Discovery of Levesquamide B through Global Natural Product Social Molecular Networking
Abstract
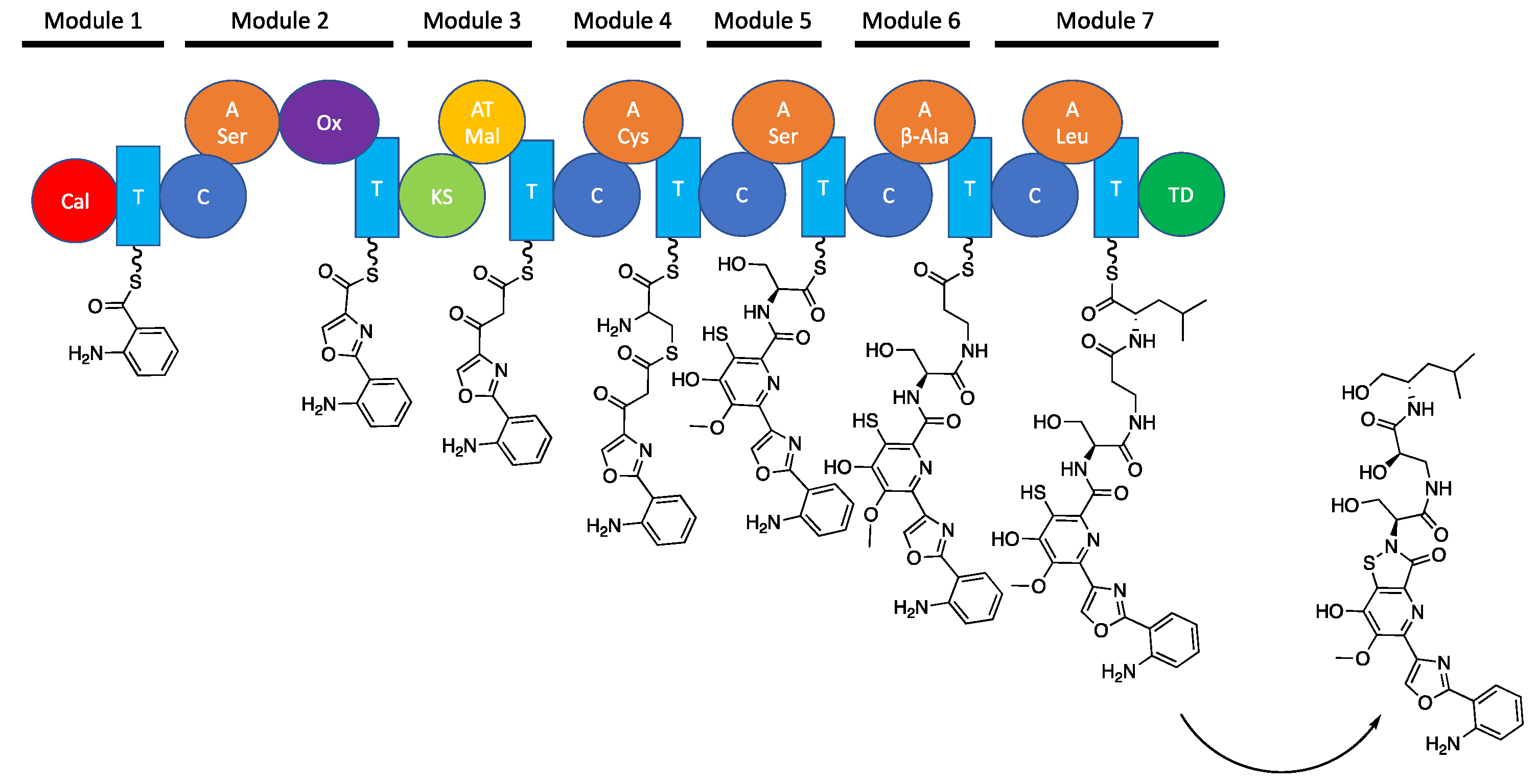
1. Introduction
2. Results & Discussion
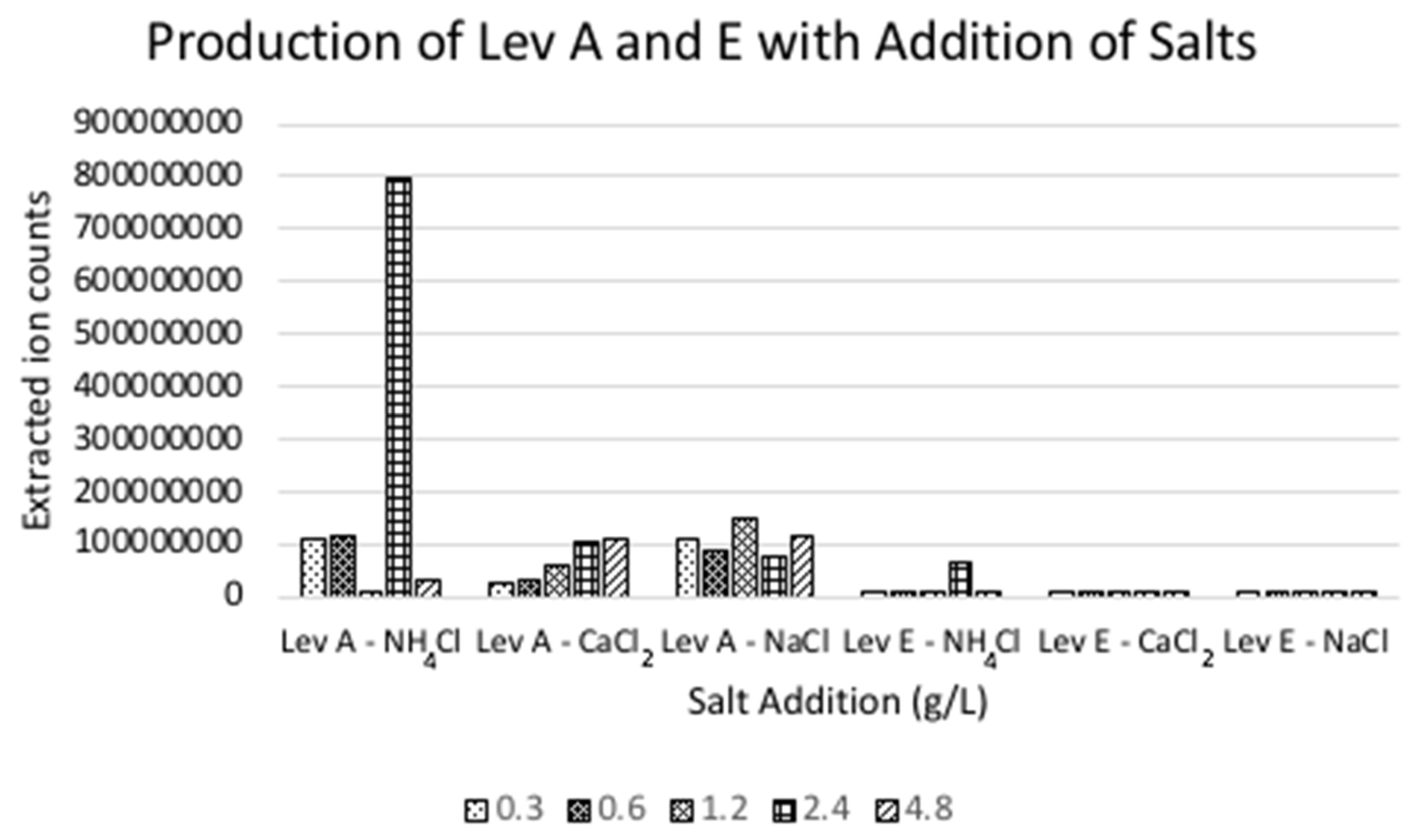
2.1. Salt Screen for Levesquamide Production
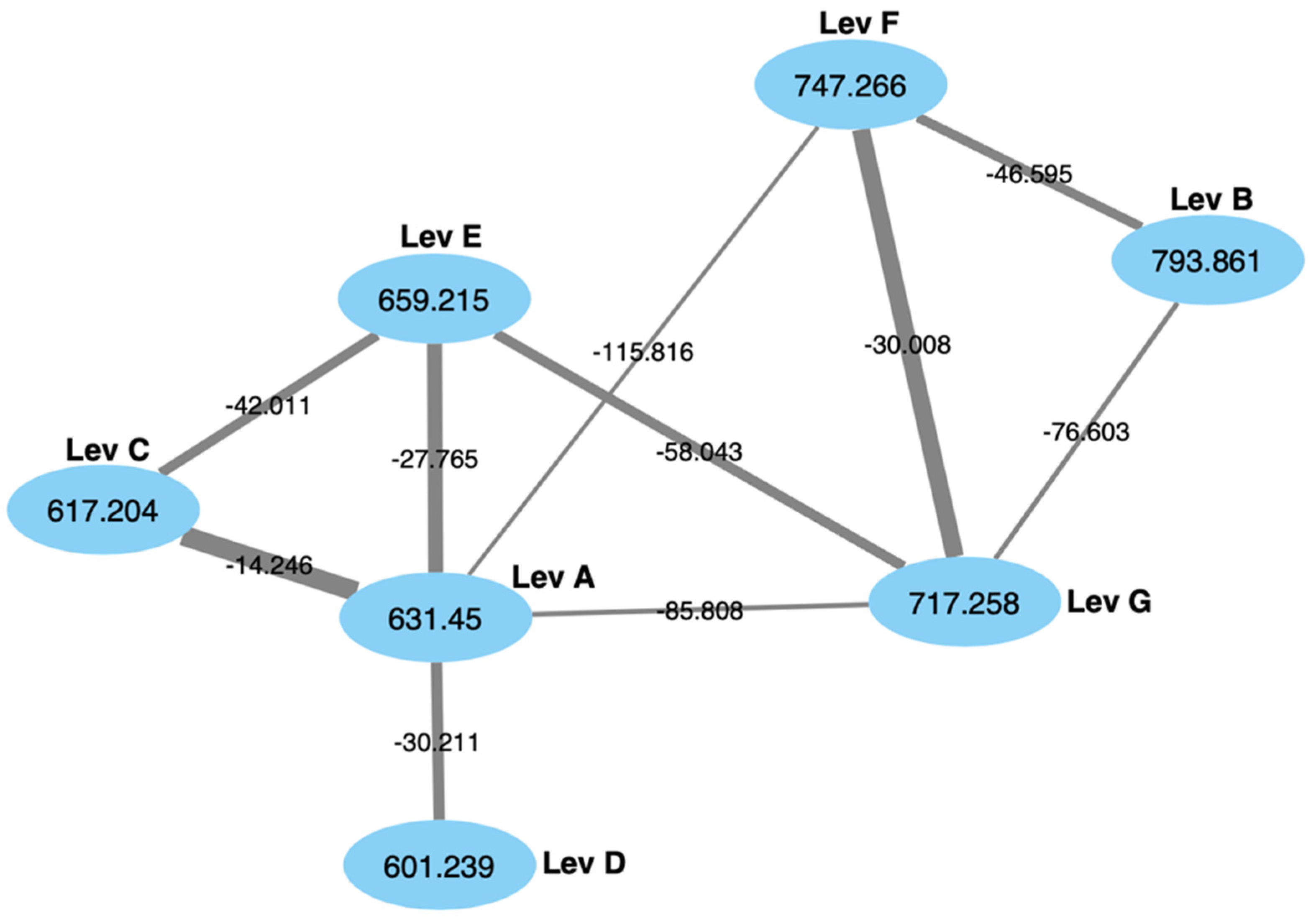
2.2. Fractionation, Molecular Network Analysis and Isolation of Levesquamide B
2.3. Structural Elucidation of Levesquamide B
2.4. MS2 Analysis of Other Levesquamide Analogues
3. Experimental
3.1. General Experimental Procedures
3.2. Salt Screen
3.3. Large Scale Fermentation and Extraction
3.4. Global Natural Product Social Networking (GNPS) Analysis of Family Members
3.5. Chromatographic Purification
3.6. Glycoside Stereochemical Analysis by Tanaka’s Method [16]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 3, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 1, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Bérdy, J. Bioactive microbial metabolites. J. Antibiot 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, D.A. Streptomyces in Nature and Medicine: The Antibiotic Makers; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Ambrosino, L.; Tangherlini, M.; Colantuono, C.; Esposito, A.; Sangiovanni, M.; Miralto, M.; Sansone, C.; Chiusano, M.L. Bioinformatics for marine products: An overview of resources, bottlenecks and perspectives. Mar. Drugs 2019, 17, 576. