A New Ratiometric Fluorescent Probe Based on BODIPY for Highly Selective Detection of Hydrogen Sulfide
Abstract
1. Introduction
2. Results and Discussion
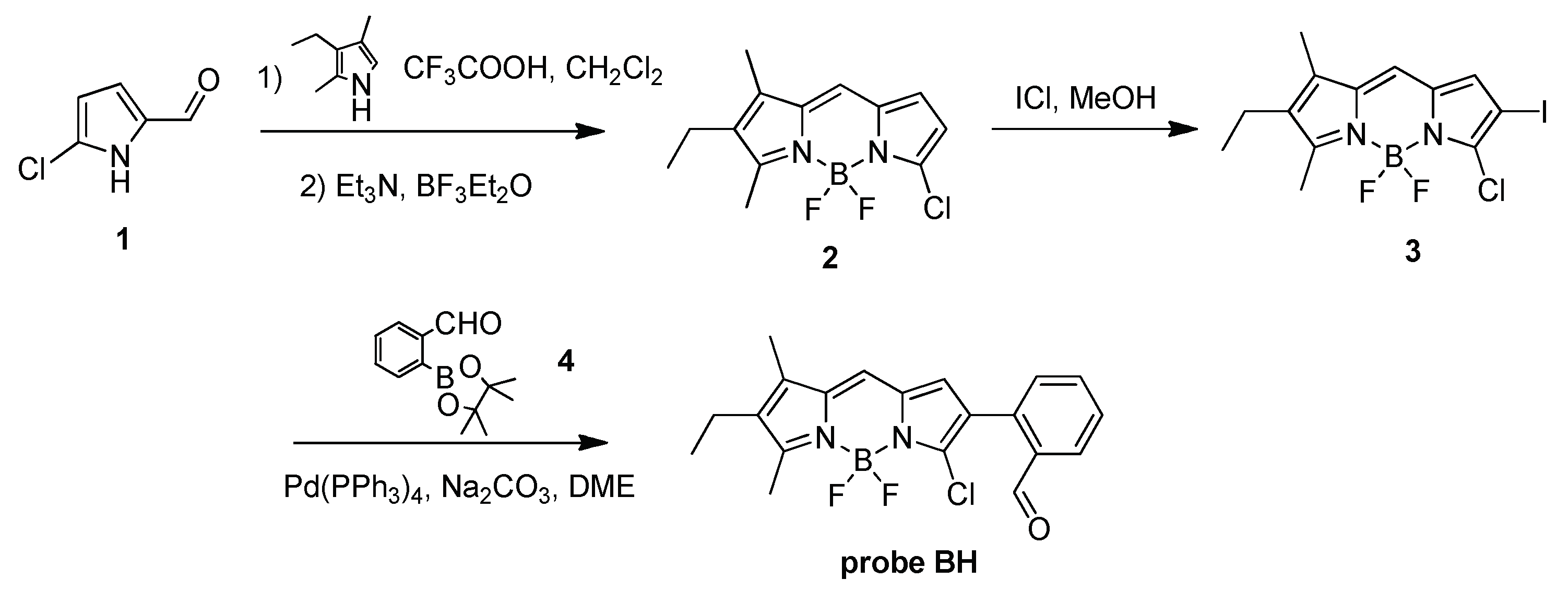
2.1. Design and Synthesis of Probe BH
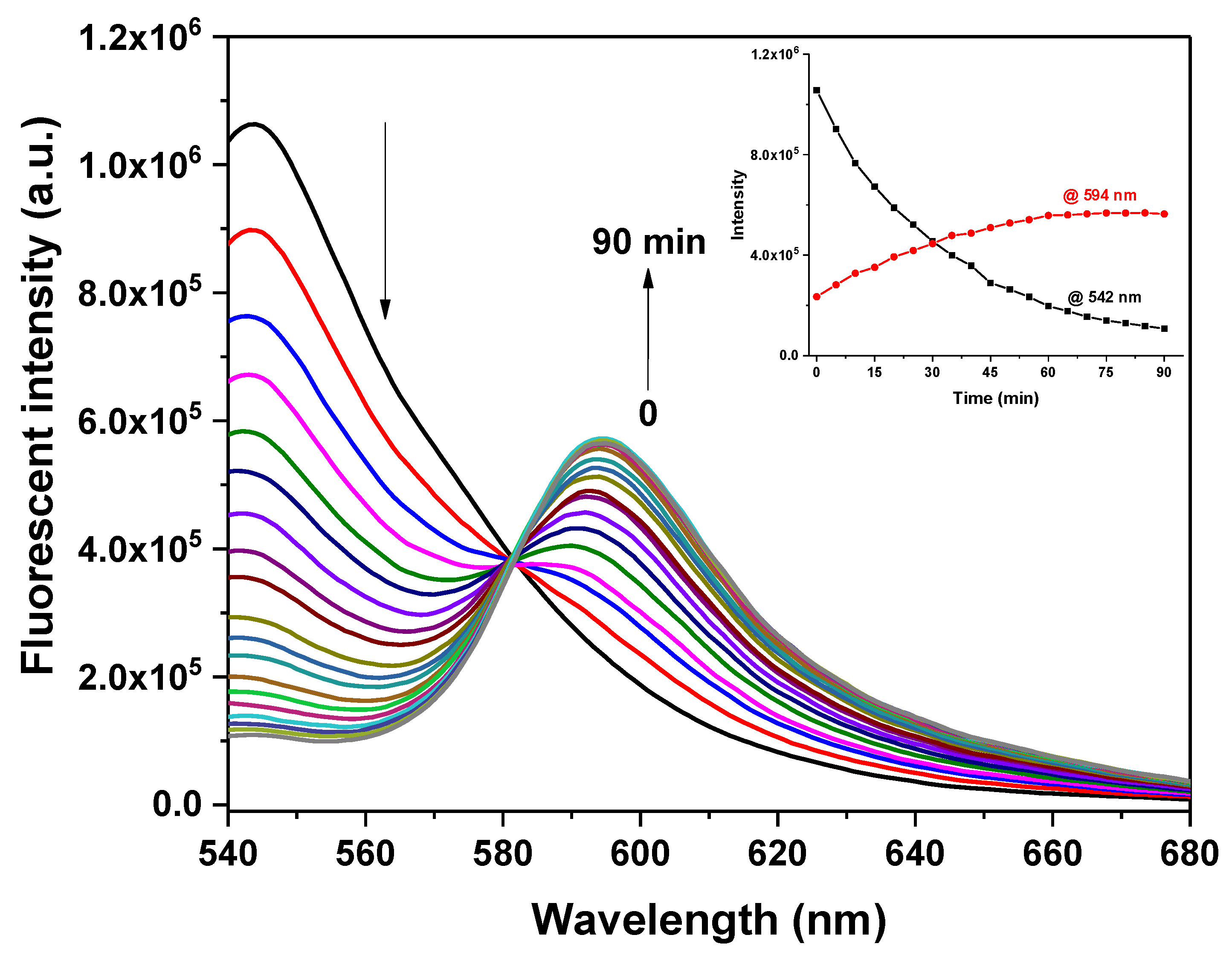
2.2. Spectral Response of Probe BH toward H2S
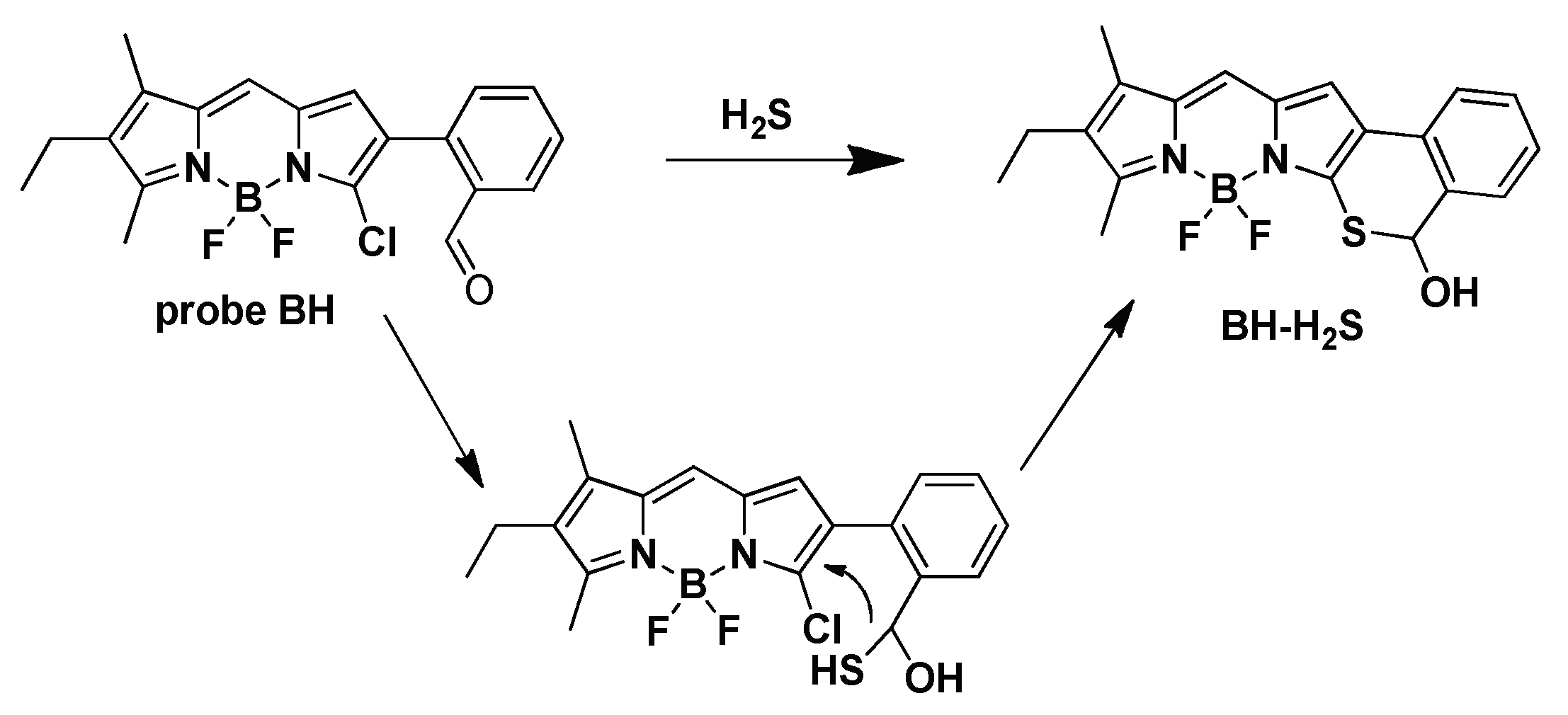
2.3. Response Mechanism Studies
2.4. Selectivity and pH Effect
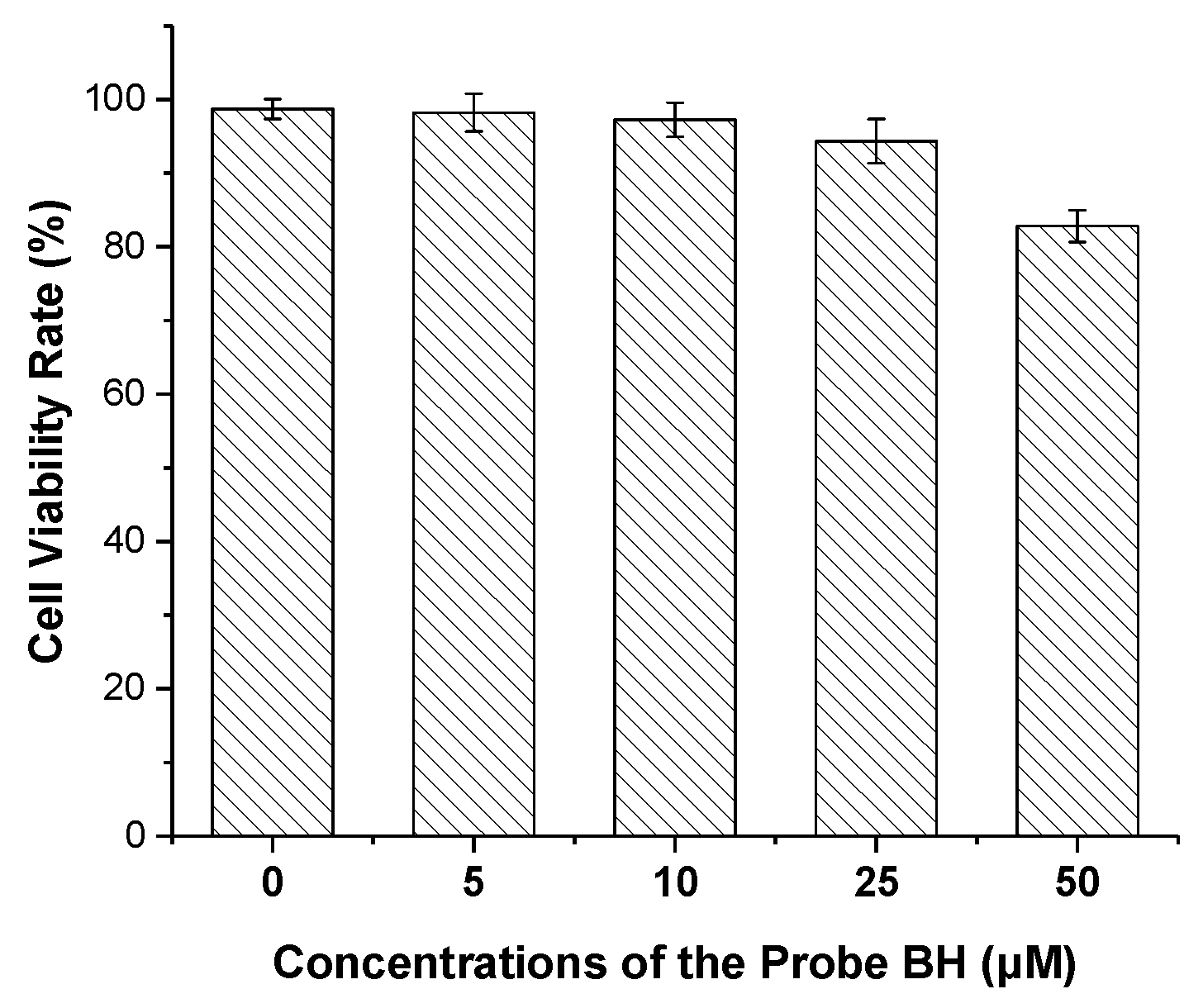
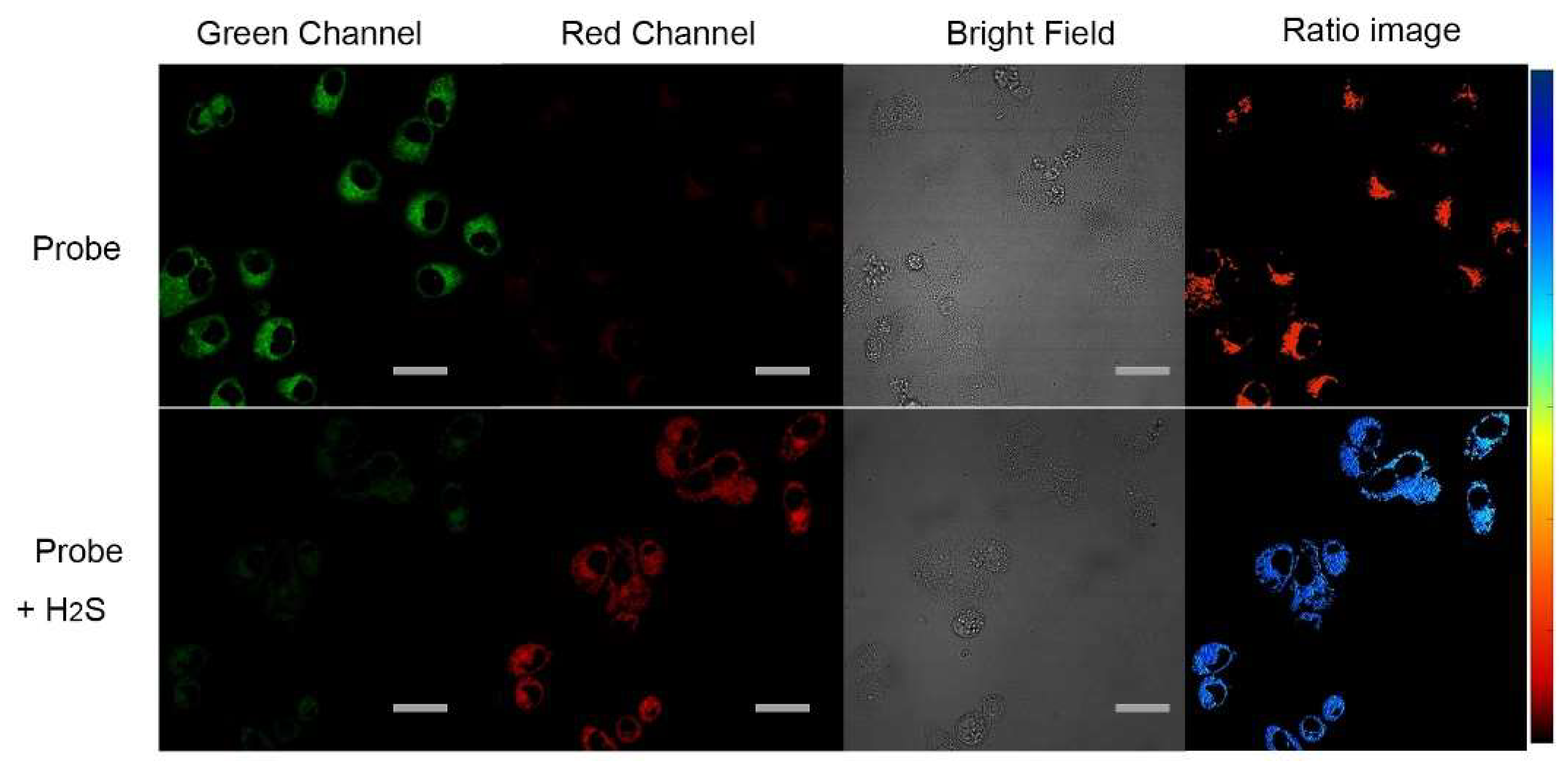
2.5. Probe Application in Cellular Imaging
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Preparation of Sample Solutions
3.3. Synthesis
3.4. Probe Application in Cellular Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Culotta, E.; Koshland, D.E. No news is good news: A startlingly simple molecule unites neuroscience, physiology, and immunology and revises. Science 1992, 258, 1862–1865. [Google Scholar] [CrossRef]
- Han, Y.; Qin, J.; Chang, X.; Yang, Z.; Du, J. Hydrogen sulfide and carbon monoxide are in synergy with each other in the pathogenesis of recurrent febrile seizures. Cell. Mol. Neurobiol. 2006, 26, 101–107. [Google Scholar] [CrossRef]
- Park, C.S.; Ha, T.H.; Choi, S.-A.; Nguyen, D.N.; Noh, S.; Kwon, O.S.; Lee, C.-S.; Yoon, H. A near-infrared “turn-on” fluorescent probe with a self-immolative linker for the in vivo quantitative detection and imaging of hydrogen sulfide. Biosens. Bioelectron. 2017, 89, 919–926. [Google Scholar] [CrossRef]
