Gastric Enteric Glial Cells: A New Contributor to the Synucleinopathies in the MPTP-Induced Parkinsonism Mouse
Abstract
1. Introduction
2. Results
2.1. The Chronic MPTP/p-Treated Mouse Exhibits Motor, Gastrointestinal and Typical Pathological Alterations, but Not Olfactory Deficits
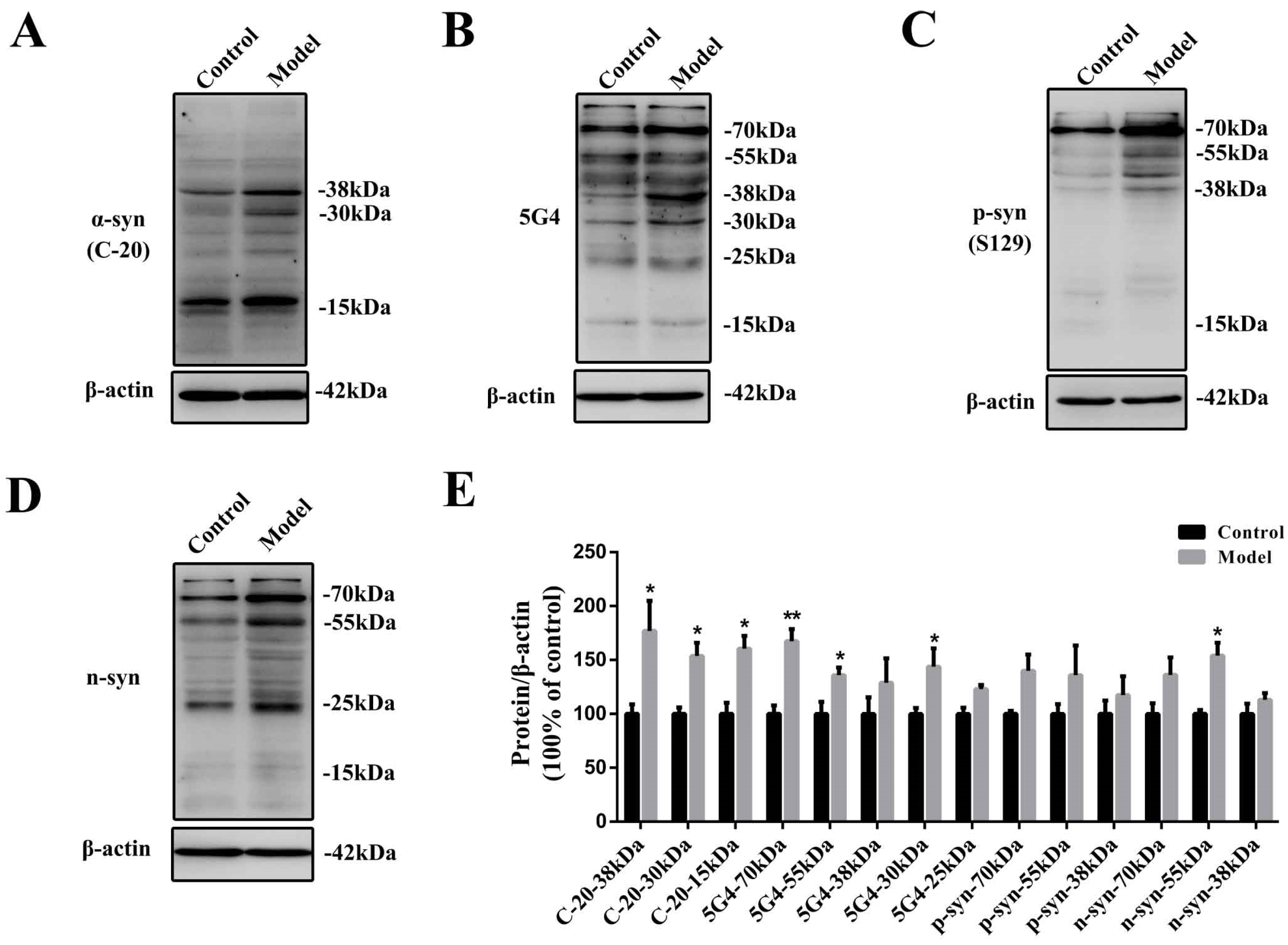
2.2. Chronic Treatment with MPTP/p Could Lead to Abnormal α-Synuclein Aggregation, Represented by Increases in Different PTMs and Aggregation Forms in the Stomach
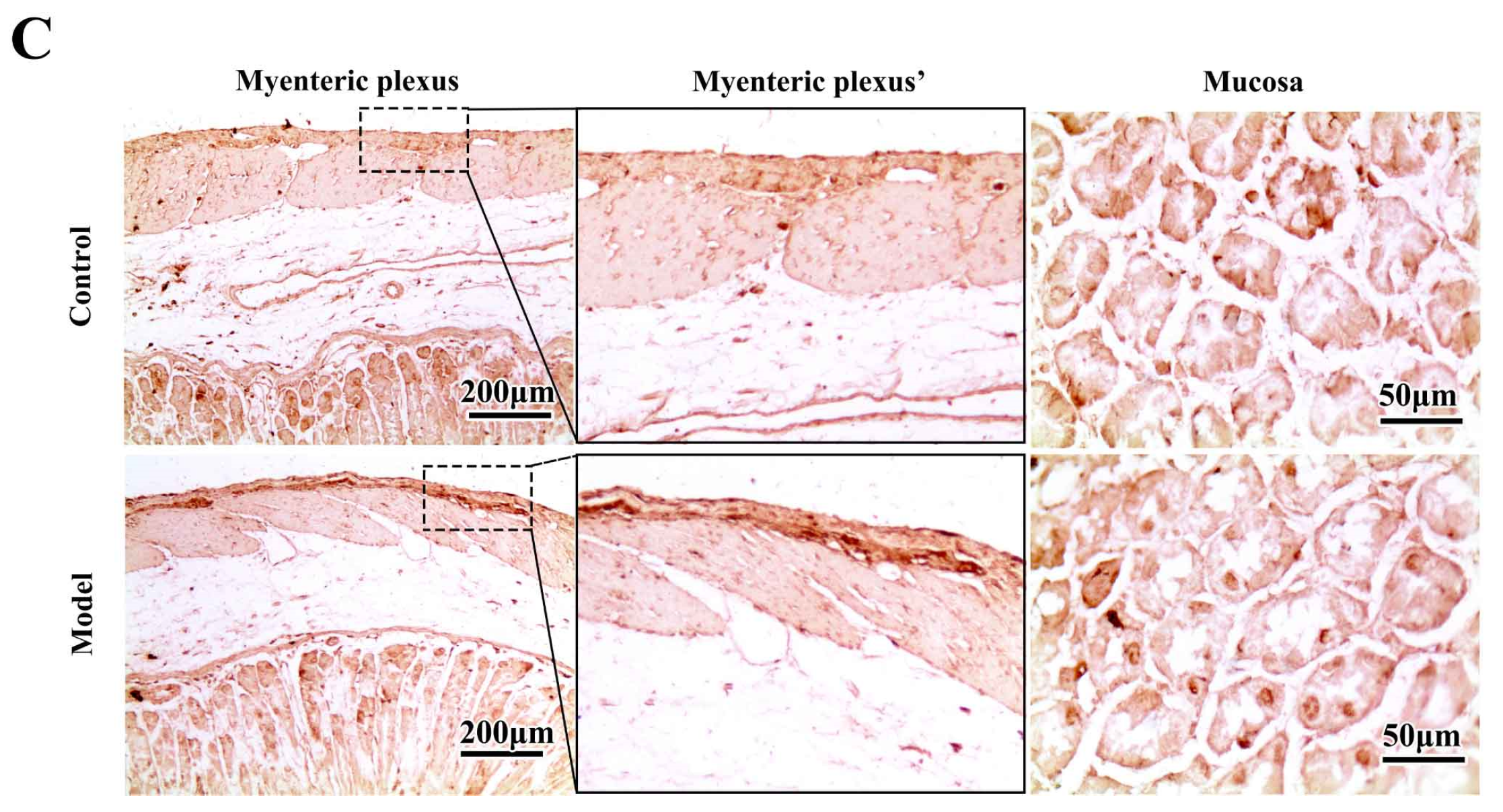
2.3. Chronic MPTP/p Exposure Induced Synucleinopathies in the Gastric Myenteric Plexus
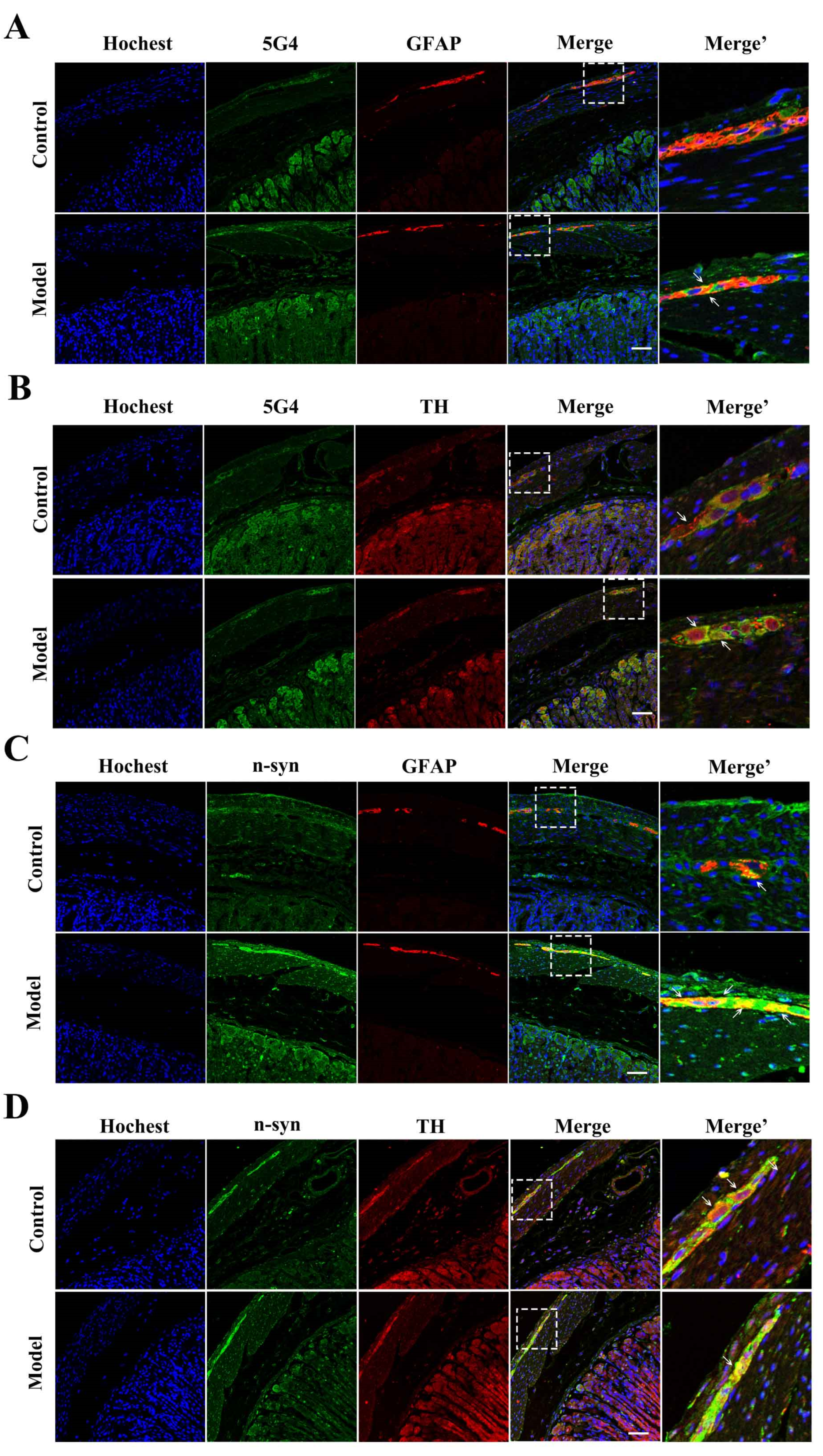
2.4. The Synucleinopathies Mainly Localized in the TH-Positive Enteric Neurons and Enteric Glial Cells (EGCs) of the Myenteric Plexus
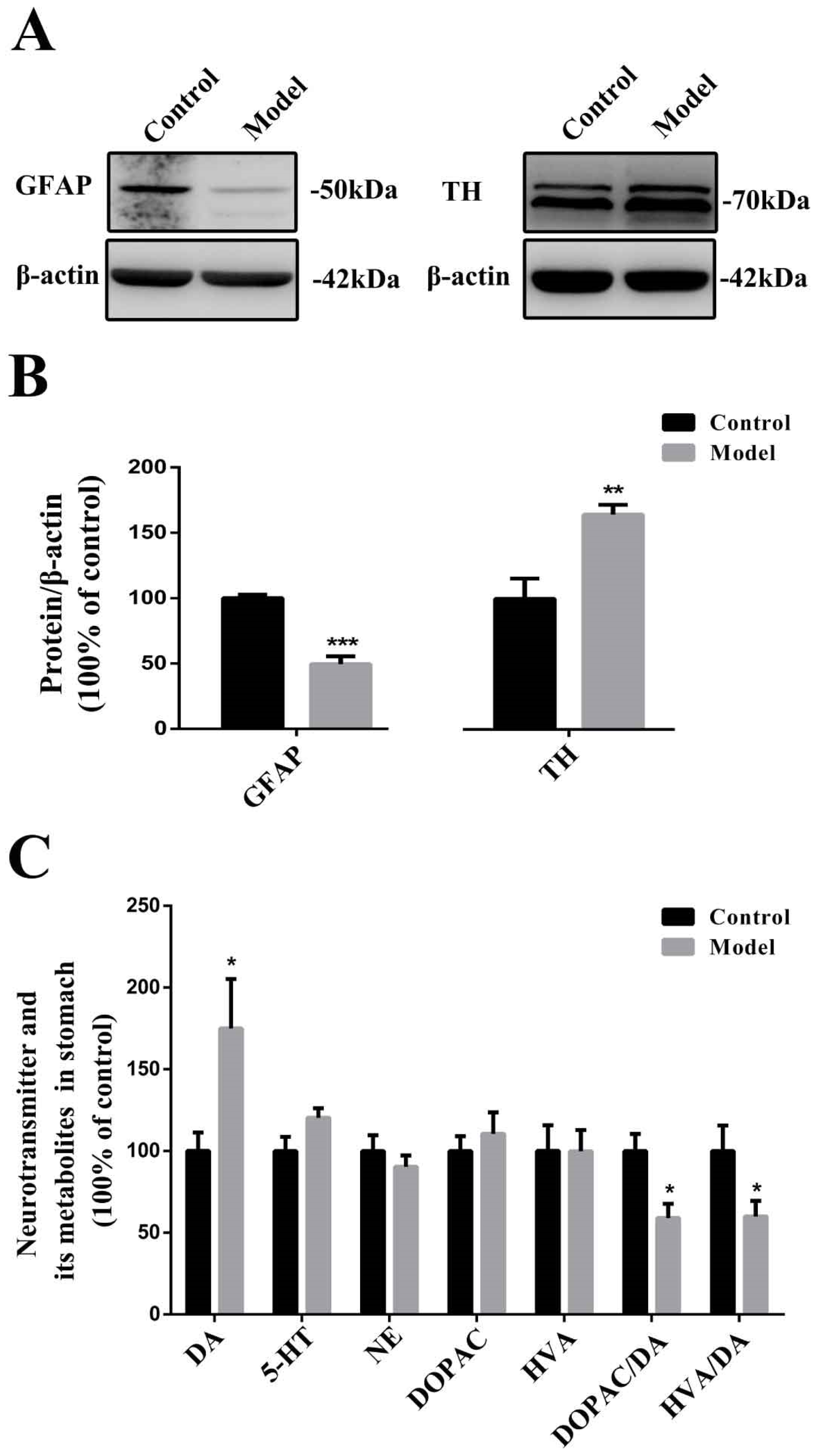
2.5. Chronic MPTP/p Exposure Influenced the Functions of EGCs and TH-Positive Neurons in the Stomach
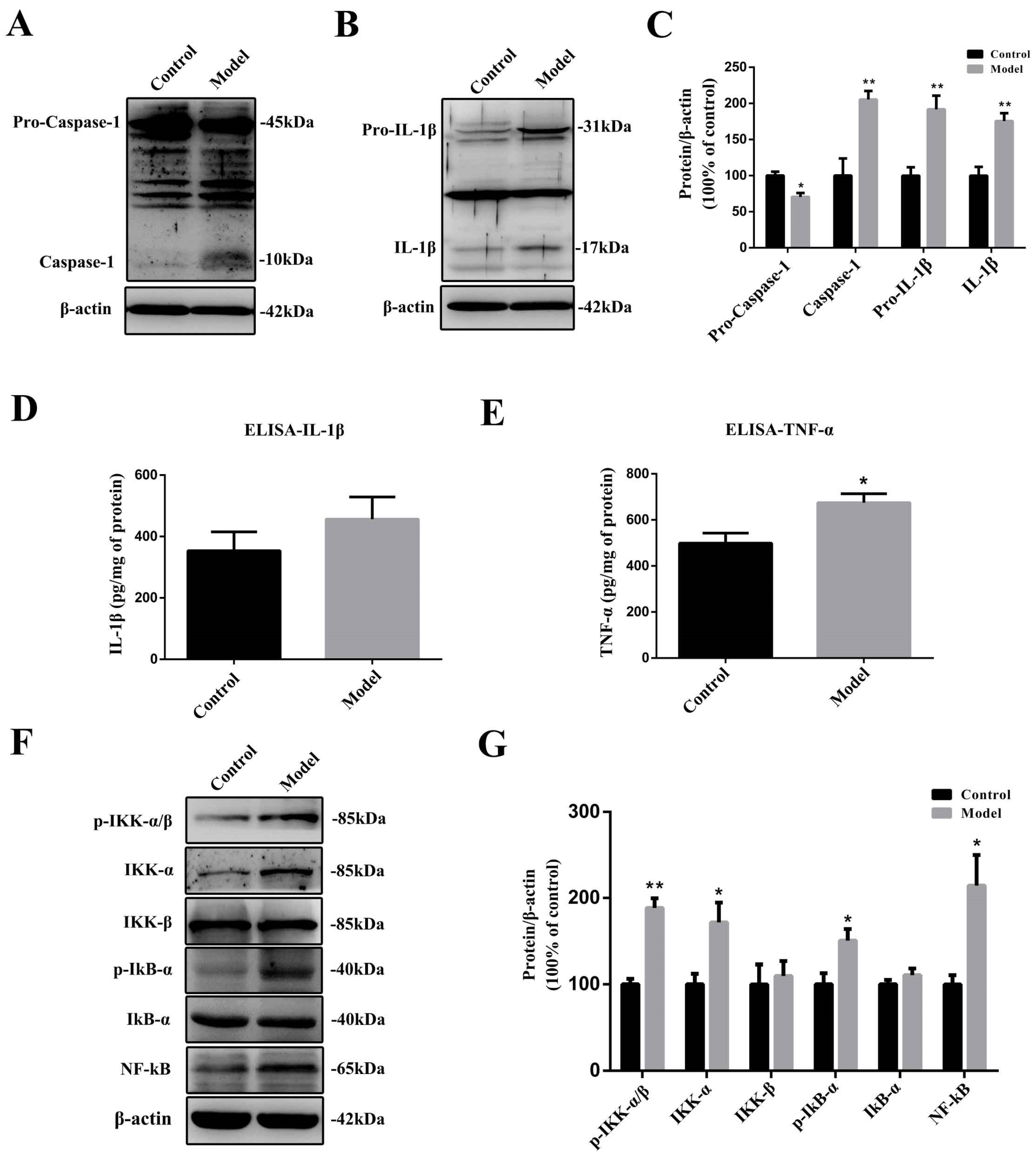
2.6. The Chronic Treatment with MPTP/p Led to a Prolonged Inflammatory State and the Activation of the Nuclear Factor-κB (NF-κB) Pathway
2.7. Naturally High Activities of MAO-B and Low Activities of SOD Make the Stomach Appear to Undergo Oxidative Stress before the SN, Which Is Commonly Thought to Be Highly Susceptible
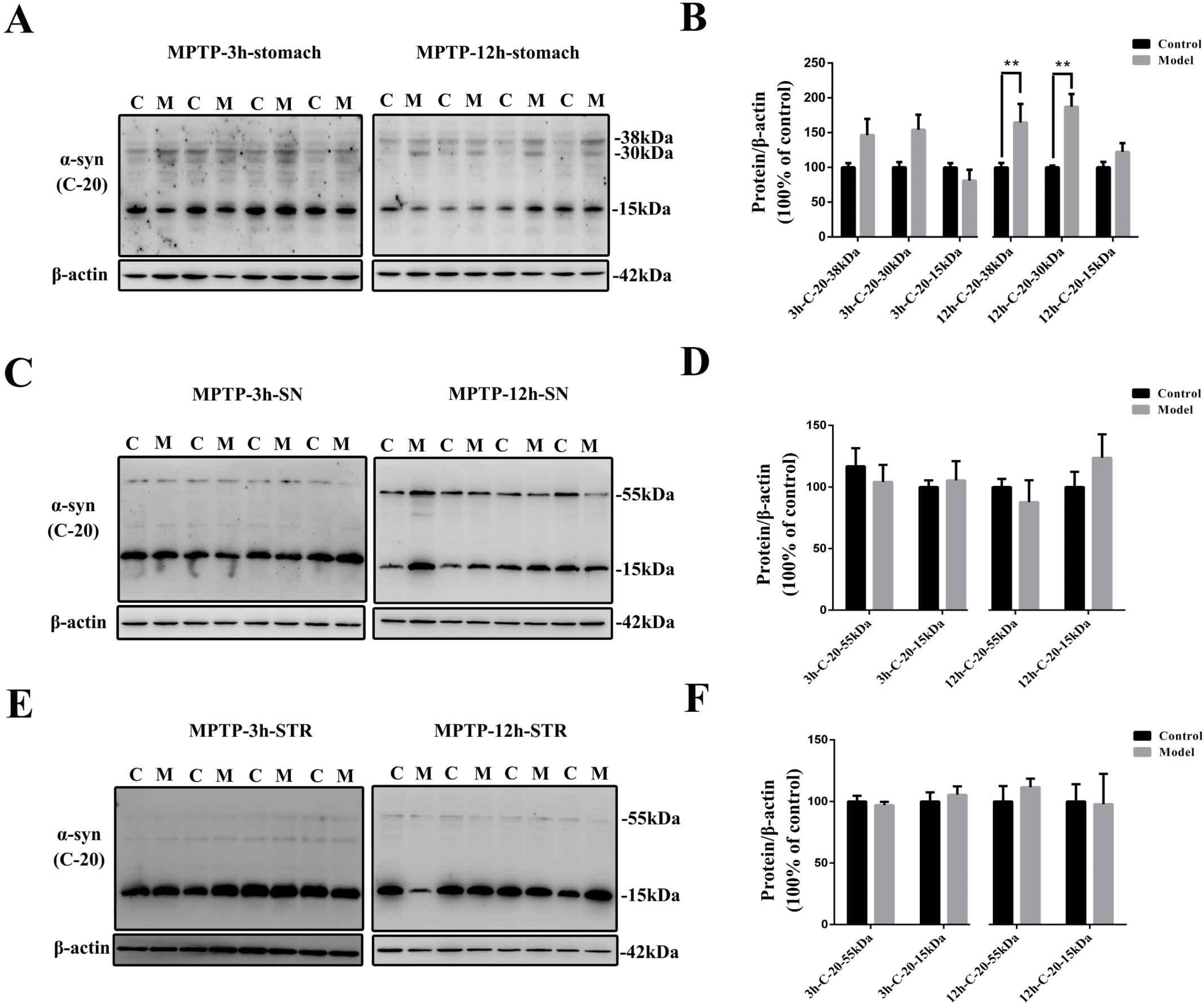
2.8. The Stomach Is More Susceptible to MPTP Than SN and STR, and EGCs Could Be the Initial Cells Contributing to the Synucleinopathies
3. Materials and Methods
3.1. Chemical Materials
3.2. Animals
3.3. MPTP Administration
3.4. Behavior Testing
3.4.1. Rotarod Test and Pole Test
3.4.2. Stool Collection Test
3.4.3. Buried Pellet Test
3.5. Tissue Preparation
3.6. Immunohistochemistry (IHC) and Histopathology
3.7. Western Blotting
3.8. Enzyme-Linked Immunosorbent Assay (ELISA)
3.9. Monoamine Oxidase Type-B (MAO-B) Activity Measurement
3.10. Reactive Oxygen Species (ROS) Detection
3.11. Superoxide Dismutase (SOD) Enzyme Activity Measurement
3.12. Measurement of Dopamine and Its Metabolites
3.13. Statistical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Zeng, Q.; Cui, M. Current Progress in the Development of Probes for Targeting alpha-Synuclein Aggregates. ACS Chem. Neurosci. 2022, 13, 552–571. [Google Scholar] [CrossRef]
- Liddle, R.A. Parkinson’s disease from the gut. Brain Res. 2018, 1693 (Pt B), 201–206. [Google Scholar] [CrossRef]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Abeliovich, A.; Rhinn, H. Parkinson’s disease: Guilt by genetic association. Nature 2016, 533, 40–41. [Google Scholar] [CrossRef]
