Chemical Profile and Antioxidant Capacity of Propolis from Tetragonula, Lepidotrigona, Lisotrigona and Homotrigona Stingless Bee Species in Vietnam
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Profile of Stingless Bee Propolis
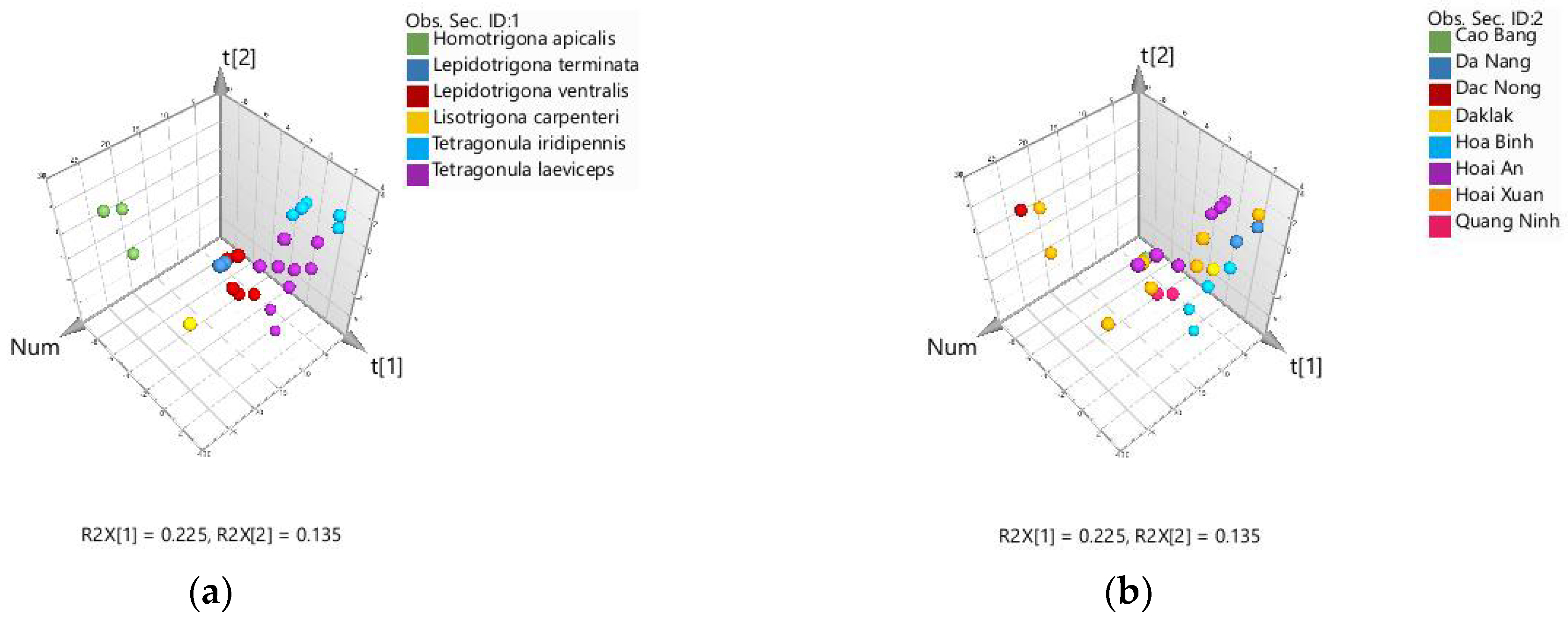
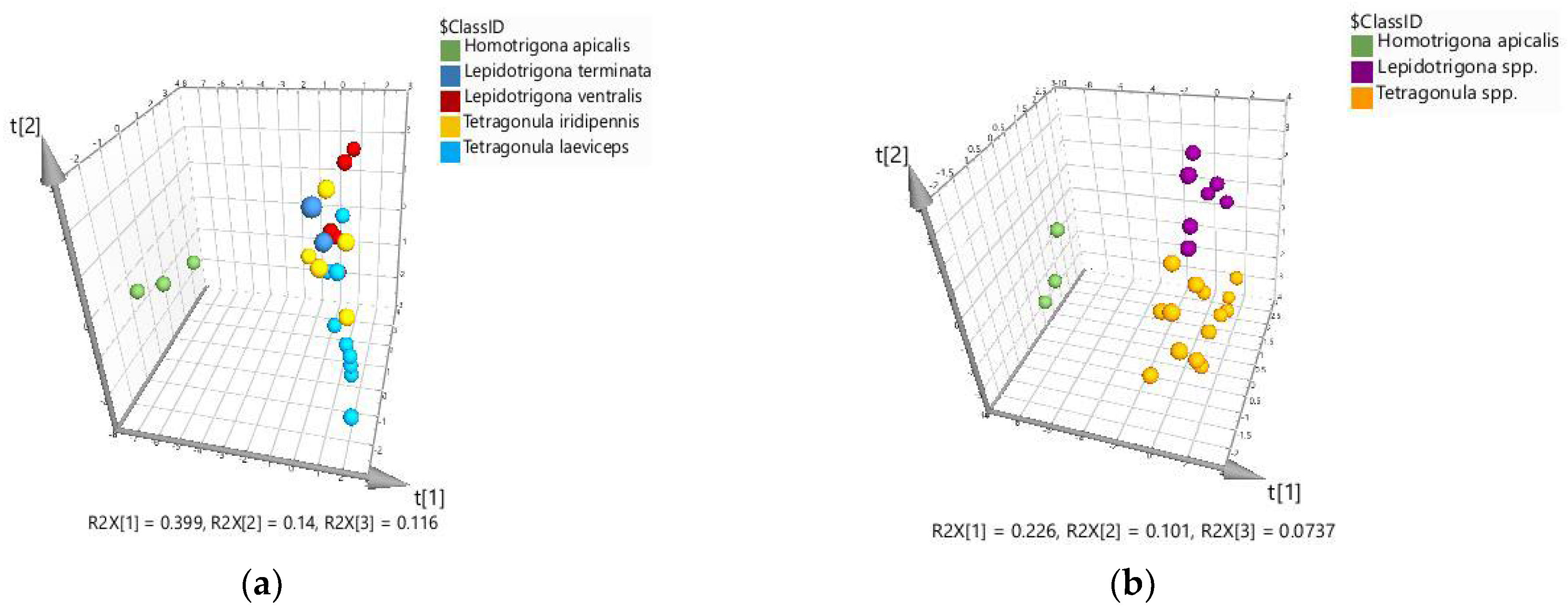
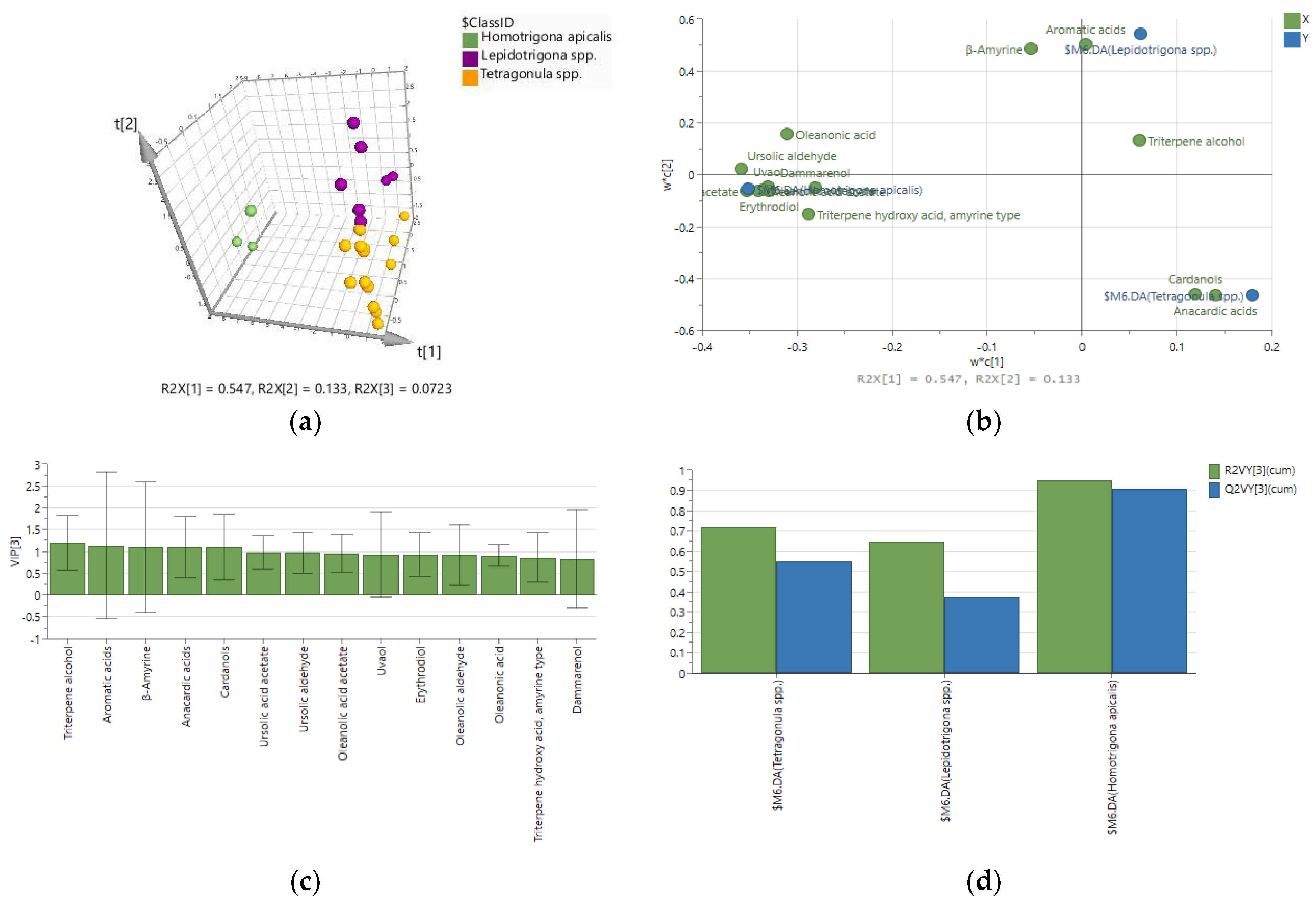
2.2. Chemometric Discrimination of Propolis Samples
2.3. Antioxidant Capacity of Stingless Bee Propolis
3. Materials and Methods
3.1. Propolis Samples
3.2. Extraction of Propolis
3.3. GC-MS Analysis and Compound Identification
3.4. Free Radical Scavenging Activity
3.5. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6. Chemometric and Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hrncir, M.; Jarau, S.; Barth, F.G.J. Stingless bees (Meliponini): Senses and behavior. J. Comp. Physiol. 2016, 202, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Grüter, C. Stingless Bees: An Overview. In Stingless Bees. Fascinating Life Sciences; Springer: Cham, Switzerland, 2020; pp. 1–42. [Google Scholar]
- Roubik, D.W. Stingless bee nesting biology. Apidologie 2006, 37, 124–143. [Google Scholar] [CrossRef]
- Rattanawannee, A.; Duangphakdee, O. Southeast Asian meliponiculture for sustainable livelihood. In Modern Beekeeping: Bases for Sustainable Production; Ranz, R.E.R., Ed.; IntechOpen: London, UK, 2020; Chapter 10; pp. 173–190. [Google Scholar]
- Michener, C.D.; Grimaldi, D.A. The oldest fossil bee: Apoid history, evolutionary stasis, and antiquity of social behavior. Proc. Natl. Acad. Sci. USA 1988, 85, 6424–6426. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Pereira, L.R.L.; Salatino, M.L.F. The emerging market of propolis of stingless bees in tropical countries. MOJ Food Process. Technol. 2019, 7, 27–29. [Google Scholar]
- Shanahan, M.; Spivak, M. Resin use by stingless bees: A review. Insects 2021, 12, 719. [Google Scholar] [CrossRef]
- Eltz, T.; Bruhl, C.A.; van der Kaars, S.; Linsenmair, K.E. Determinants of stingless bee nest density in lowland dipterocarp forest of Sabah, Malaysia. Oecologia 2002, 131, 27–34. [Google Scholar] [CrossRef]