- Xuan, W.; Sheng, C.; Cao, Y.; He, W.; Wang, W. Fluorescent probes for the detection of hydrogen sulfide in biological systems. Angew. Chem. Int. Ed. 2012, 51, 2282–2284. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Peers, C.; Bauer, C.C.; Boyle, J.P.; Scragg, J.L.; Dallas, M.L. Modulation of ion channels by hydrogen sulfide. Antioxid. Redox Signal. 2011, 17, 95–105. [Google Scholar] [CrossRef]
- Wu, L.; Ishigaki, Y.; Hu, Y.; Sugimoto, K.; Zeng, W.; Harimoto, T.; Sun, Y.; He, J.; Suzuki, T.; Jiang, X.; et al. H2S-activatable near-infrared afterglow luminescent probes for sensitive molecular imaging in vivo. Nat. Commun. 2020, 11, 446. [Google Scholar] [CrossRef]
- Jain, S.K.; Bull, R.; Rains, J.L.; Bass, P.F.; Levine, S.N.; Reddy, S.; McVie, R.; Bocchini, J.A. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation. Antioxid. Redox Signal. 2010, 12, 1333–1338. [Google Scholar] [CrossRef]
- Kamoun, P.; Belardinelli, M.C.; Chabli, A.; Lallouchi, K.; Chadefaux-Vekemans, B. Endogenous hydrogen sulfide overproduction in Down syndrome. Am. J. Med. Gen. 2003, 11, 310–311. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Wang, K.; Wang, C.; Zhang, L.; Liu, K. Ultrasensitive and facile electrochemical detection of hydrogen sulfide in rat brain microdialysate based on competitive binding reaction. Anal. Bioanal. Chem. 2017, 409, 1101–1107. [Google Scholar] [CrossRef]