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied. Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W.; Jensen, P. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat. Chem. Biol. 2006, 2, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Haltli, B.A.; Kerr, R.G. Draft Genome Sequence of Streptomyces sp. Strain RKND-216, an Antibiotic Producer Isolated from Marine Sedument in Prince Edward Island, Canada. Micro. Res. Announc. 2019, 8, 35. [Google Scholar]
- Liang, L.; Haltli, B.A.; Marchbank, D.A.; Fischer, M.; Kirby, C.W.; Correa, H.; Clark, T.N.; Gray, C.A.; Kerr, R.G. Discovery of an Isothiazolinone-Containing Antitubercular Natural Product Levesquamide. J. Org. Chem. 2020, 85, 6450–6462. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Walsh, C.T. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: Logic, machinery, and mechanisms. Chem. Rev. 2006, 106, 3468–3496. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.; Vior, N.M.; Braña, A.F.; González-Sabin, J.; Rohr, J.; Moris, F.; Méndez, C.; Salas, J.A. Elucidating the biosynthetic pathway for the polyketide-nonribosomal peptide collismycin A: Mechanism for formation of the 2, 2′-bipyridyl ring. Chem. Biol. 2012, 19, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Pang, B.; Zhang, Z.; Chen, M.; Wu, Z.; Zhao, Q.; Zhang, Q.; Wang, Y.; Liu, Y.; Liu, W. Caerulomycins and collismycins share a common paradigm for 2, 2′-bipyridine biosynthesis via an unusual hybrid polyketide−peptide assembly logic. J. Am. Chem. Soc. 2012, 134, 9038–9041. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.A.; Sanchez, L.M.; Rath, C.M.; Liu, X.; Boudreau, P.D.; Bruns, N.; Glukhov, E.; Wodtke, A.; de Felicio, R.; Fenner, A.; et al. Molecular networking as a dereplication strategy. J. Nat. Prod. 2013, 76, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.; Phelan, V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]



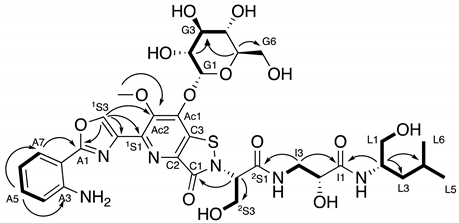 | |||||
|---|---|---|---|---|---|
| Position | δC/N, Type | δH, Mult. (J in Hz) | COSY | HMBC | |
| Anthranilate | A1 | 160.6, C | - | - | - |
| A2 | 106.6, C | - | - | ||
| A3 | 148.2, C | - | - | - | |
| A4 | 115.8, CH | 6.87, dd (1.1, 8.3) | A5 | A6, A2, A1, A3-NH2 | |
| A5 | 130.8, CH | 7.20, ddd (7.1, 8.5, 1.6) | A4, A6 | A3, A7 | |
| A6 | 115.0, CH | 6.65, dd (8.1, 7.0) | A5, A7 | A4, A5, A2, A7 | |
| A7 | 126.9, CH | 7.75, dd (1.0, 7.6) | A6 | A3, A5, A1 | |
| NH2-A3 | 64.5, NH2 | 7.00, s | - | A4 | |
| 1Serine | 1S1 | 158.4, C | - | - | - |
| 1S2 | 131.2, C | - | - | ||
| 1S3 | 137.2, CH | 8.40, s | - | A1, 1S2, 1S1, 1S2-N | |
| N-1S2 | 236.4, N | - | - | - | |
