Labeling Microplastics with Fluorescent Dyes for Detection, Recovery, and Degradation Experiments
Abstract
1. Introduction
2. Materials and Methods
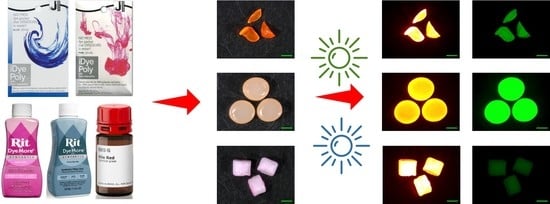
2.1. Microplastics and Dye Selection
2.2. Microplastic Staining and Study Design
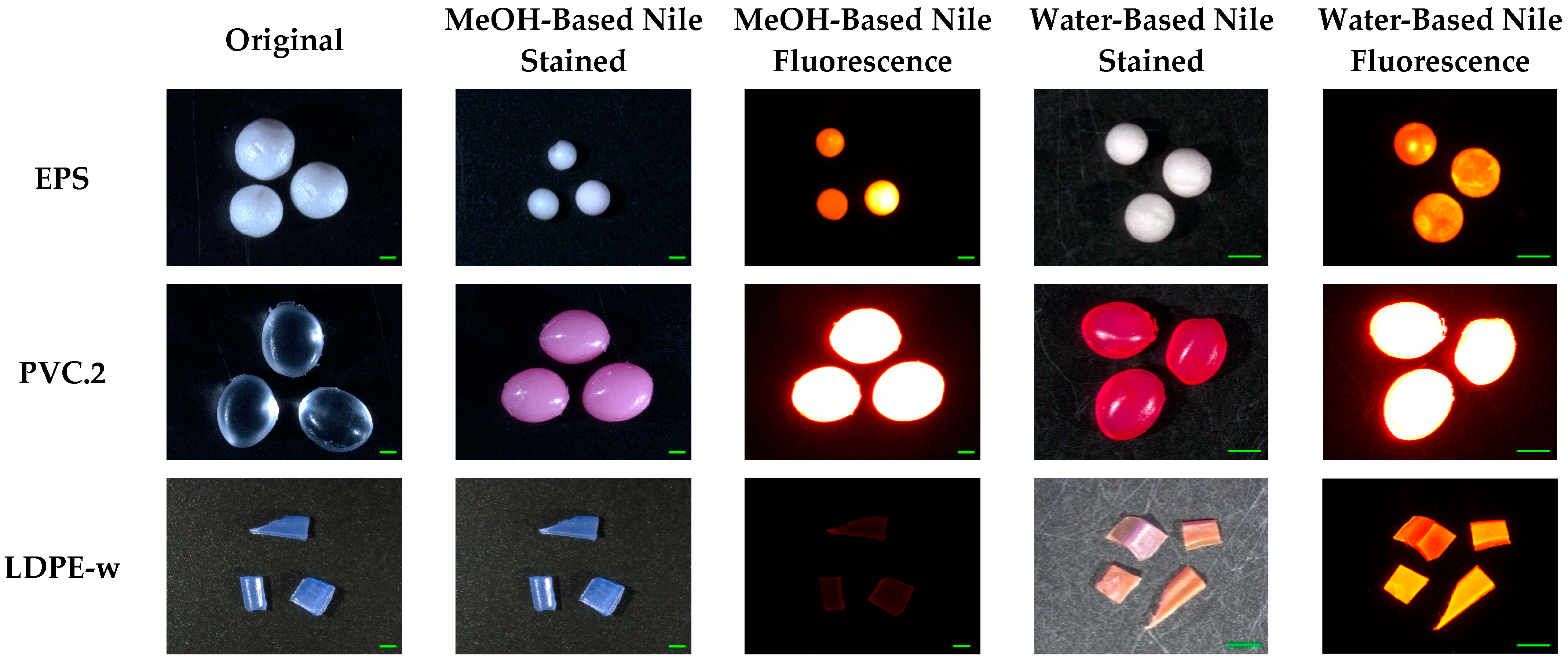
2.3. Characteristics of Stained Microplastics
3. Results and Discussion
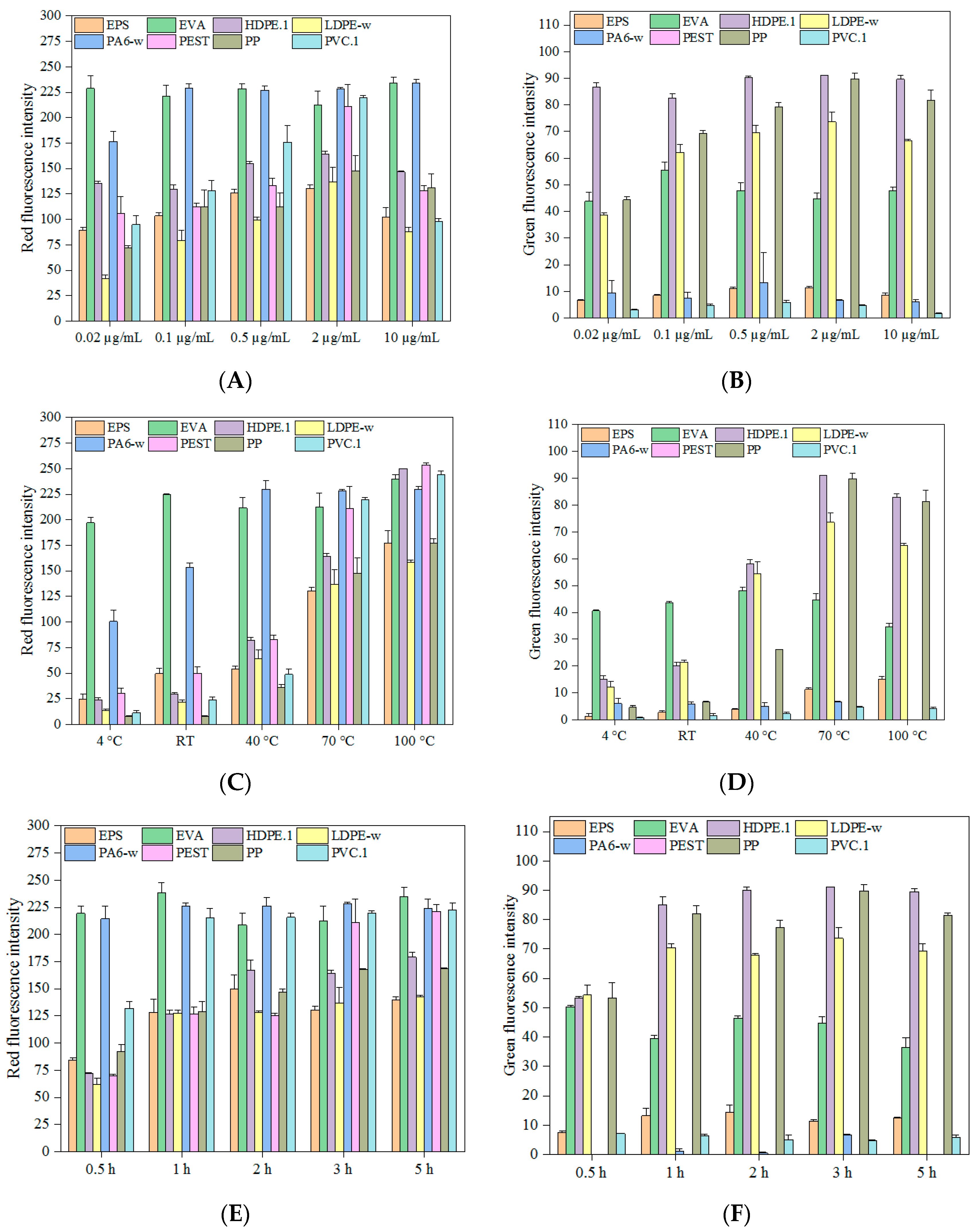
3.1. Influence of Dye Concentration
3.2. Influence of Incubation Temperature
3.3. Influence of Staining Time
3.4. Staining Efficiency on Various Polymers
3.5. Stability of Fluorescence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Nomenclature
| Ultra-low-density ethylene | ULDPE |
| Low-density polyethylene | LDPE |
| Linear low-density polyethylene | LLDPE |
| Medium-density polyethylene | MDPE |
| High-density polyethylene | HDPE |
| Polypropylene | PP |
| Polyester | PEST |
| Polyethylene terephthalate | PET |
| Ethylene-vinyl acetate | EVA |
| Acrylonitrile-butadiene-styrene | ABS |
| Expanded polystyrene foam | EPS |
| Polystyrene | PS |
| Nylon 6 | PA6 |
| Nylon 6,6 | PA66 |
| Polyvinyl chloride | PVC |
| Cellulose acetate | CA |
| Weathered polycarbonate | PC |
| Weathered low-density polyethylene | LDPE-w |
| Weathered polyethylene terephthalate | PETE-w |
| Weathered nylon 6 | PA6-w |
References
- Grbić, J.; Helm, P.; Athey, S.; Rochman, C.M. Microplastics entering northwestern Lake Ontario are diverse and linked to urban sources. Water Res. 2020, 174, 115623. [Google Scholar] [CrossRef]
- Scircle, A.; Missling, K.; Cizdziel, J.V.; Li, L.; Vianello, A. Single-Pot Method for Collection and Preparation of Natural Water for Microplastic Analyses: Microplastics in the Mississippi River System During and After Historic Flooding in 2019. Environ. Toxicol. Chem. 2020, 39, 986–995. [Google Scholar] [CrossRef]
- Uurasjärvi, E.; Pääkkönen, M.; Setälä, O.; Koistinen, A.; Lehtiniemi, M. Microplastics accumulate to thin layers in the stratified Baltic Sea. Environ. Pollut. 2021, 268, 115700. [Google Scholar] [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Gao, Z.; Wontor, K.; Cizdziel, J.V.; Lu, H. Distribution and characteristics of microplastics in beach sand near the outlet of a major reservoir in north Mississippi, USA. Microplastics Nanoplastics 2022, 2, 10. [Google Scholar] [CrossRef]