- Zeng, J.; Li, M.; Liu, A.; Feng, F.; Zeng, T.; Duan, W.; Li, M.; Gong, M.; Wen, C.Y.; Yin, Y. Au/AgI dimeric nanoparticles for highly selective and sensitive colorimetric detection of hydrogen sulfide. Adv. Funct. Mater. 2018, 28, 1800515. [Google Scholar] [CrossRef]
- Montoya, L.A.; Shen, X.; McDermott, J.J.; Kevil, G.G.; Pluth, M.D. Mechanistic investigations reveal that dibromobimane extrudes sulfur from biological sulfhydryl sources other than hydrogen sulfide. Chem. Sci. 2015, 6, 294–300. [Google Scholar] [CrossRef]
- Niu, L.Y.; Chen, Y.Z.; Zheng, H.R.; Wu, L.Z.; Tung, C.H.; Yang, Q.Z. Design strategies of fluorescent probes for selective detection among biothiols. Chem. Soc. Rev. 2015, 44, 6143–6160. [Google Scholar] [CrossRef]
- Yu, F.; Han, X.; Chen, L. Fluorescent probes for hydrogen sulfide detection and bioimaging. Chem. Commun. 2014, 50, 12234–12249. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Recent advances in the application of BODIPY in bioimaging and chemosensing. J. Mater. Chem. C 2019, 7, 11361–11405. [Google Scholar] [CrossRef]
- Kowasa, T.; Mawda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar]