| Acetate | Ac1 | 162.4, C | - | - | - |
| Ac2 | 146.0, C | - | - | - | |
| MeO-Ac2 | 57.5, CH3 | 3.90, s | - | Ac2 | |
| Glucose | G1 | 92.6, CH | 4.21, d (3.6) | G2 | |
| G2 | 73.5, CH | 3.22, m | G1,G2 | G3 | |
| G3 | 72.1, | 3.31, m | G2,G4 | ||
| G4 | 73.5, | 3.34, m | G3 | ||
| G5 | 70.6 | 3.45, m | G3,G6 | ||
| G6 | 61.1 CH2 | 3.81, m | G5 | ||
| Cysteine | C1 | 166.1, C | - | - | - |
| C2 | 134.9, C | - | - | - | |
| C3 | 132.0, C | - | - | - | |
| N-C2 | nd | - | - | - | |
| 2Serine | 2S1 | 167.6, C | - | - | - |
| 2S2 | 58.1, CH | 5.19, t (5.7) | 2S3 | C2c, C1, 2S3 | |
| 2S3 | 61.1, CH2 | 3.82, m | 2S2, 2S3-OH | 2S2, 2S1 | |
| OH-2S3 | - | 5.32, broad | 2S3 | - | |
| N-2S2 | 125.6, N | - | - | - | |
| Isoserine | I1 | 171.4, C | - | - | - |
| I2 | 70.1, CH | 3.96, m | I3, I2-OH | I3, I1 | |
| I3a | 43.4, CH2 | 3.46, m | I2, I3b, I3-NH | 2S1, I2, I1 | |
| I3b | 3.12, m | I2, I3a, I3-NH | |||
| OH-I2 | - | 5.74, d (5.1) | I2 | I3, I2, I1 | |
| NH-I3 | 111.6, NH | 8.43, t (5.7) | I3 | I3, 2S2, I2, 2S1 | |
| Leucinol | L1a | 63.7, CH2 | 3.36, m | L1b, L2, L1-OH | L3, L2 |
| L1b | 3.28, m | L1a, L2, L1-OH | |||
| L2 | 48.4, CH | 3.81, m | L1, L3, L2-NH | L4, L1, L3, I1 | |
| L3 | 40.0, CH2 | 1.31, m | L2, L4 | L2, L4, L5, L6, L1 | |
| L4 | 24.2, CH | 1.55, m | L3, L5, L6 | L2, L3, L5, L6 | |
