Abstract
HPLC-UV was used to compare the major constituents of two Pelargonium × hortorum cultivars and Pelargonium sidoides root extract. It revealed the presence of catechin and gallic acid in high concentrations and the absence of umckalin in P. × hortorum root extracts. The antibacterial activity of these extracts was screened against 19 Pseudomonas aeruginosa clinical isolates. P. × hortorum root extracts showed the lowest MIC values (512–1024 µg/mL). This activity was concluded to be attributable to the high concentrations of catechin and gallic acid. The anti-biofilm activity of catechin, gallic acid, and their combination was examined by a crystal violet assay. The combination reduced the percentage of strong and moderate biofilm-forming isolates from 52.63% to 5.26%. The impact on lasI and lasR genes expression using qRT-PCR and simultaneous docking against LasR protein was explored. The combination downregulated lasI and lasR gene expression in eight and six P. aeruginosa isolates, respectively, and showed the greatest docking score. Additionally, the in vivo protection capability of this combination in infected mice showed enhancement in the survival rate. Our study revealed the potential biofilm and quorum-sensing-inhibitory activity of the catechin and gallic acid combination as a novel alternative to inhibit bacterial pathogenicity.
1. Introduction
Pelargonium × hortorum, commonly known as zonal or garden geranium, is one of the most popular ornamental crops that is believed to be originated by cross hybridization between several wild species of genus Pelargonium section Ciconium [1,2]. It is an economically important taxon of the family Geraniaceae due to its value in the florist industry worldwide [2].
Pelargonium sidoides (Umckaloabo) is a medicinal plant belonging to the genus Pelargonium. It is used traditionally for the treatment of many disorders, including upper respiratory tract infections, such as bronchitis, common cold, and sinusitis [3]. The main constituents of P. sidoides showed antibacterial and antiviral activities against several respiratory pathogens [4,5,6]. Despite the popularity of Pelargonium × hortorum plants, and the medicinal importance of other species belonging to the same genus, the research studies investigating their phytochemical composition and medicinal uses are limited. Previous phytochemical research indicated that the therapeutic effect of P. sidoides is partly attributed to catechin, gallic acid, and highly oxygenated coumarins, including scopoletin, and umckalin [7,8]. Therefore, the current study was aimed at tracing the presence of these four biological and chemotaxonomic markers in different parts of two Pelargonium × hortorum cultivars grown in Saudi Arabia as ornamental flowering plants and comparing the obtained results to that of the root extract of the medicinal plant, P. sidoides, using the HPLC-UV technique.
Disseminating antibiotic resistance among the pathogenic bacterial isolates is regarded as a major public health concern owing to the high number of patients suffering from untreatable serious infections [9]. Pseudomonas aeruginosa is a ubiquitous opportunistic Gram-negative pathogenic bacterium. It is one of the major causes of hospital-acquired infections [10,11]. It can cause a wide range of antibiotic-resistant infections that are accompanied by high rates of morbidity and mortality [11,12]. Bacteria have various virulence factors such as biofilms which increase the bacterial ability to develop resistance to antibiotics by hindering their diffusion through the biofilm matrix [13]. In addition, biofilms can mediate the horizontal transfer of antibiotic resistance genes among bacterial cells impeded in biofilms [14,15]. Virulence quenching is an important strategy in the battle against the infections caused by multidrug-resistant bacteria [13]. This approach focuses on inhibiting bacterial quorum sensing, decreasing the bacterial pathogenesis, and enhancing the immune system of the host to destroy such pathogenic bacteria. This approach has an important advantage as it does not harm the host normal flora [16].
LasI/LasR is a major pathway used by P. aeruginosa for the regulation of quorum sensing using autoinducers such as acyl-homoserine lactones which activate the transcriptional regulators in the bacterial cells and induce gene transcription. Therefore, the inhibition of quorum sensing (QS) by targeting LasI and LasR enzymes could help in decreasing the antibiotic resistance and pathogenicity of P. aeruginosa [17,18].
In this study, the antibacterial activity of the methanolic extracts of different parts of Pelargonium × hortorum cultivars and their major biological markers against P. aeruginosa clinical isolates was investigated. We also elucidated the anti-biofilm and anti-quorum-sensing potential of the tested biological markers. Moreover, their mechanism of action was investigated using qRT-PCR and in silico docking studies against the main targets involved in the coordination of QS in P. aeruginosa.
2. Results and Discussion
2.1. Chromatographic Analysis
Pelargonium × hortorum and P. sidoides are important plants belonging to the same genus in the family Geraniaceae. However, there is no established analytical method that could compare the phytochemical profile of both plants. In this study, two P. × hortorum cultivars, with pink flowers (PPH), Figure 1a, and white-to-rose flowers (WPH), Figure 1b, were collected. The different parts of the collected plants were separated into roots, flowers, leaves, and stems. On the other hand, P. sidoides root extract was obtained from the commercial kalobin® solution. The method used for the comparative study is based on the presence of four reported biomarkers, including catechin, gallic acid, scopoletin, and umckalin (Figure 2) in the root extract of P. sidoides [6,19].

Figure 1.
Pelargonium × hortorum (PH) cultivars; (a) with pink flowers (PPH); (b) with white-to-rose flowers (WPH).
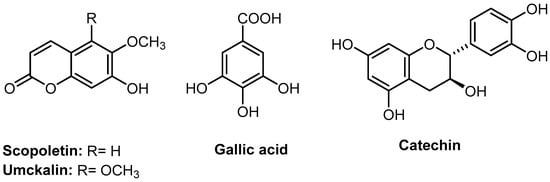
Figure 2.
Analytical standards used in the phytochemical distinction between Pelargonium × hortorum cultivars and Pelargonium sidoides.
