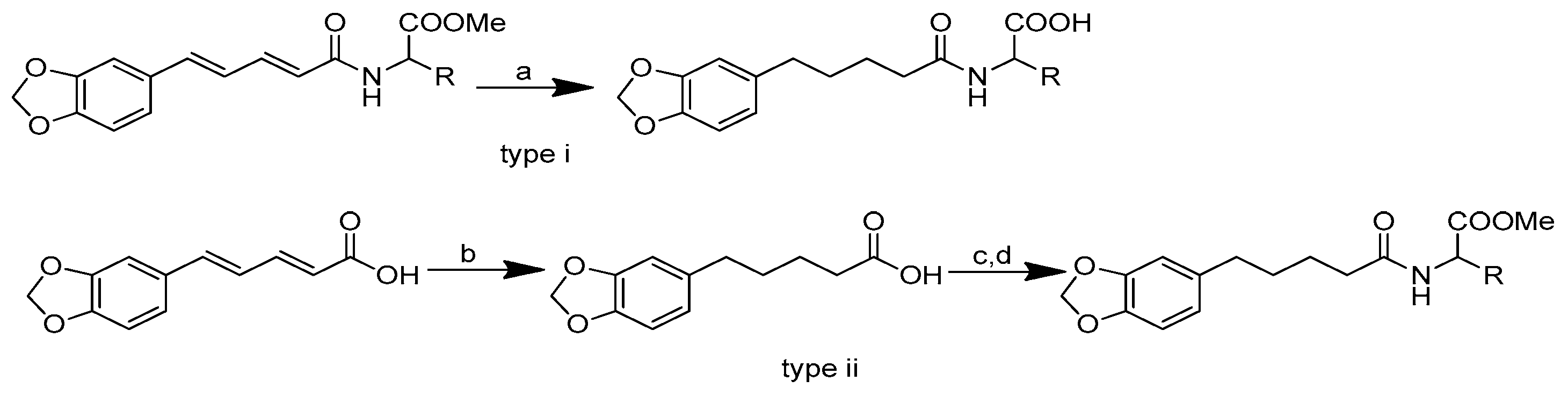
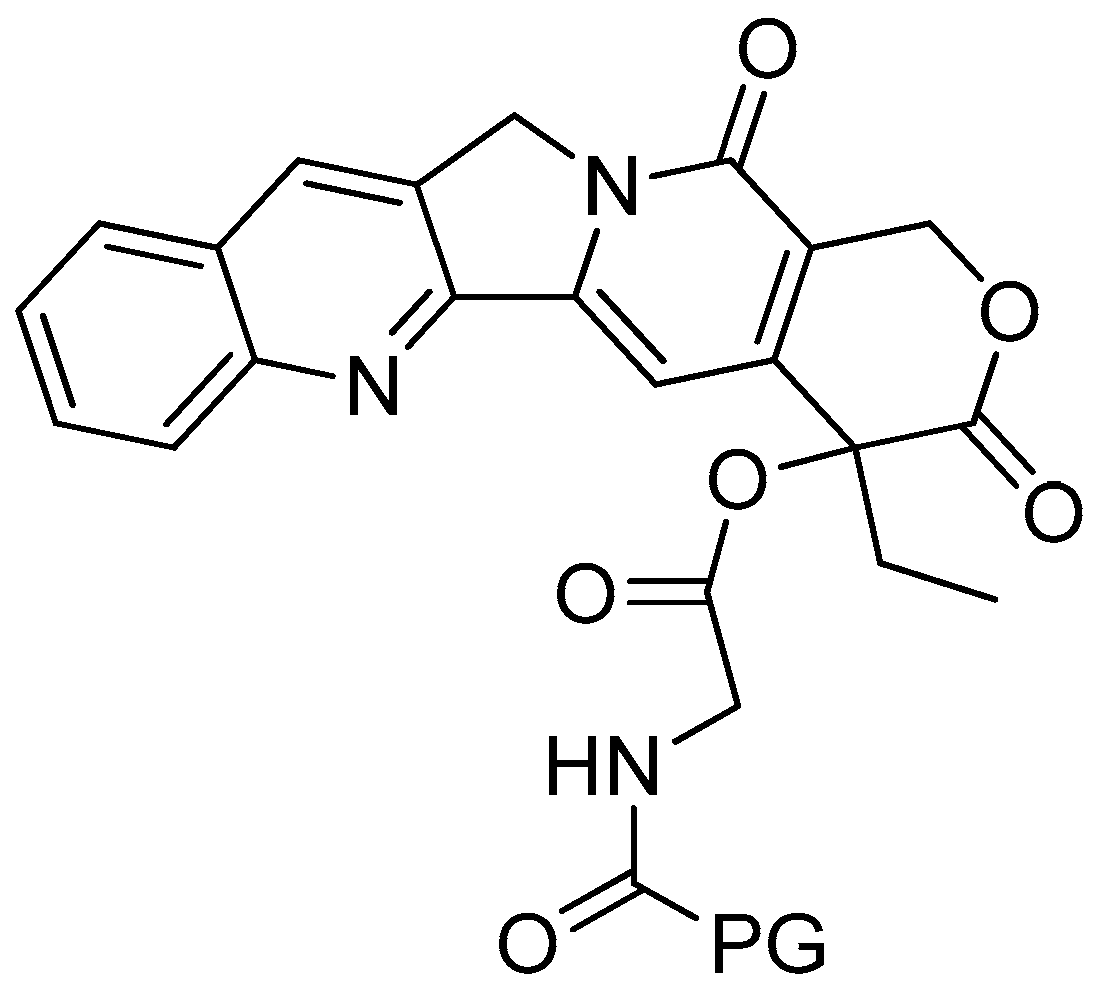
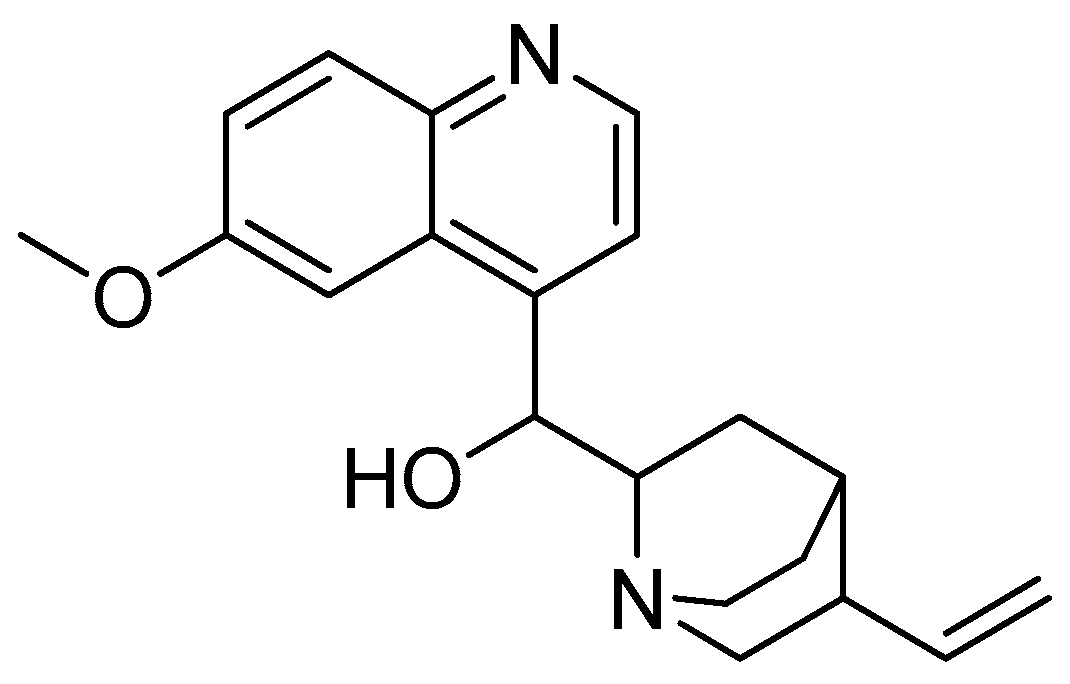
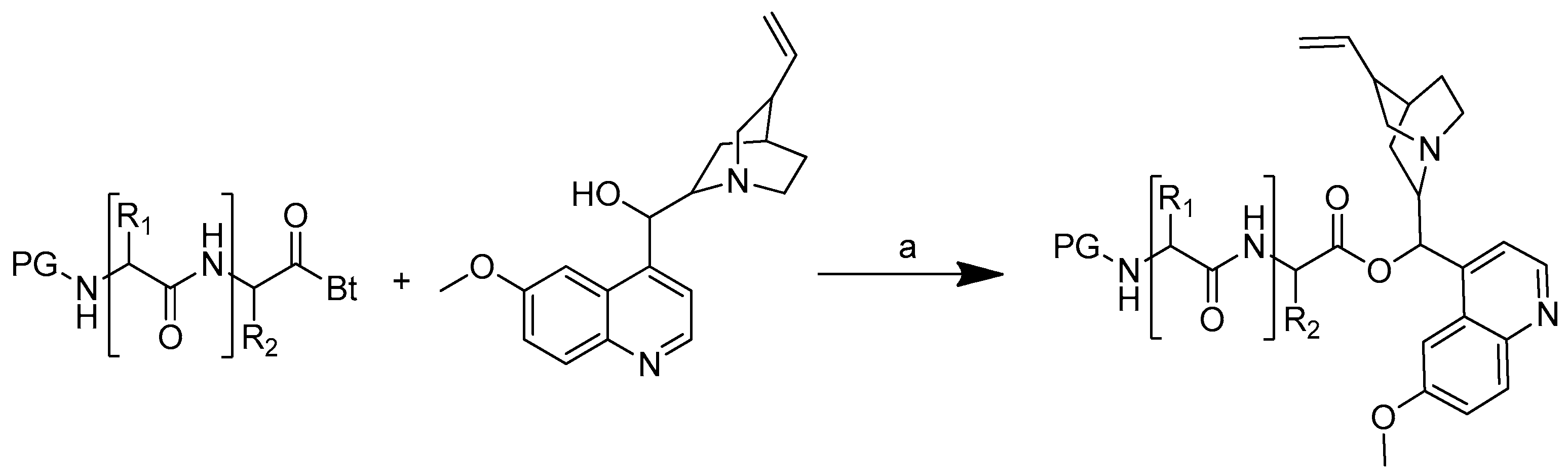
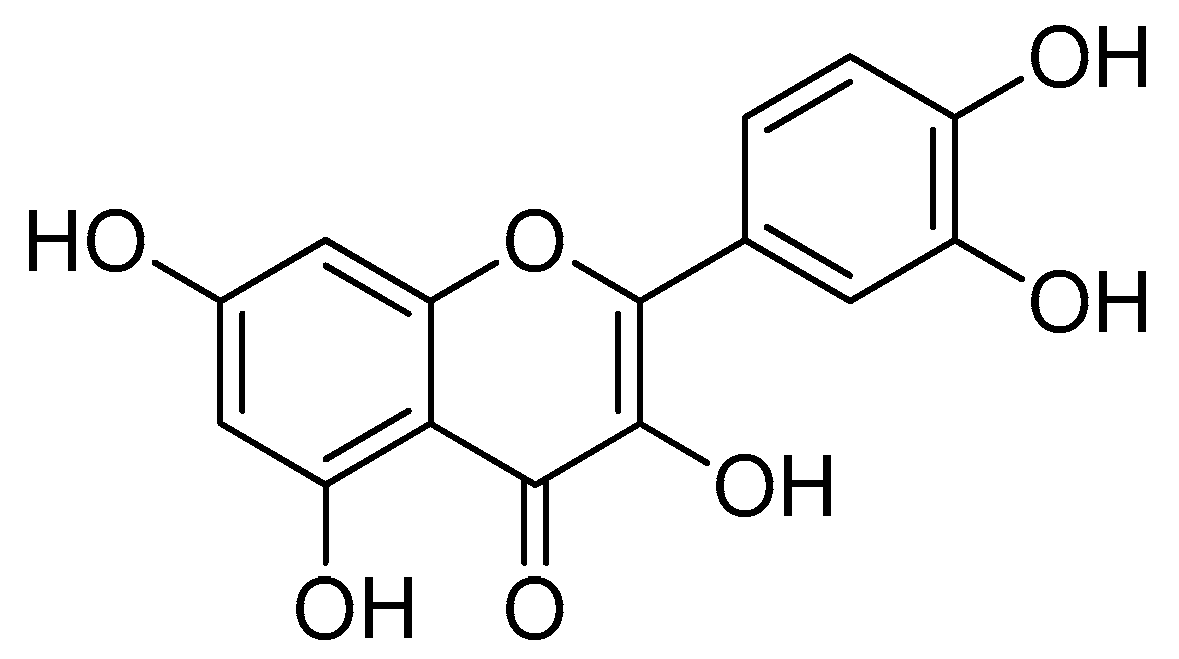
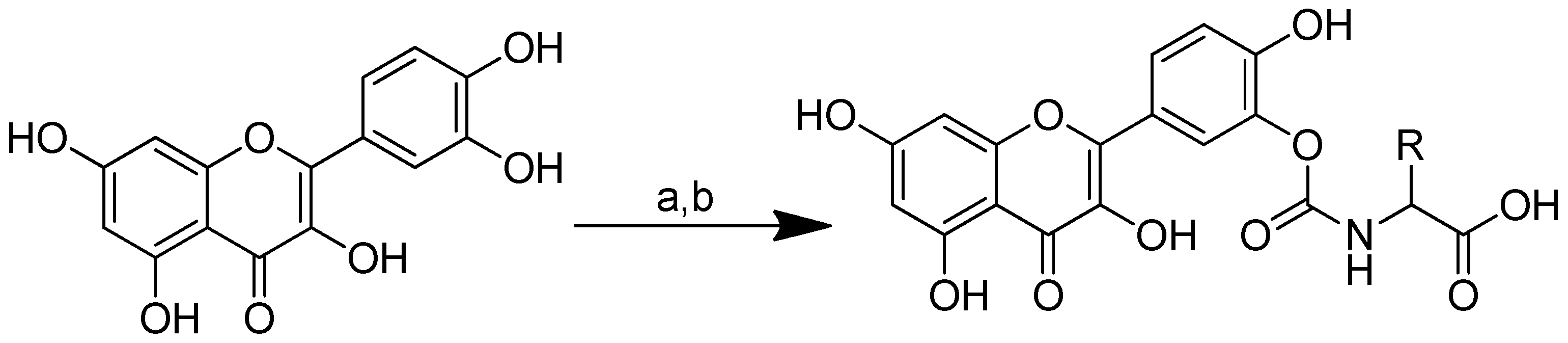
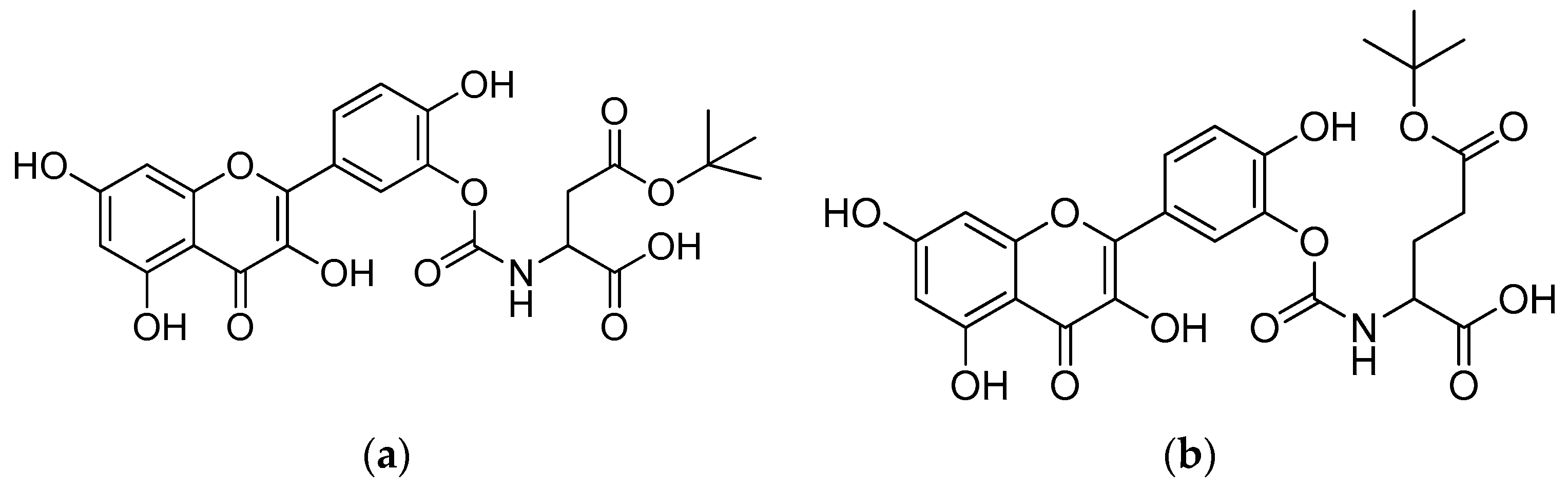
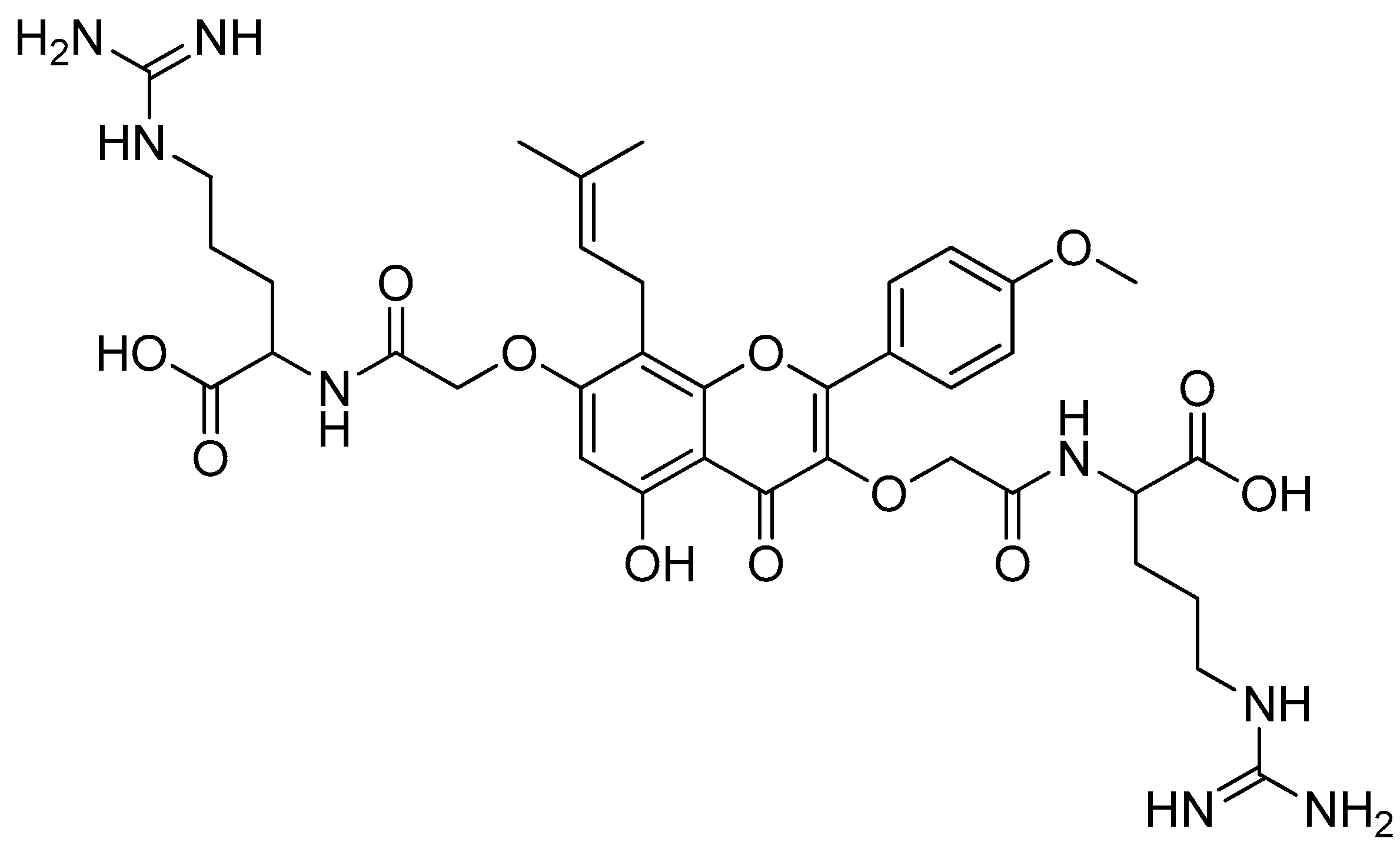
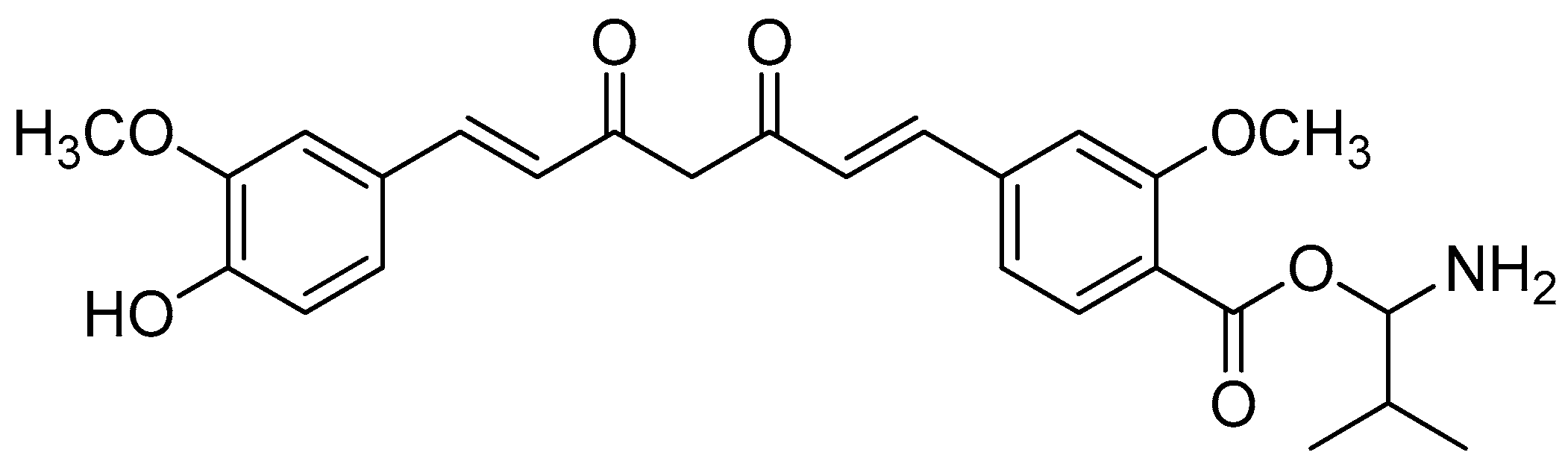
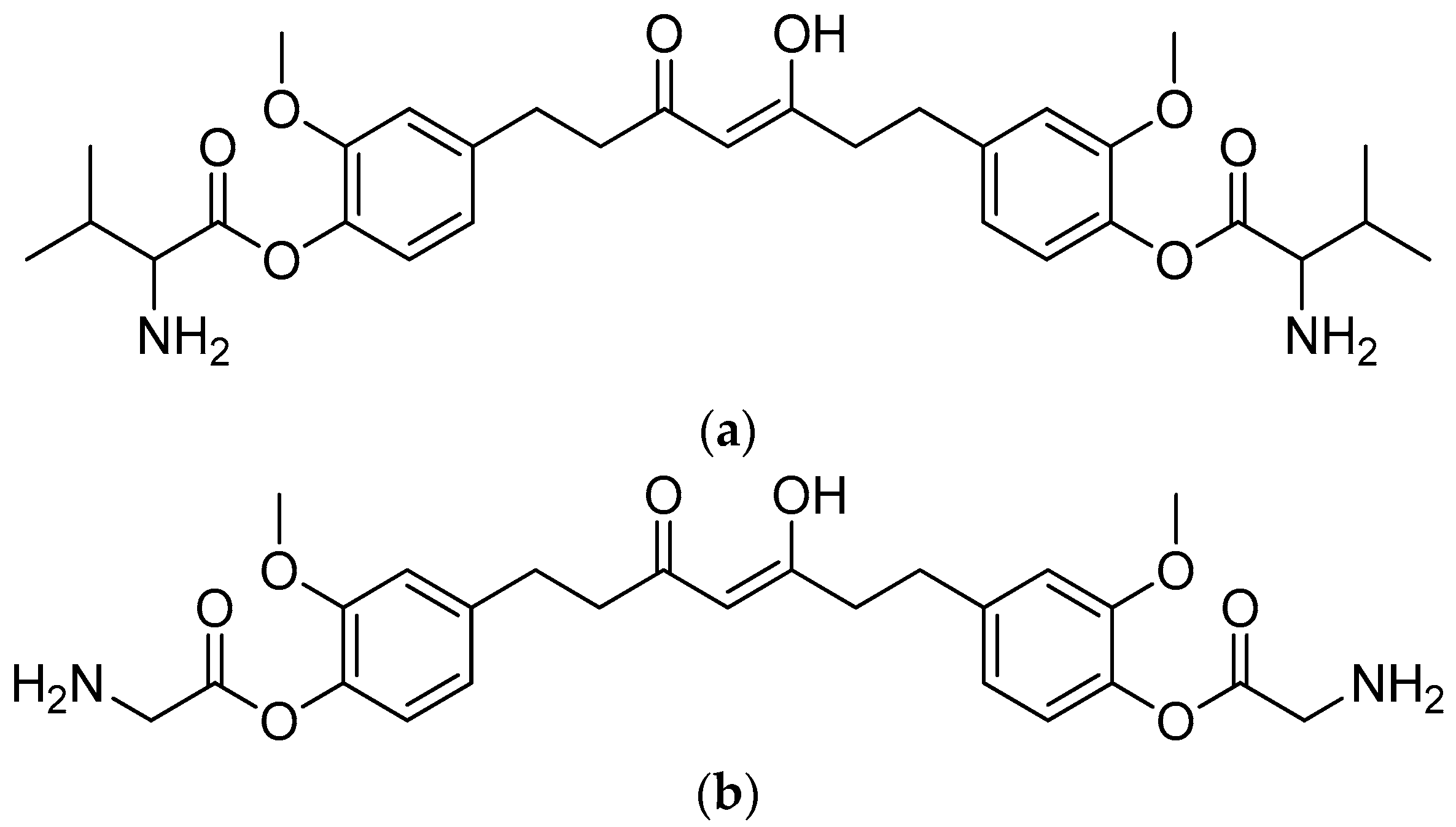
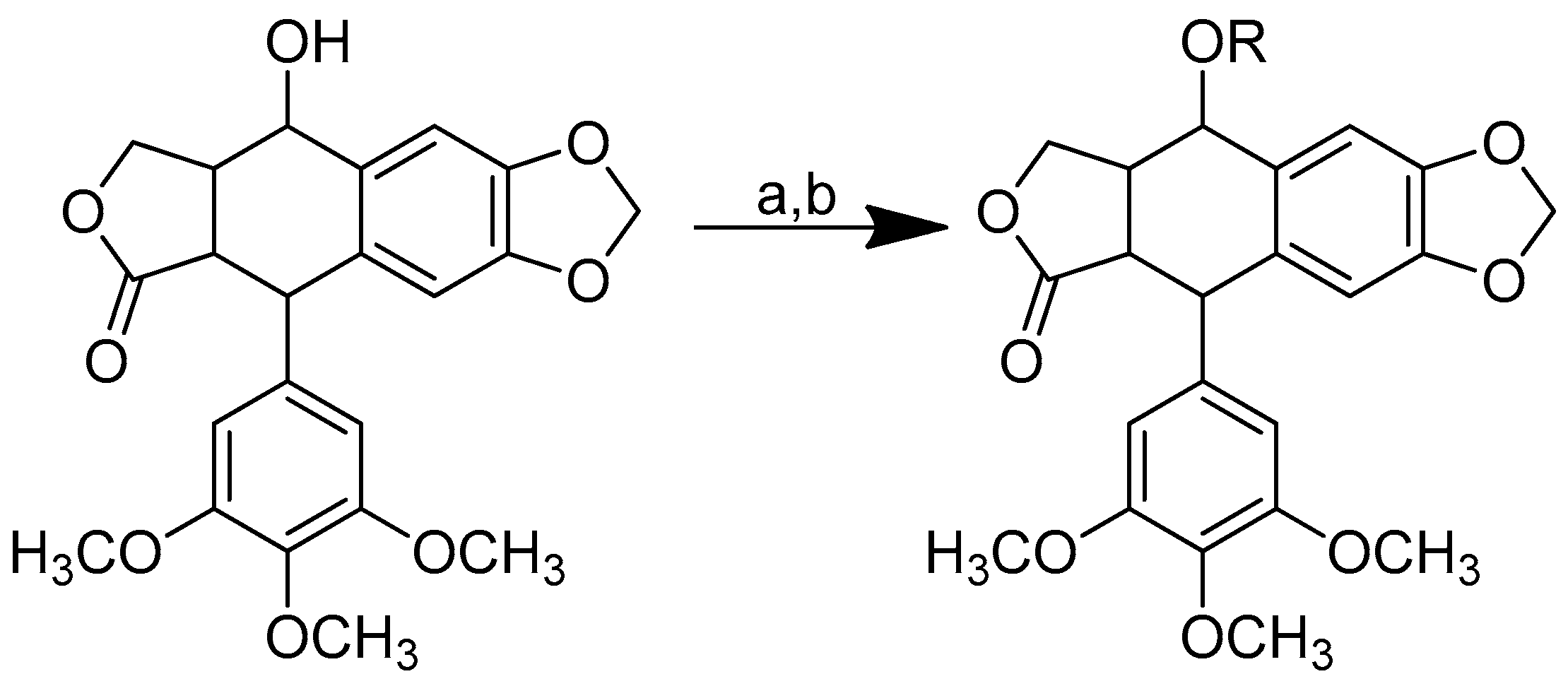
Scheme 1.
Reagents and conditions: (a) 20% KOH, CH3OH, 80°C, 3 days, 88%; (b) CH3SO2Cl, Et3N, DCM, 0 °C, 30 min, 85%; (c) amino acid methyl ester, Et3N, DCM, RT, 40–75%; (d) KF-Al2O3 (40%), microwave, 1000 W, 3–4 min, 70–80%. R in order: L-phenylalanine; L-tyrosine; L-valine; L-methionine; L-tryptophan.
Scheme 1.
Reagents and conditions: (a) 20% KOH, CH3OH, 80°C, 3 days, 88%; (b) CH3SO2Cl, Et3N, DCM, 0 °C, 30 min, 85%; (c) amino acid methyl ester, Et3N, DCM, RT, 40–75%; (d) KF-Al2O3 (40%), microwave, 1000 W, 3–4 min, 70–80%. R in order: L-phenylalanine; L-tyrosine; L-valine; L-methionine; L-tryptophan.
