Simultaneous Determination of 108 Pesticide Residues in Three Traditional Chinese Medicines Using a Modified QuEChERS Mixed Sample Preparation Method and HPLC-MS/MS
Abstract
1. Introduction
2. Results and Discussion
2.1. MS/MS Conditions Optimization
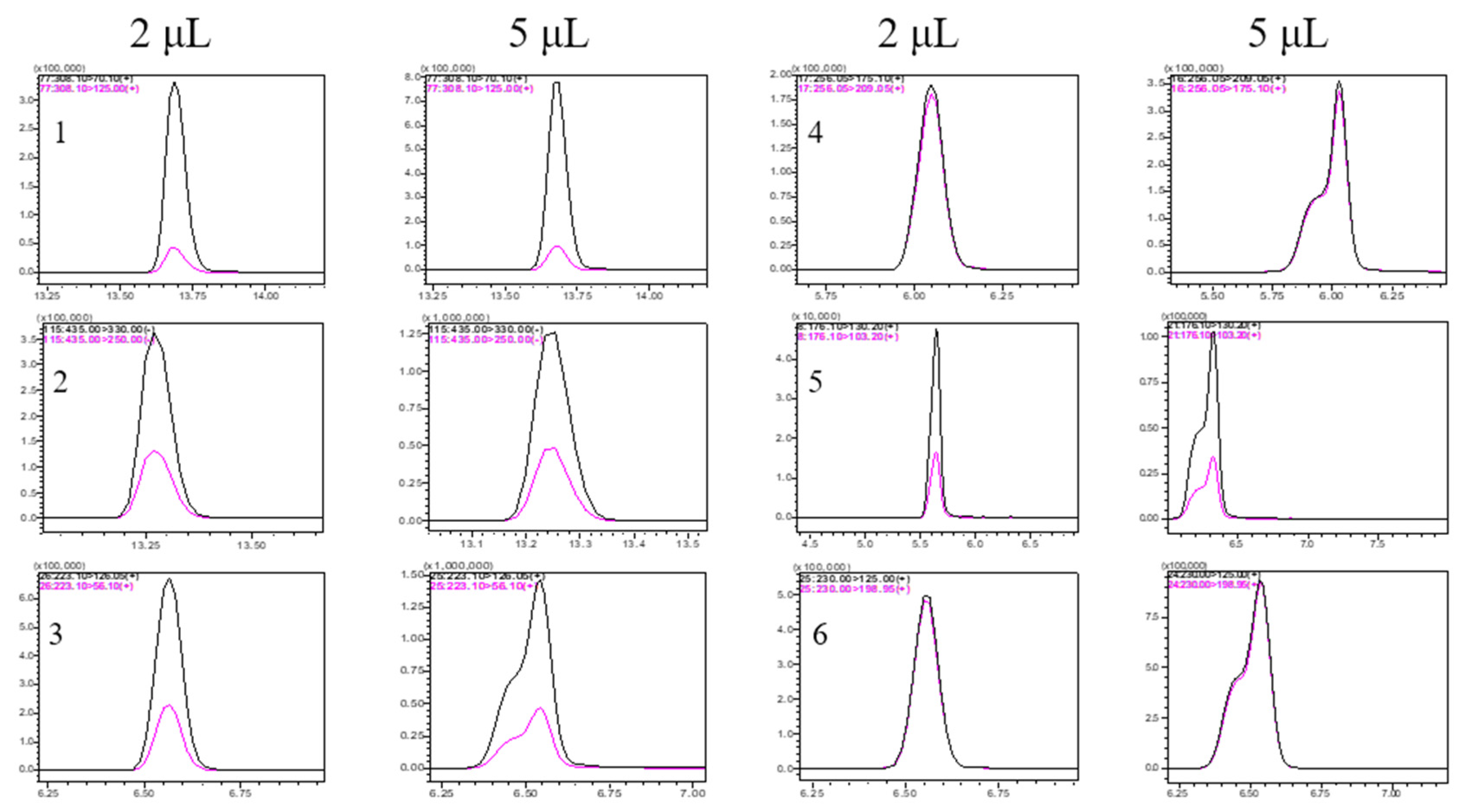
2.2. Optimization Chromatographic Conditions
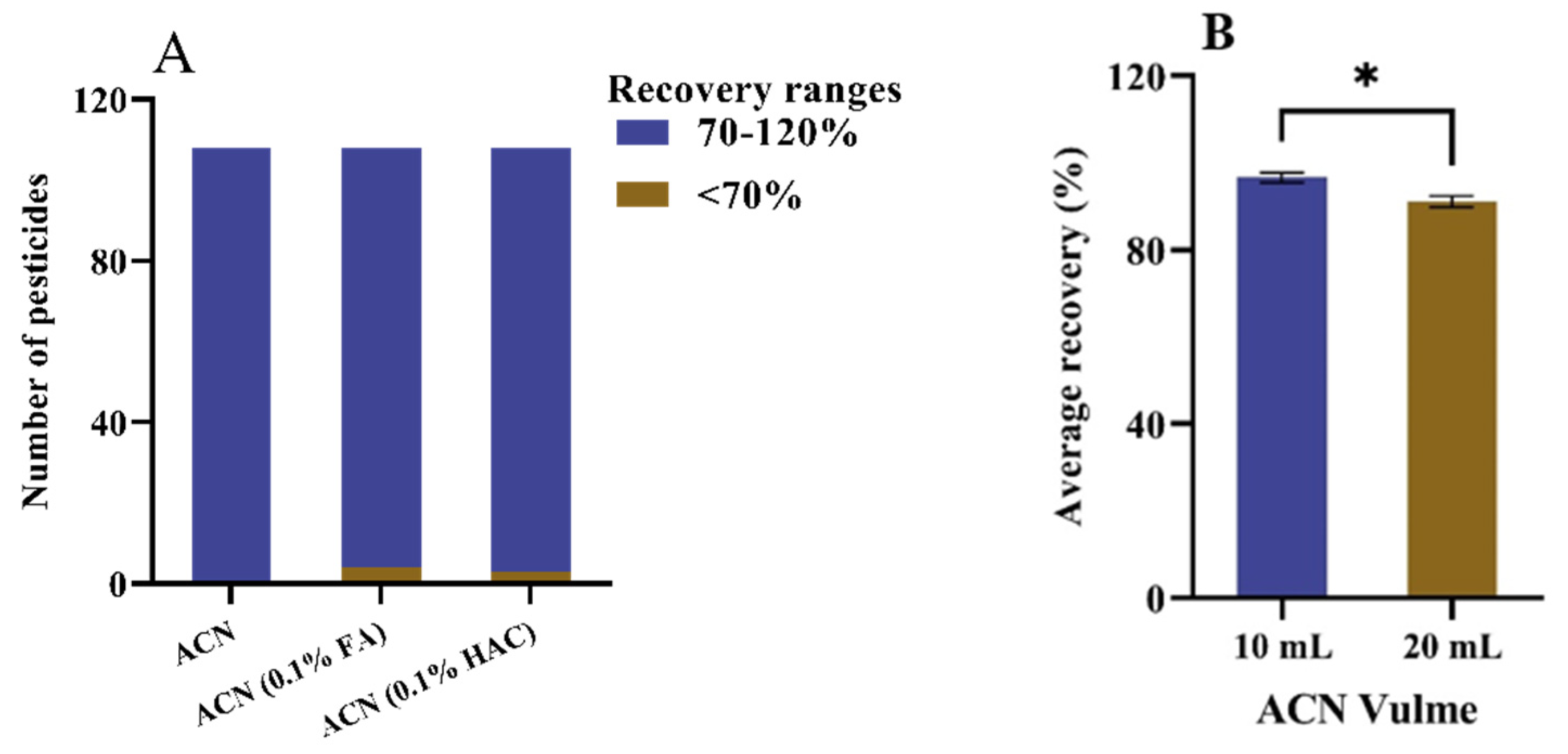
2.3. Extraction Method Optimization
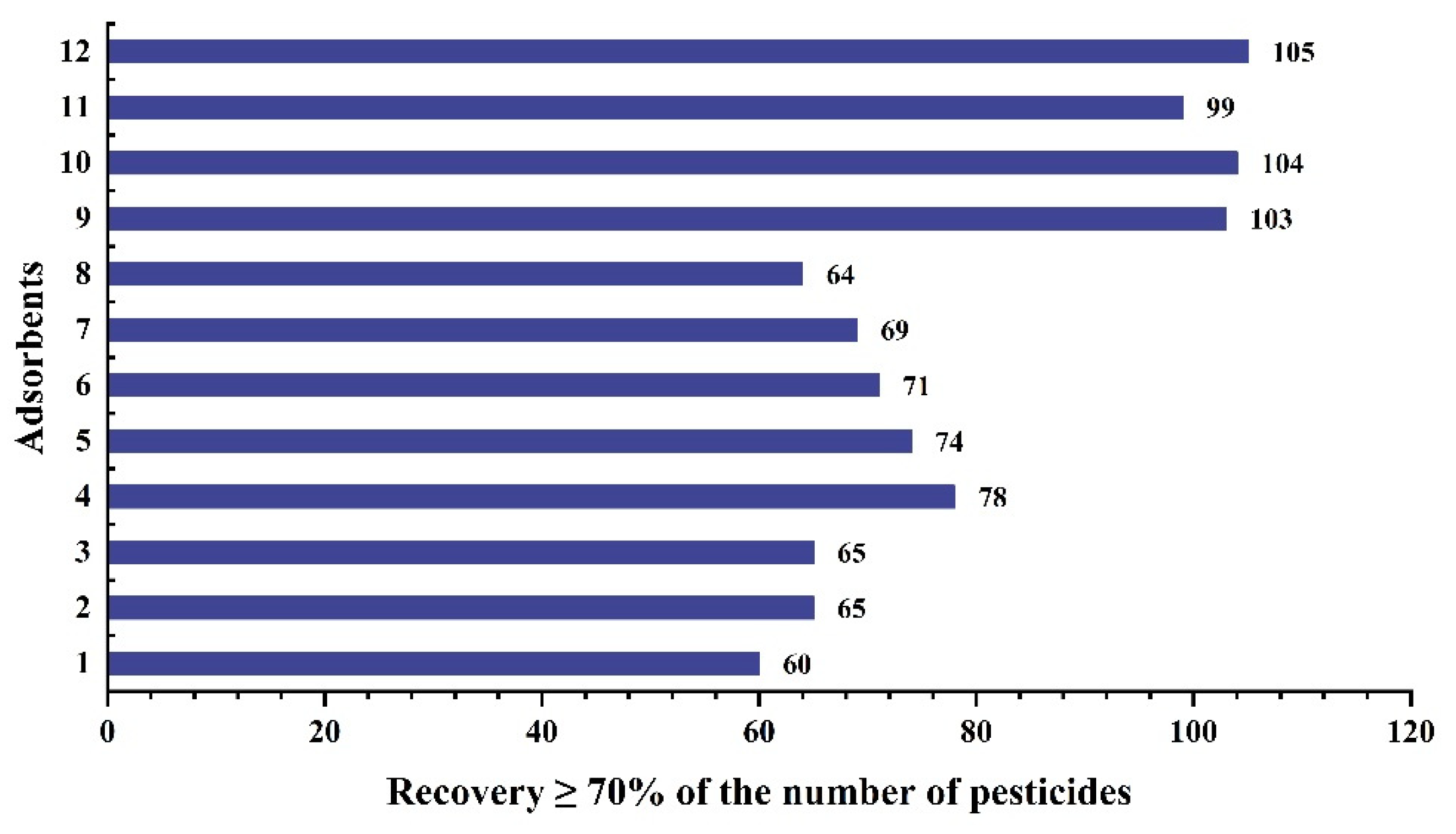
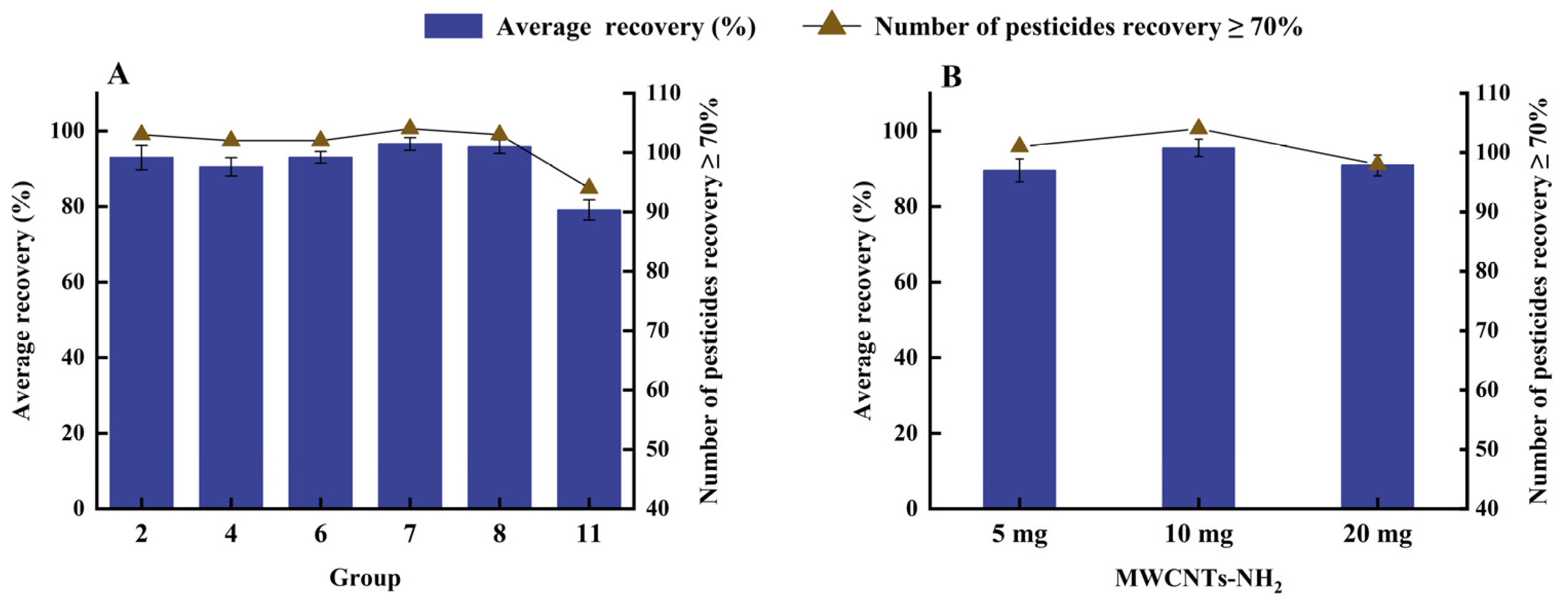
2.4. Clean Up Method Optimization
2.5. Matrix Effect
2.6. Method Validation
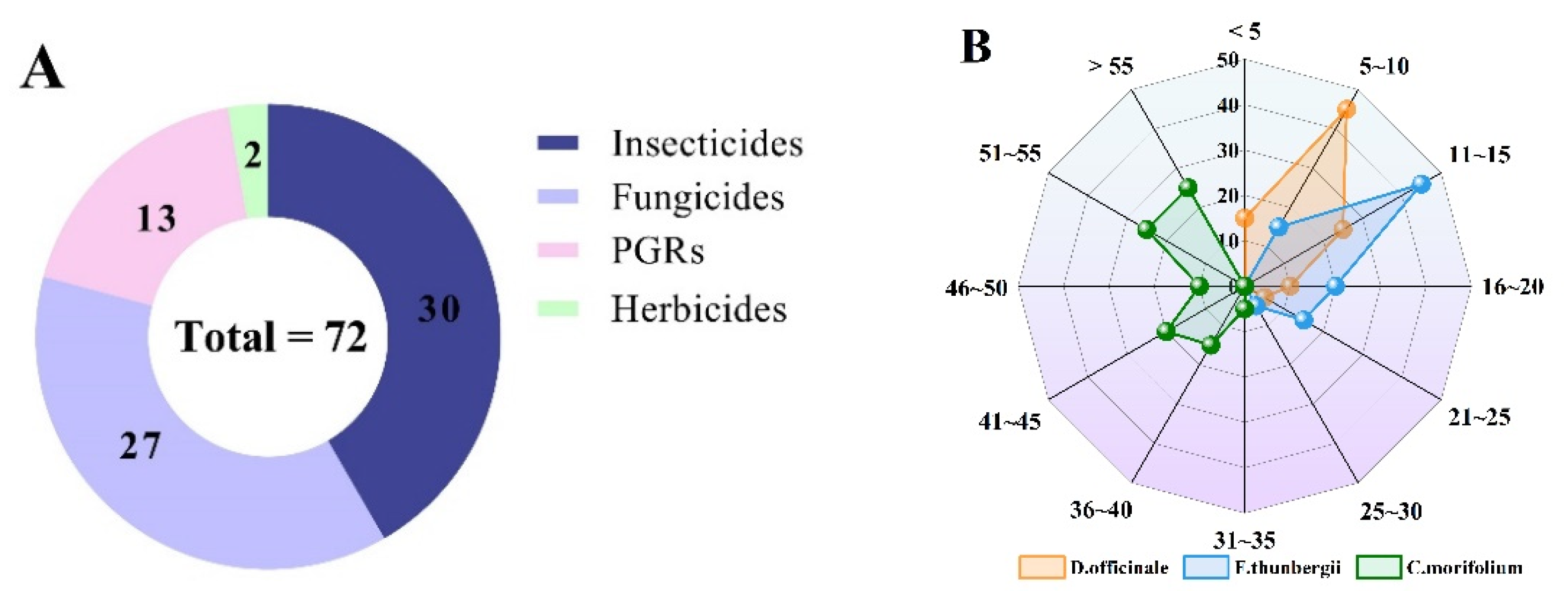
2.7. Application to Real Samples
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. HPLC-MS/MS Conditions
3.3. Sample Pretreatment
3.4. Sample Preparation
3.5. Method Validation
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ding, Y.L.; Lin, L.Y.; Chen, D.Q.; Xu, H.; Wang, Z.T. Content determination of six flavonoids in Dendrobium officinale stems from different producing areas, cultivation and processing methods by QAMS combined with dual-wavelength method. China J. Chin. Mater. Med. 2021, 46, 3605–3613. [Google Scholar]
- Yao, D.Z.; Min, H.; He, H.H.; Chen, L.; Qiao, H.X.; Ye, J.F. Determination and Comparison of Total Alkaloids Content of Fritiliariae Thunbergh Bulbus from Different Habitats. Chin. J. Mod. Appl. Pharm. 2014, 31, 1249–1251. [Google Scholar]