- Kim, C.Y.; Alcalay, R.N. Genetic Forms of Parkinson’s Disease. Semin. Neurol. 2017, 37, 135–146. [Google Scholar] [CrossRef]
- Masuda-Suzukake, M.; Nonaka, T.; Hosokawa, M.; Oikawa, T.; Arai, T.; Akiyama, H.; Man, D.M.A.; Hasegawa, M. Prion-like spreading of pathological alpha-synuclein in brain. Brain J. Neurol. 2013, 136 Pt 4, 1128–1138. [Google Scholar] [CrossRef]
- Henderson, M.X.; Trojanowski, J.Q.; Lee, V.M. alpha-Synuclein pathology in Parkinson’s disease and related alpha-synucleinopathies. Neurosci. Lett. 2019, 709, 134316. [Google Scholar] [CrossRef]
- Wood, H. Parkinson disease: Peripheral alpha-synuclein deposits—Prodromal markers for Parkinson disease? Nat. Rev. Neurol. 2016, 12, 249. [Google Scholar] [CrossRef]
- Burre, J.; Sharma, M.; Sudhof, T.C. Cell Biology and Pathophysiology of alpha-Synuclein. Cold Spring Harb. Perspect. Med. 2018, 8, a024091. [Google Scholar] [CrossRef]
- Dogra, N.; Mani, R.J.; Katare, D.P. The Gut-Brain Axis: Two Ways Signaling in Parkinson’s Disease. Cell Mol. Neurobiol. 2022, 42, 315–332. [Google Scholar] [CrossRef]
- Pan-Montojo, F.; Schwarz, M.; Winkler, C.; Arnhold, M.; O’Sullivan, G.A.; Pal, A.; Said, J.W.; Marsico, G.; Verbavatz, J.-M.; Rodrigo-Angulo, M.; et al. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci. Rep. 2012, 2, 898. [Google Scholar] [CrossRef]
- Holmqvist, S.; Chutna, O.; Bousset, L.; Aldrin-Kirk, P.; Li, W.; Bjorklund, T.; Wang, Z.-Y.; Roybon, L.; Melki, R.; Li, J.-Y. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014, 128, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Dehay, B.; Bourdenx, M.; Gorry, P.; Przedborski, S.; Vila, M.; Hunot, S.; Singleton, A.; Olanow, C.W.; Merchant, K.M.; Bezard, E.; et al. Targeting alpha-synuclein for treatment of Parkinson’s disease: Mechanistic and therapeutic considerations. Lancet Neurol. 2015, 14, 855–866. [Google Scholar] [CrossRef]
- Ghosh, D.; Mehra, S.; Sahay, S.; Singh, P.K.; Maji, S.K. alpha-synuclein aggregation and its modulation. Int. J. Biol. Macromol. 2017, 100, 37–54. [Google Scholar] [CrossRef]
- He, S.; Wang, F.; Yung, K.K.L.; Zhang, S.; Qu, S. Effects of alpha-Synuclein-Associated Post-Translational Modifications in Parkinson’s Disease. ACS Chem. Neurosci. 2021, 12, 1061–1071. [Google Scholar] [CrossRef]
- Breydo, L.; Wu, J.W.; Uversky, V.N. Alpha-synuclein misfolding and Parkinson’s disease. Biochim. Biophys. Acta 2012, 1822, 261–285. [Google Scholar] [CrossRef]
- Burmann, B.M.; Gerez, J.A.; Matecko-Burmann, I.; Campioni, S.; Kumari, P.; Ghosh, D.; Mazur, A.; Aspholm, E.E.; Šulskis, D.; Wawrzyniuk, M.; et al. Regulation of alpha-synuclein by chaperones in mammalian cells. Nature 2020, 577, 127–132. [Google Scholar] [CrossRef]