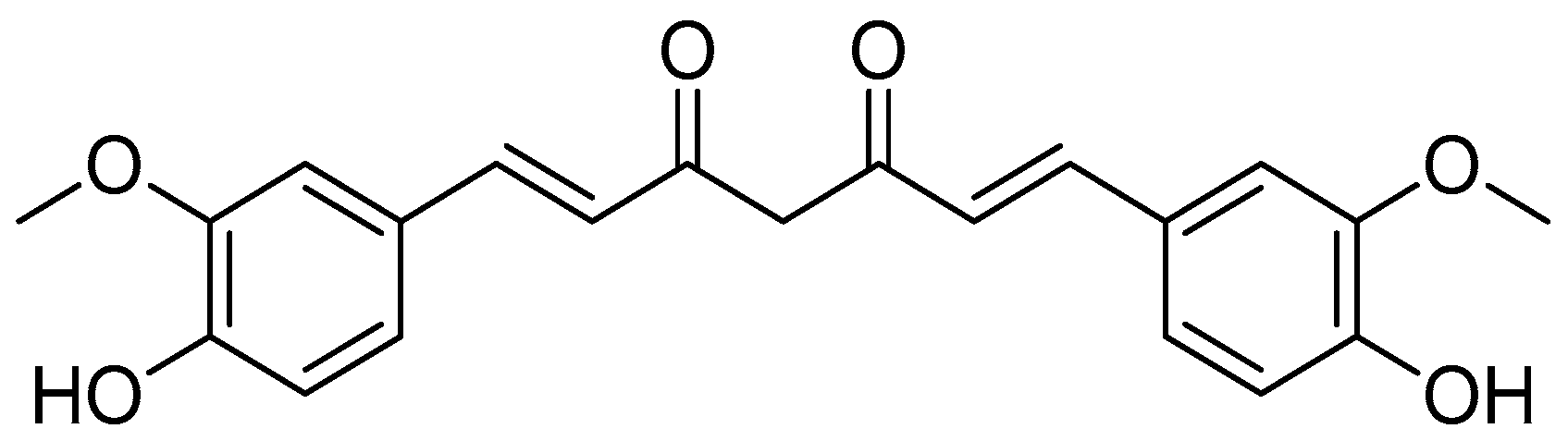
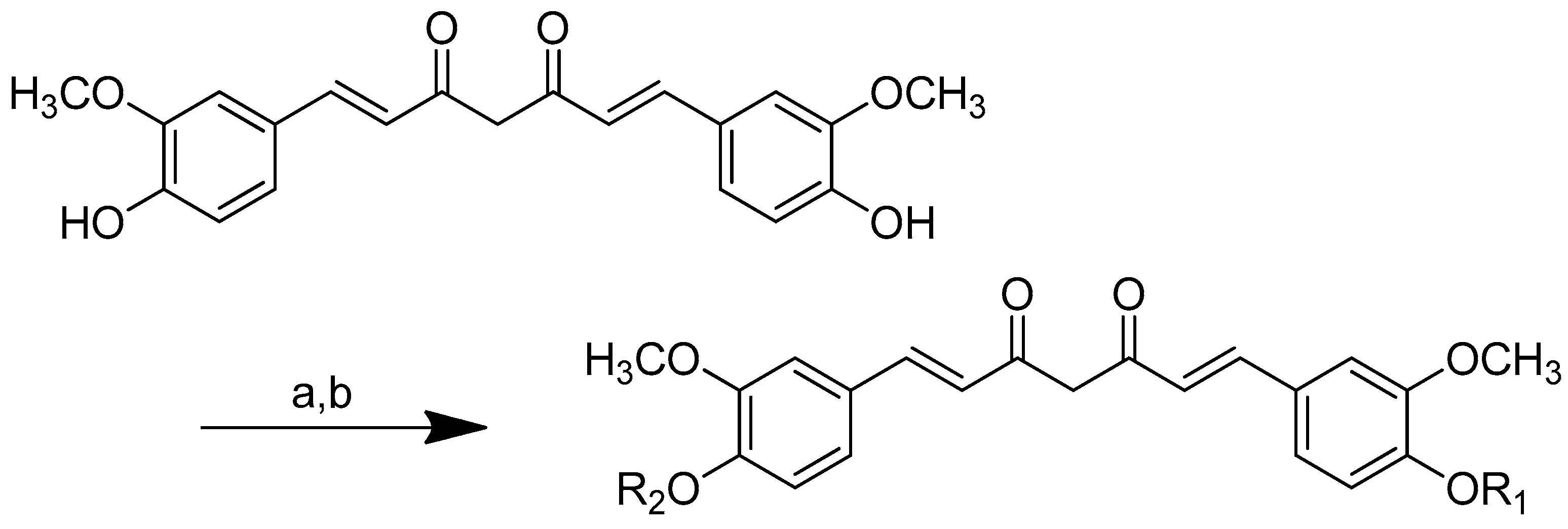
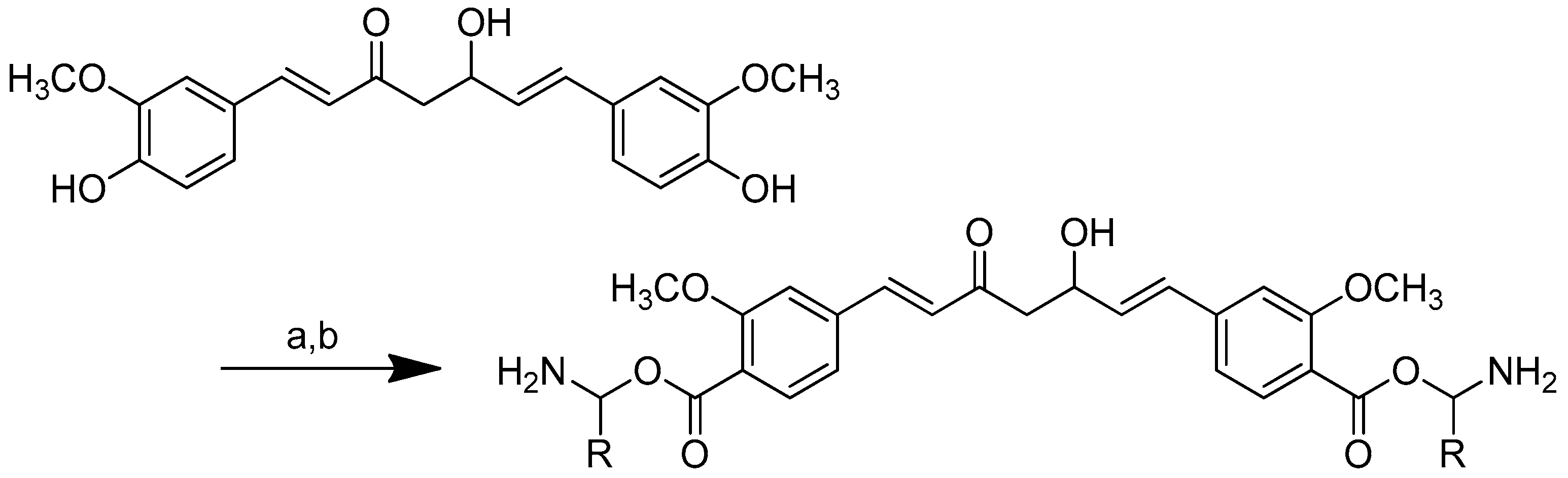
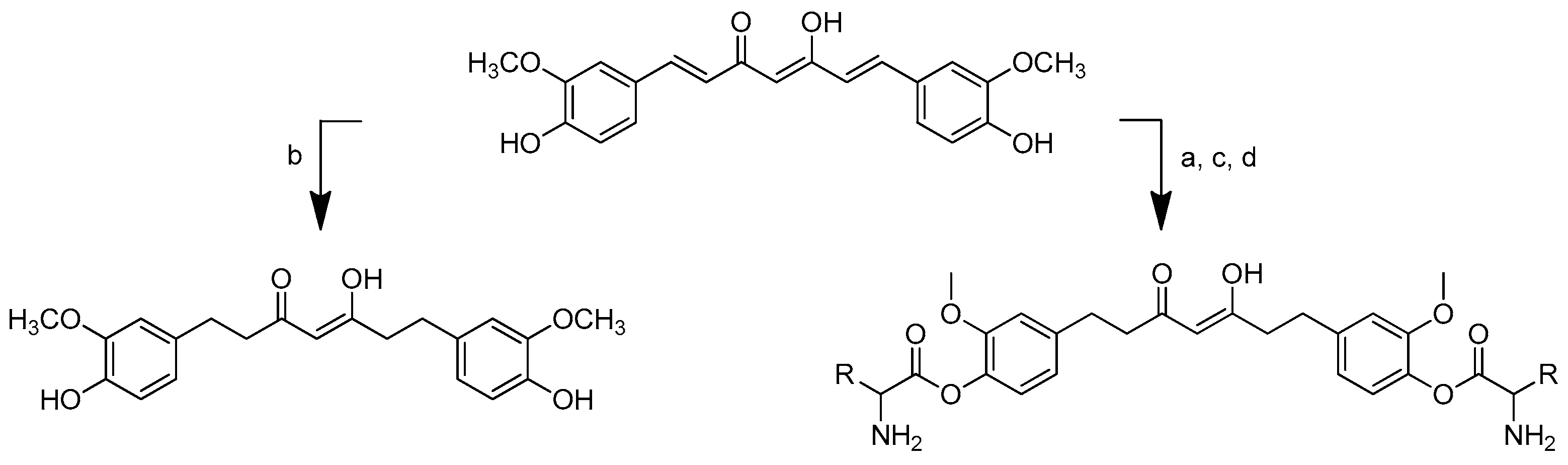
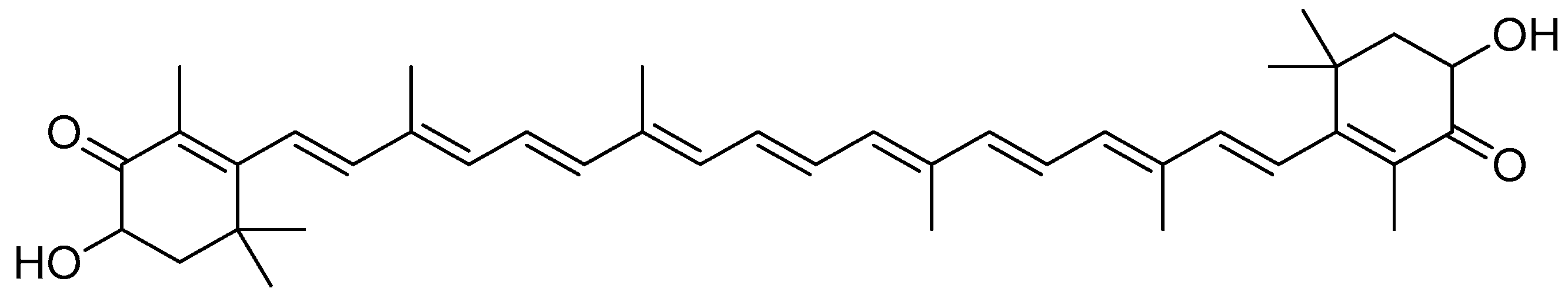
Scheme 2.
Reagents and conditions: type i (a) 5% Pd/C, H2 (40 Psi), MeOH, 30 min; type ii (b) 5% Pd/C, H2 (40 Psi), MeOH, 30 min; (c) CH3SO2Cl, Et3N, DCM, 0 °C, 30 min, 85%; (d) amino acid methyl ester, Et3N, DCM, RT.
Scheme 2.
Reagents and conditions: type i (a) 5% Pd/C, H2 (40 Psi), MeOH, 30 min; type ii (b) 5% Pd/C, H2 (40 Psi), MeOH, 30 min; (c) CH3SO2Cl, Et3N, DCM, 0 °C, 30 min, 85%; (d) amino acid methyl ester, Et3N, DCM, RT.