The conditions of the simultaneous chromatographic separation were optimized by changing one parameter while maintaining other parameters constant at a time. These parameters included the mobile phase, stationary phase, wavelength, and samples preparation method. Several trials were performed with separate values of acetonitrile-water ratio and pH. The most appropriate chromatographic settings for the current study were using the acetonitrile-water ratio, 20:80 v/v (pH 3.0), a flow rate of 1 mL/min, an injection volume of 20 µL, a run time of 25 min, on a Waters XBridge® C18 column (200 mm L. × 4.6 mm W., and a particle size of 5 µm) employed at 30 °C and a wavelength of 210 nm. This analytical method is fully validated in accordance with the guidelines of the International Council for Harmonization (ICH) to show that it is linear, accurate, precise, and fast.
The four investigated biomolecules were simultaneously eluted yielding symmetrical peaks within 25 min as a minimum time of analysis, Figure 3. The retention time values were 3.3, 4.0, 8.4, and 19.2 min, for gallic acid, catechin, scopoletin, and umckalin, respectively (Figure 3a).
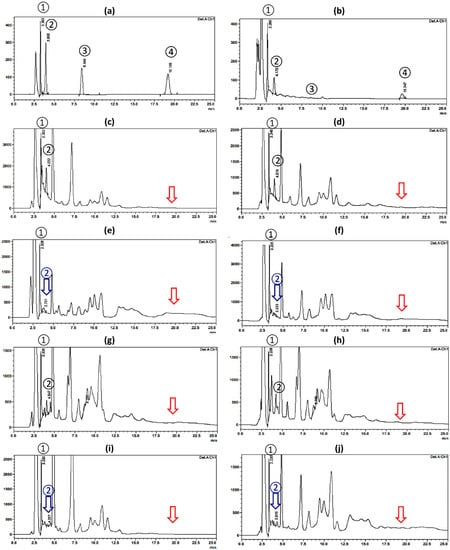
Figure 3.
HPLC-UV chromatograms (at 210 nm) on a RP-C18 column with reference standards peaks; (1) Gallic acid, (2) Catechin, (3) Scoploletin, and (4) Umckalin. (a) Mixture of reference standards biomolecules; and different plant extracts at a concentration of 10 mg/mL of; (b) Pelargonium sidoides root extract, (c) Pelargonium × hortorum with pink flowers (PPH) root, (d) Pelargonium × hortorum with white flowers (WPH) root, (e) PPH flower, (f) WPH flower, (g) PPH leaf, (h) WPH leaf, (i) PPH stem, and (j) WPH stem. The blue arrows refer to undetected catechin peaks and the red arrows refer to undetected umckalin peaks.
The HPLC-UV chromatogram of P. sidoides showed the presence of detectable peaks corresponding to the investigated biomolecules with concentrations of 22, 10.96, 0, and 3.09 μg/mL for gallic acid, catechin, scopoletin, and umckalin, respectively (Table 1 and Figure 3b).

Table 1.
HPLC-UV assay results of detected catechin, gallic acid, scopoletin, and umckalin content at a wavelength of 210 nm using RP-C18 column, in pelargonium sidoides and two Pelargonium × hortorum cultivars; with pink flowers (PPH) and with white-to-rose flowers (WPH).
The root extracts of PPH and WPH showed the presence of appreciable amounts of gallic acid (163.69 and 98.75 μg/mL, respectively) and catechin (172.56 and 104.59 μg/mL, respectively) with observable higher concentrations in PPH as displayed in Figure 3c,d, and Table 1. The presence of large amounts of catechin in the roots of PPH and WPH was further confirmed by TLC against the reference sample that showed an orange spot (Rf 0.11) on silica gel GF254, using the solvent system; CHCl3-CH3OH, 90:10 v/v (Figure S1). Similarly, the PPH and WPH leaf extracts showed the presence of gallic acid (92.29 and 82.62 μg/mL) and catechin (44.10 and 47.98 μg/mL), respectively, but in much lower concentrations than that of the root extracts. The flower and the stem extracts of both cultivars showed the absence of clear peaks for catechin. However, they showed the presence of major peaks for gallic acid in relatively high concentrations in PPH and WPH flower extracts (124.99 and 213.50 μg/mL, respectively) than in stem extracts (133.96 and 85.11 in PPH and WPH, respectively). Regarding scopoletin, although ambiguous peaks at Rt range from 8.2 to 8.6 min were detected in different extracts of P. × hortorum cultivars, TLC chromatograms against the reference standard revealed the absence of fluorescent peaks at UV254 and UV336 lights corresponding to this compound confirming its absence from these plants; Figure S1a,b (See Supplementary Materials). Moreover, none of the investigated P. × hortorum extracts showed the presence of peaks corresponding to the coumarin biomarker, umckalin; Figure 3c–j.
Therefore, by comparing the chromatograms (Figure 3) and the detected concentrations of the studied biomolecules (Table 1), it could be clearly concluded that the extracts of P. × hortorum cultivars can be distinguished from that of the medicinally used P. sidoides species by the absence of the prominent characteristic peak for umckalin. This result is in full agreement with the conclusion drawn by Harborne and Williams [19] which indicated that umckalin alone is sufficient to be used as a chemotaxonomic marker to distinguish P. sidoides from garden geraniums. We can also conclude the presence of catechin and gallic acid combination in higher concentrations in P. × hortorum root extracts in comparison to P. sidoides root extract. The root extract of Pelargonium × hortorum, especially the cultivar with the pink flowers (PPH), can be considered as a rich source for catechin and gallic acid phytochemical combination.