| L5 | 23.4, CH3 | 0.86, d (6.7) | L4 | L6, L3 | |
| L6 | 21.2, CH3 | 0.83, d (6.5) | L4 | L5, L3 | |
| OH-L1 | - | 4.67, s | L1 | - | |
| NH-L2 | 121.5, NH | 7.38, d (8.8) | L2 | L3, L2, L1, I2, I1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
LeClair, M.M.; Maw, Z.A.; Grunwald, A.L.; Kelly, J.R.; Haltli, B.A.; Kerr, R.G.; Cartmell, C. Discovery of Levesquamide B through Global Natural Product Social Molecular Networking. Molecules 2022, 27, 7794. https://doi.org/10.3390/molecules27227794
LeClair MM, Maw ZA, Grunwald AL, Kelly JR, Haltli BA, Kerr RG, Cartmell C. Discovery of Levesquamide B through Global Natural Product Social Molecular Networking. Molecules. 2022; 27(22):7794. https://doi.org/10.3390/molecules27227794
Chicago/Turabian StyleLeClair, Mary M., Zacharie A. Maw, Alyssa L. Grunwald, Joshua R. Kelly, Bradley A. Haltli, Russell G. Kerr, and Christopher Cartmell. 2022. "Discovery of Levesquamide B through Global Natural Product Social Molecular Networking" Molecules 27, no. 22: 7794. https://doi.org/10.3390/molecules27227794
APA StyleLeClair, M. M., Maw, Z. A., Grunwald, A. L., Kelly, J. R., Haltli, B. A., Kerr, R. G., & Cartmell, C. (2022). Discovery of Levesquamide B through Global Natural Product Social Molecular Networking. Molecules, 27(22), 7794. https://doi.org/10.3390/molecules27227794









