Interaction of Destruxin A with Three Silkworm Proteins: BmCRT, BmDPP3, and BmPDIA5
Abstract
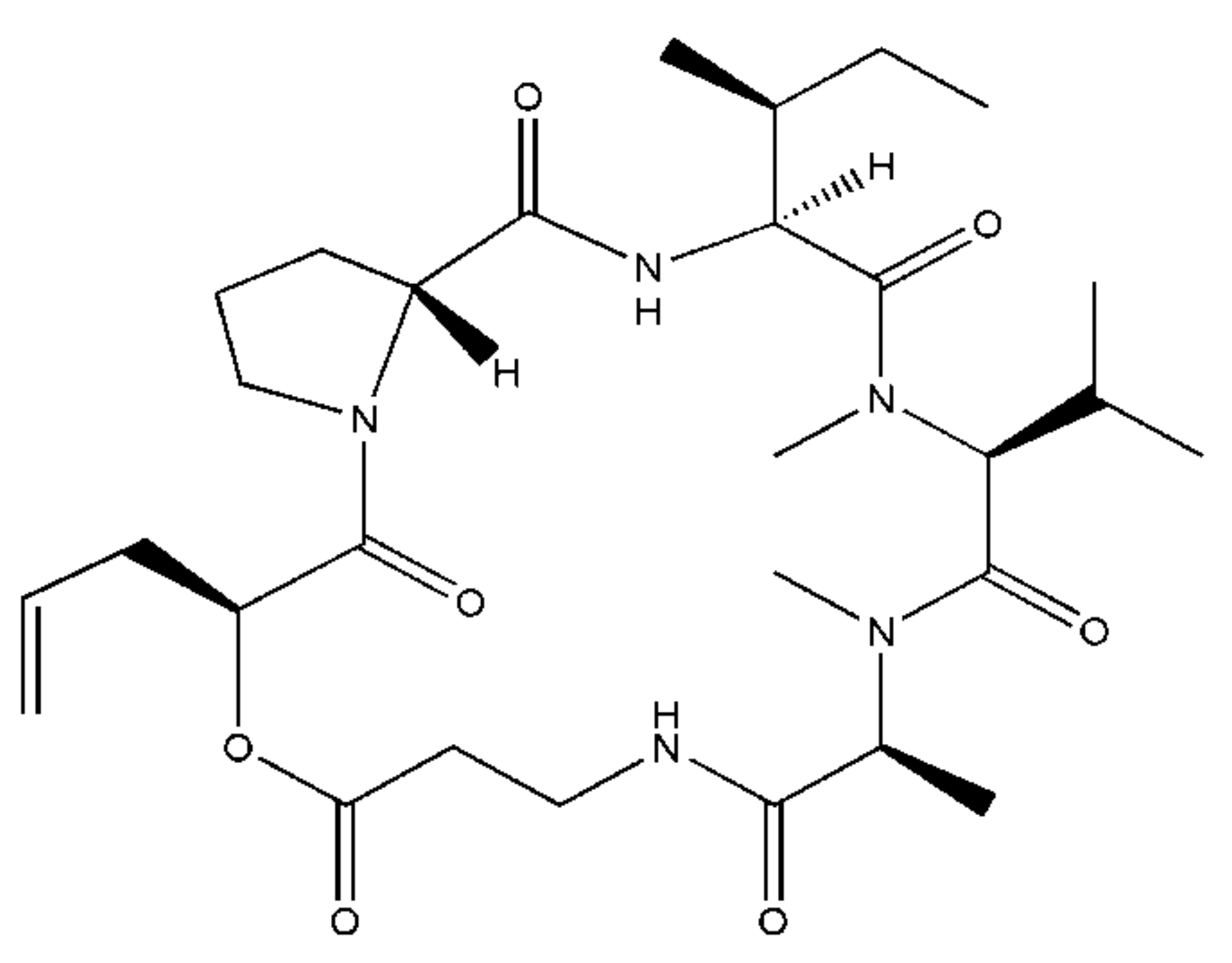
1. Introduction
2. Results
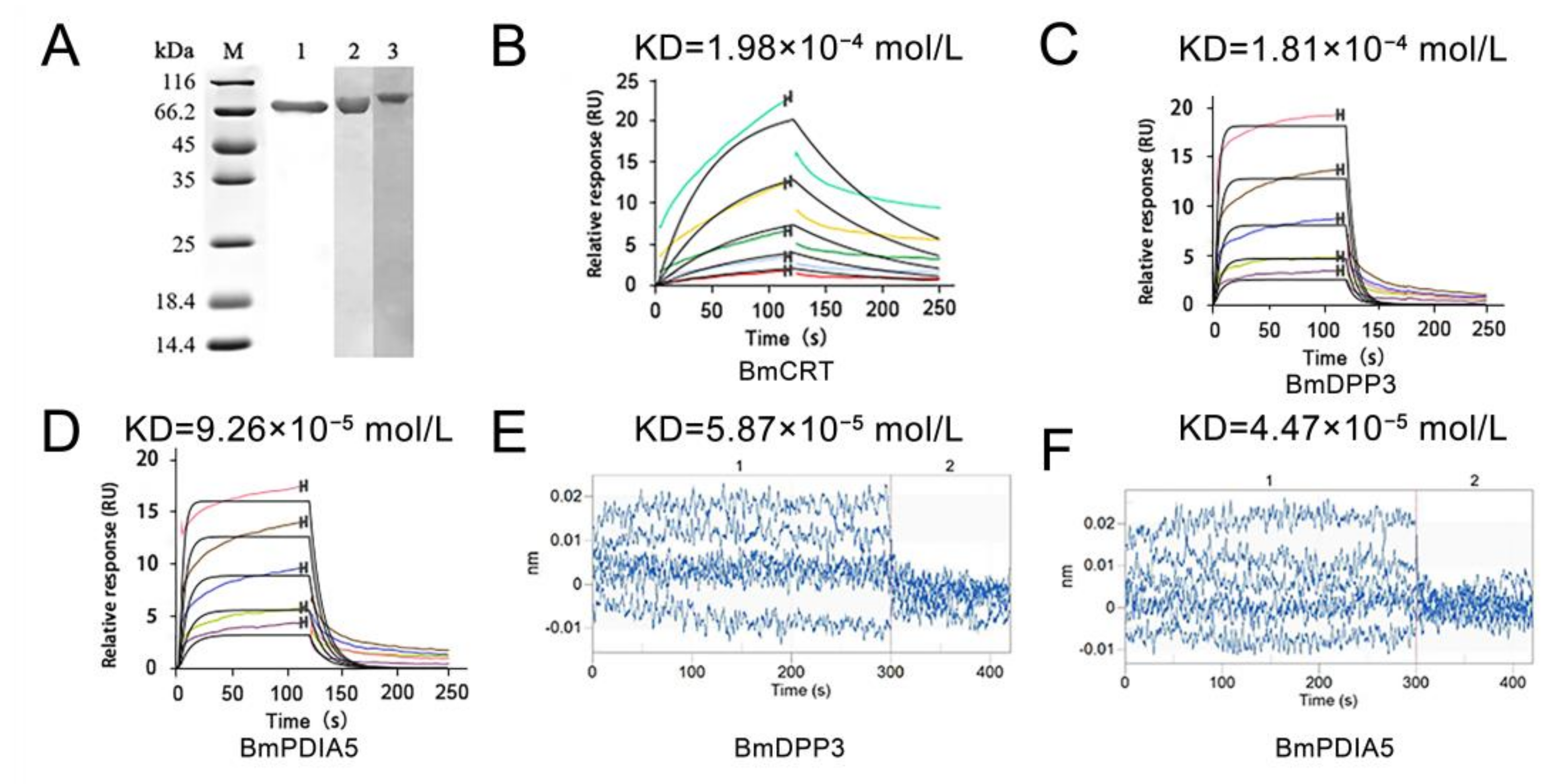
2.1. Binding Affinity of DA with Three Proteins Determined by Surface Plasmon Resonance (SPR) and Bio-Layer Interferometry (BLI) In Vitro
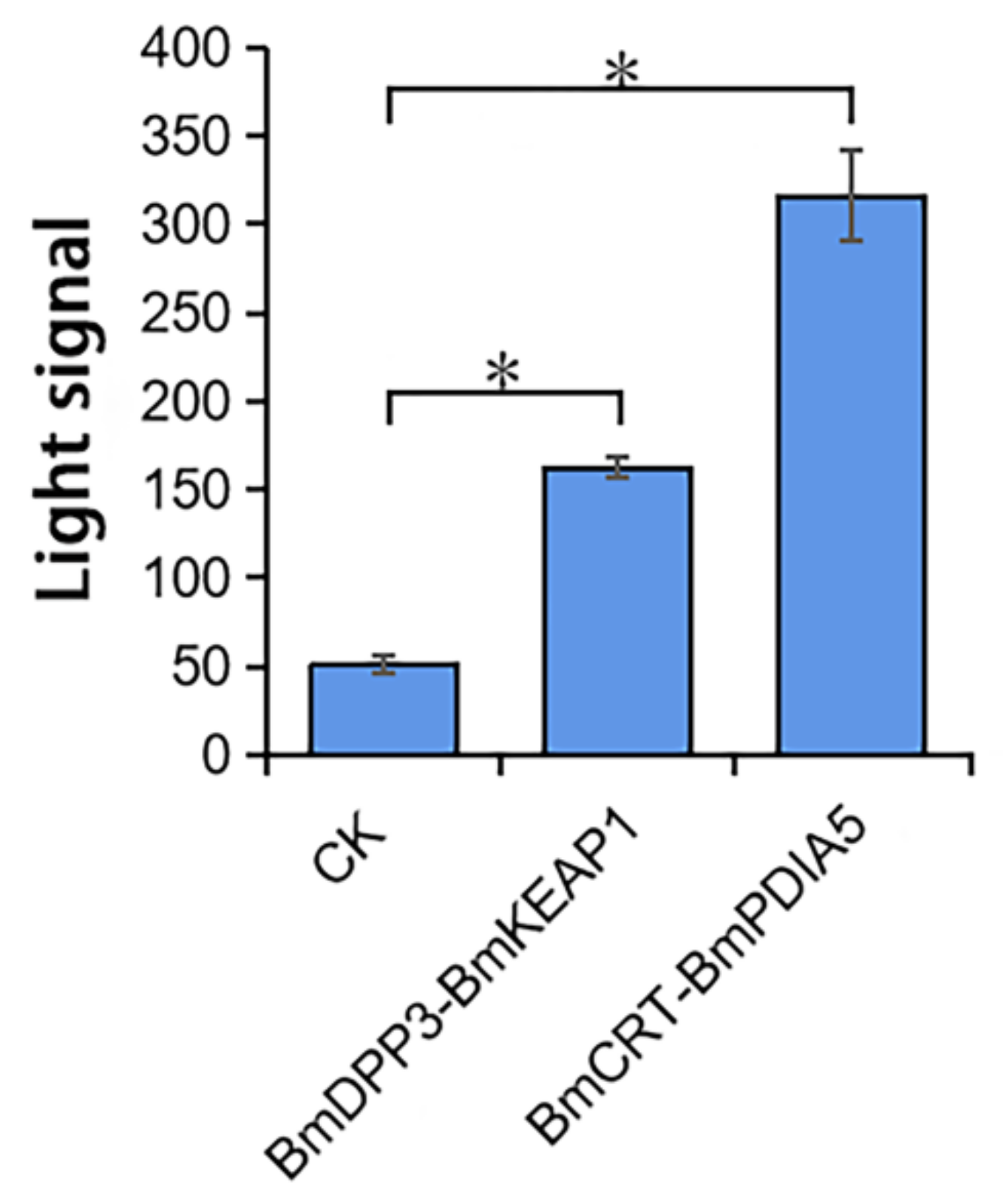
2.2. The Interactions between DA and Three Proteins Determined by Insect Two-Hybrid (I2H) In Vivo
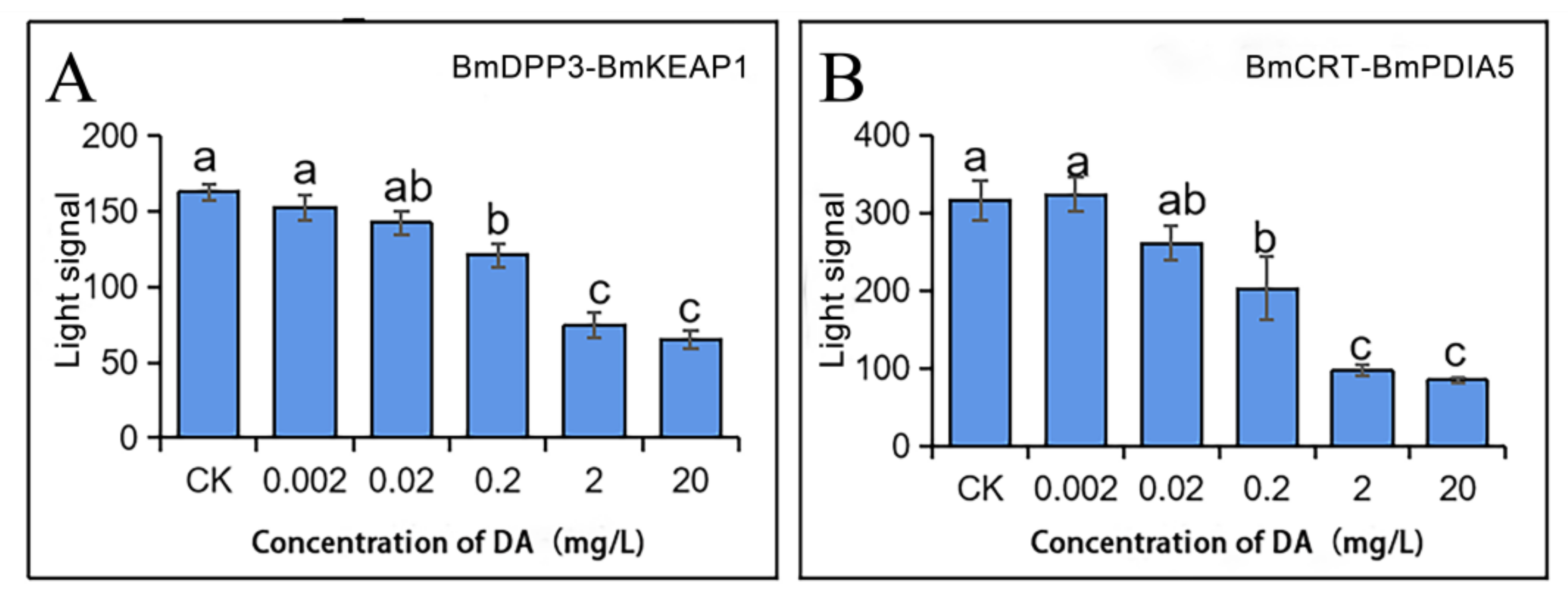
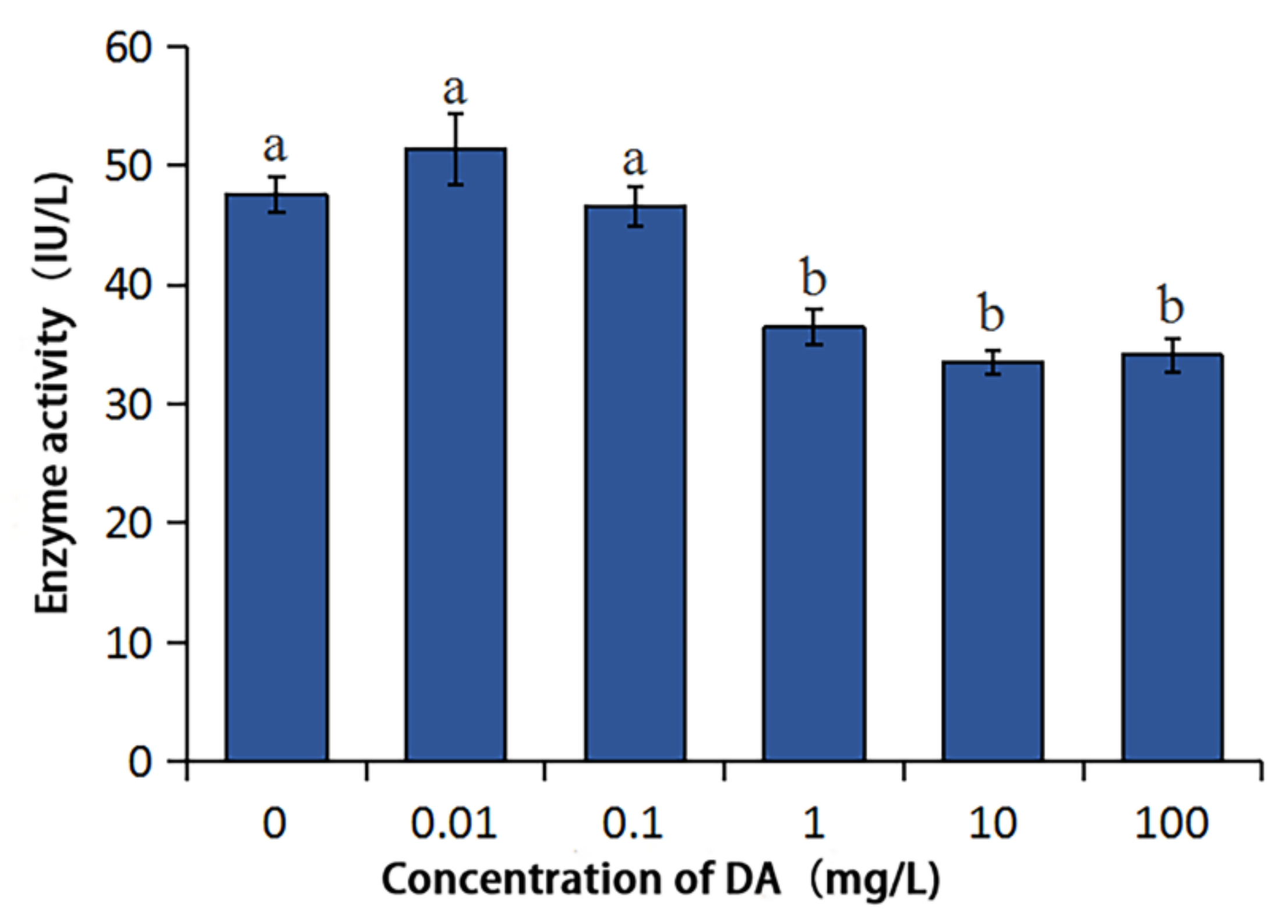
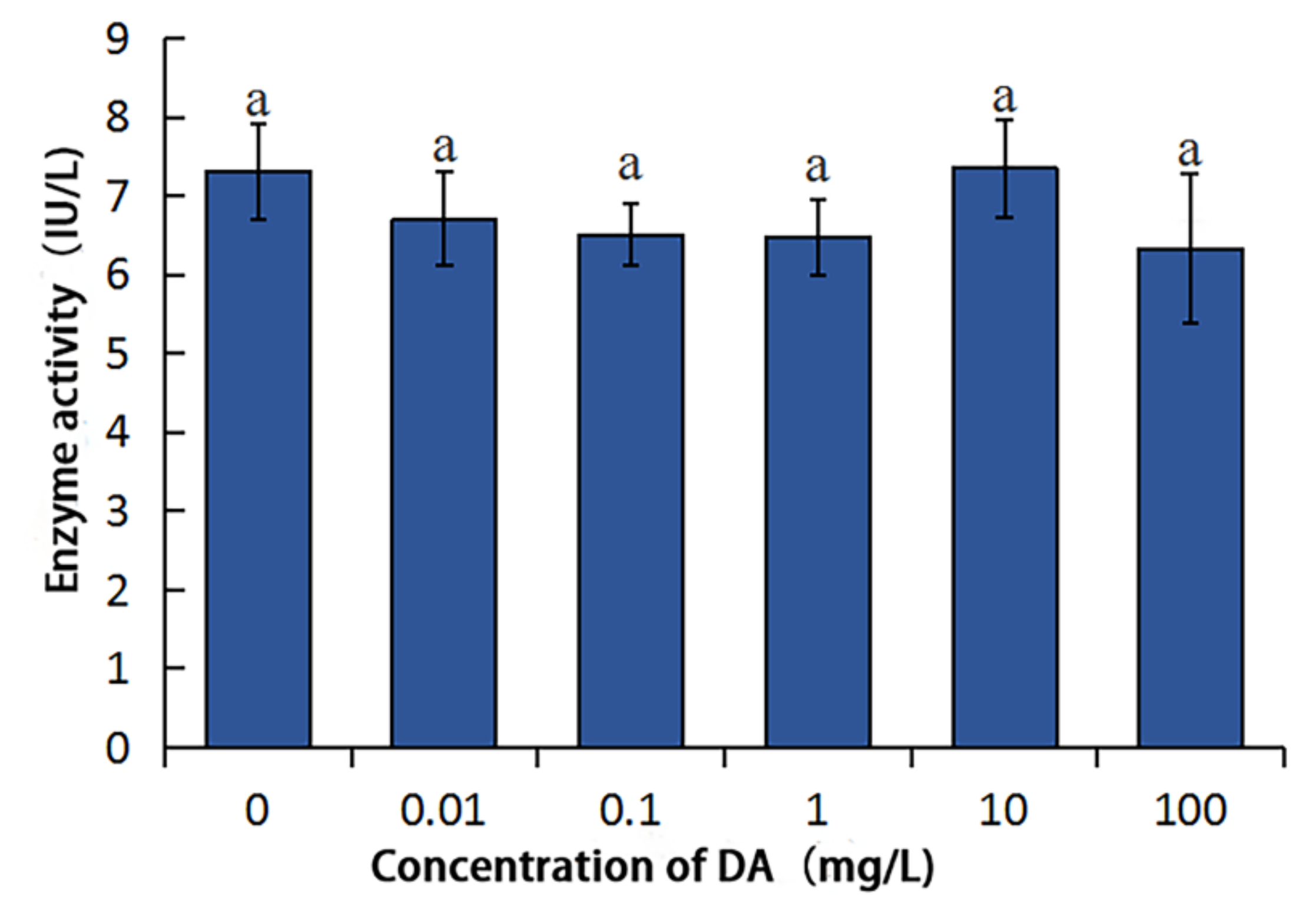
2.3. Effects of DA on the Enzymatic Activities of BmDPP3 and BmPDIA5
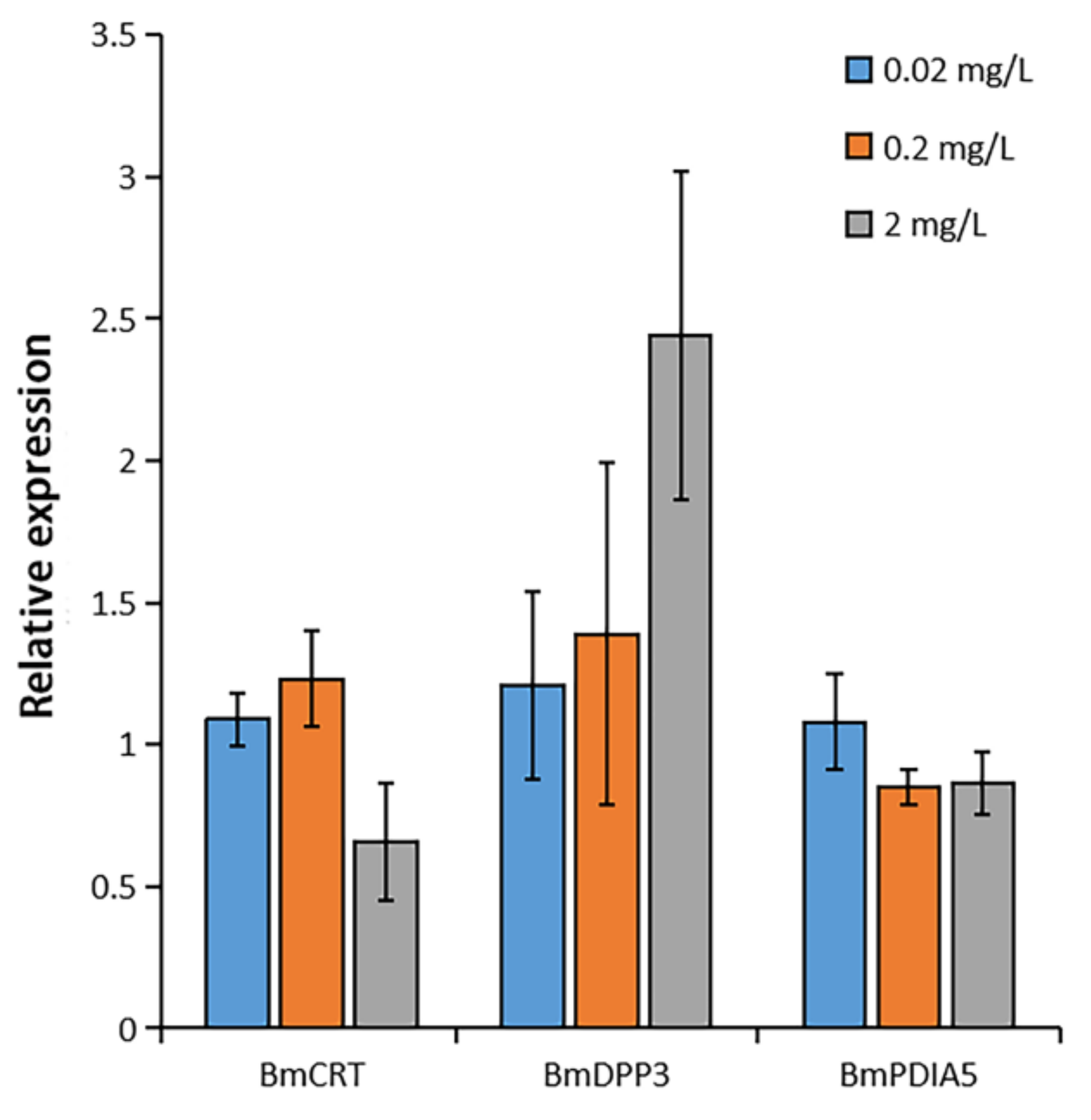
2.4. Effects of DA on the Gene Expression of Three Proteins
3. Discussion
4. Materials and Methods
4.1. Cell Culture and DA
4.2. The Expression, Isolation and Purification of Proteins
4.3. Surface Plasmon Resonance (SPR)
4.4. Bio-Layer Interferometry (BLI)
4.5. Effects of DA on Enzyme Activities of BmDPP3 and BmPDIA5
4.6. Insect Two-Hybrid (I2H)