- Langston, J.W. The MPTP Story. J. Park. Dis. 2017, 7, S11–S19. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef]
- Biju, K.C.; Evans, R.C.; Shrestha, K.; Carlisle, D.C.B.; Gelfond, J.; Clark, R.A. Methylene Blue Ameliorates Olfactory Dysfunction and Motor Deficits in a Chronic MPTP/Probenecid Mouse Model of Parkinson’s Disease. Neuroscience 2018, 380, 111–122. [Google Scholar] [CrossRef]
- Kin, K.; Yasuhara, T.; Kameda, M.; Date, I. Animal Models for Parkinson’s Disease Research: Trends in the 2000s. Int. J. Mol. Sci. 2019, 20, 5402. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.M.; De Miranda, B.; Sanders, L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation-An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef]
- Li, X.; Yang, W.; Li, X.; Chen, M.; Liu, C.; Li, J.; Yu, S. Alpha-synuclein oligomerization and dopaminergic degeneration occur synchronously in the brain and colon of MPTP-intoxicated parkinsonian monkeys. Neurosci. Lett. 2020, 716, 134640. [Google Scholar] [CrossRef] [PubMed]
- Gibrat, C.; Saint-Pierre, M.; Bousquet, M.; Levesque, D.; Rouillard, C.; Cicchetti, F. Differences between subacute and chronic MPTP mice models: Investigation of dopaminergic neuronal degeneration and alpha-synuclein inclusions. J. Neurochem. 2009, 109, 1469–1482. [Google Scholar] [CrossRef]
- McQuade, R.M.; Singleton, L.M.; Wu, H.; Lee, S.; Constable, R.; Di Natale, M.; Ringuet, M.T.; Berger, J.P.; Kauhausen, J.; Parish, C.L.; et al. The association of enteric neuropathy with gut phenotypes in acute and progressive models of Parkinson’s disease. Sci. Rep. 2021, 11, 7934. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Wagner, U.; Dumont, B.; Pikkarainen, M.; Osman, A.A.; Streichenberger, N.; Leisser, I.; Verchère, J.; Baron, T.; Alafuzoff, I.; et al. An antibody with high reactivity for disease-associated alpha-synuclein reveals extensive brain pathology. Acta Neuropathol. 2012, 124, 37–50. [Google Scholar] [CrossRef]
- Heng, Y.; Zhang, Q.S.; Mu, Z.; Hu, J.F.; Yuan, Y.H.; Chen, N.H. Ginsenoside Rg1 attenuates motor impairment and neuroinflammation in the MPTP-probenecid-induced parkinsonism mouse model by targeting alpha-synuclein abnormalities in the substantia nigra. Toxicol. Lett. 2016, 243, 7–21. [Google Scholar] [CrossRef]
- Chavarria, C.; Souza, J.M. Oxidation and nitration of alpha-synuclein and their implications in neurodegenerative diseases. Arch. Biochem. Biophys. 2013, 533, 25–32. [Google Scholar] [CrossRef]