- Yu, M.; Yao, F.; Zhang, H.; Wang, J.; Qi, P.P.; Wang, X.Q. Determination of 30 organophosphorus pesticides in eight traditional Chinese medicine in Zhejiang (Zhebawei) by liquid chromatography-tandem mass spectrometry. Acta Pharm. Sim. 2022, 24, 591–600. [Google Scholar]
- Xu, W.; Shun-Wan, C.; Jiang, M.; Ping, L.; Pang-Chui, S.; Ge, L. Investigation of association of chemical profiles with the tracheobronchial relaxant activity of Chinese medicinal herb Beimu derived from various Fritillaria species. J. Ethnopharmacol. 2018, 210, 39–46. [Google Scholar]
- Cui, M.; Chen, S.; Wang, H.; Li, Z.; Chen, H.; Chen, Y.; Zhou, H.; Li, X.; Chen, J. Metabolic profiling investigation of Fritillaria thunbergii Miq. By gas chromatography–mass spectrometry. J. Food Drug Anal. 2018, 26, 337–347. [Google Scholar] [CrossRef]
- Lin, L.; Harnly, J.M. Identification of the phenolic components of chrysanthemum flower (Chrysanthemum morifolium Ramat). Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, Y.; Yang, J.; Shao, X. Dendrobium candidum extract inhibits proliferation and induces apoptosis of liver cancer cells by inactivating Wnt/β-catenin signaling pathway. Biomed. Pharmacother. 2019, 110, 371–379. [Google Scholar] [CrossRef]