- Zhang, J.; Wang, N.N.; Ji, X.; Tao, Y.F.; Wang, J.M.; Zhao, W.L. BODIPY-based fluorescent probes for biothiols. Chem. Eur. J. 2020, 26, 4172–4192. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Wu, H.; Guo, X.-F.; Wang, H. Novel BODIPY-based fluorescent probes with large Stokes shift for imaging hydrogen sulfide. Dyes Pigments 2019, 165, 400–407. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Zhao, M.; Hong, Y.; Jin, Q.; Yao, S.; Zheng, C.; Quan, Y.-Y.; Ye, X.; Huang, Z.-S. A NIR fluorescent probe for the detection and visualization of hydrogen sulfide in colorectal cancer cell. Sens. Actuator B Chem. 2019, 298, 126898. [Google Scholar] [CrossRef]
- Yue, J.; Tao, Y.; Zhang, J.; Wang, H.; Wang, N.; Zhao, W. BODIPY-based Fluorescent Probe for Fast Detection of Hydrogen Sulfide and Lysosome-targeting Applications in Living Cells. Chem. Asian J. 2021, 16, 850–855. [Google Scholar] [CrossRef]
- Wang, R.; Gao, W.; Gao, J.; Xu, G.; Zhu, T.; Gu, X.; Zhao, C. A forster resonance energy transfer switchable fluorescent probe with H2S-activated second near-infrared emission for bioimaging. Front. Chem. 2019, 7, 2296–2646. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Gong, D.; Teng, Z.; Wang, J.; Cao, T.; Iqbal, K.; Liu, W.; Iqbal, A.; Qin, W.; Guo, H. 2-Vinylfuran substituted BODIPY H2S fluorescent turn on probe based on hydrolysis of furfural and nucleophilic addition of double bond. Sens. Actuator B Chem. 2019, 297, 126712. [Google Scholar] [CrossRef]