Phenolic compounds, such as flavonoids, anthocyanins, coumarins, and hydroxycinnamic acids, are biosynthesized in plants via the shikimate pathway [20,21]. Phenylalanine ammonia-lyase (PAL) is a key enzyme involved in this biosynthetic pathway where it catalyzes the non-oxidative deamination of L-phenylalanine to trans-cinnamic acid and ammonium [20,21]. Several studies revealed that the difference in the protein expression of PAL is related to the level of biosynthesis of phenolic compounds in plants [22,23]. The two studied P. × hortorum plants are cultivated varieties “cultivars” that refer to man-made variations within the plant species to obtain desirable characteristics. Creating new cultivars with superior traits is crucial, especially in the field of growing medicinal plants [24]. In this study, the cultivar with pink flowers (PPH) showed higher concentrations of both gallic acid and catechin. This may be attributable to the high expression of the genes responsible for PAL protein biosynthesis in this cultivar.
2.2. Biological Activity
2.2.1. Antibacterial Activity
P. aeruginosa can cause severe life-threatening infections, particularly in patients who are suffering from a weak immune system such as AIDS and cancer patients. Unfortunately, many isolates of P. aeruginosa can form biofilm which enables them to be much more resistant to the available antibiotics [25]. Consequently, it is essential to discover novel alternatives to the currently used antibiotics. Plants are a rich source for various bioactive agents which possess a wide range of biological potentials. Here, we explored the antibacterial activity of the methanolic extracts of different parts of Pelargonium × hortorum cultivars and P. sidoides root extract against P. aeruginosa isolates using the agar well diffusion method. Interestingly, all of the tested plant extracts exhibited antibacterial activity as they resulted in the appearance of inhibition zones around the wells. Thus, their MIC values were detected using the broth dilution method. Among the tested extracts, the root extracts of PPH and WPH showed the most promising antibacterial activity against the tested P. aeruginosa isolates. Noticeably, the root extract of PPH showed better results than that of WPH. The PPH root extract exhibited minimum inhibitory concentration (MIC) values ranging from 512 to 1024 µg/mL against 18 isolates, while WPH root extract exhibited MIC values within the same range against only 14 isolates, Table S1 (See Supplementary Materials). Other plant extracts including P. sidoides root extract exhibited higher MIC values of ≥2048 µg/mL against most of the tested isolates.
Catechin and gallic acid are polyphenolic compounds that are widely distributed in numerous foods and plants and they are renowned for their various biological activities [26,27]. Both biomolecules have been reported to exhibit antimicrobial activity against several bacterial species [28,29,30]. Therefore, it was concluded that the antibacterial activity of the most active PPH root extract could be attributed to the presence of catechin and gallic acid in high concentration as revealed by the HPLC-UV analytical study.
The biological activity of the herbal plant extracts is usually attributed to the presence of combination of multiple compounds [31]. Hence, the antibacterial activity of the two biomolecules, catechin and gallic acid, individually or in combination, were also tested. Remarkably, both catechin and gallic acid exhibited better antibacterial activity with lower MIC values ranging from 64 to 1024 µg/mL as depicted in Table S2 (See Supplementary Materials). In order to elucidate the relevance of the antibacterial activity of catechin and gallic acid, we compared their MICs to the MICs of the standard antibiotic (ciprofloxacin). Remarkably, their MICs were relatively close to the MICs of ciprofloxacin which had MIC values ranging from 16 to 256 µg/mL as shown in Table S2 (See Supplementary Materials). Additionally, their combination exhibited a synergistic activity as they demonstrated fractional inhibitory concentration index (FICI) value lower than 0.5. The FICI lower than one means synergy, as in this case, less concentration of the tested drugs would be required for production of the same effect as the drugs alone [32]. Therefore, both biomolecules are recommended to be used in a combination therapy which can result in reducing the ability of bacteria to develop resistance and decreasing the dose and the adverse effects of each compound in the combination [33].
2.2.2. Anti-Biofilm and Anti-Quorum-Sensing Activity
Biofilm represents an attractive target for the anti-virulence compounds because biofilm eradication can produce a negative impact on the progression of infections [34]. Hence, it is considered a promising strategy for preventing multidrug-resistant bacteria. Therefore, we explored the anti-biofilm potential of catechin, gallic acid, and their combination using a crystal violet assay. Previous studies explored the anti-quorum sensing and anti-biofilm activities of catechin or gallic acid alone [35,36,37]. However, to our knowledge, this is the first time to explore the activity of their combination. Interestingly, the combination of catechin and gallic acid exhibited more potent anti-biofilm activity in comparison to the individually tested molecules. The combination exhibited a remarkable anti-biofilm activity as it reduced the percentage of the strong and moderate biofilm-forming isolates from 52.63% to 5.26% as revealed in Table 2.

Table 2.
Impact of catechin, gallic acid and their combination on the biofilm-forming capability of P. aeruginosa isolates.
During the biofilm formation, bacterial cells communicate with each other via the QS system which induces biofilm formation, regulates the metabolic activity of the planktonic cells, and increases their virulence. LasI/LasR is a major pathway used by P. aeruginosa for the regulation of quorum sensing using autoinducers such as acyl-homoserine lactones which activate the transcriptional regulators in the bacterial cells and induce gene transcription. As the combination of catechin and gallic acid revealed the best anti-biofilm activity, we tested its impact on the gene expression of the QS genes (lasI and lasR) in ten P. aeruginosa isolates which had a strong and moderate biofilm formation ability before treatment using qRT-PCR. Remarkably, the combinatorial treatment revealed a significant downregulation in lasI and lasR gene expression in eight and six P. aeruginosa isolates, respectively, as revealed in Figure 4.
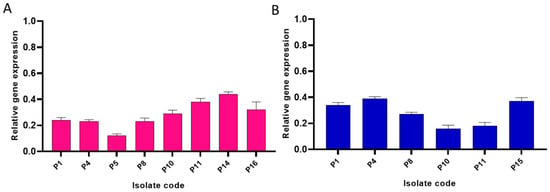
Figure 4.