- Wu, T.; Qi, P.; Wang, J.; Wang, Z.; Di, S.; Xu, H.; Zhao, H.; Zhao, C.; Wang, X. Simultaneous determination of 114 pesticides in complex Chinese herbal medicine Fritillaria using ordered mesoporous carbon CMK-3 as a reversed-dispersive solid phase extraction sorbent. RSC Adv. 2021, 11, 4129–4137. [Google Scholar] [CrossRef]
- Lu, X.; Ouyang, Y.; Zeng, W.; Lin, C.; Xiao, L.; Luo, G.; Zhan, R.; Yan, P. Effect of Pretreatment on Detection of 37 Pesticide Residues in Chrysanthemum indicum. J. Anal. Methods Chem. 2021, 2021, 8854025. [Google Scholar] [CrossRef]
- Chen, W.; Lu, J.; Zhang, J.; Wu, J.; Yu, L.; Qin, L.; Zhu, B. Traditional Uses, Phytochemistry, Pharmacology, and Quality Control of Dendrobium officinale Kimura et. Migo. Front. Pharmacol. 2021, 12, 2026. [Google Scholar] [CrossRef]
- Gao, S.M.; Liu, J.S.; Sun, T.; Liu, F.S.; Jing, H.; Qi, Y.D.; Zhang, B.G.; Liu, H.T.; Xiao, P.G. Influence of different cultivation measures on chemical quality of Codonopsis Radix. China Pharm. J. 2016, 41, 3753–3760. [Google Scholar]
- Pytlakowska, K.; Kita, A.; Janoska, P.; Połowniak, M.; Kozik, V. Multi-element analysis of mineral and trace elements in medicinal herbs and their infusions. Food Chem. 2012, 135, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.E.; Brickel, J.A.; Bhat, V.S. Chemical-specific maximum allowable levels for pesticide residues in dietary supplements. Food Chem. Toxicol. 