- Kwapong, P.K.; Aidoo, K.; Combey, R.; Karikari, A.S. Stingless Bees: Importance, Management and Utilization; Unimax Macmilland Press: Accra North, Ghana, 2010. [Google Scholar]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73 (Suppl. 1), S1–S6. [Google Scholar] [CrossRef]
- Farooqui, T.; Farooqui, A.A. Beneficial effects of propolis on human health and neurological diseases. Front. Biosci. 2012, 4, 779–793. [Google Scholar] [CrossRef]
- Choudhari, M.K.; Punekar, S.A.; Ranade, R.V.; Paknikar, K.M. Antimicrobial activity of stingless bee (Trigona sp.) propolis used in the folk medicine of Western Maharashtra, India. J. Ethnopharmacol. 2012, 141, 363–367. [Google Scholar] [CrossRef]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its role and efficacy in human health and diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef]
- Salatino, A.; Fernandes-Silva, C.C.; Righi, A.A.; Salatino, M.L.F. Propolis research and the chemistry of plant products. Nat. Prod. Rep. 2011, 28, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Popova, M.; Trusheva, B. The phytochemistry of the honeybee. Phytochemistry 2018, 155, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Drescher, N.; Wallace, H.M.; Katouli, M.; Massaro, C.F.; Leonhardt, S.D. Diversity matters: How bees benefit from different resin sources. Oecologia 2014, 176, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, K.; Popova, M.; Dimitrova, L.; Trusheva, B.; Thanh, L.N.; Phuong, D.T.L.; Lien, N.T.; Najdenski, H.; Bankova, V. Phytochemical analysis of Vietnamese propolis produced by the stingless bee Lisotrigona cacciae. PLoS ONE 2019, 14, e0216074. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Bankova, V. Propolis of stingless bees: A phytochemist’s guide through the jungle of tropical biodiversity. Phytomedicine 2021, 86, 153098. [Google Scholar] [CrossRef]
- Bankova, V.; Bertelli, D.; Borba, R.; Conti, B.J.; da Silva Cunha, I.B.; Danert, C.; Eberlin, M.N.; Falcão, S.I.; Isla, M.I.; Moreno, M.I.N.; et al. Standard methods for Apis mellifera propolis research. J. Apic. Res. 2019, 58, 1–49. [Google Scholar] [CrossRef]