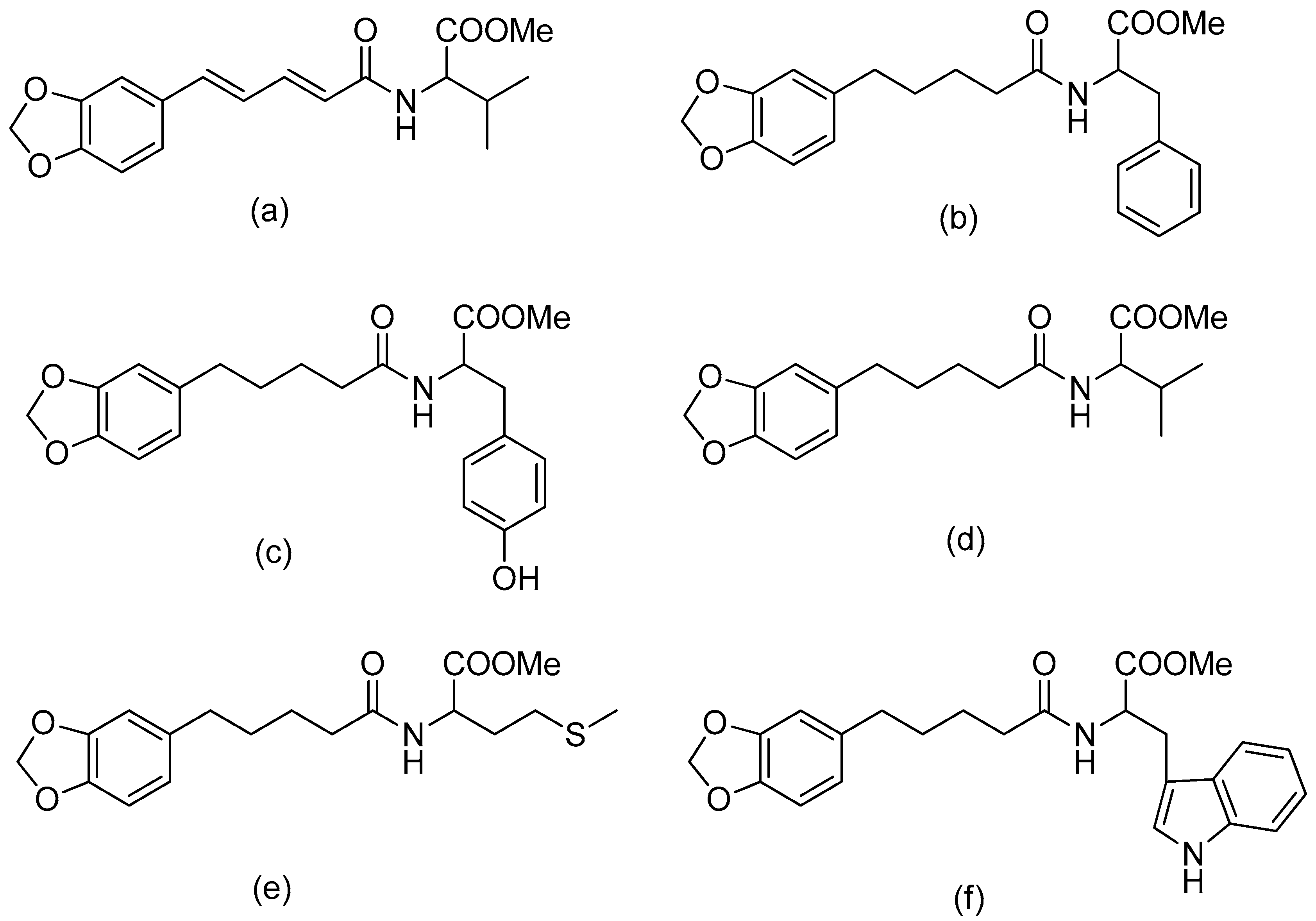
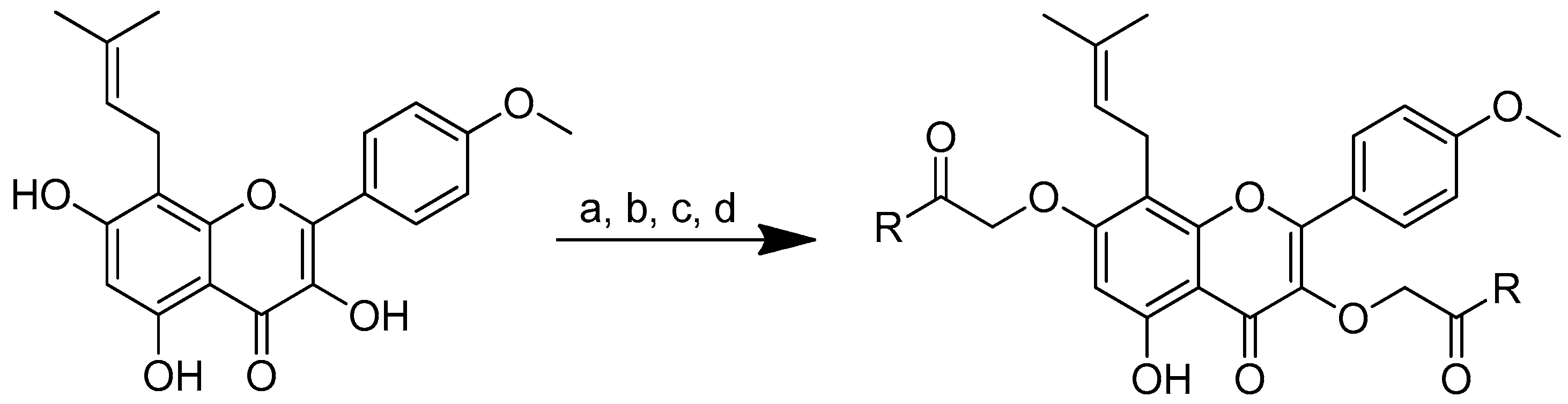
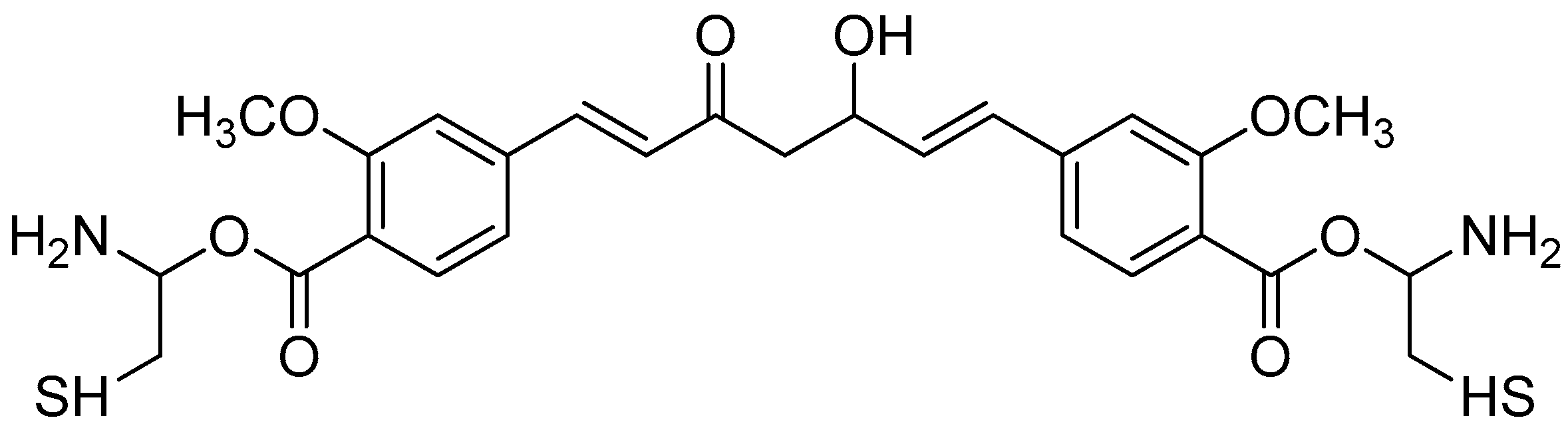
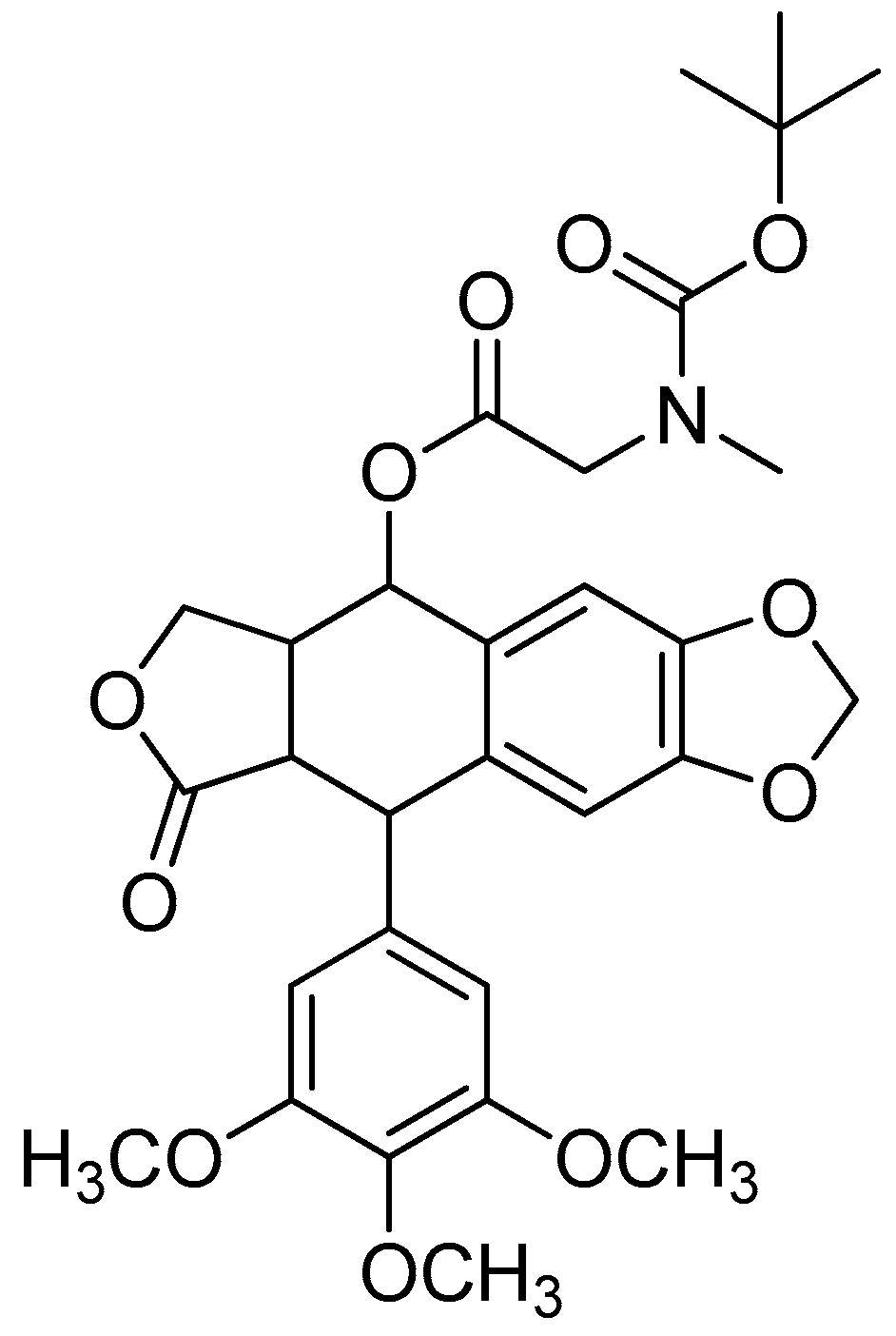
Figure 2.
(a) Piperoyl-L-valine methyl ester; (b) tetrahydropiperoyl-L-phenylalanine methyl ester; (c) tetrahydropiperoyl-L-tyrosine methyl ester; (d) tetrahydropiperoyl-L-valine methyl ester; (e) tetrahydropiperoyl-L-methionine methyl ester; (f) tetrahydropiperoyl-L-tryptophan methyl ester.
Figure 2.
(a) Piperoyl-L-valine methyl ester; (b) tetrahydropiperoyl-L-phenylalanine methyl ester; (c) tetrahydropiperoyl-L-tyrosine methyl ester; (d) tetrahydropiperoyl-L-valine methyl ester; (e) tetrahydropiperoyl-L-methionine methyl ester; (f) tetrahydropiperoyl-L-tryptophan methyl ester.
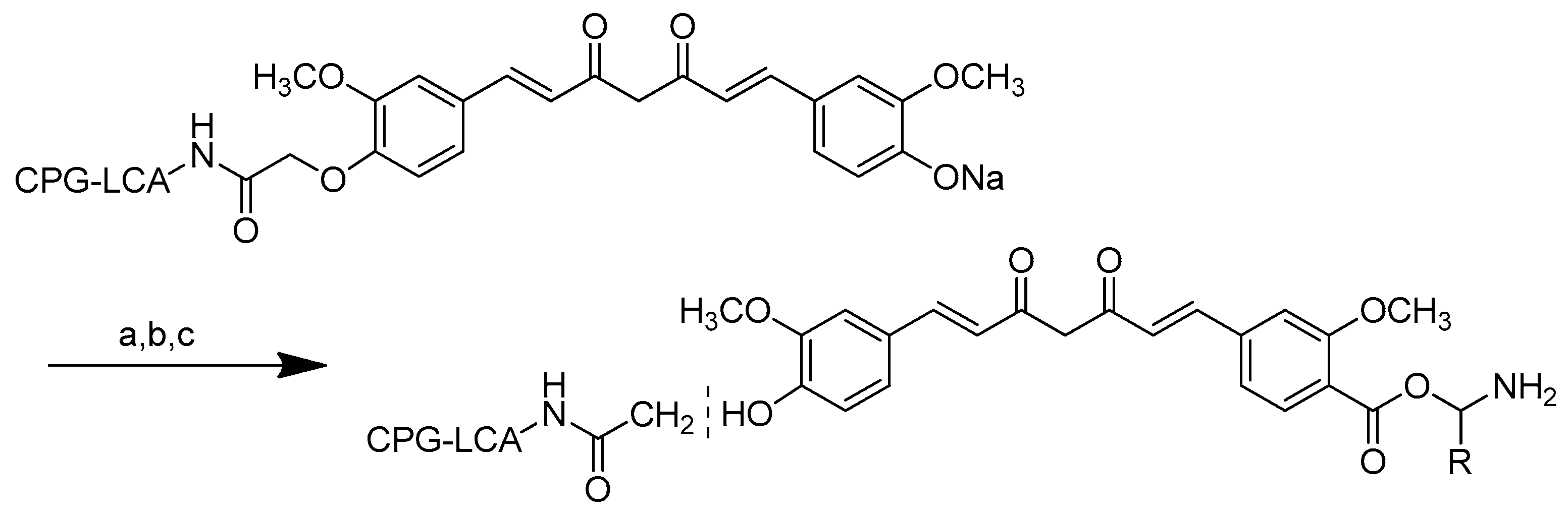
Scheme 3.
Synthesis of the PG conjugates of S-camptothecin. Reagents and conditions: (a) amino acid linker (R), DIPC, DMAP, DMF; (b) 50% TFA-CH2Cl2; (c) poly-R-(L-glutamic acid) (PG), DIPC, DMAP, DMF. R is the following amino acids: conjugate no. (2): L-glycine; (3) L-alanine; (4) β-alanine; (5) 4-NH-butyryl; (6) 2-O-acetyl; (7) 4-O-butyryl; (8) γ-glutamic acid.
Scheme 3.
Synthesis of the PG conjugates of S-camptothecin. Reagents and conditions: (a) amino acid linker (R), DIPC, DMAP, DMF; (b) 50% TFA-CH2Cl2; (c) poly-R-(L-glutamic acid) (PG), DIPC, DMAP, DMF. R is the following amino acids: conjugate no. (2): L-glycine; (3) L-alanine; (4) β-alanine; (5) 4-NH-butyryl; (6) 2-O-acetyl; (7) 4-O-butyryl; (8) γ-glutamic acid.
Figure 4.
Poly-R-(L-glutamic acid)-L-glycine-camptothecin.
Figure 4.
Poly-R-(L-glutamic acid)-L-glycine-camptothecin.
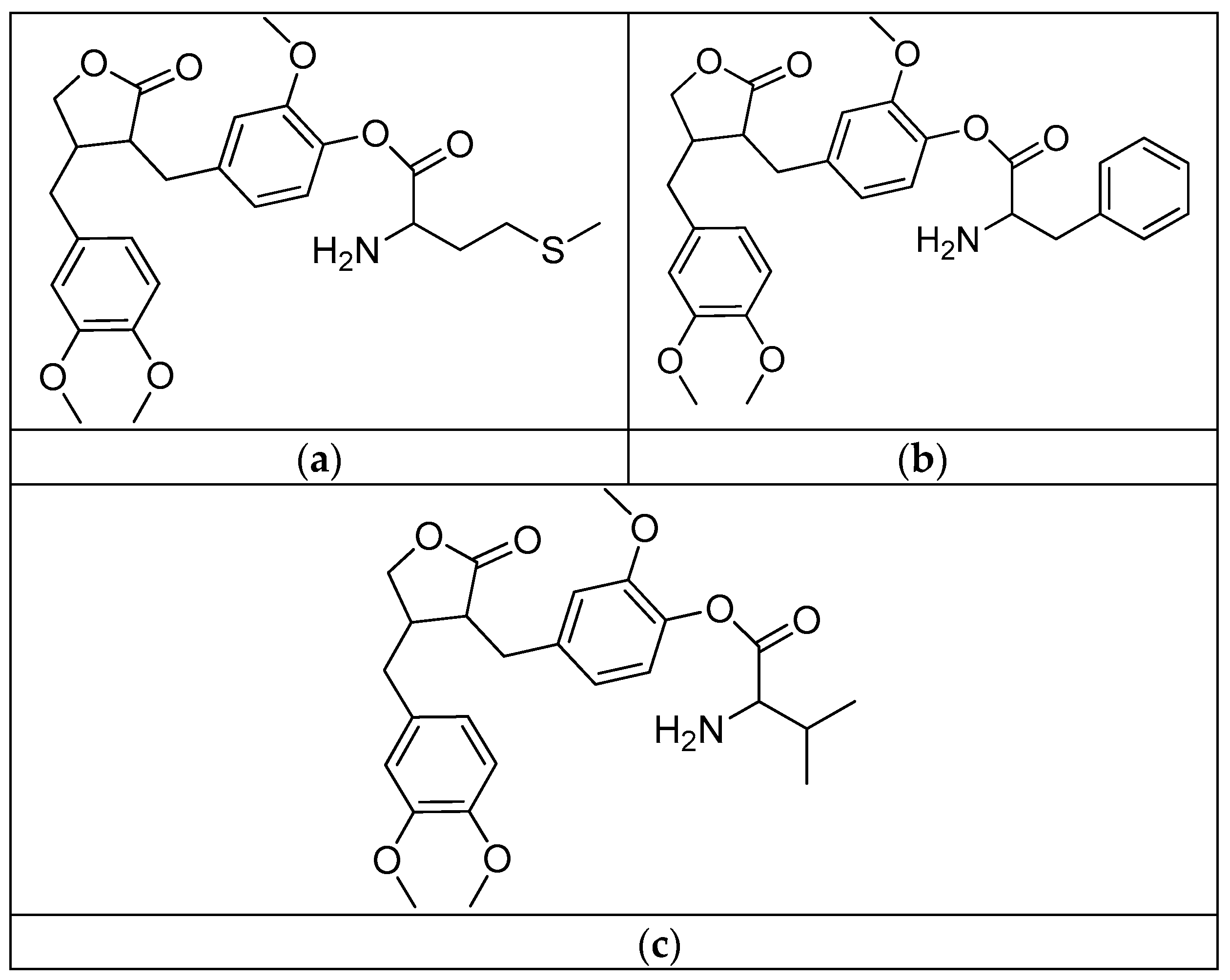
Scheme 4.