- Bagheri, T.; Gholizadeh, M.; Abarghouei, S.; Zakeri, M.; Hedayati, A.; Rabaniha, M.; Aghaeimoghadam, A.; Hafezieh, M. Microplastics distribution, abundance and composition in sediment, fishes and benthic organisms of the Gorgan Bay, Caspian Sea. Chemosphere 2020, 257, 127201. [Google Scholar] [CrossRef]
- Mercogliano, R.; Avio, C.G.; Regoli, F.; Anastasio, A.; Colavita, G.; Santonicola, S. Occurrence of microplastics in commercial seafood under the perspective of the human food chain. A review. J. Agric. Food Chem. 2020, 68, 5296–5301. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, X.; Huang, W.; Li, J.; Wang, C.; Zhang, D.; Zhang, C. Microplastic pollution in deep-sea sediments and organisms of the Western Pacific Ocean. Environ. Pollut. 2020, 259, 113948. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Tagg, A.S.; Harrison, J.P.; Ju-Nam, Y.; Sapp, M.; Bradley, E.L.; Sinclair, C.J.; Ojeda, J.J. Fenton’s reagent for the rapid and efficient isolation of microplastics from wastewater. Chem. Commun. 2017, 53, 372–375. [Google Scholar] [CrossRef]
- Long, Z.; Pan, Z.; Wang, W.; Ren, J.; Yu, X.; Lin, L.; Lin, H.; Chen, H.; Jin, X. Microplastic abundance, characteristics, and removal in wastewater treatment plants in a coastal city of China. Water Res. 2019, 155, 255–265. [Google Scholar] [CrossRef]
- Karakolis, E.G.; Nguyen, B.; You, J.B.; Rochman, C.M.; Sinton, D. Fluorescent dyes for visualizing microplastic particles and fibers in laboratory-based studies. Environ. Sci. Technol. Lett. 2019, 6, 334–340. [Google Scholar] [CrossRef]
- Sundbaek, K.B.; Koch, I.D.W.; Villaro, C.G.; Rasmussen, N.S.; Holdt, S.L.; Hartmann, N.B. Sorption of fluorescent polystyrene microplastic particles to edible seaweed Fucus vesiculosus. J. Appl. Phycol. 2018, 30, 2923–2927. [Google Scholar] [CrossRef]
- Bringer, A.; Cachot, J.; Prunier, G.; Dubillot, E.; Clérandeau, C. Thomas, H. Experimental ingestion of fluorescent microplastics by pacific oysters, Crassostrea gigas, and their effects on the behavior and development at early stages. Chemosphere 2020, 254, 126793. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, H.J.; Kim, S.K.; Kim, H.J. Global pattern of microplastics (MPs) in commercial food-grade salts: Sea salt as an indicator of seawater MP pollution. Environ. Sci. Technol. 2018, 52, 12819–12828. [Google Scholar] [CrossRef]