4.7. Effects of DA on the Gene Expression of Three Proteins
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruiz-Sanchez, E.; Orchard, I.; Lange, A.B. Effects of the cyclopeptide mycotoxin destruxin a on the malpighian tubules of rhodnius prolixus (stål). Toxicon 2010, 55, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; St. Leger, R.J.; Wu, L.P. Fungal peptide destruxin a plays a specific role in suppressing the innate immune response in drosophila melanogaster. J. Biol. Chem. 2007, 282, 8969–8977. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tzeng, Y. Development and applications of destruxins: A review. Biotechnol. Adv. 2012, 30, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kang, Q.; Lu, Y.; Bai, L.; Wang, C. Unveiling the biosynthetic puzzle of destruxins in metarhizium species. Proc. Natl. Acad. Sci. USA 2016, 113, E4578. [Google Scholar] [CrossRef]
- Hunt, V.L.; Charnley, A.K. The inhibitory effect of the fungal toxin, destruxin a, on behavioural fever in the desert locust. J. Insect Physiol. 2011, 57, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.R.; Hu, Q.B.; Yu, X.Q.; Ren, S.X. Effects of destruxins on free calcium and hydrogen ions in insect hemocytes. Insect Sci. 2014, 21, 31–38. [Google Scholar] [CrossRef]
- Fan, J.Q.; Chen, X.R.; Qiong-Bo, H.U. Effects of destruxin a on hemocytes morphology of bombyx mori. J. Integr. Agr. 2013, 12, 1042–1048. [Google Scholar] [CrossRef]
- Hu, W.; He, G.; Wang, J.; Hu, Q. The effects of destruxin a on relish and rel gene regulation to the suspected immune-related genes of silkworm. Molecules 2017, 22, 41. [Google Scholar] [CrossRef]
- Wang, J.; Berestetskiy, A.; Hu, Q. Destruxin a interacts with aminoacyl trna synthases in bombyx mori. J. Fungi 2021, 7, 593. [Google Scholar] [CrossRef]
- Wang, J.; Weng, Q.; Yin, F.; Hu, Q. Interactions of destruxin a with silkworms’ arginine trna synthetase and lamin-c proteins. Toxins 2020, 12, 137. [Google Scholar] [CrossRef]
- Wang, L.; Fang, Q.; Zhu, J.; Wang, F.; Rean Akhtar, Z.; Ye, G. Molecular cloning and functional study of calreticulin from a lepidopteran pest, pieris rapae. Dev. Comp. Immunol. 