Synthesis of quinine conjugates. PG: protected groups. (a): K2CO3, DMF. R is the following amino acids: conjugate no. (2) R1: L-glycine; (3) R1: L-alanine; (4) R1: L-phenylalanine; (5) R1: L-isoleucine; (6) R1: L-histidine; (7) R1: L-serine; (8) R1: L-glutamic acid; (9) R1: L-lysine; (10) R1: L-aspartic acid; (11) R1: L-cysteine; (12) R1: L-alanine, R2: L-phenylalanine; (13) R1: L-valine, R2: L-leucine; (14) R1: L-isoleucine, R2: L-glycine.
Scheme 4.
Synthesis of quinine conjugates. PG: protected groups. (a): K2CO3, DMF. R is the following amino acids: conjugate no. (2) R1: L-glycine; (3) R1: L-alanine; (4) R1: L-phenylalanine; (5) R1: L-isoleucine; (6) R1: L-histidine; (7) R1: L-serine; (8) R1: L-glutamic acid; (9) R1: L-lysine; (10) R1: L-aspartic acid; (11) R1: L-cysteine; (12) R1: L-alanine, R2: L-phenylalanine; (13) R1: L-valine, R2: L-leucine; (14) R1: L-isoleucine, R2: L-glycine.
Figure 6.
(a) Acylbenzotriazoles-L-aspartic acid-quinine; (b) acylbenzotriazoles-L-isoleucine-glycine-quinine.
Figure 6.
(a) Acylbenzotriazoles-L-aspartic acid-quinine; (b) acylbenzotriazoles-L-isoleucine-glycine-quinine.
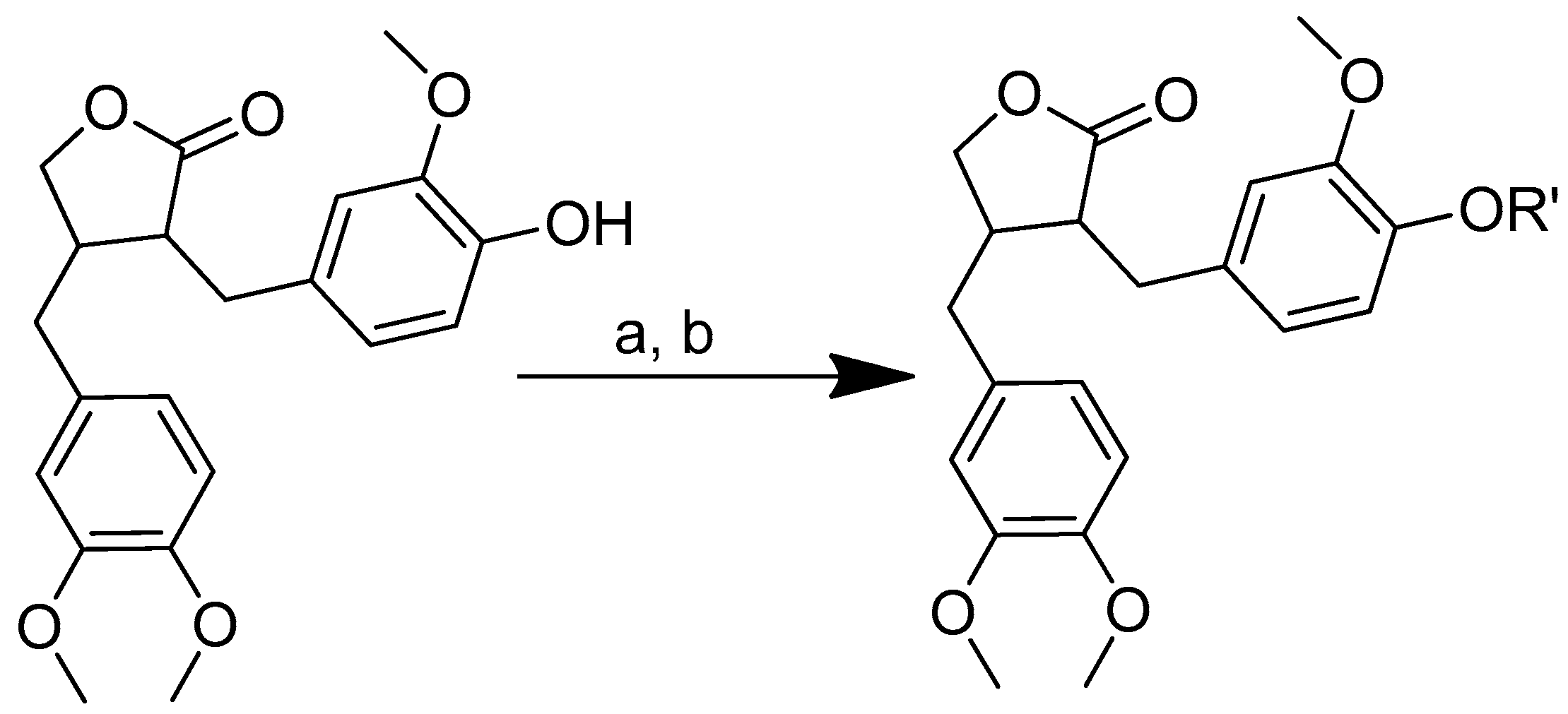
Scheme 5.
Syntheses of the quercetin analogues. (a): (4-NO2-PhO)2CO, DIPEA, DMF, 0 °C to rt; (b): TFA, CH2Cl2, 0 °C, rt. R in order: (2) L-alanine; (3) L-valine; (4) L-lysine; (5) L-phenylalanine; (6) L-aspartic acid; (7) L-methionine; (8) L-glutamic acid; (9) L-alanine-L-aspartic acid; (10) L-alanine-L-glutamic acid.
Scheme 5.
Syntheses of the quercetin analogues. (a): (4-NO2-PhO)2CO, DIPEA, DMF, 0 °C to rt; (b): TFA, CH2Cl2, 0 °C, rt. R in order: (2) L-alanine; (3) L-valine; (4) L-lysine; (5) L-phenylalanine; (6) L-aspartic acid; (7) L-methionine; (8) L-glutamic acid; (9) L-alanine-L-aspartic acid; (10) L-alanine-L-glutamic acid.
Figure 8.
(a) Quercetin–aspartic acid conjugate; (b) quercetin–glutamic acid conjugate.
Figure 8.
(a) Quercetin–aspartic acid conjugate; (b) quercetin–glutamic acid conjugate.
Scheme 6.
Synthesis of amino-acid-conjugated flavone compounds. (a) Ethyl iodoacetate, K2CO3, acetone, reflux, 12 h; (b) LiOH, THF, H2O, RT, 1.5 h; (c) corresponding basic amino acid, DIC, HOBt, anhydrous DMF, RT, overnight; (d) corresponding basic amino acid, HATU, DIPEA, anhydrous DMF, RT, overnight. R in order: (2) L-histidine; (3) L-arginine; (4) L-lysine.
Scheme 6.
Synthesis of amino-acid-conjugated flavone compounds. (a) Ethyl iodoacetate, K2CO3, acetone, reflux, 12 h; (b) LiOH, THF, H2O, RT, 1.5 h; (c) corresponding basic amino acid, DIC, HOBt, anhydrous DMF, RT, overnight; (d) corresponding basic amino acid, HATU, DIPEA, anhydrous DMF, RT, overnight. R in order: (2) L-histidine; (3) L-arginine; (4) L-lysine.
Figure 10.
Icaritin–arginine conjugate.
Figure 10.
Icaritin–arginine conjugate.
Scheme 7.
Synthesis of (2) 4,40-(di-O-glutamoyl)-curcumin, (3) 4,40-(di-O-valinoyl) curcumin, and (4) 4,40-(di-O-glycinoyl) curcumin. (a): Pyridine/N-Phathloyl (glutamoyl/valinoyl/glycinoyl) chloride/6 h/r.t.; (b): NH3:Pyridine (9:1 v/v).
Scheme 7.
Synthesis of (2) 4,40-(di-O-glutamoyl)-curcumin, (3) 4,40-(di-O-valinoyl) curcumin, and (4) 4,40-(di-O-glycinoyl) curcumin. (a): Pyridine/N-Phathloyl (glutamoyl/valinoyl/glycinoyl) chloride/6 h/r.t.; (b): NH3:Pyridine (9:1 v/v).
Scheme 8.
Synthesis of monoester curcumin on CPG-LCAA. (a): i. N-Phthaloyl ((5) glycinoyl/(6) valinoyl) chloride, DMAP, overnight, rt; (b): ammonia:pyridine (9:1 v/v), 5 min; (c): i. HI, 37%, 2 mL.
Scheme 8.
Synthesis of monoester curcumin on CPG-LCAA. (a): i. N-Phthaloyl ((5) glycinoyl/(6) valinoyl) chloride, DMAP, overnight, rt; (b): ammonia:pyridine (9:1 v/v), 5 min; (c): i. HI, 37%, 2 mL.
Figure 12.
Monovalinoyl curcumin.
Figure 12.
Monovalinoyl curcumin.
Scheme 9.
Synthesis of curcumin–amino acid conjugates. (a) DCC/DMAP/TEA, dioxane, N2, 25–30 °C; (b) TFA (10% solution, DCM), 10 min.
Scheme 9.
Synthesis of curcumin–amino acid conjugates. (a) DCC/DMAP/TEA, dioxane, N2, 25–30 °C; (b) TFA (10% solution, DCM), 10 min.
Figure 13.
Biscysteinoyl curcumin.
Figure 13.
Biscysteinoyl curcumin.
Scheme 10.
Synthesis of tetrahydrocurcumin–amino acid conjugates. (a) DCC, DMAP, dioxane, rt, 7–12 h, inert; (b) Pd/BaSO4; (c) 10% TFA in DCM, 0 °C, 3–5.5 h; (d) H2, Pd/BaSO4, 30 psi, 0.5–1.5 h. R in order: (2) L-alanine; (3) L-isoleucine; (4) L-proline; (5) L-valine; (6) L-phenylalanine; (7) L-glycine; (8) L-leucine.
Scheme 10.
Synthesis of tetrahydrocurcumin–amino acid conjugates. (a) DCC, DMAP, dioxane, rt, 7–12 h, inert; (b) Pd/BaSO4; (c) 10% TFA in DCM, 0 °C, 3–5.5 h; (d) H2, Pd/BaSO4, 30 psi, 0.5–1.5 h. R in order: (2) L-alanine; (3) L-isoleucine; (4) L-proline; (5) L-valine; (6) L-phenylalanine; (7) L-glycine; (8) L-leucine.
Figure 14.
(a) Tetrahydrocurcumin–L-glycine; (b) tetrahydrocurcumin–L-valine.
Figure 14.
(a) Tetrahydrocurcumin–L-glycine; (b) tetrahydrocurcumin–L-valine.
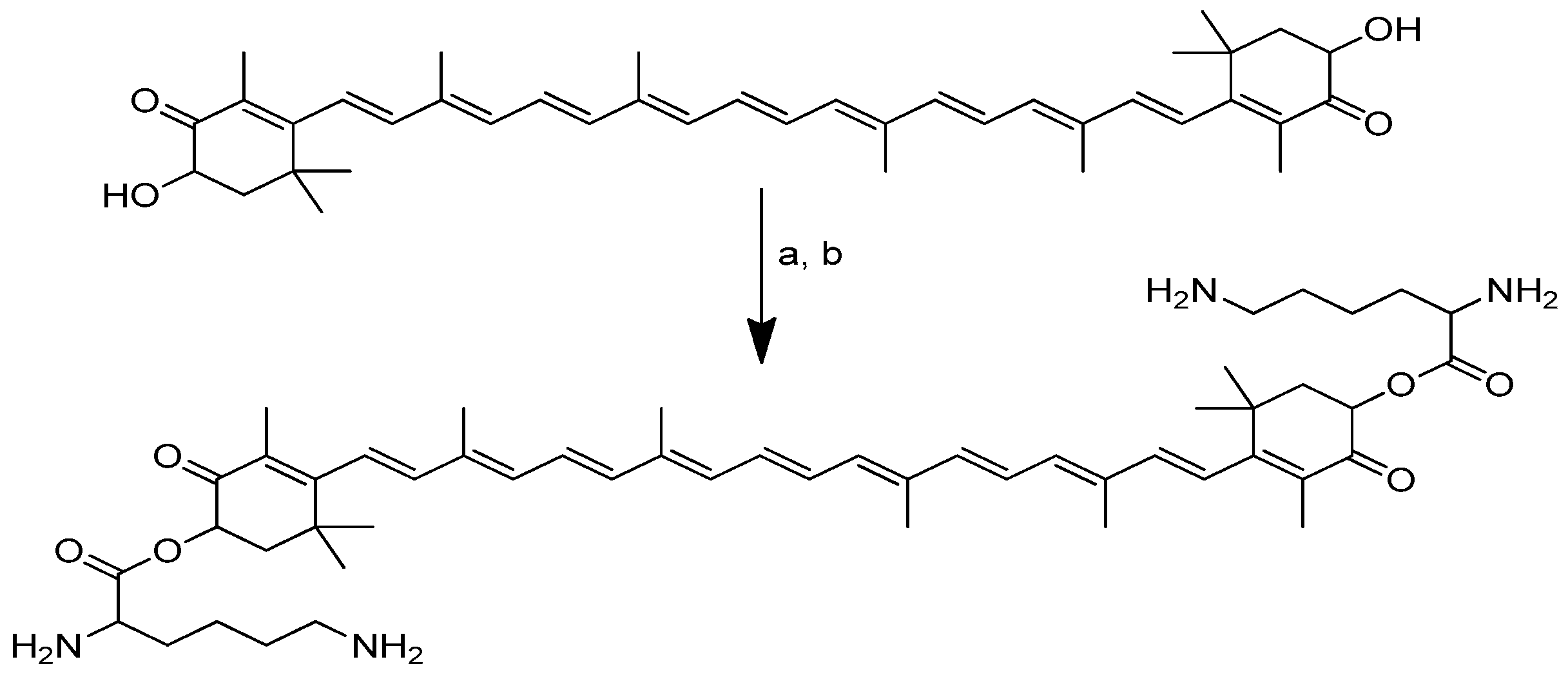
Scheme 11.
Synthesis of the dilysinate diester of astaxanthin. (a) Boc-protected lysine, DMAP, DIC, CH2CL2; (b) anhydrous HCl in dioxane.
Scheme 11.
Synthesis of the dilysinate diester of astaxanthin. (a) Boc-protected lysine, DMAP, DIC, CH2CL2; (b) anhydrous HCl in dioxane.