- Zhao, Q.; Yin, C.X.; Kang, J.; Wen, Y.; Huo, F.J. A viscosity sensitive azide-pyridine BODIPY-based fluorescent dye for imaging of hydrogen sulfide in living cells. Dyes Pigments 2018, 159, 166–172. [Google Scholar] [CrossRef]
- Jia, Y.; Xia, L.-J.; Chen, L.; Guo, X.-F.; Wang, H.; Zhang, H.-J. A novel BODIPY-based fluorescent probe for selective detection of hydrogen sulfide in living cells and tissues. Talanta 2018, 181, 104–111. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, X.; Zhou, J.; Dong, X.; Chen, Z.; Zhao, W. Pyridinium substituted BODIPY as NIR fluorescent probe for simultaneous sensing of hydrogen sulfide/glutathione and cysteine/homocysteine. Sens. Actuator B Chem. 2018, 257, 1076–1082. [Google Scholar] [CrossRef]
- Quan, Y.Y.; Fan, L.N.; Shen, H.Y.; Wu, B.N.; Kong, S.N.; Luo, Y.S.; Huang, Z.S.; Ye, X.X. A multifunctional BODIPY based fluorescent probe for hydrogen sulfide detection and photodynamic anticancer therapy in HCT116 colon cancer cell. Dyes Pigments 2022, 197, 109897. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Li, Q.; Shao, S. A BODIPY-based turn-on fluorescent probe for the selective detection of hydrogen sulfide in solution and in cells. Talanta 2015, 144, 763–768. [Google Scholar] [CrossRef]
- Fang, T.; Jiang, X.-D.; Sun, C.; Li, Q. BODIPY-based naked-eye fluorescent on-off probe with high selectivity for H2S based on thiolysis of dinitrophenyl ether. Sens. Actuator B Chem. 2019, 290, 551–557. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, X.; Li, K.; Zhu, S.; Guo, Z.; Zhang, L.; Wang, F.; Fei, Q.; Luo, S.; Shi, P.; et al. Förster resonance energy transfer switchable self-assembled micellar nanoprobe: Ratiometric fluorescent trapping of endogenous H2S generation via fluvastatin-stimulated upregulation. J. Am. Chem. Soc. 2015, 137, 8490–8498. [Google Scholar] [CrossRef]
- Gao, M.; Wang, R.; Yu, F.B.; You, J.; Chen, L.X. A near-infrared fluorescent probe for the detection of hydrogen polysulfides biosynthetic pathways in living cells and in vivo. Analyst 2015, 140, 3766–3772. [Google Scholar] [CrossRef]
- Liu, K.; Liu, C.; Shang, H.; Ren, M.; Lin, W. A novel red light emissive two-photon fluorescent probe for hydrogen sulfide (H2S) in nucleolus region and its application for H2S detection in zebrafish and live mice. Sens. Actuators B Chem. 2018, 256, 342–350. [Google Scholar] [CrossRef]
- Feng, S.; Xia, Q.; Feng, G. Iminocoumarin-based red to near-infrared fluorescent turn-on probe with a large Stokes shift for imaging H2S in living cells and animals. Dyes Pigments 2019, 163, 447–453. [Google Scholar] [CrossRef]
- Shu, W.; Zang, S.; Wang, C.; Gao, M.; Jing, J.; Zhang, X. An endoplasmic reticulum targeted ratiometric fluorescent probe for sensing of hydrogen sulfide in living cells and zebrafish. Anal. Chem. 2020, 92, 9982–9988. [Google Scholar] [CrossRef]