2019, 123, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.P.; Ascencio, S.Y.C.; Oyarzun, J.C.G.; Hernandez, A.C.; Alavarado, P.V. Pesticides: Classification, uses and toxicity. Measures of exposure and genotoxic risks. J. Res. Environ. Sci. Toxicol. 2012, 1, 279–293. [Google Scholar]
- Luo, L.; Dong, L.; Huang, Q.; Ma, S.; Fantke, P.; Li, J.; Jiang, J.; Fitzgerald, M.; Yang, J.; Jia, Z.; et al. Detection and risk assessments of multi-pesticides in 1771 cultivated herbal medicines by LC/MS-MS and GC/MS-MS. Chemosphere 2021, 262, 127477. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Chen, C.; Xin, W.; Zhang, W. Simultaneous determination of 74 pesticide residues in Panax notoginseng by QuEChERS coupled with gas chromatography tandem mass spectrometry. Food Sci. Hum. Wellness 2021, 10, 241–250. [Google Scholar] [CrossRef]
- Fang, N.; Zhang, C.; Lu, Z.; Lu, Z.; Zhang, Z.; Wang, B.; Hou, Z.; Zhao, X. Dissipation, processing factors and dietary risk assessment for flupyradifurone residues in ginseng. Molecules 2022, 27, 5473. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, K.; Guo, L.; Wang, C.; Zhong, D.; Shang, L.; Nian, H.; Cui, X.; Huang, S. Rapid determination and dietary intake risk assessment of 249 pesticide residues in Panax notoginseng. Ecotoxicol. Environ. Saf. 2022, 233, 113348. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Sutcharitchan, C.; Miao, S.; Li, W.; Liu, J.; Zhou, H.; Ma, Y.; Ji, S.; Cui, Y. High performance liquid chromatography-tandem mass spectrometry method for residue determination of 39 plant growth regulators in root and rhizome Chinese herbs. Food Chem. 2020, 322, 126766. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of grephitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Chen, R.; Xue, X.; Wang, G.; Wang, J. Determination and dietary intake risk assessment of 14 pesticide residues in apples of China. Food Chem. 2021, 351, 129266. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Song, F.; Liu, Z.; Zheng, Z.; Xing, J.; Liu, S. Multi-residue method for fast determination of pesticide residues in plants used in traditional chinese medicine by ultra-high-performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2012, 1225, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.S.; Soliman, A.S.; El-Gammal, H.A.; Amer, M.E.; Attallah, E.R. Development and validation of a multiresidue method for the determination of 323 pesticide residues in dry herbs using QuEChERS method and LC-ESI-MS/MS. Int. J. Environ. Anal. Chem. 2017, 97, 1003–1023. [Google Scholar] [CrossRef]
- Li, R.; Li, M.; Wang, T.; Wang, T.; Chen, J.; Francis, F.; Fan, B.; Kong, Z.; Dai, X. Screening of pesticide residues in Traditional Chinese Medicines using modified QuEChERS sample preparation procedure and LC-MS/MS analysis. J. Chromatogr. B 2020, 1152, 122224. [Google Scholar] [CrossRef]
- Wang, S.; Qi, P.; Di, S.; Wang, J.; Wu, S.; Wang, X.; Wang, Z.; Wang, Q.; Wang, X.; Zhao, C.; et al. Significant role of supercritical fluid chromatography—Mass spectrometry in improving the matrix effect and analytical efficiency during multi-pesticides residue analysis of complex chrysanthemum samples. Anal. Chim. Acta 2019, 1074, 108–116. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, F.; Lou, Z.; Lu, M.; Chen, Z. Simultaneous and enantioselective determination of cis-epoxiconazole and indoxacarb residues in various teas, tea infusion and soil samples by chiral high performance liquid chromatography coupled with tandem quadrupole-time-of-flight mass spectrometry. J. Chromatogr. A 2014, 1359, 212–223. [Google Scholar] [CrossRef]
- Ferrer, I.; García-Reyes, J.F.; Mezcua, M.; Thurman, E.M.; Fernández-Alba, A.R. Multi-residue pesticide analysis in fruits and vegetables by liquid chromatography–time-of-flight mass spectrometry. J. Chromatogr. A 2005, 1082, 81–90. [Google Scholar] [CrossRef]