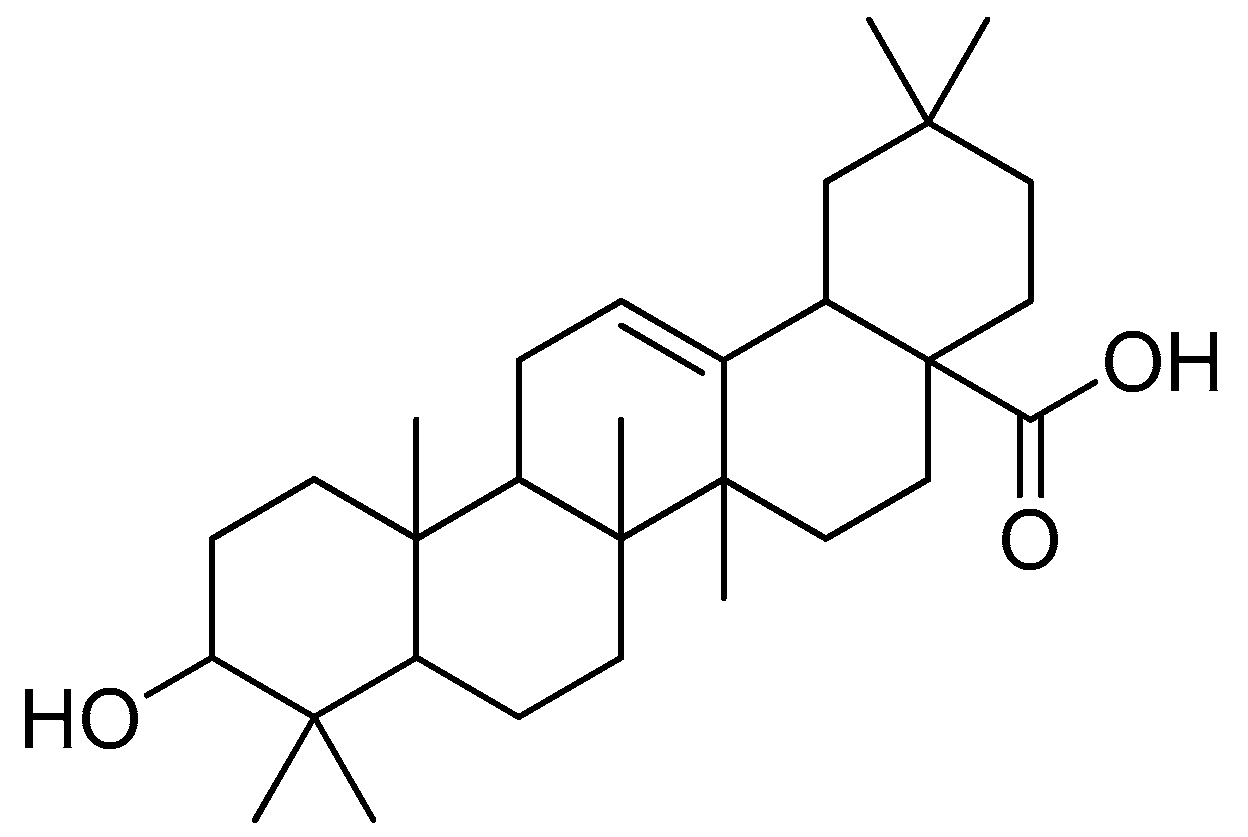
Figure 16.
Oleanolic acid.
Figure 16.
Oleanolic acid.
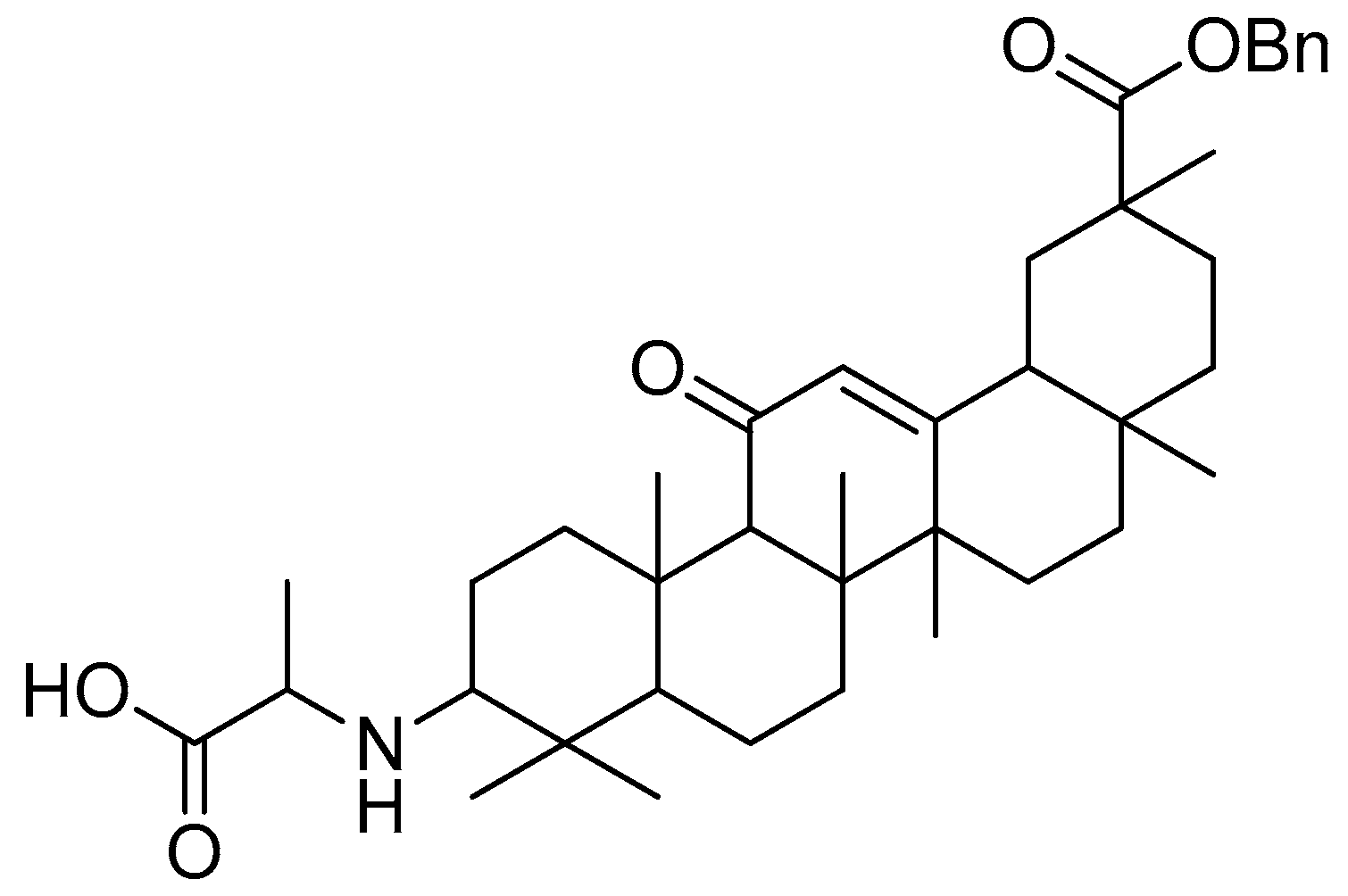
Scheme 12.
Synthesis of oleanolic conjugates. (a) Benzyl bromide, K2CO3, DMF, reflux 2 h, 85 °C; (b) Cbz-(L-)-amide acids, EDCI, DMAP, dry DCM, r.t. 12 h; (c) Pd(OH)2/C, H2, MeOH, r.t. 12 h.
Scheme 12.
Synthesis of oleanolic conjugates. (a) Benzyl bromide, K2CO3, DMF, reflux 2 h, 85 °C; (b) Cbz-(L-)-amide acids, EDCI, DMAP, dry DCM, r.t. 12 h; (c) Pd(OH)2/C, H2, MeOH, r.t. 12 h.
Figure 17.
Oleanolic acid–lysine conjugate.
Figure 17.
Oleanolic acid–lysine conjugate.
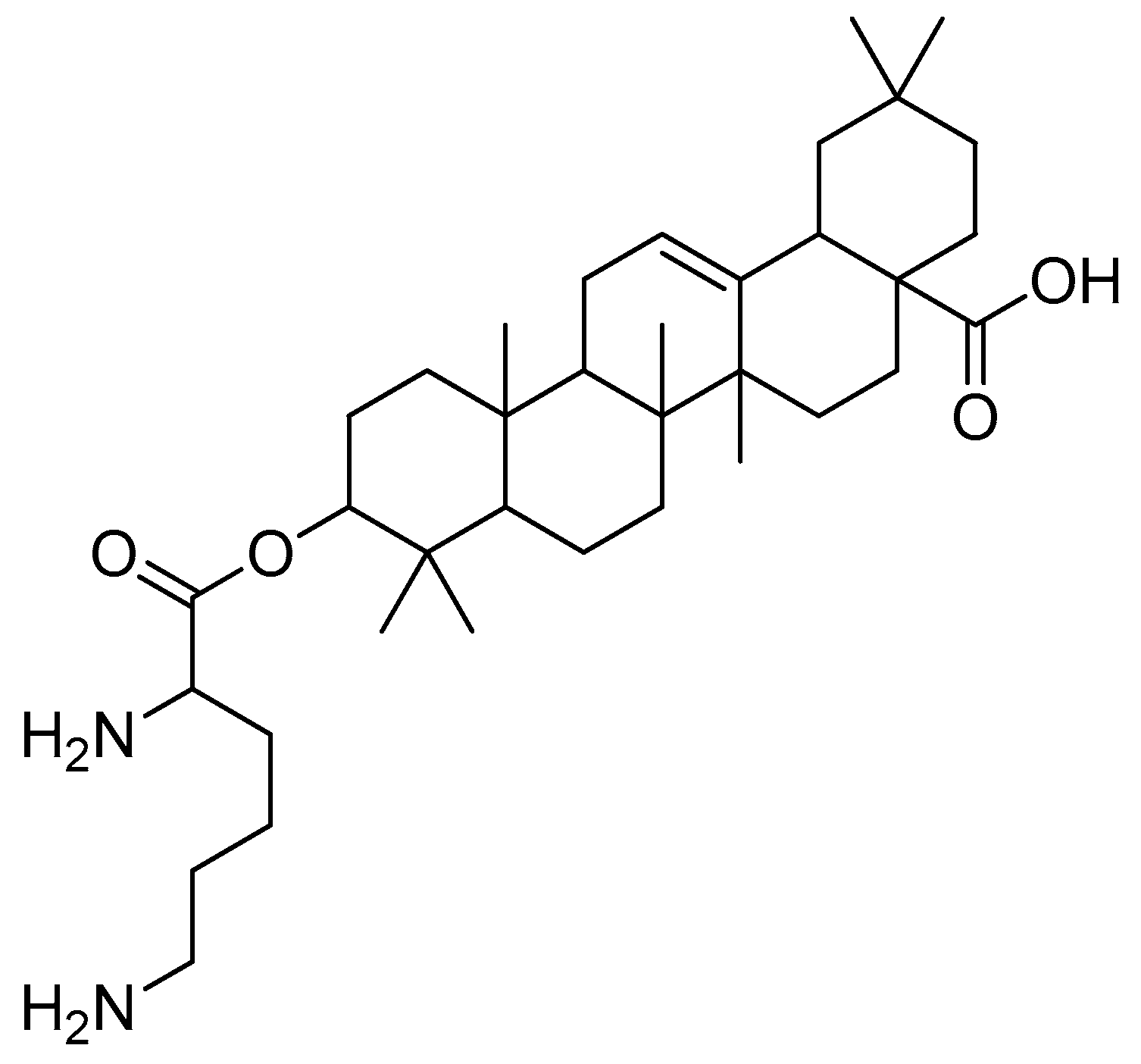
Scheme 13.
The synthesis of monosubstituted betulin esters containing L-amino acids. (a) Boc-amino acids, CDI, THF, 24 h; (b) HCl in methanol. R in order: L-lysine; L-ornithine.
Scheme 13.
The synthesis of monosubstituted betulin esters containing L-amino acids. (a) Boc-amino acids, CDI, THF, 24 h; (b) HCl in methanol. R in order: L-lysine; L-ornithine.
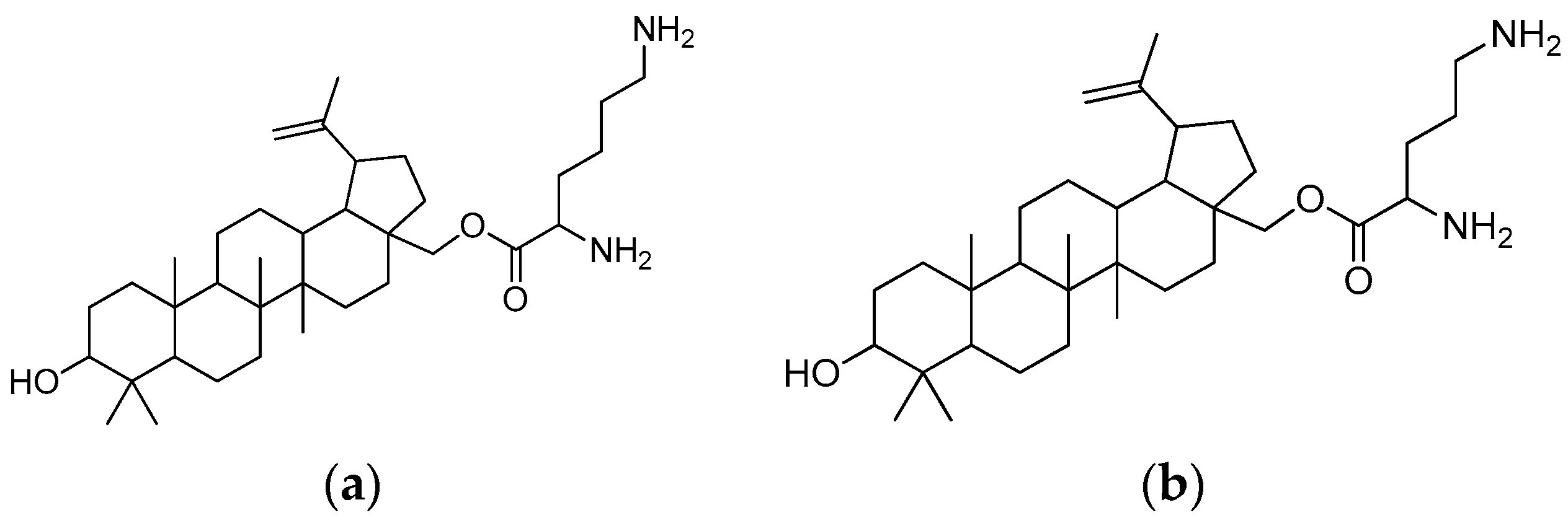
Figure 18.
(a) Betulin-L-lysine; (b) betulin-L-ornithine.
Figure 18.
(a) Betulin-L-lysine; (b) betulin-L-ornithine.
Figure 19.
Glycyrrhetinic acid.
Figure 19.
Glycyrrhetinic acid.
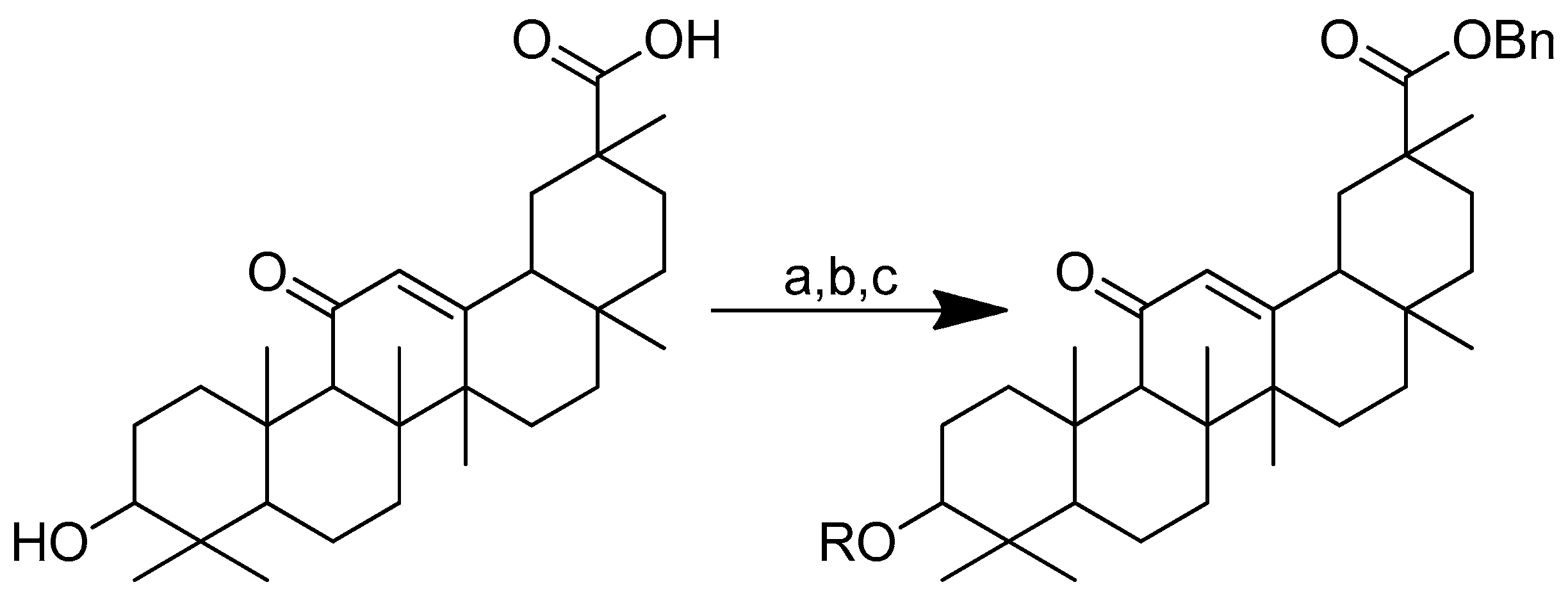
Scheme 14.