- Wu, Q.; Yin, C.; Wen, Y.; Zhang, Y.; Huo, F. An ICT lighten ratiometric and NIR fluorogenic probe to visualize endogenous/exogenous hydrogen sulphide and imaging in mice. Sens. Actuator B Chem. 2019, 288, 507–511. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Ru, J.; Yang, Y.; Feng, Y.; Cao, C.; Wang, K.; Zhang, G.; Liu, W. A mitochondrial-targeted red fluorescent probe for detecting endogenous H2S in cells with high selectivity and development of a visual paper-based sensing platform. Sens. Actuator B Chem. 2020, 312, 127982. [Google Scholar] [CrossRef]
- Xia, S.; Shen, J.; Wang, J.; Wang, H.; Zhou, H.; Tanasova, M. Ratiometric fluorescent and colorimetric BODIPY-based sensor for zinc ions in solution and living cells. Sens. Actuator B Chem. 2018, 258, 1279–1286. [Google Scholar] [CrossRef]
- Pang, Z.; Ye, H.; Ma, D.; Tu, X.; Yi, L.; Xi, Z. A H2S-specific ultrasensitive fluorogenic probe reveals TMV-induced H2S production to limit virus replication. ChemBioChem 2021, 22, 2292–2299. [Google Scholar] [CrossRef]
- Paul, N.; Sarkar, R.; Sarkar, R.; Barui, A.; Sarkar, S. Detection of hydrogen sulfide using BODIPY based colorimetric and fluorescent on-off chemosensor. J. Chem. Sci. 2020, 132, 21. [Google Scholar] [CrossRef]
- Gong, D.Y.; Zhu, X.T.; Tian, Y.J.; Han, S.C.; Deng, M.; Iqbal, A.; Liu, W.S.; Qin, W.; Guo, H.C. A Phenylselenium-Substituted BODIPY Fluorescent Turn-off Probe for Fluorescence Imaging of Hydrogen Sulfide in Living Cells. Anal. Chem. 2017, 89, 1801–1807. [Google Scholar] [CrossRef]