Bar chart showing downregulation of the expression of (A) lasI and (B) lasR genes. In the tested isolates by combinatorial treatment with catechin and gallic acid.
2.2.3. In Vivo Assay
Moreover, the protective effect of catechin and gallic acid combination was investigated in a mice model to evaluate its in vivo antibacterial activity. The survival curve of the experimental groups was constructed as revealed in Figure 5. There was no death among the non-infected normal mice (group I). Regarding group II, representing the mice infected with untreated P. aeruginosa, four mice died after two days, four mice died after four days, and the rest died after one week. Interestingly, one mouse only died after eight days in group III representing the mice infected with P. aeruginosa treated with gallic acid and catechin combination and the remaining mice remained alive till the end of the experiment. The combination revealed a significant (p < 0.05) decline in the killing capability of the treated isolates when compared to the non-treated ones.
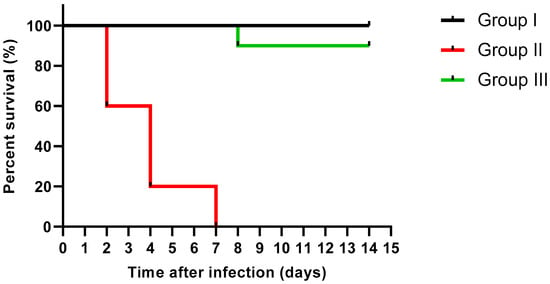
Figure 5.
Kaplan–Meier survival curve of the experimental groups after treatment with catechin and gallic acid combination.
2.3. Docking Study
In this article, we performed a molecular docking study against the transcriptional activator receptor protein (LasR) that is essential for QS in P. aeruginosa to investigate the mechanism of action of gallic acid, catechin, and their combination. Simultaneous docking is a new function that has been implemented into the new version of AutoDock Vina 1.2.0 which enables the docking of multiple ligands to the same target simultaneously [38]. We have used this functionality for the first time to dock catechin and gallic acid to the active site of LasR simultaneously. Interestingly, the docked combination showed the highest docking score (−13.07 kcal/mol, Table 3) which is greater than the individually tested molecules and the co-crystallized autoinducer, 3-oxo-C12-acylhomoserine lactone. Visualization of the molecular model of binding showed that catechin was docked into the ligand-binding domain (LBD) of LasR and formed hydrogen bonds with Thr-115, and Ser-129 amino acid residues that are reported to be involved in the interaction with the autoinducer ligand [39]. It also showed hydrogen bonding interaction with Tyr-64, Thr-75, and Leu-125 amino acid residues in the LBD (Figure 6). While gallic acid was highly fitted into a different pocket close to the LBD and formed hydrogen bonds with Gln-81, Ile-92, and Gln-98 amino acid residues (Figure 6) that may be involved in the dimerization of LasR protein. The obtained results support the high potential of catechin and gallic acid combination in inhibiting the LasR enzyme and interrupting QS and biofilm formation in P. aeruginosa.

Table 3.
Docking scores of catechin, gallic acid, and their combination against the transcriptional activator receptor (LasR) using AutoDock Vina 1.2.3.
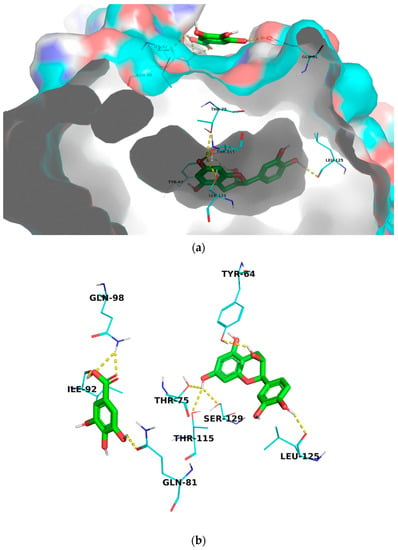
Figure 6.
Molecular model binding of simultaneously docked catechin and gallic acid to the transcriptional activator receptor (LasR, PDB code: 2UVO); (a) LasR protein in surface presentation demonstrating the docking sites of catechin and gallic acid (b) Line presentation of LasR amino acid residues involved in interaction with catechin and gallic acid.
3. Material and Methods
3.1. Plant Material
Two Pelargonium × hortorum cultivars were selected for this study; one with pink flowers (PPH), Figure 1a, and the other with white-to-rose flowers (WPH), Figure 1b. The identity of these plants was confirmed by Prof. Ibrahim Mashaly, Professor of Plant Ecology, Faculty of Science, Mansoura University, Egypt. The different parts of the plants were separated into leaves, flowers, stems, and roots to be used in the comparative HPLC-UV analytical study against P. sidoides.
3.2. Extraction
The different dried powdered parts of WPH, including 15 g of leaves, 1.9 g of flowers, 3.5 g of stems, and 3.5 g of roots, in addition to 12 g of leaves, 3 g of flowers, 6 g of roots, and 9.5 g of stems of PPH were extracted separately by rinsing with CH3OH (3 × 100 mL). The CH3OH extracts were combined and concentrated using a rotary evaporator at 45 °C then allowed to dry at room temperature. The dried total CH3OH extracts of WPH yielded leaf extract (2730.2 mg, 18.2% w/w), flower extract (309.2 mg, 16.27% w/w), root extract (468.6 mg, 13.38% w/w), and stem extract (704.2 mg, 20.12% w/w). While the dried total CH3OH extracts of PPH yielded leaf extract (2360.3 gm, 19.66% w/w), flower (308.2 mg, 10.27% w/w), root extract (798.9, 13.31% gm w/w), and stem extract (1363.9, 14.35% gm w/w). The obtained dry extracts were kept in closed containers in a refrigerator to be used in HPLC analyses.