Synthesis of the glycyrrhetinic acid derivatives with esters at the C-3 position: (a) Bn-Br, dry DMF, dry K2CO3, 85 °C, reflux, 3 h; (b) Boc-amino acids, DCM, DMAP, EDCI, rt, 12 h; (c) TFA in dry DCM. R in order: (3) L-glycine; (4) L-alanine; (5) L-phenylalanine; (6) L-proline; (7) L-sarine; (8) L-leucine; (9) L-isoleucine; (10) L-methionine.
Scheme 14.
Synthesis of the glycyrrhetinic acid derivatives with esters at the C-3 position: (a) Bn-Br, dry DMF, dry K2CO3, 85 °C, reflux, 3 h; (b) Boc-amino acids, DCM, DMAP, EDCI, rt, 12 h; (c) TFA in dry DCM. R in order: (3) L-glycine; (4) L-alanine; (5) L-phenylalanine; (6) L-proline; (7) L-sarine; (8) L-leucine; (9) L-isoleucine; (10) L-methionine.
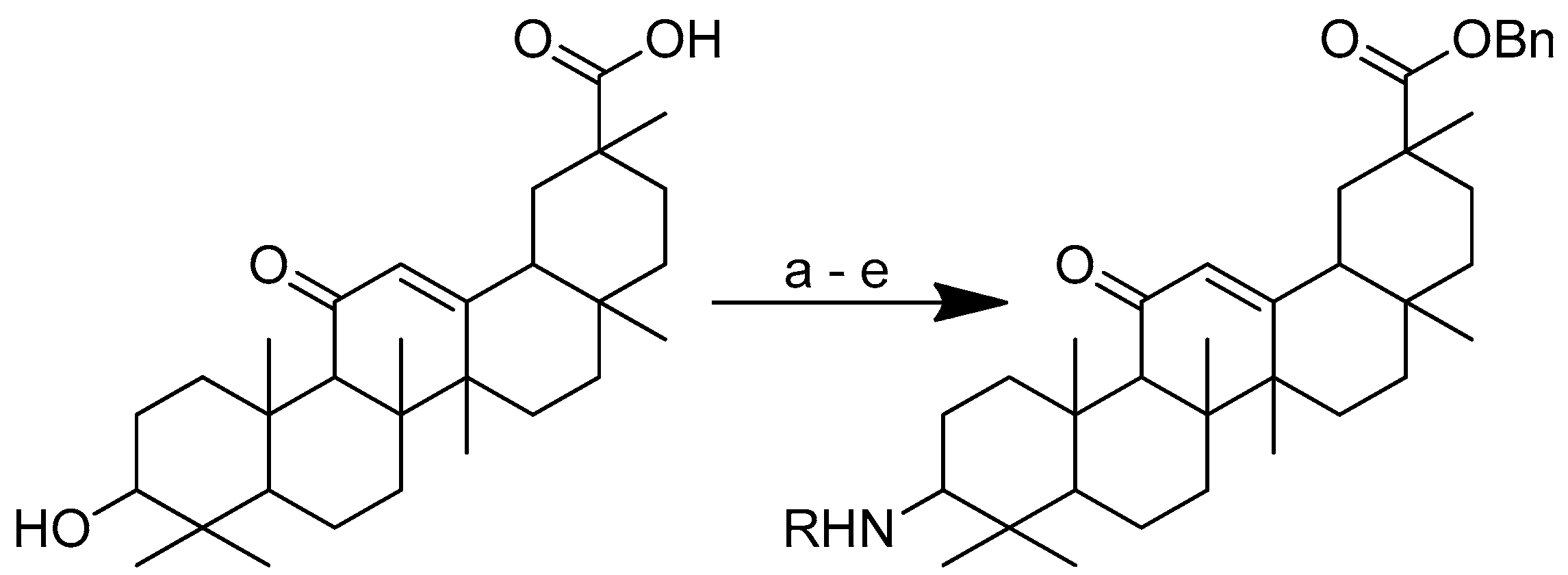
Scheme 15.
Synthesis of the glycyrrhetinic acid with amide linkages at the C-3 position. Reagents and conditions: (a) Bn-Br, dry DMF, dry K2CO3, 80 °C, reflux, 3 h; (b) CrO3/H2SO4, CH3COCH3, 0 °C, 1 h; (c) NaCNBH3, CH3COONH4, CH3OH, rt, 12 h; (d) Boc-amino acids, DCM, HOBt, EDCI, DIPEA, rt, 12 h; (e) TFA in dry DCM, 0 °C, 4 h. R in order: (3) L-glycine; (4) L-alanine; (5) L-phenylalanine; (6) L-proline; (7) L-sarine; (8) L-leucine; (9) L-isoleucine; (10) L-methionine.
Scheme 15.
Synthesis of the glycyrrhetinic acid with amide linkages at the C-3 position. Reagents and conditions: (a) Bn-Br, dry DMF, dry K2CO3, 80 °C, reflux, 3 h; (b) CrO3/H2SO4, CH3COCH3, 0 °C, 1 h; (c) NaCNBH3, CH3COONH4, CH3OH, rt, 12 h; (d) Boc-amino acids, DCM, HOBt, EDCI, DIPEA, rt, 12 h; (e) TFA in dry DCM, 0 °C, 4 h. R in order: (3) L-glycine; (4) L-alanine; (5) L-phenylalanine; (6) L-proline; (7) L-sarine; (8) L-leucine; (9) L-isoleucine; (10) L-methionine.
Figure 20.
Glycyrrhetinic acid–alanine with amide linkage.
Figure 20.
Glycyrrhetinic acid–alanine with amide linkage.
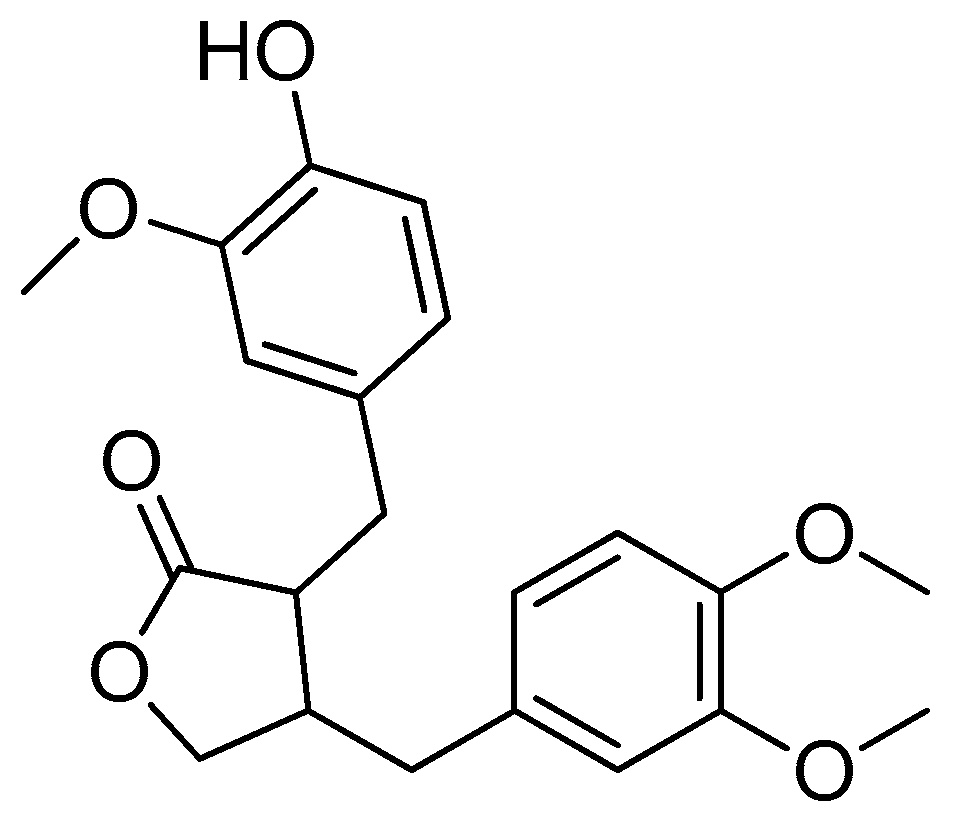
Scheme 16.
Synthesis of amino acid–arctigenin conjugates. (a) C2H3N, EDCI, DMAP; (b) CH3COOCH2CH3, HCl. R in order: (3) L-methionine; (4) L-phenylalanine; (5) L-valine.
Scheme 16.
Synthesis of amino acid–arctigenin conjugates. (a) C2H3N, EDCI, DMAP; (b) CH3COOCH2CH3, HCl. R in order: (3) L-methionine; (4) L-phenylalanine; (5) L-valine.
Figure 22.
(a) Arctigenin-L-methionine; (b) arctigenin-L-phenylalanine; (c) arctigenin-L-valine.
Figure 22.
(a) Arctigenin-L-methionine; (b) arctigenin-L-phenylalanine; (c) arctigenin-L-valine.
Scheme 17.
Synthesis of the podophyllotoxin–amino acid conjugates. Reagents and conditions: (a) Boc-amino acids, EDCI, DMAP, room temperature, 12 h; (b) TFA in dry DCM, 0 °C, 4 h. R in order: (1) Boc-L-glycine; (2) Boc-L-sarcosine; (3) Boc-L-alanine; (4) Boc-L-phenylalanine; (5) L-glycine; (6) L-sarcosine; (7) L-alanine; (8) L-phenylalanine.
Scheme 17.
Synthesis of the podophyllotoxin–amino acid conjugates. Reagents and conditions: (a) Boc-amino acids, EDCI, DMAP, room temperature, 12 h; (b) TFA in dry DCM, 0 °C, 4 h. R in order: (1) Boc-L-glycine; (2) Boc-L-sarcosine; (3) Boc-L-alanine; (4) Boc-L-phenylalanine; (5) L-glycine; (6) L-sarcosine; (7) L-alanine; (8) L-phenylalanine.
Figure 23.
Podophyllotoxin-Boc-L-sarcosine.
Figure 23.
Podophyllotoxin-Boc-L-sarcosine.
Scheme 18.
Synthetic pathway for α-mangostin-conjugated amino acids. (a) BrCH2CO2Me, KOH, anhydrous EtOH, reflux, 24 h; (b) LiOH, THF, H2O, rt, 2 h; (c) for lysine-OMe-mangostin: H-Lys(Fmoc)-OMe·HCl, DIC, HOBt, anhydrous DMF, rt, overnight; then piperidine, DMF, rt, 20 min; (d) for 3–7: corresponding basic amino acid, DIC, HOBt, anhydrous DMF, rt, overnight. R in order: (2) L-Lysine-OMe; (3) L-Histidine-OMe; (4) L-Arginine-OMe; (5) L-Arginine-OEt; (6) L-Arginine-NH2; (7) L-Arginine-OtBu.
Scheme 18.
Synthetic pathway for α-mangostin-conjugated amino acids. (a) BrCH2CO2Me, KOH, anhydrous EtOH, reflux, 24 h; (b) LiOH, THF, H2O, rt, 2 h; (c) for lysine-OMe-mangostin: H-Lys(Fmoc)-OMe·HCl, DIC, HOBt, anhydrous DMF, rt, overnight; then piperidine, DMF, rt, 20 min; (d) for 3–7: corresponding basic amino acid, DIC, HOBt, anhydrous DMF, rt, overnight. R in order: (2) L-Lysine-OMe; (3) L-Histidine-OMe; (4) L-Arginine-OMe; (5) L-Arginine-OEt; (6) L-Arginine-NH2; (7) L-Arginine-OtBu.
Figure 25.
Arginine-OMe-mangostin.
Figure 25.
Arginine-OMe-mangostin.
Table 1.
Antileishmanial activity and cytotoxicity of piperoyl–amino acid conjugates. (1) Piperine; (2) piperic acid; (3) piperoyl-L-phenylalanine methyl ester; (4) piperoyl-L-tyrosine methyl ester; (5) piperoyl-L-valine methyl ester; (6) piperoyl-L-methionine methyl ester; (7) piperoyl-L-tryptophan methyl ester; (8) piperoyl-L-phenylalanine; (9) piperoyl-L-tyrosine; (10) piperoyl-L-valine; (11) piperoyl-L-methionine; (12) piperoyl-L-tryptophan; (13) tetrahydropiperoyl-L-phenylalanine methyl ester; (14) tetrahydropiperoyl-L-tyrosine methyl ester; (15) tetrahydropiperoyl-L-valine methyl ester; (16) tetrahydropiperoyl-L-methionine methyl ester; (17) tetrahydropiperoyl-L-tryptophan methyl ester.
Table 1.
Antileishmanial activity and cytotoxicity of piperoyl–amino acid conjugates. (1) Piperine; (2) piperic acid; (3) piperoyl-L-phenylalanine methyl ester; (4) piperoyl-L-tyrosine methyl ester; (5) piperoyl-L-valine methyl ester; (6) piperoyl-L-methionine methyl ester; (7) piperoyl-L-tryptophan methyl ester; (8) piperoyl-L-phenylalanine; (9) piperoyl-L-tyrosine; (10) piperoyl-L-valine; (11) piperoyl-L-methionine; (12) piperoyl-L-tryptophan; (13) tetrahydropiperoyl-L-phenylalanine methyl ester; (14) tetrahydropiperoyl-L-tyrosine methyl ester; (15) tetrahydropiperoyl-L-valine methyl ester; (16) tetrahydropiperoyl-L-methionine methyl ester; (17) tetrahydropiperoyl-L-tryptophan methyl ester.
| Compound | IC50 (mM) |
|---|
| Amastigotes | Promastigotes |
|---|
| 1 | 0.75 | 2.56 |
| 2 | 0.39 | 1.76 |
| 3 | 0.19 | 0.83 |
| 4 | 0.21 | 0.79 |
| 5 | 0.07 | 0.88 |
| 6 | 0.17 | 0.82 |
| 7 | 0.12 | 0.86 |
| 8 | 0.26 | 0.93 |
| 9 | 0.25 | 0.76 |
| 10 | 0.31 | 1.03 |
| 11 | 0.24 | 1.31 |
| 12 | 0.24 | 1.03 |
| 13 | 0.21 | 0.67 |
| 14 | 0.20 | 0.54 |
| 15 | 0.24 | 0.72 |
| 16 | 0.22 | 0.61 |
| 17 | 0.18 | 0.47 |
Table 2.
Effects of poly-R-(L-glutamic acid) conjugates of camptothecin on the in vivo growth of subcutaneous B-16 melanomas. TGD: tumour growth delays. (1) Poly-R-(L-glutamic acid)-camptothecin; (2) poly-R-(L-glutamic acid)-glycine-camptothecin; (3) poly-R-(L-glutamic acid)-alanine-camptothecin; (4) poly-R-(L-glutamic acid)-(β-alanine)-camptothecin; (5) poly-R-(L-glutamic acid)-(4-NH-butyryl)-camptothecin; (6) poly-R-(L-glutamic acid)-(2-O-acetyl)-camptothecin; (7) poly-R-(L-glutamic acid)-(4-O-butyryl)-camptothecin; (8) poly-R-(L-glutamic acid)-(γ-glu)-camptothecin.
