, 237, 124431. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, L.; Huang, T.; Li, W.; Wang, Y.; Wang, Z. Decolorization of azo dye by peroxymonosulfate activated by carbon nanotube: Radical versus non-radical mechanism. J. Hazard. Mater. 2016, 320, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.; Liu, G.; Zhou, H. Preparation of N-doped biochar from sewage sludge and melamine for peroxymonosulfate activation: N-functionality and catalytic mechanisms. Sci. Total Environ. 2020, 744, 140862. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Ding, Z.; Zhao, Z.; Xu, X.; Fang, Z. Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye. Ultrason. Sonochem. 2017, 34, 953–959. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Zhang, T.; Xu, Z.; Li, Y.; Li, B.; Tian, S. UV facilitated synergistic effects of polymetals in ore catalyst on peroxymonosulfate activation: Implication for the degradation of bisphenol S. Chem. Eng. J. 2021, 431, 133989. [Google Scholar] [CrossRef]
- Kiejza, D.; Kotowska, U.; Polińska, W.; Karpińska, J. Peracids—New oxidants in advanced oxidation processes: The use of peracetic acid, peroxymonosulfate, and persulfate salts in the removal of organic micropollutants of emerging concern—A review. Sci. Total Environ. 2021, 790, 148195. [Google Scholar] [CrossRef]
- Ma, M.; Chen, L.; Zhao, J.; Liu, W.; Ji, H. Efficient activation of peroxymonosulfate by hollow cobalt hydroxide for degradation of ibuprofen and theoretical study. Chin. Chem. Lett. 2019, 30, 2191–2195. [Google Scholar] [CrossRef]
- Fadaei, S.; Noorisepehr, M.; Pourzamani, H.; Salari, M.; Moradnia, M.; Darvishmotevalli, M.; Mengelizadeh, N. Heterogeneous activation of peroxymonosulfate with Fe3O4 magnetic nanoparticles for degradation of Reactive Black 5: Batch and column study. J. Environ. Chem. Eng. 2021, 9, 105414. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Naderi, M.; Boczkaj, G.; Jorfi, S.; Khataee, A. Hybrid metal and non-metal activation of Oxone by magnetite nanostructures co-immobilized with nano-carbon black to degrade tetracycline: Fenton and electrochemical enhancement with bio-assay. Sep. Purif. Technol. 2021, 274, 119055. [Google Scholar] [CrossRef]
- Li, J.; Lin, H.; Zhu, K.; Zhang, H. Degradation of Acid Orange 7 using peroxymonosulfate catalyzed by granulated activated carbon and enhanced by electrolysis. Chemosphere 2017, 188, 139–147. [Google Scholar] [CrossRef]
- Sajjadi, S.; Khataee, A.; Soltani, R.D.C.; Hasanzadeh, A. N, S co-doped graphene quantum dot–decorated Fe3O4 nanostructures: Preparation, characterization and catalytic activity. J. Phys. Chem. Solids 2019, 127, 140–150. [Google Scholar]
- Zhang, J.; Chen, P.; Gao, W.; Wang, W.; Tan, F.; Wang, X.; Qiao, X.; Wong, P.K. Melamine-cyanurate supramolecule induced graphitic N-rich graphene for singlet oxygen-dominated peroxymonosulfate activation to efficiently degrade organic pollutants. Sep. Purif. Technol. 2021, 265, 118474. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Y.; Zhou, M.; Xing, K.; Rao, L.; Lv, W.; Yao, Y. Enhanced peroxymonosulfate activation process based on homogenously dispersed iron and nitrogen active sites on a three-dimensional porous carbon framework. Chem. Eng. J. 2020, 404, 126537. [Google Scholar] [CrossRef]
- Yang, S.; Xu, S.; Tong, J.; Ding, D.; Wang, G.; Chen, R.; Jin, P.; Wang, X.C. Overlooked role of nitrogen dopant in carbon catalysts for peroxymonosulfate activation: Intrinsic defects or extrinsic defects? Appl. Catal. B Environ. 2021, 295, 120291. [Google Scholar] [CrossRef]