3.3. Chromatographic Analysis
3.3.1. Mobile Phases
Acetonitrile, CH3CN (HPLC grade, ≥99.9%, SIGMA-ALDRICH®, St. Louis, MO, USA), methanol, CH3OH (HPLC grade, ≥99.9%, Fisher Chemical, Waltham, MA, USA), orthophosphoric acid, H3PO4 (HPLC, 85–90%, FLUKA®, Seelze, Germany) were used in the preparation of the mobile phase. Nylon membrane filters of 0.45 μm pore size were used in solvent filtration (HNWP, MERCK MILLIPORE® Ltd., Billerica, MA, USA). Ultra-pure (HPLC grade) water was obtained using a Milli-Q water purification system.
3.3.2. Instrument Panel
HPLC chromatographic investigation was achieved utilizing a UFLC-SHIMADZU 1200 series UFLC system (Santa Clara, CA, USA) provided with a binary pump, an autosampler, and an online degasser. Separation was achieved on a C18 column, 200 mm L. × 4.6 mm W., and 5 µm particle size (Waters XBridge®, Waters, Ireland). The system was supplied with an HPLC photodiode-array detector (SPD-20 A) set at a wavelength of 210 nm. The HPLC instrument was interfaced with a computer installed with LabSolutions software on a Microsoft Windows 7.0 operating system.
3.3.3. Chromatographic Conditions
Isocratic elution was applied using a mobile phase composed of CH3CN-H2O (25:75, v/v) with an adjusted pH at 3.0 by adding drops of 10% H3PO4. The mobile phase was then filtered by a 0.45 µm membrane filter and degassed by a sonicator for 15 min. The column was set at 30 °C as the operating temperature. An injection volume of 20 µL, a flow rate of 1 mL/min, and a total run time of 25 min were maintained during investigations.
3.3.4. Analytical Standards
Four standards were used in this study, including umckalin (HPLC purity ≥ 95.0%, CAS #: 43053-62-9, SIGMA-ALDRICH), scopoletin (HPLC purity ≥ 97.0%, CAS #: 92-61-5, SIGMA-ALDRICH), (+)-catechin (HPLC purity ≥ 99.0%, CAS #: 154-23-4, SIGMA-ALDRICH), and gallic acid (HPLC purity ≥ 99.0%, CAS Number: 149-91-7, SIGMA-ALDRICH), Figure 2.
3.3.5. Method Development
Preparation of Standard Stock Solutions and Calibration Curves
The standard stock solutions of catechin, gallic acid, scopoletin, and umckalin were prepared by dissolving 10 mg of each reference standard in 10 mL of CH3OH to obtain 1 mg/mL solution. Then, each solution was further diluted with CH3OH to obtain a 100 µg/mL final concentration. To prepare a serial dilution, the obtained stock solution, in each case, was further diluted with CH3OH to obtain different concentrations ranging from 0.1 to 1 µg/mL.
Preparation of Standard Reference Solution
The working standard solution was obtained by mixing 5 mL of each standard stock solution in a 25 mL volumetric flask, adjusting the volume with CH3OH to obtain 200 µg/mL concentration, followed by transferring 8.75 mL of the last solution to a 25 mL volumetric flask and adjusting the volume with (CH3CN:CH3OH, 1:1 v/v) to obtain 70 µg/mL concentration. Aliquots of 20 µL of this working solution were injected, the chromatograms were recorded, and the responses of the peak areas were calculated. Percentages of catechin, gallic acid, scopoletin, and umckalin in different parts of the investigated plants were recorded; then, means of the % content were calculated.
Preparation of Test Solutions
Different extracts of Pelargonium × hortorum cultivars (100 mg) were dissolved in CH3OH in a 10 mL volumetric flask, sonicated, filtered through a 0.45 µm membrane filter, and the first few mL of the filtrate was discarded to afford a 10 mg/mL solution. Aliquots of 20 µL of sample solutions were injected in triplicate and the peak areas were measured.
Preparations of Pelargonium sidoides Test Solution
For HPLC analysis, 1.1 mL of Kalobin® solution, which approximately contains 100 mg of P. sidoides dry root extract, were diluted with methanol in a 10 mL volumetric flask, sonicated, and filtered through a 0.45 µm membrane filter to prepare a 10 mg/mL solution. Aliquots of 20 µL of sample solution were injected in triplicate and the peak areas were measured.
3.4. In Vitro Antibacterial Activity
3.4.1. Bacteria
Nineteen P. aeruginosa clinical isolates were acquired from the culture collection of the Department of Pharmaceutical Microbiology, Faculty of Pharmacy, Tanta University, Egypt. Pseudomonas aeruginosa ATCC 27853 was the reference isolate.
3.4.2. Susceptibility Testing
The agar well diffusion method was utilized for testing the susceptibility of P. aeruginosa isolates to the tested agents [13]. The plates containing Muller–Hinton agar (MHA) were inoculated with the bacterial suspensions; then, wells were made and filled with the tested agents, ciprofloxacin as a positive control, and 10% dimethyl sulfoxide (DMSO) as a negative control. The plates were incubated overnight at 37 °C and they were inspected for appearance of the inhibition zones around the wells which indicates possessing antibacterial activity. Then, the MIC values were detected by a broth microdilution assay in 96-well microtitration plates as previously described using the previously mentioned positive and negative controls [40]. In order to determine the potential synergy between catechin and gallic acid, the chequerboard assay was used as previously reported [41]. We calculated the FIC according to the following formula:
The FICI of catechin and gallic acid in the combination was calculated according to the following formula:
The two compounds were considered to have a synergetic interaction in the combination if the FICI was equal to or less than 0.5.