- Inui, S.; Hosoya, T.; Kumazawa, S. Hawaiian propolis: Comparative analysis and botanical origin. Nat. Prod. Commun. 2014, 9, 165–166. [Google Scholar] [CrossRef]
- Boonnak, N.; Karalai, C.; Chantrapromma, S.; Ponglimanont, C.; Fun, H.-K.; Kanjana-Opas, A.; Chantrapromma, K.; Kato, S. Anti-Pseudomonas aeruginosa xanthones from the resin and green fruits of Cratoxylum cochinchinense. Tetrahedron 2009, 65, 3003–3013. [Google Scholar] [CrossRef]
- Chailap, B.; Nuanyai, T.; Puthong, S.; Buakeaw, A. Chemical constituents of fruits and leaves of Cratoxylum cochinchinense and their cytotoxic activities. Naresuan Univ. J. Sci. Technol. 2017, 25, 22–30. [Google Scholar]
- Oanh, V.T.K.; Thoa, H.T.; Hang, N.T.M.; Phuong, D.T.L.; Lien, N.T.P.; Popova, M.; Trusheva, B.; Bankova, V.; Le, T.N. New dihydrochromene and xanthone derivatives from Lisotrigona furva propolis. Fitoterapia 2021, 149, 104821. [Google Scholar] [CrossRef]
- Bisset, N.G.; Chavanel, V.; Lantz, J.-P.; Wolffer, E. Constituants sesquiterpéniques et triterpéniques des resins du genre Shorea. Phytochemistry 1971, 10, 2451–2463. [Google Scholar] [CrossRef]
- Burger, P.; Charrie-Duhaut, A.; Connan, J.; Flecker, M.; Albrecht, P. Archaeological resinous samples from Asian wrecks: Taxonomic characterization by GC-MS. Anal. Chim. Acta 2009, 648, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Sanpa, S.; Popova, M.; Bankova, V.; Tunkasiri, T.; Eitssayeam, S.; Panuwan, S. Antibacterial compounds from propolis of Tetragonula laeviceps and Tetrigona melanoleuca (Hymenoptera: Apidae) from Thailand. PLoS ONE 2015, 10, e0126886. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar]
- Chen, Y.; Zhu, S.-B.; Xie, M.-Y.; Nie, S.-P.; Liu, W.; Li, C.; Gong, X.-F.; Wang, Y.-X. Quality control and original discrimination of Ganoderma lucidum based on high-performance liquid chromatographic fingerprints and combined chemometrics methods. Anal. Chim. Acta 2008, 623, 146–156. [Google Scholar] [CrossRef]