2012, 38, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Schmidt, O. Is cell surface calreticulin involved in phagocytosis by insect hemocytes? J. Insect Physiol. 2003, 49, 545–550. [Google Scholar] [CrossRef]
- Choi, J.Y.; Whitten, M.M.; Cho, M.Y.; Lee, K.Y.; Kim, M.S.; Ratcliffe, N.A.; Lee, B.L. Calreticulin enriched as an early-stage encapsulation protein in wax moth galleria mellonella larvae. Dev. Comp. Immunol. 2002, 26, 335–343. [Google Scholar] [CrossRef]
- Zhang, G.; Schmidt, O.; Asgari, S. A calreticulin-like protein from endoparasitoid venom fluid is involved in host hemocyte inactivation. Dev. Comp. Immunol. 2006, 30, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Opas, M.; Szewczenko-Pawlikowski, M.; Jass, G.K.; Mesaeli, N.; Michalak, M. Calreticulin modulates cell adhesiveness via regulation of vinculin expression. J. Cell Biol. 1996, 135, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Gelebart, P.; Opas, M.; Michalak, M. Calreticulin, a ca2+-binding chaperone of the endoplasmic reticulum. Int. J. Biochem. Cell Biol. 2005, 37, 260–266. [Google Scholar] [CrossRef]