- Wang, G.; Chen, S.; Quan, X.; Yu, H.; Zhang, Y. Enhanced activation of peroxymonosulfate by nitrogen doped porous carbon for effective removal of organic pollutants. Carbon 2017, 115, 730–739. [Google Scholar] [CrossRef]
- Wu, S.; Liu, H.; Yang, C.; Li, X.; Lin, Y.; Yin, K.; Sun, J.; Teng, Q.; Du, C.; Zhong, Y. High-performance porous carbon catalysts doped by iron and nitrogen for degradation of bisphenol F via peroxymonosulfate activation. Chem. Eng. J. 2019, 392, 123683. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Miraftabi, Z.; Mahmoudi, M.; Jorfi, S.; Boczkaj, G.; Khataee, A. Stone cutting industry waste-supported zinc oxide nanostructures for ultrasonic assisted decomposition of an anti-inflammatory non-steroidal pharmaceutical compound. Ultrason. Sonochem. 2019, 58, 104669. [Google Scholar] [CrossRef]
- Sepyani, F.; Soltani, R.D.C.; Jorfi, S.; Godini, H.; Safari, M. Implementation of continuously electro-generated Fe3O4 nanoparticles for activation of persulfate to decompose amoxicillin antibiotic in aquatic media: UV254 and ultrasound intensification. J. Environ. Manag. 2018, 224, 315–326. [Google Scholar] [CrossRef]
- Wacławek, S.; Grübel, K.; Černík, M. Simple spectrophotometric determination of monopersulfate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 928–933. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Rezaee, A.; Khataee, A.; Godini, H. Optimisation of the operational parameters during a biological nitrification process using response surface methodology. Can. J. Chem. Eng. 2014, 92, 13–22. [Google Scholar] [CrossRef]
- Jiang, X.; Li, J.; Fang, J.; Gao, L.; Cai, W.; Li, X.; Xu, A.; Ruan, X. The photocatalytic performance of g-C3N4 from melamine hydrochloride for dyes degradation with peroxymonosulfate. J. Photochem. Photobiol. A Chem. 2017, 336, 54–62. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Deng, Y.; Deng, J.; Zhou, S.; Li, J.; Xin, X. Radical induced degradation of acetaminophen with Fe3O4 magnetic nanoparticles as heterogeneous activator of peroxymonosulfate. J. Hazard. Mater. 2014, 276, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Tojeira, A.; Vaz, D.B.D.M.C.; Mendes, A.; Bártolo, P. Preparation and Characterization of Films Based on Alginate and Aloe Vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Voo, W.-P.; Lee, B.-B.; Idris, A.; Islam, A.; Tey, B.-T.; Chan, E.-S. Production of ultra-high concentration calcium alginate beads with prolonged dissolution profile. RSC Adv. 2015, 5, 36687–36695. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, X.; Chen, X.; Yu, C.; Yin, Q.; Yan, H.; Lin, Q. Fabrication and evaluation of melamine-formaldehyde resin crosslinked PVA composite coating membranes with enhanced oxygen barrier properties for food packaging. RSC Adv. 2021, 11, 14295–14305. [Google Scholar] [CrossRef]
- Chirita, M.; Banica, R.; Ieta, A.; Grozescu, I. Superparamagnetic Unusual Behavior of Micrometric Magnetite Monodisperse Monocrystals Synthesized by Fe-EDTA Thermal Decomposition. Part. Sci. Technol. 2012, 30, 354–363. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Rezaee, A.; Khorramabadi, G.S.; Yaghmaeian, K. Optimization of lead (II) biosorption in an aqueous solution using chemically modified aerobic digested sludge. Water Sci. Technol. 2011, 63, 129–135. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Jafari, A.J.; Khorramabadi, G.S. Investigation of cadmium (II) ions biosorption onto pretreated dried activated sludge. Am. J. Environ. Sci. 2009, 5, 41. [Google Scholar]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Zhou, X.; Wu, Q.; Zheng, H.; Chang, J.; Ren, N. Selective degradation of sulfonamide antibiotics by peroxymonosulfate alone: Direct oxidation and nonradical mechanisms. Chem. Eng. J. 2018, 334, 2539–2546. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Wang, Y.; Kang, J.; Wang, S. N-Doping-Induced Nonradical Reaction on Single-Walled Carbon Nanotubes for Catalytic Phenol Oxidation. ACS Catal. 2015, 5, 553–559. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Ma, F.; Wang, L.; Duan, X.; Wang, S. Catalytic Removal of Aqueous Contaminants on N-Doped Graphitic Biochars: Inherent Roles of Adsorption and Nonradical Mechanisms. Environ. Sci. Technol. 2018, 52, 8649–8658. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.I.; Shivashankar, S.A. Single crystalline magnetite, maghemite, and hematite nanoparticles with rich coercivity. RSC Adv. 2014, 4, 4105–4113. [Google Scholar] [CrossRef]
- Ji, F.; Li, C.; Wei, X.; Yu, J. Efficient performance of porous Fe2O3 in heterogeneous activation of peroxymonosulfate for decolorization of Rhodamine B. Chem. Eng. J. 2013, 231, 434–440. [Google Scholar] [CrossRef]
- Yap, P.-S.; Lim, T.-T. Effect of aqueous matrix species on synergistic removal of bisphenol-A under solar irradiation using nitrogen-doped TiO2/AC composite. Appl. Catal. B Environ. 2011, 101, 709–717. [Google Scholar] [CrossRef]
- Zhao, L.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q.; Yin, X. Simultaneous removal of bisphenol A and phosphate in zero-valent iron activated persulfate oxidation process. Chem. Eng. J. 2016, 303, 458–466. [Google Scholar] [CrossRef]
- Cai, C.; Wang, L.; Gao, H.; Hou, L.; Zhang, H. Ultrasound enhanced heterogeneous activation of peroxydisulfate by bimetallic Fe-Co/GAC catalyst for the degradation of Acid Orange 7 in water. J. Environ. Sci. 2014, 26, 1267–1273. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darvishi Cheshmeh Soltani, R.; Asgari, F.; Hassani, N.; Yoon, Y.; Khataee, A. Treatment of Water Contaminated with Non-Steroidal Anti-Inflammatory Drugs Using Peroxymonosulfate Activated by Calcined Melamine@magnetite Nanoparticles Encapsulated into a Polymeric Matrix. Molecules 2022, 27, 7845. https://doi.org/10.3390/molecules27227845
Darvishi Cheshmeh Soltani R, Asgari F, Hassani N, Yoon Y, Khataee A. Treatment of Water Contaminated with Non-Steroidal Anti-Inflammatory Drugs Using Peroxymonosulfate Activated by Calcined Melamine@magnetite Nanoparticles Encapsulated into a Polymeric Matrix. Molecules. 2022; 27(22):7845. https://doi.org/10.3390/molecules27227845
Chicago/Turabian StyleDarvishi Cheshmeh Soltani, Reza, Fatemeh Asgari, Negin Hassani, Yeojoon Yoon, and Alireza Khataee. 2022. "Treatment of Water Contaminated with Non-Steroidal Anti-Inflammatory Drugs Using Peroxymonosulfate Activated by Calcined Melamine@magnetite Nanoparticles Encapsulated into a Polymeric Matrix" Molecules 27, no. 22: 7845. https://doi.org/10.3390/molecules27227845
APA StyleDarvishi Cheshmeh Soltani, R., Asgari, F., Hassani, N., Yoon, Y., & Khataee, A. (2022). Treatment of Water Contaminated with Non-Steroidal Anti-Inflammatory Drugs Using Peroxymonosulfate Activated by Calcined Melamine@magnetite Nanoparticles Encapsulated into a Polymeric Matrix. Molecules, 27(22), 7845. https://doi.org/10.3390/molecules27227845