- Nugitrangson, P.; Puthong, S.; Iempridee, T.; Pimtong, W.; Pornpakakul, S.; Chanchao, C. In vitro and in vivo characterization of the anticancer activity of Thai stingless bee (Tetragonula laeviceps) cerumen. Exp. Biol. Med. 2015, 241, 166–176. [Google Scholar] [CrossRef]
- Ishizu, E.; Honda, S.; Vongsak, B.; Kumazawa, S. Identification of plant origin of propolis from Thailand stingless bees by comparative analysis. Nat. Prod. Commun. 2018, 13, 973–975. [Google Scholar] [CrossRef]
- Roubik, D.W. Nest structure: Stingless bees. In Encyclopedia of Social Insects; Starr, S.K., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–6. [Google Scholar]
- Jalil, A.H. Beescape for Meliponines: Conservation of Indo-Malayan Stingless Bees; Partridge Singapore: Singapore, 2014. [Google Scholar]
- Mohamed, W.A.S.; Ismail, N.Z.; Omar, E.A.; Samad, N.A.; Adam, S.K.; Mohamad, S. GC-MS evaluation, antioxidant content, and cytotoxic activity of propolis extract from Peninsular Malaysian stingless bees, Tetrigona apicalis. Evid. Based Complement. Altern. Med. 2020, 2020, 8895262. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Blüthgen, N. A sticky affair: Resin collection by Bornean stingless bees. Biotropica 2009, 41, 730–736. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Blüthgen, N.; Schmitt, T. Smelling like resin: Terpenoids account for species-specific cuticular profiles in Southeast-Asian stingless bees. Insect. Soc. 2009, 56, 157–170. [Google Scholar] [CrossRef]
- Nghia, N.H. Dipterocarp Species in Vietnam. In Proceedings of the Seven Round-table Conference on Dipterocarp, Kuala Lumpur, Malaysia, 7–10 October 2002; Aminak, H., Ani, S., Sim, H.C., Krishnapillay, B., Eds.; pp. 73–79. [Google Scholar]
- Miguel-Chávez, R.S. Phenolic antioxidant capacity: A review of the state of the art. In Phenolic Compounds—Biological Activities; Soto-Hernandez, M., Garcia-Mateos, M.R., Eds.; InTechc: Zagreb, Croatia, 2017; Chapter 4; pp. 59–74. [Google Scholar]