- Diaz, J.R.; Ramírez, C.A.; Nocua, P.A.; Guzman, F.; Requena, J.M.; Puerta, C.J. Dipeptidyl peptidase 3, a novel protease from leishmania braziliensis. PLoS ONE 2018, 13, e190618. [Google Scholar] [CrossRef]
- Jha, S.; Taschler, U.; Domenig, O.; Poglitsch, M.; Bourgeois, B.; Pollheimer, M.; Pusch, L.M.; Malovan, G.; Frank, S.; Madl, T.; et al. Dipeptidyl peptidase 3 modulates the renin–angiotensin system in mice. J. Biol. Chem. 2020, 295, 13711–13723. [Google Scholar] [CrossRef]
- Ren, X.; Yu, J.; Guo, L.; Ma, H. Dipeptidyl-peptidase 3 protects oxygen-glucose deprivation/reoxygenation-injured hippocampal neurons by suppressing apoptosis, oxidative stress and inflammation via modulation of keap1/nrf2 signaling. Int. Immunopharmacol. 2021, 96, 107595. [Google Scholar] [CrossRef]
- Vinaik, R.; Kozlov, G.; Gehring, K. Structure of the non-catalytic domain of the protein disulfide isomerase-related protein (pdir) reveals function in protein binding. PLoS ONE 2013, 8, e62021. [Google Scholar] [CrossRef]