- EU (Eupropean Commission). Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed (SANTE/11813/2017); Eupropean Commission: Luxembourg, 2017. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Tang, T.; Du, S.; Sang, N.; Huang, H.; Zhang, C.; Zhao, X. Simultaneous Determination of 108 Pesticide Residues in Three Traditional Chinese Medicines Using a Modified QuEChERS Mixed Sample Preparation Method and HPLC-MS/MS. Molecules 2022, 27, 7636. https://doi.org/10.3390/molecules27217636
Fan X, Tang T, Du S, Sang N, Huang H, Zhang C, Zhao X. Simultaneous Determination of 108 Pesticide Residues in Three Traditional Chinese Medicines Using a Modified QuEChERS Mixed Sample Preparation Method and HPLC-MS/MS. Molecules. 2022; 27(21):7636. https://doi.org/10.3390/molecules27217636
Chicago/Turabian StyleFan, Xuyan, Tao Tang, Song Du, Ningning Sang, Hao Huang, Chenghui Zhang, and Xueping Zhao. 2022. "Simultaneous Determination of 108 Pesticide Residues in Three Traditional Chinese Medicines Using a Modified QuEChERS Mixed Sample Preparation Method and HPLC-MS/MS" Molecules 27, no. 21: 7636. https://doi.org/10.3390/molecules27217636
APA StyleFan, X., Tang, T., Du, S., Sang, N., Huang, H., Zhang, C., & Zhao, X. (2022). Simultaneous Determination of 108 Pesticide Residues in Three Traditional Chinese Medicines Using a Modified QuEChERS Mixed Sample Preparation Method and HPLC-MS/MS. Molecules, 27(21), 7636. https://doi.org/10.3390/molecules27217636