- Jemli, M.E.; Kamal, R.; Marmouzi, I.; Zerrouki, A.; Cherrah, Y.; Alaoui, K. Radical scavenging activity and ferric reducing ability of Juniperus thurifera (L.), J. Oxycedrus (L.), J. phoenicea (L.) and Tetraclinis articulata (L.). Adv. Pharmacol. Sci. 2016, 2016, 6392656. [Google Scholar] [PubMed]
- Petrov, P.D.; Tsvetanov, C.B.; Mokreva, P.; Yoncheva, K.; Konstantinov, S.; Trusheva, B.; Popova, M.; Bankova, V. Novel micellar form of poplar propolis with high cytotoxic activity. RSC Adv. 2016, 6, 30728–30731. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Dezmirean, D.S.; Paşca, C.; Moise, A.R.; Bobiş, A. Plant sources responsible for the chemical composition and main bioactive properties of poplar-type propolis. Plants 2021, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Masuoka, N.; Ha, T.J.; Tsujimoto, K. Antioxidant activity of anacardic acids. Food Chem. 2006, 99, 555–562. [Google Scholar] [CrossRef]
- Masuoka, N.; Tamsampaoloet, K.; Chavasiri, W.; Kubo, I. Superoxide anion scavenging activity of alk(en)yl phenol compounds by using PMS-NADH system. Heliyon 2016, 2, e00169. [Google Scholar] [CrossRef]
- Tsujimoto, K.; Hayashi, A.; Ha, T.J.; Kubo, I. Anacardic acids and ferric ion chelation. Z. Naturforsch. 2008, 62c, 710–716. [Google Scholar] [CrossRef]
- Trevisan, M.T.; Pfundstein, B.; Haubner, R.; Würtele, G.; Spiegelhalder, B.; Bartsch, H.; Owen, R.W. Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. Food Chem. Toxicol. 2006, 44, 188–197. [Google Scholar] [CrossRef]
- Nafi, N.E.M.; Zin, N.B.M.; Pauzi, N.; Khadar, A.S.A.; Anisava, A.R.; Badiazaman, A.A.M.; Khamsah Mohd, K.S. Cytotoxicity, antioxidant and phytochemical screening of propolis extracts from four different Malaysian stingless bee species. Malays. J. Fundam. Appl. Sci. 2018, 15, 307–312. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH) tests. J. Am. Oil Chem. Soc. 2002, 79, 1191–1195. [Google Scholar] [CrossRef]
- Tomasina, F.; Carabio, C.; Celano, L.; Thomson, L. Analysis of two methods to evaluate antioxidants. Biochem. Mol. Biol. Educ. 2012, 40, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 77–106. [Google Scholar]
| Code | Geographical Location | Bee Species | Time of Collection (Month/Year) |