3.4.3. Anti-Biofilm Activity
P. aeruginosa isolates were screened for their ability to form biofilm by a crystal violet assay as previously described [42,43] via determining the optical density (OD) at 590 nm using ELISA reader (Sunrise Tecan, Austria). The cut-off value (ODc) was calculated. It is defined as three standard deviations (SD) above the mean OD of the negative control. Then, the OD of each isolate was compared to the ODc to determine its biofilm-forming ability. The isolates were grouped into four classes (non-biofilm-forming, weak, moderate, and strong). Then, they were treated with catechin and gallic acid alone and in combination at sub-MIC values (1/4× MIC) and their biofilm-forming ability was explored again.
3.4.4. Anti-Quorum-Sensing Activity by Quantitative Real-Time PCR (qRT-PCR)
The impact of the sub-inhibitory concentration of gallic acid and catechin combination on the QS genes (lasI and lasR) [44] was investigated by qRT-PCR using oprL gene as a housekeeping gene [41]. Overnight cultures of P. aeruginosa (1.0 McFarland) were added to fresh 5 mL Luria–Bertani (LB) broth containing the tested compounds and grown at 37 °C for eight hours with shaking. Then, total RNA was extracted by GeneJET RNA kit (Thermo Scientific, Waltham, MA, USA) and it was converted to cDNA by the cDNA synthesis kit (iNtRON Biotechnology, Seongnam-si, Gyeonggi-do, South Korea) according to the instructions of the manufacturer. The cDNA was amplified using SYBR® Green master mix (Thermo Scientific, MA, USA). The primer sequences are listed in Table S3 (See Supplementary Materials). The 2−ΔΔCt method [45] was followed for the calculation of the relative QS gene expression.
3.5. In Vivo Protection Assay
3.5.1. Animals
We obtained thirty male albino mice from the animal house of Cairo University, Egypt. They weighed 22 g to 27 g, and they were allowed to access freely both filtered water and a standard pellet meal. Additionally, they were retained in room temperature and a 12 h light/dark cycle [46]. They were left for one week for acclimation before the beginning of the in vivo study. Our experiment was performed according to the guide of standard laboratory animal care and service. The allocated approval code was TP/RE/08-22P-0031 by the Research Ethical Committee, Faculty of Pharmacy, Tanta University, Egypt.
3.5.2. Experimental Protocol
The survival rate was determined in the different experimental groups to evaluate the ability of the combination to protect the mice against the infection caused by P. aeruginosa [9]. In brief, suspensions of P. aeruginosa were prepared (1 × 108 CFU/mL) with and without treatment with sub-inhibitory concentrations of the combination. They were intraperitoneally injected into the animals. Then, mice were randomly grouped into three groups (n = 10 mice): group I non-infected normal group, group II infected with untreated P. aeruginosa, and group III infected with treated P. aeruginosa. Survival of the mice was monitored for two weeks.
3.5.3. Statistics
Experiments were conducted in triplicate and the results are presented as mean ± standard deviation (SD). Two-way ANOVA was utilized for evaluating the statistical significance at p < 0.05. A Kaplan–Meier survival curve was established for assessing the survival rate of the animals. We used Graph Pad Prism software for the statistical analysis (Version 8, GraphPad Software Inc., San Diego, CA, USA).
3.6. Docking Study
The molecular docking study was executed using the new version of Autodock vina 1.2.3 [38,47] as described in Data S1 (See Supplementary Materials).
4. Conclusions
In this article, an HPLC-UV study has been developed for comparing the main phytochemical constituents of the different plant parts of two Pelargonium × hortorum cultivars with the medicinally used P. sidoides root extract. Umckalin was found to be characteristic for P. sidoides root extract and absent in all other tested extracts. P. × hortorum root extracts can be considered as a rich source for catechin and gallic acid combination and showed the best antibacterial activity against the tested P. aeruginosa isolates. Our study revealed that the combination between catechin and gallic acid could be a future medical approach for the treatment of the infections caused by P. aeruginosa due to its anti-biofilm and quorum-quenching activities. It was demonstrated that these activities could be attributed to the downregulation of LasR and LasI genes that are essential for quorum sensing in P. aeruginosa, in addition to inhibiting the LasR protein as predicted by the simultaneous docking study. More research studies are recommended for confirming the predicted interactions of catechin and gallic acid and the effect of their binding on the activity of LasR protein and to illuminate the anti-biofilm potential of this combination against other bacterial species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27227841/s1, Figure S1: TLC chromatograms of investigated plant extracts against reference standards; Table S1: Minimum inhibitory concentration (MIC) of investigated extracts; Table S2: Minimum inhibitory concentration (MIC) values of catechin and gallic acid against P. aeruginosa isolates; Table S3: Sequences of the utilized primers; Data S1: docking study procedure [38,47,48,49].
Author Contributions
Conceptualization, F.M.A.B.; methodology, M.A.A., E.E., A.A.K. and M.H.E.; formal analysis, M.A.A., M.A.A.A., M.H.E. and E.E.; investigation, M.A.A., E.E., A.A.K. and M.H.E.; resources, F.M.A.B. and E.M.; data curation, M.A.A., E.E., M.H.E. and E.M.; writing—original draft preparation, M.H.E., M.A.A., E.M., E.E. and A.A.K.; writing—review and editing, F.M.A.B., M.H.E. and E.E.; visualization, M.A.A., E.E. and M.H.E.; supervision, F.M.A.B.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The Deanships of Graduate Studies and Scientific Research at Prince Sattam bin Abdulaziz University are acknowledged for supporting this research.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence this work.
Sample Availability Statement
Samples of the compounds are not available from the authors.