- Du, A.; McCracken, K.W.; Walp, E.R.; Terry, N.A.; Klein, T.J.; Han, A.; Wells, J.M.; May, C.L. Arx is required for normal enteroendocrine cell development in mice and humans. Dev. Biol. 2012, 365, 175–188. [Google Scholar] [CrossRef] [PubMed]
- De Zoete, M.R.; Palm, N.W.; Zhu, S.; Flavell, R.A. Inflammasomes. Cold Spring Harb. Perspect. Biol. 2014, 6, a016287. [Google Scholar] [CrossRef] [PubMed]
- Hafner-Bratkovic, I.; Bencina, M.; Fitzgerald, K.A.; Golenbock, D.; Jerala, R. NLRP3 inflammasome activation in macrophage cell lines by prion protein fibrils as the source of IL-1beta and neuronal toxicity. Cell. Mol. Life Sci. CMLS 2012, 69, 4215–4228. [Google Scholar] [CrossRef] [PubMed]
- Farrell, K.F.; Krishnamachari, S.; Villanueva, E.; Lou, H.; Alerte, T.N.; Peet, E.; Drolet, R.E.; Perez, R.G. Non-motor parkinsonian pathology in aging A53T alpha-synuclein mice is associated with progressive synucleinopathy and altered enzymatic function. J. Neurochem. 2014, 128, 536–546. [Google Scholar] [CrossRef]
- Akbari, A.; Li, Y.; Kilani, R.T.; Ghahary, A. Red blood cell lysate modulates the expression of extracellular matrix proteins in dermal fibroblasts. Mol. Cell. Biochem. 2012, 370, 79–88. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.D.; Song, L.K.; Li, J.; Chu, S.F.; Yuan, Y.H.; Chen, N.-H. Environment-contact administration of rotenone: A new rodent model of Parkinson’s disease. Behav. Brain Res. 2015, 294, 149–161. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, S.Y.; Song, X.Y.; Xia, C.Y.; Yang, Y.N.; Zhang, P.C.; Chen, N.-H. Protective effects of Forsythia suspense extract with antioxidant and anti-inflammatory properties in a model of rotenone induced neurotoxicity. Neurotoxicology 2015, 52, 72–83. [Google Scholar] [CrossRef]
- Vaquer-Alicea, J.; Diamond, M.I. Propagation of Protein Aggregation in Neurodegenerative Diseases. Annu. Rev. Biochem. 2019, 88, 785–810. [Google Scholar] [CrossRef]
- Levin, J.; Schmidt, F.; Boehm, C.; Prix, C.; Botzel, K.; Ryazanov, S.; Leonov, A.; Griesinger, C.; Giese, A. The oligomer modulator anle138b inhibits disease progression in a Parkinson mouse model even with treatment started after disease onset. Acta Neuropathol. 2014, 127, 779–780. [Google Scholar] [CrossRef]
- Candelise, N.; Scaricamazza, S.; Salvatori, I.; Ferri, A.; Valle, C.; Manganelli, V.; Garofalo, T.; Sorice, M.; Misasi, R. Protein Aggregation Landscape in Neurodegenerative Diseases: Clinical Relevance and Future Applications. Int. J. Mol. Sci. 2021, 22, 6016. [Google Scholar] [CrossRef]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, Z.; Chen, S. Alpha-Synuclein Nitration and Its Implications in Parkinson’s Disease. ACS Chem. Neurosci. 2019, 10, 777–782. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, B.R.; Rocha, E.M.; Bai, Q.; El Ayadi, A.; Hinkle, D.; Burton, E.A.; Greenamyre, J.T. Astrocyte-specific DJ-1 overexpression protects against rotenone-induced neurotoxicity in a rat model of Parkinson’s disease. Neurobiol. Dis. 2018, 115, 101–114. [Google Scholar] [CrossRef]