|---|---|---|---|
| V-1 | Da Nang | Tetragonula iridipennis | 07/2018 |
| V-2 | Da Nang | Tetragonula laeviceps | 07/2018 |
| V-3 | Hoa Binh | Tetragonula laeviceps | 11/2018 |
| V-4 | Hoa Binh | Tetragonula laeviceps | 11/2018 |
| V-5 | Hoa Binh | Tetragonula laeviceps | 11/2018 |
| V-6 | Quang Ninh | Lepidotrigona ventralis | 11/2018 |
| V-7 | Quang Ninh | Lepidotrigona ventralis | 11/2018 |
| V-8 | Daklak | Tetragonula laeviceps | 11/2018 |
| V-9 | Hoa Binh | Tetragonula laeviceps | 11/2018 |
| V-10 | Hoai Xuan | Lisotrigona carpenteri | 09/2019 |
| V-11 | Hoai Xuan | Tetragonula laeviceps | 09/2019 |
| V-12 | Hoai Xuan | Tetragonula iridipennis | 09/2019 |
| V-13 | Hoai Xuan | Tetragonula laeviceps | 09/2019 |
| V-14 | Hoai Xuan | Lepidotrigona ventralis | 09/2019 |
| V-15 | Hoai Xuan | Homotrigona apicalis | 09/2019 |
| V-16 | Hoai Xuan | Lepidotrigona terminata | 09/2019 |
| V-17 | Hoai Xuan | Homotrigona apicalis | 09/2019 |
| V-18 | Hoai An | Tetragonula iridipennis | 09/2019 |
| V-19 | Hoai An | Tetragonula iridipennis | 09/2019 |
| V-20 | Hoai An | Tetragonula iridipennis | 09/2019 |
| V-21 | Hoai An | Lepidotrigona ventralis | 09/2019 |
| V-22 | Hoai An | Tetragonula laeviceps | 09/2019 |
| V-23 | Hoai An | Lepidotrigona terminata | 09/2019 |
| V-24 | Cao Bang | Lepidotrigona ventralis | 06/2019 |
| V-25 | Dac Nong | Homotrigona apicalis | 04/2019 |
| Sample | DPPH, %RSA | FRAP, μmol/L |
|---|---|---|
| Tetragonula iridipennis | ||
| V-1 | 5.43 ± 0.69 | 150.63 ± 3.77 |
| V-12 | 9.15 ± 0.13 | 149.09 ± 10.47 |
| V-18 | 3.79 ± 0.18 | 187.90 ± 6.64 |
| V-19 | 6.80 ± 0.46 | 246.29 ± 9.50 |
| V-20 | 2.45 ± 0.11 | 145.01 ± 4.96 |
| Tetragonula laeviceps | ||
| V-2 | 9.03 ± 0.25 | 267.70 ± 3.33 |
| V-3 | 12.71 ± 0.89 | 186.22 ± 2.06 |
| V-4 | 46.19 ± 0.18 | 706.29 ± 1.70 |
| V-5 | 32.21 ± 0.04 | 696.23 ± 8.84 |
| V-8 | 9.90 ± 0.19 | 128.81 ± 2.19 |
| V-9 | 19.09 ± 0.04 | 403.87 ± 3.23 |
| V-11 | 6.79 ± 0.07 | 168.95 ± 2.14 |
| V-13 | 12.26 ± 0.21 | 258.70 ± 15.09 |
| V-22 | 34.40 ± 0.37 | 456.71 ± 7.49 |
| Lepidotrigona ventralis | ||