References
- Harney, P.M. A chromatographic study of species presumed ancestral to Pelargonium × hortorum Bailey. Can. J. Genet. Cytol. 1966, 8, 780–787. [Google Scholar] [CrossRef]
- Hondo, K.; Iwasaka, C.; Kakihara, F. Characteristics of progeny obtained from the cross between geraniums (Pelargonium × hortorum) and wild species in section Ciconium, including heat tolerance and odor of leaves. Environ. Control. Biol. 2014, 52, 37–43. [Google Scholar] [CrossRef]
- Timmer, A.; Günther, J.; Motschall, E.; Rücker, G.; Antes, G.; Kern, W.V. Pelargonium sidoides extract for treating acute respiratory tract infections. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Careddu, D.; Pettenazzo, A. Pelargonium sidoides extract EPs 7630: A review of its clinical efficacy and safety for treating acute respiratory tract infections in children. Int. J. Gen. Med. 2018, 11, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kayser, O.; Kolodziej, H. Antibacterial activity of extracts and constituents of Pelargonium sidoides and Pelargonium reniforme. Planta Med. 1997, 63, 508–510. [Google Scholar] [CrossRef]
- Alossaimi, M.A.; Alzeer, M.A.; Abdel Bar, F.M.; ElNaggar, M.H. Pelargonium sidoides Root Extract: Simultaneous HPLC Separation, Determination, and Validation of Selected Biomolecules and Evaluation of SARS-CoV-2 Inhibitory Activity. Pharmaceuticals 2022, 15, 1184. [Google Scholar] [CrossRef] [PubMed]
- Colling, J.; Groenewald, J.-H.; Makunga, N. Genetic alterations for increased coumarin production lead to metabolic changes in the medicinally important Pelargonium sidoides DC (Geraniaceae). Metab. Eng. 2010, 12, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H. Fascinating metabolic pools of Pelargonium sidoides and Pelargonium reniforme, traditional and phytomedicinal sources of the herbal medicine Umckaloabo®. Phytomedicine 2007, 14, 9–17. [Google Scholar] [CrossRef]
- Khayat, M.T.; Abbas, H.A.; Ibrahim, T.S.; Khayyat, A.N.; Alharbi, M.; Darwish, K.M.; Elhady, S.S.; Khafagy, E.S.; Safo, M.K.; Hegazy, W.A.H. Anti-Quorum Sensing Activities of Gliptins against Pseudomonas aeruginosa and Staphylococcus aureus. Biomedicines 2022, 10, 1169. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef]
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef]
- Montero, M.M.; Lopez Montesinos, I.; Knobel, H.; Molas, E.; Sorlí, L.; Siverio-Parés, A.; Prim, N.; Segura, C.; Duran-Jordà, X.; Grau, S. Risk factors for mortality among patients with Pseudomonas aeruginosa bloodstream infections: What is the influence of XDR phenotype on outcomes? J. Clin. Med. 2020, 9, 514. [Google Scholar] [CrossRef]
- Alotaibi, B.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Elharty, M.E.; Saleh, A.; Abdelkader, D.H.; Mokhtar, F.A. Antibacterial activity of nano zinc oxide green-synthesised from Gardenia thailandica triveng. Leaves against Pseudomonas aeruginosa clinical isolates: In vitro and in vivo study. Artif. Cells Nanomed. Biotechnol. 2022, 50, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, B.; Mokhtar, F.A.; El-Masry, T.A.; Elekhnawy, E.; Mostafa, S.A.; Abdelkader, D.H.; Elharty, M.E.; Saleh, A.; Negm, W.A. Antimicrobial Activity of Brassica rapa L. Flowers Extract on Gastrointestinal Tract Infections and Antiulcer Potential Against Indomethacin-Induced Gastric Ulcer in Rats Supported by Metabolomics Profiling. J. Inflamm. Res. 2021, 14, 7411–7430. [Google Scholar] [CrossRef] [PubMed]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Ford, C.A.; Hurford, I.M.; Cassat, J.E. Antivirulence Strategies for the Treatment of Staphylococcus aureus Infections: A Mini Review. Front. Microbiol. 2020, 11, 632706. [Google Scholar] [CrossRef]
- Markus, V.; Golberg, K.; Teralı, K.; Ozer, N.; Kramarsky-Winter, E.; Marks, R.S.; Kushmaro, A. Assessing the molecular targets and mode of action of furanone C-30 on Pseudomonas aeruginosa quorum sensing. Molecules 2021, 26, 1620. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wu, G. Can biofilm be reversed through quorum sensing in Pseudomonas aeruginosa? Front. Microbiol. 2019, 10, 1582. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Phytochemistry of the genus Geranium. In Geranium and Pelargonium; The genera Geranium and Pelargonium; Lis-Balchin, M., Ed.; Taylor & Francis: London, UK; New York, NY, USA, 2002; pp. 20–29. [Google Scholar]
- Jones, D.H. Phenylalanine ammonia-lyase: Regulation of its induction, and its role in plant development. Phytochemistry 1984, 23, 1349–1359. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Ortega-García, F.; Peragón, J. Phenylalanine ammonia-lyase, polyphenol oxidase, and phenol concentration in fruits of Olea europaea L. cv. Picual, Verdial, Arbequina, and Frantoio during ripening. J. Agric. Food Chem. 2009, 57, 10331–10340. [Google Scholar] [CrossRef]
- Sun, J.; Peng, H.; Zhang, E.; You, X.; Huang, M.; Xu, L.; Wang, J. Comparison of enzymes and flavonoids related to postharvest browning in three litchi (Litchi chinensis SONN.) cultivars. J. Food Biochem. 2011, 35, 1603–1611. [Google Scholar] [CrossRef]
- Hamany Djande, C.Y.; Pretorius, C.; Tugizimana, F.; Piater, L.A.; Dubery, I.A. Metabolomics: A Tool for Cultivar Phenotyping and Investigation of Grain Crops. Agronomy 2020, 10, 831. [Google Scholar] [CrossRef]