- Miller, J.A.; Trout, B.R.; Sullivan, K.A.; Bialecki, R.A.; Roberts, R.A.; Tjalkens, R.B. Low-dose 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine causes inflammatory activation of astrocytes in nuclear factor-kappaB reporter mice prior to loss of dopaminergic neurons. J. Neurosci. Res. 2011, 89, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Bove, J.; Perier, C. Neurotoxin-based models of Parkinson’s disease. Neuroscience 2012, 211, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Im, M.J.; Park, C.H.; Lee, C.J.; Choi, S.; Yoon, B.J. Remodeling of the dendritic structure of the striatal medium spiny neurons accompanies behavioral recovery in a mouse model of Parkinson’s disease. Neurosci. Lett. 2013, 557, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhu, M.; Liang, Z. (-)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease. Mol. Med. Rep. 2018, 17, 4883–4888. [Google Scholar] [CrossRef]
- Sokolowski, A.L.; Larsson, B.S.; Lindquist, N.G. Distribution of 1-(3H)-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (3H-MPTP) in the frog: Uptake in neuromelanin. Pharmacol. Toxicol. 1990, 66, 252–258. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Heng, Y.; Mou, Z.; Huang, J.Y.; Yuan, Y.H.; Chen, N.H. Reassessment of subacute MPTP-treated mice as animal model of Parkinson’s disease. Acta Pharmacol. Sin. 2017, 38, 1317–1328. [Google Scholar] [CrossRef]
- Muller, T.; Mohr, J.D. Pharmacokinetics of monoamine oxidase B inhibitors in Parkinson’s disease: Current status. Expert Opin. Drug Metab. Toxicol. 2019, 15, 429–435. [Google Scholar] [CrossRef]
- Tripathi, R.K.P.; Ayyannan, S.R. Monoamine oxidase-B inhibitors as potential neurotherapeutic agents: An overview and update. Med. Res. Rev. 2019, 39, 1603–1706. [Google Scholar] [CrossRef] [PubMed]
- Visanji, N.P.; Brooks, P.L.; Hazrati, L.N.; Lang, A.E. The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol. Commun. 2013, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Ghebremedhin, E.; Rub, U.; Bratzke, H.; Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004, 318, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic alpha-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e7. [Google Scholar] [CrossRef]