- Kanemura, S.; Matsusaki, M.; Inaba, K.; Okumura, M. Pdi family members as guides for client folding and assembly. Int. J. Mol. Sci. 2020, 21, 9351. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Okada, K.; Imaoka, S. Interaction between bisphenol derivatives and protein disulphide isomerase (pdi) and inhibition of pdi functions: Requirement of chemical structure for binding to pdi. J. Biochem. 2008, 144, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Baksh, S.; Burns, K.; Andrin, C.; Michalak, M. Interaction of calreticulin with protein disulfide isomerase. J. Biol. Chem. 1995, 270, 31338–31344. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Alcivar, A.L.; Ma, J.; Foo, T.K.; Zwyea, S.; Mahdi, A.; Huo, Y.; Kensler, T.W.; Gatza, M.L.; Xia, B. Nrf2 induction supporting breast cancer cell survival is enabled by oxidative stress-induced dpp3-keap1 interaction. Cancer Res. 2017, 77, 2016–2204. [Google Scholar] [CrossRef]
- Molinari, M.; Eriksson, K.K.; Calanca, V.; Galli, C.; Cresswell, P.; Michalak, M.; Helenius, A. Contrasting functions of calreticulin and calnexin in glycoprotein folding and er quality control. Mol. Cell 2004, 13, 125–135. [Google Scholar] [CrossRef]