- Klein, M.; Fischer, E.K. Microplastic abundance in atmospheric deposition within the Metropolitan area of Hamburg, Germany. Sci. Total Environ. 2019, 685, 96–103. [Google Scholar] [CrossRef]
- Alfonso, M.B.; Arias, A.H.; Ronda, A.C.; Piccolo, M.C. Continental microplastics: Presence, features, and environmental transport pathways. Sci. Total Environ. 2021, 799, 149447. [Google Scholar] [CrossRef]
- Björnsdotter, M. Leaching of Residual Monomers, Oligomers and Additives from Polyethylene, Polypropylene, Polyvinyl Chloride, High-Density Polyethylene and Polystyrene Virgin Plastics. Bachelor’s Thesis, Örebro University, Örebro, Sweden, 2015. [Google Scholar]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a method for extracting microplastics from complex, organic-rich, environmental matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef]
- Raju, S.; Carbery, M.; Kuttykattil, A.; Senthirajah, K.; Lundmark, A.; Rogers, Z.; Suresh, S.C.B.; Evans, G.; Palanisami, T. Improved methodology to determine the fate and transport of microplastics in a secondary wastewater treatment plant. Water Res. 2020, 173, 115549. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Cizdziel, J.V.; Wontor, K.; Lu, H. Are Rural and Small Community Aerated Wastewater Stabilization Ponds a Neglected Source of Microplastic Pollution? Water 2021, 13, 2833. [Google Scholar] [CrossRef]
- Simon, M.; van Alst, N.; Vollertsen, J. Quantification of microplastic mass and removal rates at wastewater treatment plants applying Focal Plane Array (FPA)-based Fourier Transform Infrared (FT-IR) imaging. Water Res. 2018, 142, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. Trends Analyt. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Sturm, M.T.; Horn, H.; Schuhen, K. The potential of fluorescent dyes—Comparative study of Nile red and three derivatives for the detection of microplastics. Anal. Bioanal. Chem. 2021, 413, 1059–1071. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution–Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef]
- Capolupo, M.; Franzellitti, S.; Valbonesi, P.; Lanzas, C.S.; Fabbri, E. Uptake and transcriptional effects of polystyrene microplastics in larval stages of the Mediterranean mussel Mytilus galloprovincialis. Environ. Pollut. 2018, 241, 1038–1047. [Google Scholar] [CrossRef]