- Radad, K.; Al-Shraim, M.; Al-Emam, A.; Wang, F.; Kranner, B.; Rausch, W.D.; Moldzio, R. Rotenone: From modelling to implication in Parkinson’s disease. Folia Neuropathol. 2019, 57, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.M.; Xie, Z.; Stout, K.A.; Zampese, E.; Burbulla, L.F.; Shih, J.C.; Kondapalli, J.; Patriarchi, T.; Tian, L.; Brichta, L.; et al. Dopamine metabolism by a monoamine oxidase mitochondrial shuttle activates the electron transport chain. Nat. Neurosci. 2020, 23, 15–20. [Google Scholar] [CrossRef]
- Werner-Allen, J.W.; DuMond, J.F.; Levine, R.L.; Bax, A. Toxic dopamine metabolite DOPAL forms an unexpected dicatechol pyrrole adduct with lysines of alpha-synuclein. Angew. Chem. 2016, 55, 7374–7378. [Google Scholar] [CrossRef]
- Burke, W.J.; Kumar, V.B.; Pandey, N.; Panneton, W.M.; Gan, Q.; Franko, M.W.; O’Dell, M.; Li, S.W.; Pan, Y.; Chung, H.D.; et al. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008, 115, 193–203. [Google Scholar] [CrossRef]
- Plotegher, N.; Berti, G.; Ferrari, E.; Tessari, I.; Zanetti, M.; Lunelli, L.; Greggio, E.; Bisaglia, M.; Veronesi, M.; Girotto, S.; et al. DOPAL derived alpha-synuclein oligomers impair synaptic vesicles physiological function. Sci. Rep. 2017, 7, 40699. [Google Scholar] [CrossRef]
- Choi, J.G.; Huh, E.; Ju, I.G.; Kim, N.; Yun, J.; Oh, M.S. 1-Methyl-4-phenyl-1,2,3,6 tetrahydropyridine/probenecid impairs intestinal motility and olfaction in the early stages of Parkinson’s disease in mice. J. Neurol. Sci. 2018, 392, 77–82. [Google Scholar] [CrossRef]
- Kim, W.S.; Kagedal, K.; Halliday, G.M. Alpha-synuclein biology in Lewy body diseases. Alzheimer’s Res. Ther. 2014, 6, 73. [Google Scholar] [CrossRef]
- Schildknecht, S.; Gerding, H.R.; Karreman, C.; Drescher, M.; Lashuel, H.A.; Outeiro, T.F.; Di Monte, D.A.; Leist, M. Oxidative and nitrative alpha-synuclein modifications and proteostatic stress: Implications for disease mechanisms and interventions in synucleinopathies. J. Neurochem. 2013, 125, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, R.E.; Helwig, M.; Bae, E.J.; Aboutalebi, H.; Lee, S.J.; Ulusoy, A.; Di Monte, D.A. Oxidative stress in vagal neurons promotes parkinsonian pathology and intercellular alpha-synuclein transfer. J. Clin. Investig. 2019, 129, 3738–3753. [Google Scholar] [CrossRef]