- Mancino, L.; Rizvi, S.M.; Lapinski, P.E.; Raghavan, M. Calreticulin recognizes misfolded hla-a2 heavy chains. Proc. Natl. Acad. Sci. USA 2002, 99, 5931–5936. [Google Scholar] [CrossRef]
- Higa, A.; Taouji, S.; Lhomond, S.; Jensen, D.; Fernandez-Zapico, M.E.; Simpson, J.C.; Pasquet, J.M.; Schekman, R.; Chevet, E. Endoplasmic reticulum stress-activated transcription factor atf6α requires the disulfide isomerase pdia5 to modulate chemoresistance. Mol. Cell. Biol. 2014, 34, 1839–1849. [Google Scholar] [CrossRef]
- Podversnik, H.; Jha, S.; Macheroux, P.; Breinbauer, R. Design and synthesis of efficient fluororethylene-peptidomimetic inhibitors of dipeptidyl peptidase iii (dpp3). Bioorgan. Med. Chem. 2022, 67, 116831. [Google Scholar] [CrossRef]
- Noiva, R. Protein disulfide isomerase: The multifunctional redox chaperone of the endoplasmic reticulum. Semin. Cell Dev. Biol. 1999, 10, 481–493. [Google Scholar] [CrossRef]
- Wang, J.; Hu, W.; Hu, Q. Bmtudor-sn is a binding protein of destruxin a in silkworm bm12 cells. Toxins 2019, 11, 67. [Google Scholar] [CrossRef]