- Fei, Q.; Li, M.M.; Chen, J.; Shi, B.; Xu, G.; Zhao, C.C.; Gu, X.F. Design of BODIPY-based near-infrared fluorescent probes for H2S. J. Photochem. Photobiol. A Chem. 2018, 355, 305–310. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, H.; Ye, T.; Li, Y.; Lin, Y.; Liu, D.; Zhou, H.; Wang, J.; Li, L. A New Ratiometric Fluorescent Probe Based on BODIPY for Highly Selective Detection of Hydrogen Sulfide. Molecules 2022, 27, 7499. https://doi.org/10.3390/molecules27217499
Xiang H, Ye T, Li Y, Lin Y, Liu D, Zhou H, Wang J, Li L. A New Ratiometric Fluorescent Probe Based on BODIPY for Highly Selective Detection of Hydrogen Sulfide. Molecules. 2022; 27(21):7499. https://doi.org/10.3390/molecules27217499
Chicago/Turabian StyleXiang, Huan, Tianqing Ye, Yanbo Li, Yanfei Lin, Dan Liu, Hongwei Zhou, Jianbo Wang, and Lei Li. 2022. "A New Ratiometric Fluorescent Probe Based on BODIPY for Highly Selective Detection of Hydrogen Sulfide" Molecules 27, no. 21: 7499. https://doi.org/10.3390/molecules27217499
APA StyleXiang, H., Ye, T., Li, Y., Lin, Y., Liu, D., Zhou, H., Wang, J., & Li, L. (2022). A New Ratiometric Fluorescent Probe Based on BODIPY for Highly Selective Detection of Hydrogen Sulfide. Molecules, 27(21), 7499. https://doi.org/10.3390/molecules27217499