- McCormack, A.L.; Mak, S.K.; Di Monte, D.A. Increased alpha-synuclein phosphorylation and nitration in the aging primate substantia nigra. Cell Death Dis. 2012, 3, e315. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, Y.; Chen, J. Transcriptome sequencing in a 6-hydroxydopamine rat model of Parkinson’s disease. Genes Genet. Syst. 2019, 94, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.F.; Song, J.; Fan, R.F.; Chen, C.L.; Ren, Q.Z.; Zhang, X.L.; Feng, X.-Y.; Zhang, Y.; Li, L.-S.; Zhu, J.-X. The role of the vagal pathway and gastric dopamine in the gastroparesis of rats after a 6-hydroxydopamine microinjection in the substantia nigra. Acta Physiol. 2014, 211, 434–446. [Google Scholar] [CrossRef]
- Neunlist, M.; Rolli-Derkinderen, M.; Latorre, R.; Van Landeghem, L.; Coron, E.; Derkinderen, P.; De Giorgio, R. Enteric glial cells: Recent developments and future directions. Gastroenterology 2014, 147, 1230–1237. [Google Scholar] [CrossRef]
- Sharkey, K.A. Emerging roles for enteric glia in gastrointestinal disorders. J. Clin. Investig. 2015, 125, 918–925. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Klingelhoefer, L.; Reichmann, H. Pathogenesis of Parkinson disease—The gut—Brain axis and environmental factors. Nat. Rev. Neurol. 2015, 11, 625–636. [Google Scholar] [CrossRef]
- Grubisic, V.; Gulbransen, B.D. Enteric glia: The most alimentary of all glia. J. Physiol. 2016, 595, 557–570. [Google Scholar] [CrossRef]
- McClain, J.L.; Grubisic, V.; Fried, D.; Gomez-Suarez, R.A.; Leinninger, G.M.; Sevigny, J.; Parpura, V.; Gulbransen, B.D. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 2014, 146, 497–507.e1. [Google Scholar] [CrossRef] [PubMed]
- McClain, J.L.; Fried, D.E.; Gulbransen, B.D. Agonist-evoked Ca signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Buttner, S.; Faes, L.; Reichelt, W.N.; Broeskamp, F.; Habernig, L.; Benke, S.; Kourtis, N.; Ruli, D.; Carmona-Gutierrez, D.; Eisenberg, T.; et al. The Ca2+/Mn2+ ion-pump PMR1 links elevation of cytosolic Ca(2+) levels to alpha-synuclein toxicity in Parkinson’s disease models. Cell Death Differ. 2013, 20, 465–477. [Google Scholar] [CrossRef]
- Nath, S.; Goodwin, J.; Engelborghs, Y.; Pountney, D.L. Raised calcium promotes alpha-synuclein aggregate formation. Mol. Cell. Neurosci. 2011, 46, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, A.; Strosznajder, J.B. Alpha-synuclein potentiates Ca2+ influx through voltage-dependent Ca2+ channels. Neuroreport 2006, 17, 1883–1886. [Google Scholar] [CrossRef]
- Rcom-H’cheo-Gauthier, A.; Goodwin, J.; Pountney, D.L. Interactions between calcium and alpha-synuclein in neurodegeneration. Biomolecules 2014, 4, 795–811. [Google Scholar] [CrossRef]






| Antigen | Antibody | Dilution | Source | |
|---|---|---|---|---|
| Western Blot | IHC/IF | |||
| TH | Rabbit polyclonal | 1:500 | 1:100 | Santa Cruz |
| α-synuclein (C-20) | Rabbit polyclonal | 1:500 | N/A | Santa Cruz |