| V-6 | 21.93 ± 0.27 | 487.16 ± 4.22 |
| V-7 | 35.86 ± 0.98 | 633.57 ± 1.10 |
| V-14 | 10.36 ± 0.57 | 460.79 ± 3.49 |
| V-21 | 66.83 ± 0.67 | 1013.30 ± 1.31 |
| V-24 | 40.61 ± 0.33 | 1156.47 ± 8.12 |
| Lepidotrigona terminata | ||
| V-16 | 27.50 ± 0.53 | 415.76 ± 0.80 |
| V-23 | 6.86 ± 0.37 | 323.52 ± 0.73 |
| Homotrigona apicalis | ||
| V-15 | 2.48 ± 0.14 | 103.58 ± 6.65 |
| V-17 | 3.94 ± 0.32 | 117.45 ± 2.03 |
| V-25 | 9.84 ± 0.41 | 283.30 ± 2.34 |
| Lisotrigona carpenteri | ||
| V-10 | 37.90 ± 0.22 | 367.01 ± 10.15 |
| Apis mellifera b (Bulgarian propolis) | 21.68 ± 0.20 | 410.19 ± 3.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, M.; Trusheva, B.; Chimshirova, R.; Antonova, D.; Gechovska, K.; Thanh, L.N.; Lien, N.T.P.; Phuong, D.T.L.; Bankova, V. Chemical Profile and Antioxidant Capacity of Propolis from Tetragonula, Lepidotrigona, Lisotrigona and Homotrigona Stingless Bee Species in Vietnam. Molecules 2022, 27, 7834. https://doi.org/10.3390/molecules27227834
Popova M, Trusheva B, Chimshirova R, Antonova D, Gechovska K, Thanh LN, Lien NTP, Phuong DTL, Bankova V. Chemical Profile and Antioxidant Capacity of Propolis from Tetragonula, Lepidotrigona, Lisotrigona and Homotrigona Stingless Bee Species in Vietnam. Molecules. 2022; 27(22):7834. https://doi.org/10.3390/molecules27227834
Chicago/Turabian StylePopova, Milena, Boryana Trusheva, Ralitsa Chimshirova, Daniela Antonova, Kamelia Gechovska, Le Nguyen Thanh, Nguyen Thi Phuong Lien, Diep Thi Lan Phuong, and Vassya Bankova. 2022. "Chemical Profile and Antioxidant Capacity of Propolis from Tetragonula, Lepidotrigona, Lisotrigona and Homotrigona Stingless Bee Species in Vietnam" Molecules 27, no. 22: 7834. https://doi.org/10.3390/molecules27227834
APA StylePopova, M., Trusheva, B., Chimshirova, R., Antonova, D., Gechovska, K., Thanh, L. N., Lien, N. T. P., Phuong, D. T. L., & Bankova, V. (2022). Chemical Profile and Antioxidant Capacity of Propolis from Tetragonula, Lepidotrigona, Lisotrigona and Homotrigona Stingless Bee Species in Vietnam. Molecules, 27(22), 7834. https://doi.org/10.3390/molecules27227834