- Tagg, A.S.; do Sul, J.A.I. Is this your glitter? An overlooked but potentially environmentally-valuable microplastic. Mar. Pollut. Bull. 2019, 146, 50–53. [Google Scholar] [CrossRef]
- Desforges, J.P.W.; Galbraith, M.; Dangerfield, N.; Ross, P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014, 79, 94–99. [Google Scholar] [CrossRef]
- Rumin, J.; Bonnefond, H.; Saint-Jean, B.; Rouxel, C.; Sciandra, A.; Bernard, O.; Cadoret, J.P. Bougaran, G. The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae. Biotechnol. Biofuels 2015, 8, 42. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but found with Nile Red: A novel method for detecting and quantifying small microplastics (1 mm to 20 μm) in environmental samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef] [PubMed]
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C.; Henry, T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018, 237, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Scircle, A.; Cizdziel, J.V. Detecting and quantifying microplastics in bottled water using fluorescence microscopy: A new experiment for instrumental analysis and environmental chemistry courses. J. Chem. Educ. 2019, 97, 234–238. [Google Scholar] [CrossRef]
- Kang, H.; Park, S.; Lee, B.; Ahn, J.; Kim, S. Modification of a Nile Red Staining Method for Microplastics Analysis: A Nile Red Plate Method. Water 2020, 12, 3251. [Google Scholar] [CrossRef]
- Lv, L.; Qu, J.; Yu, Z.; Chen, D.; Zhou, C.; Hong, P.; Sun, S.; Li, C. A simple method for detecting and quantifying microplastics utilizing fluorescent dyes-Safranine T, fluorescein isophosphate, Nile red based on thermal expansion and contraction property. Environ. Pollut. 2019, 255, 113283. [Google Scholar] [CrossRef]
- Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D.; Kutralam-Muniasamy, G. Analyzing microplastics with Nile Red: Emerging trends, challenges, and prospects. J. Hazard. Mater. 2022, 423, 127171. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar. Pollut. Bull. 2012, 64, 2782–2789. [Google Scholar] [CrossRef]
- Tamminga, M.; Hengstmann, E.; Fischer, E.K. Microplastic analysis in the South Funen Archipelago, Baltic Sea, implementing manta trawling and bulk sampling. Mar. Pollut. Bull. 2018, 128, 601–608. [Google Scholar] [CrossRef]