Table 2.
Effects of poly-R-(L-glutamic acid) conjugates of camptothecin on the in vivo growth of subcutaneous B-16 melanomas. TGD: tumour growth delays. (1) Poly-R-(L-glutamic acid)-camptothecin; (2) poly-R-(L-glutamic acid)-glycine-camptothecin; (3) poly-R-(L-glutamic acid)-alanine-camptothecin; (4) poly-R-(L-glutamic acid)-(β-alanine)-camptothecin; (5) poly-R-(L-glutamic acid)-(4-NH-butyryl)-camptothecin; (6) poly-R-(L-glutamic acid)-(2-O-acetyl)-camptothecin; (7) poly-R-(L-glutamic acid)-(4-O-butyryl)-camptothecin; (8) poly-R-(L-glutamic acid)-(γ-glu)-camptothecin.
| Compound | Dose (mg) | B-16 TGD (Days) |
|---|
| 1 | 48 | 4 |
| 2 | 35 | 2 |
| 3 | 62 | 2 |
| 4 | 67 | 1 |
| 5 | 60 | 1 |
| 6 | 75 | 4 |
| 7 | 35 | 3 |
| 8 | 41 | 1 |
Table 3.
In vitro antimalarial activities of compounds against the chloroquine-sensitive 3D7 strain of Plasmodium falciparum. (1) Quinine; (2) boc-glycine-quinine; (3) boc-L-alanine-quinine; (4) boc-L-phenylalanine-quinine; (5) boc-L-isoleucine-quinine; (6) boc-L-histidine-quinine; (7) boc-L-serine-quinine; (8) boc-L-glutamic acid-quinine; (9) Z-L-lysine-quinine; (10) Z-L-aspartic acid-quinine; (11) Z-L-cysteine-quinine; (12) Z-L-alanine-L-phenylalanine-quinine; (13) Z-L-valine-L-leucine-quinine; (14) Z-L-isoleucine-glycine-quinine.
Table 3.
In vitro antimalarial activities of compounds against the chloroquine-sensitive 3D7 strain of Plasmodium falciparum. (1) Quinine; (2) boc-glycine-quinine; (3) boc-L-alanine-quinine; (4) boc-L-phenylalanine-quinine; (5) boc-L-isoleucine-quinine; (6) boc-L-histidine-quinine; (7) boc-L-serine-quinine; (8) boc-L-glutamic acid-quinine; (9) Z-L-lysine-quinine; (10) Z-L-aspartic acid-quinine; (11) Z-L-cysteine-quinine; (12) Z-L-alanine-L-phenylalanine-quinine; (13) Z-L-valine-L-leucine-quinine; (14) Z-L-isoleucine-glycine-quinine.
| Compound | IC50 (nM) |
|---|
| 1 | 18 |
| 2 | 27 |
| 3 | 36 |
| 4 | 38 |
| 5 | 550 |
| 6 | 42 |
| 7 | 95 |
| 8 | 76 |
| 9 | 71 |
| 10 | 17 |
| 11 | 40 |
| 12 | 120 |
| 13 | 74 |
| 14 | 23 |
Table 4.
Solubility of quercetin and quercetin–amino acid conjugates in PBS buffer. (1) Quercetin; (2) quercetin-alanine; (3) quercetin-valine; (4) quercetin-lysine; (5) quercetin-phenylalanine; (6) quercetin-aspartic acid; (7) quercetin-methionine; (8) quercetin-glutamic acid; (9) quercetin-alanine-aspartic acid; (10) quercetin-alanine-glutamic acid.
Table 4.
Solubility of quercetin and quercetin–amino acid conjugates in PBS buffer. (1) Quercetin; (2) quercetin-alanine; (3) quercetin-valine; (4) quercetin-lysine; (5) quercetin-phenylalanine; (6) quercetin-aspartic acid; (7) quercetin-methionine; (8) quercetin-glutamic acid; (9) quercetin-alanine-aspartic acid; (10) quercetin-alanine-glutamic acid.
| Compound | Water Solubility (μM) | Fold Increase |
|---|
| 1 | 50 | 1.0 |
| 2 | 1290 | 25.8 |
| 3 | 767 | 15.3 |
| 4 | 338 | 6.8 |
| 5 | 1766 | 35.3 |
| 6 | 2262 | 45.2 |
| 7 | 675 | 13.5 |
| 8 | 2649 | 53.0 |
| 9 | 2093 | 41.9 |
| 10 | 2628 | 52.6 |
Table 5.
In vitro antibacterial activity of amino-acid-modified flavone compounds. (1) Icaritin; (2) icaritin-L-histidine; (3) icaritin-L-arginine; (4) icaritin-L-lysine.
Table 5.
In vitro antibacterial activity of amino-acid-modified flavone compounds. (1) Icaritin; (2) icaritin-L-histidine; (3) icaritin-L-arginine; (4) icaritin-L-lysine.
| Compound | Bacterial Strains, µg/mL (µM) |
|---|
| S. aureus | B. cereus |
|---|
| 1 | 50 | 50 |
| 2 | >50 | >50 |
| 3 | 1.56 | 3.13 |
| 4 | 25 | 25 |
Table 6.
Antibacterial activity of curcumin bioconjugates (values represent the zone of inhibition in mm) against multi-resistant bacterial strains. (-) Resistant. (1) curcumin; (2) 4,40-(di-O-glutamoyl)-curcumin; (3) 4,40-(di-O-valinoyl) curcumin; (4) 4,40-(di-O-glycinoyl) curcumin; (5) monoglycinoyl curcumin; (6) monovalinoyl curcumin.
Table 6.
Antibacterial activity of curcumin bioconjugates (values represent the zone of inhibition in mm) against multi-resistant bacterial strains. (-) Resistant. (1) curcumin; (2) 4,40-(di-O-glutamoyl)-curcumin; (3) 4,40-(di-O-valinoyl) curcumin; (4) 4,40-(di-O-glycinoyl) curcumin; (5) monoglycinoyl curcumin; (6) monovalinoyl curcumin.
| Bacteria | Compound |
|---|
| 1 | 2 | 3 | 4 | 5 | 6 |
|---|
| Inhibition Zone (mm) |
|---|
| Micrococci | - | 18 | 16 | 15 | - | 26 |
| K. aeruginosa | - | 9 | - | - | 18 | 18 |
| S. saprophyticus | - | - | 14 | 14 | - | - |
| E. cloacae | - | - | 20 | 12 | - | 25 |
| E. coli | 10 | - | 20 | - | 12 | 13 |
Table 7.
MICs of THC–amino acid conjugates against Gram-positive and Gram-negative bacteria. (1) THC, (2) THC-L-alanine, (3) THC-L-isoleucine, (4) THC-L-proline, (5) THC-L-valine, (6) THC-L-phenylalanine, (7) THC-L-glycine, and (8) THC-L-leucine.
Table 7.
MICs of THC–amino acid conjugates against Gram-positive and Gram-negative bacteria. (1) THC, (2) THC-L-alanine, (3) THC-L-isoleucine, (4) THC-L-proline, (5) THC-L-valine, (6) THC-L-phenylalanine, (7) THC-L-glycine, and (8) THC-L-leucine.
| Compound | MIC (µM) |
|---|
| B. cereus | S. aureus | E. coli | Y. enterocolitica |
|---|
| 1 | 1066 | 1329 | 1723 | 2114 |
| 2 | 340 | 437 | 583 | 777 |
| 3 | 334 | 459 | 543 | 585 |
| 4 | 309 | 353 | 485 | 618 |
| 5 | 263 | 482 | 526 | 657 |
| 6 | 337 | 487 | 600 | 750 |
| 7 | 257 | 360 | 514 | 617 |
| 8 | 334 | 376 | 501 | 585 |
Table 8.
IC50 evaluated after 72 h incubation in A431 cancer cell lines and human keratinocytes as the control. (1) Betulin; (2) betulinic acid; (3) betulin-lysine; (4) betulin-ornithine.
Table 8.
IC50 evaluated after 72 h incubation in A431 cancer cell lines and human keratinocytes as the control. (1) Betulin; (2) betulinic acid; (3) betulin-lysine; (4) betulin-ornithine.
| Compound | IC50 (μM) |
|---|
| HaCat Cells, 72 h | A431 Cells, 72 h |
|---|
| 1 | 150.2 | 45.2 |
| 2 | 160.2 | 35.6 |
| 3 | 145.2 | 2.3 |
| 4 | 146.3 | 4.5 |
Table 9.
The in vitro cytotoxicity of glycyrrhetinic acid conjugates with esters in the C-3 position against cancer cell lines. (1) Glycyrrhetinic acid; (2) cisplatin; (3) glycyrrhetinic acid-L-glycine; (4) glycyrrhetinic acid-L-alanine; (5) glycyrrhetinic acid-L-phenylalanine; (6) glycyrrhetinic acid-L-proline; (7) glycyrrhetinic acid-L-sarine; (8) glycyrrhetinic acid-L-leucine; (9) glycyrrhetinic acid-L-isoleucine; (10) glycyrrhetinic acid-L-methionine.
Table 9.
The in vitro cytotoxicity of glycyrrhetinic acid conjugates with esters in the C-3 position against cancer cell lines. (1) Glycyrrhetinic acid; (2) cisplatin; (3) glycyrrhetinic acid-L-glycine; (4) glycyrrhetinic acid-L-alanine; (5) glycyrrhetinic acid-L-phenylalanine; (6) glycyrrhetinic acid-L-proline; (7) glycyrrhetinic acid-L-sarine; (8) glycyrrhetinic acid-L-leucine; (9) glycyrrhetinic acid-L-isoleucine; (10) glycyrrhetinic acid-L-methionine.
| Compound | IC50 (µM) |
|---|
| A549 | MCF7 |
|---|
| 1 | >40 | >40 |
| 2 | 9.0 | 6.8 |
| 3 | 2.8 | 3.8 |
| 4 | 3.1 | 4.3 |
| 5 | 16.9 | 14.4 |
| 6 | 4.7 | 5.2 |
| 7 | 4.9 | 3.8 |
| 8 | 22.9 | 11.9 |
| 9 | 4.9 | 10.0 |
| 10 | 7.5 | 8.0 |
Table 10.
The in vitro cytotoxicity of glycyrrhetinic acid conjugates with amide linkages in the C-3 position against cancer cell lines. (1) Glycyrrhetinic acid; (2) cisplatin; (3) glycyrrhetinic acid-L-glycine; (4) glycyrrhetinic acid-L-alanine; (5) glycyrrhetinic acid-L-phenylalanine; (6) glycyrrhetinic acid-L-proline; (7) glycyrrhetinic acid-L-sarine; (8) glycyrrhetinic acid-L-leucine; (9) glycyrrhetinic acid-L-isoleucine; (10) glycyrrhetinic acid-L-methionine.
Table 10.
The in vitro cytotoxicity of glycyrrhetinic acid conjugates with amide linkages in the C-3 position against cancer cell lines. (1) Glycyrrhetinic acid; (2) cisplatin; (3) glycyrrhetinic acid-L-glycine; (4) glycyrrhetinic acid-L-alanine; (5) glycyrrhetinic acid-L-phenylalanine; (6) glycyrrhetinic acid-L-proline; (7) glycyrrhetinic acid-L-sarine; (8) glycyrrhetinic acid-L-leucine; (9) glycyrrhetinic acid-L-isoleucine; (10) glycyrrhetinic acid-L-methionine.
| Compound | IC50 (µM) |
|---|
| A549 | MCF7 |
|---|
| 1 | >40 | >40 |
| 2 | 9.0 | 6.8 |
| 3 | 2.4 | 2.8 |
| 4 | 2.1 | 2.1 |
| 5 | 3.0 | 3.3 |
| 6 | 3.3 | 7.6 |
| 7 | 3.3 | 3.4 |
| 8 | 3.3 | 6.1 |
| 9 | 3.4 | 4.7 |
| 10 | 6.9 | 7.0 |
Table 11.
Effects of arctigenin and its amino acid derivatives on tumour growth in H22 tumour-bearing mice. (1) Cyclophosphamide; (2) arctigenin; (3) arctigenin-L-methionine; (4) arctigenin-L-phenylalanine; (5) arctigenin-L-valine.
Table 11.
Effects of arctigenin and its amino acid derivatives on tumour growth in H22 tumour-bearing mice. (1) Cyclophosphamide; (2) arctigenin; (3) arctigenin-L-methionine; (4) arctigenin-L-phenylalanine; (5) arctigenin-L-valine.
| Compound | Dosage (mg/kg) | Inhibitory Rate (%) |
|---|
| 1 | 25 | 72.06 |
| 2 | 40 | 26.26 |
| 3 | 40 | 55.87 |
| 4 | 40 | 51.40 |
| 5 | 40 | 69.27 |
Table 12.
Cytotoxic activity of podophyllotoxin–amino acid conjugates in vitro (IC50, nM). (1) Podophyllotoxin-Boc-L-glycine; (2) podophyllotoxin-Boc-L-sarcosine; (3) podophyllotoxin-Boc-L-alanine; (4) podophyllotoxin-Boc-L-phenylalanine; (5) podophyllotoxin-L-glycine; (6) podophyllotoxin-L-sarcosine; (7) podophyllotoxin-L-alanine; (8) podophyllotoxin-L-phenylalanine; (9) doxorubicin.
Table 12.
Cytotoxic activity of podophyllotoxin–amino acid conjugates in vitro (IC50, nM). (1) Podophyllotoxin-Boc-L-glycine; (2) podophyllotoxin-Boc-L-sarcosine; (3) podophyllotoxin-Boc-L-alanine; (4) podophyllotoxin-Boc-L-phenylalanine; (5) podophyllotoxin-L-glycine; (6) podophyllotoxin-L-sarcosine; (7) podophyllotoxin-L-alanine; (8) podophyllotoxin-L-phenylalanine; (9) doxorubicin.
| Compound | IC50 (nM) |
|---|
| A549 | MCF-7 | HepG2 | L-02 |
|---|
| 1 | 1.6 | 11.3 | 26.6 | 7.3 |
| 2 | 9.5 | 132.6 | 96.4 | 160.2 |
| 3 | 17.3 | 106.9 | 81.5 | 109.5 |
| 4 | 30.2 | 95.3 | 87.1 | 78.4 |
| 5 | 12.9 | 7.2 | 17.5 | 14.5 |
| 6 | 6.8 | 17.5 | 24.9 | 9.6 |
| 7 | 13.6 | 18.5 | 17.8 | 10.8 |
| 8 | 3.8 | 15.6 | 27.3 | 8.9 |
| 9 | 228.2 | 75.6 | 693.1 | 53.3 |
Table 13.
In vitro antibacterial activity of amino-acid-modified α-mangostin. (1) α-Mangostin; (2) α-mangostin-OMe-lysine; (3) α-mangostin-OMe-histidine; (4) α-mangostin-OMe-arginine; (5) α-mangostin-OEt-arginine; (6) α-mangostin-NH2-arginine; (7) α-mangostin-OtBu-arginine.
Table 13.
In vitro antibacterial activity of amino-acid-modified α-mangostin. (1) α-Mangostin; (2) α-mangostin-OMe-lysine; (3) α-mangostin-OMe-histidine; (4) α-mangostin-OMe-arginine; (5) α-mangostin-OEt-arginine; (6) α-mangostin-NH2-arginine; (7) α-mangostin-OtBu-arginine.
| Compound | MIC Values, µg/mL (µM) |
|---|
| S. aureus | B. cereus |
|---|
| 1 | 2 | 2 |
| 2 | 6 | 12 |
| 3 | >50 | >50 |
| 4 | 2 | 2 |
| 5 | 6 | 12 |
| 6 | 6 | 6 |
| 7 | 12 | 12 |