| 5G4 antibody | Mouse monoclonal | 1:1000 | 1:250 | Millipore |
| phosphorylated-α-synuclein (Ser129) | Rabbit polyclonal | 1:1000 | N/A | Abcam |
| Nitrated-α-synuclein (nTyr125+Tyr133) | Mouse monoclonal | 1:1000 | 1:200 | Thermo Fisher |
| β-actin | Mouse monoclonal | 1:5000 | N/A | Sigma |
| GFAP | Rabbit monoclonal | 1:1000 | 1:200 | Abcam |
| Caspase-1(14F468) | Mouse monoclonal | 1:500 | N/A | Santa Cruz |
| IL-1β (H-153) | Rabbit polyclonal | 1:500 | N/A | Santa Cruz |
| p-IKK-α/β (Ser176/180) | Rabbit monoclonal | 1:1000 | N/A | Cell signaling |
| IKK-α | Rabbit polyclonal | 1:1000 | N/A | Cell signaling |
| IKK-β (2C8) | Rabbit monoclonal | 1:1000 | N/A | Cell signaling |
| p-IkB-α (Ser32) | Rabbit monoclonal | 1:1000 | N/A | Cell signaling |
| IkB-α (L35A5) | Mouse monoclonal | 1:1000 | N/A | Cell signaling |
| NF-kB (F-6) | Mouse monoclonal | 1:500 | N/A | Santa Cruz |
| Antigen | Conjugation | Dilution | Source | |
|---|---|---|---|---|
| Western Blot | IHC/IF | |||
| Goat anti-rabbit IgG | HRP | 1:5000 | 1:350 | KPL |
| Mouse IgG | HRP | 1:5000 | 1:350 | KPL |
| Biotinylated Anti-Mouse IgG (H+L) Antibody | Biotin | 1:5000 | N/A | KPL |
| Biotinylated Anti-Rabbit IgG (H+L) Antibody | Biotin | 1:2000 | N/A | KPL |
| Peroxidase-labeled Streptavidin | HRP | 1:5000 | N/A | KPL |
| Donkey anti-mouse IgG | Alexa Fluor 488 | N/A | 1:400 | Life technologies |
| Donkey anti-rabbit IgG | Alexa Fluor 546 | N/A | 1:400 | Life technologies |
| Donkey anti-goat IgG | Alexa Fluor 488 | N/A | 1:400 | Life technologies |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heng, Y.; Li, Y.-Y.; Wen, L.; Yan, J.-Q.; Chen, N.-H.; Yuan, Y.-H. Gastric Enteric Glial Cells: A New Contributor to the Synucleinopathies in the MPTP-Induced Parkinsonism Mouse. Molecules 2022, 27, 7414. https://doi.org/10.3390/molecules27217414
Heng Y, Li Y-Y, Wen L, Yan J-Q, Chen N-H, Yuan Y-H. Gastric Enteric Glial Cells: A New Contributor to the Synucleinopathies in the MPTP-Induced Parkinsonism Mouse. Molecules. 2022; 27(21):7414. https://doi.org/10.3390/molecules27217414
Chicago/Turabian StyleHeng, Yang, Yan-Yan Li, Lu Wen, Jia-Qing Yan, Nai-Hong Chen, and Yu-He Yuan. 2022. "Gastric Enteric Glial Cells: A New Contributor to the Synucleinopathies in the MPTP-Induced Parkinsonism Mouse" Molecules 27, no. 21: 7414. https://doi.org/10.3390/molecules27217414
APA StyleHeng, Y., Li, Y.-Y., Wen, L., Yan, J.-Q., Chen, N.-H., & Yuan, Y.-H. (2022). Gastric Enteric Glial Cells: A New Contributor to the Synucleinopathies in the MPTP-Induced Parkinsonism Mouse. Molecules, 27(21), 7414. https://doi.org/10.3390/molecules27217414