- Alam, K.; Farraj, D.A.A.; Mah, E.F.S.; Yameen, M.A.; Elshikh, M.S.; Alkufeidy, R.M.; Mustafa, A.; Bhasme, P.; Alshammari, M.K.; Alkubaisi, N.A.; et al. Anti-biofilm activity of plant derived extracts against infectious pathogen-Pseudomonas aeruginosa PAO1. J. Infect. Public Health 2020, 13, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 1–10. [Google Scholar] [CrossRef]
- Oriakhi, K.; Orumwensodia, K.O. Combinatorial effect of gallic acid and catechin on some biochemical and pro-inflammatory markers in CCl4-mediated hepatic damage in rats. Phytomed. Plus 2021, 1, 100017. [Google Scholar] [CrossRef]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial activity of gallic acid against food-related Pseudomonas strains and its use as biocontrol tool to improve the shelf life of fresh black truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Sarjit, A.; Wang, Y.; Dykes, G.A. Antimicrobial activity of gallic acid against thermophilic Campylobacter is strain specific and associated with a loss of calcium ions. Food Microbiol. 2015, 46, 227–233. [Google Scholar] [CrossRef]
- Bai, L.; Takagi, S.; Ando, T.; Yoneyama, H.; Ito, K.; Mizugai, H.; Isogai, E. Antimicrobial activity of tea catechin against canine oral bacteria and the functional mechanisms. J. Vet. Med. Sci. 2016, 78, 1439–1445. [Google Scholar] [CrossRef]
- Kolodziej, H.; Kiderlen, A.F. In vitro evaluation of antibacterial and immunomodulatory activities of Pelargonium reniforme, Pelargonium sidoides and the related herbal drug preparation EPs 7630. Phytomedicine 2007, 14 (Suppl. 6), 18–26. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Pournaras, S.; Roilides, E.; Walsh, T.J. Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2010, 54, 602–609. [Google Scholar] [CrossRef]
- Shang, D.; Liu, Y.; Jiang, F.; Ji, F.; Wang, H.; Han, X. Synergistic Antibacterial Activity of Designed Trp-Containing Antibacterial Peptides in Combination With Antibiotics Against Multidrug-Resistant Staphylococcus epidermidis. Front. Microbiol. 2019, 10, 2719. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Das, M.C.; Sandhu, P.; Das, N.; Tribedi, P.; De, U.C.; Akhter, Y.; Bhattacharjee, S. Antibiofilm activity of Parkia javanica against Pseudomonas aeruginosa: A study with fruit extract. RSC Adv. 2017, 7, 5497–5513. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Mukherjee, I.; Ghosh, S.; Dey, A.; Banerjee, R.; Ray, R.R. Catechin as the Most Efficient Bioactive Compound from Azadirachta indica with Antibiofilm and Anti-quorum Sensing Activities Against Dental Biofilm: An In vitro and in silico Study. Appl. Biochem. Biotechnol. 2021, 193, 1617–1630. [Google Scholar] [CrossRef]
- Santos, C.A.; Lima, E.M.F.; Franco, B.D.G.d.M.; Pinto, U.M. Exploring phenolic compounds as quorum sensing inhibitors in foodborne bacteria. Front. Microbiol. 2021, 12, 735931. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; El Jaziri, M.; Baucher, M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010, 76, 243–253. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2. 0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Chowdhury, N.; Bagchi, A. Molecular insight into the activity of LasR protein from Pseudomonas aeruginosa in the regulation of virulence gene expression by this organism. Gene 2016, 580, 80–87. [Google Scholar] [CrossRef]
- Attallah, N.G.; Al-Fakhrany, O.M.; Elekhnawy, E.; Hussein, I.A.; Shaldam, M.A.; Altwaijry, N.; Alqahtani, M.J.; Negm, W.A. Anti-Biofilm and Antibacterial Activities of Cycas media R. Br Secondary Metabolites: In silico, in vitro, and in vivo Approaches. Antibiotics 2022, 11, 993. [Google Scholar] [CrossRef]
- Bahari, S.; Zeighami, H.; Mirshahabi, H.; Roudashti, S.; Haghi, F. Inhibition of Pseudomonas aeruginosa quorum sensing by subinhibitory concentrations of curcumin with gentamicin and azithromycin. J. Glob. Antimicrob. Resist. 2017, 10, 21–28. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Adeyemo, R.O.; Famuyide, I.M.; Dzoyem, J.P.; Lyndy Joy, M. Anti-Biofilm, Antibacterial, and Anti-Quorum Sensing Activities of Selected South African Plants Traditionally Used to Treat Diarrhoea. Evid.-Based Complement. Altern. Med. 2022, 2022, 1307801. [Google Scholar] [CrossRef]
- Naga, N.G.; Zaki, A.A.; El-Badan, D.E.; Rateb, H.S.; Ghanem, K.M.; Shaaban, M.I. Methoxyisoflavan derivative from Trigonella stellata inhibited quorum sensing and virulence factors of Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2022, 38, 156. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Negm, W.A.; El-Kadem, A.H.; Elekhnawy, E.; Attallah, N.G.; Al-Hamoud, G.A.; El-Masry, T.A.; Zayed, A. Wound-Healing Potential of Rhoifolin-Rich Fraction Isolated from Sanguisorba officinalis Roots Supported by Enhancing Re-Epithelization, Angiogenesis, Anti-Inflammatory, and Antimicrobial Effects. Pharmaceuticals 2022, 15, 178. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar]
- Bottomley, M.J.; Muraglia, E.; Bazzo, R.; Carfì, A. Molecular Insights into Quorum Sensing in the Human Pathogen Pseudomonas aeruginosa from the Structure of the Virulence Regulator LasR Bound to Its Autoinducer. J. Biol. Chem. 2007, 282, 13592–13600. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).