- Godoy, V.; Prata, J.C.; Blázquez, G.; Almendros, A.I.; Duarte, A.C.; Rocha-Santos, T.; Calero, M.; Martín-Lara, M.Á. Effects of distance to the sea and geomorphological characteristics on the quantity and distribution of microplastics in beach sediments of Granada (Spain). Sci. Total Environ. 2020, 746, 142023. [Google Scholar] [CrossRef]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef]
- Stanton, T.; Johnson, M.; Nathanail, P.; Gomes, R.L.; Needham, T.; Burson, A. Exploring the efficacy of Nile red in microplastic quantification: A costaining approach. Environ. Sci. Technol. Lett. 2019, 6, 606–611. [Google Scholar] [CrossRef]
- Martinez, V.; Henary, M. Nile red and Nile blue: Applications and syntheses of structural analogues. Chem.-Eur. J. 2016, 22, 13764–13782. [Google Scholar] [CrossRef] [PubMed]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef] [PubMed]


| Influencing Factors | Dyes | Series | Polymers |
|---|---|---|---|
| Dye concentration (1) | Nile red | 0.02, 0.1, 0.5, 2, 10 (µg/mL) | EPS, EVA, HDPE.1, LDPE-w, PA6-w, PEST, PP, PVC.1 |
| Rit pink dye | 1:500, 1:200, 1:100, 1:50, 1:20, 1:1 or 2.2, 5.5, 11, 22, 55, 1100 (mg/mL) | ||
| Rit blue dye | |||
| iDye pink dye | 0.2, 0.5, 1, 2, 5, 100 (mg/mL) | ||
| iDye Blue dye | |||
| Incubation temperature (2) | Nile red | 4° C, RT (21 °C), 40 °C, 70 °C and 100 °C for 3 h | |
| Rit pink dye | |||
| Rit blue dye | |||
| iDye pink dye | |||
| iDye blue dye | |||
| Incubation duration (2) | Nile red | 0.5, 1, 2, 3, 5 h at 70°C | |
| Rit pink dye | |||
| Rit blue dye | |||
| iDye pink dye | |||
| iDye blue dye |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Wontor, K.; Cizdziel, J.V. Labeling Microplastics with Fluorescent Dyes for Detection, Recovery, and Degradation Experiments. Molecules 2022, 27, 7415. https://doi.org/10.3390/molecules27217415
Gao Z, Wontor K, Cizdziel JV. Labeling Microplastics with Fluorescent Dyes for Detection, Recovery, and Degradation Experiments. Molecules. 2022; 27(21):7415. https://doi.org/10.3390/molecules27217415
Chicago/Turabian StyleGao, Zhiqiang, Kendall Wontor, and James V. Cizdziel. 2022. "Labeling Microplastics with Fluorescent Dyes for Detection, Recovery, and Degradation Experiments" Molecules 27, no. 21: 7415. https://doi.org/10.3390/molecules27217415
APA StyleGao, Z., Wontor, K., & Cizdziel, J. V. (2022). Labeling Microplastics with Fluorescent Dyes for Detection, Recovery, and Degradation Experiments. Molecules, 27(21), 7415. https://doi.org/10.3390/molecules27217415