- Wang, J.; Weng, Q.; Hu, Q. Effects of destruxin a on silkworm’s immunophilins. Toxins 2019, 11, 349. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Peng, H.; Weng, Q.; Hu, Q.; Wang, J. Interaction of Destruxin A with Three Silkworm Proteins: BmCRT, BmDPP3, and BmPDIA5. Molecules 2022, 27, 7713. https://doi.org/10.3390/molecules27227713
Yin X, Peng H, Weng Q, Hu Q, Wang J. Interaction of Destruxin A with Three Silkworm Proteins: BmCRT, BmDPP3, and BmPDIA5. Molecules. 2022; 27(22):7713. https://doi.org/10.3390/molecules27227713
Chicago/Turabian StyleYin, Xuyu, Haitao Peng, Qunfang Weng, Qiongbo Hu, and Jingjing Wang. 2022. "Interaction of Destruxin A with Three Silkworm Proteins: BmCRT, BmDPP3, and BmPDIA5" Molecules 27, no. 22: 7713. https://doi.org/10.3390/molecules27227713
APA StyleYin, X., Peng, H., Weng, Q., Hu, Q., & Wang, J. (2022). Interaction of Destruxin A with Three Silkworm Proteins: BmCRT, BmDPP3, and BmPDIA5. Molecules, 27(22), 7713. https://doi.org/10.3390/molecules27227713




