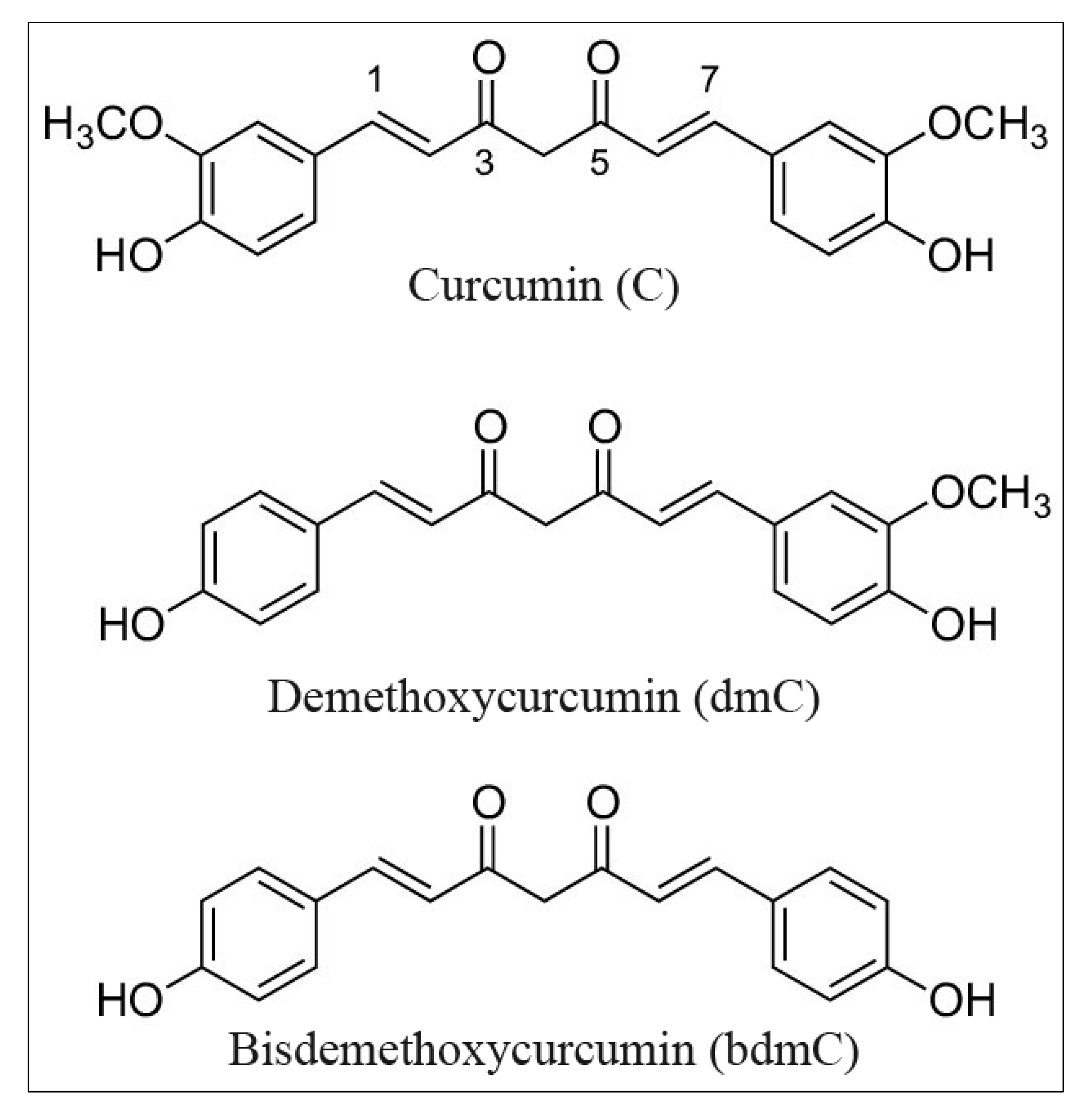
Curcumin and Its Derivatives Induce Apoptosis in Human Cancer Cells by Mobilizing and Redox Cycling Genomic Copper Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Stock Solutions of Curcumins
2.2. Isolation of Lymphocytes
2.3. Viability Assessment of Lymphocytes
2.4. Alkaline Single-Cell Gel Electrophoresis (Comet Assay)
2.5. Cell Lines and Reagents
2.6. Cell Growth Inhibition Studies by 3-(4,5-Dimethylthiazol-2-yl)-2,5 Diphenyltetra-Zolium (MTT) Assay
2.7. Detection of Apoptosis Using Histone/DNA ELISA
2.8. Soft Agar Colonization Assay
2.9. Cell Migration Assay
2.10. Real-Time Reverse Transcriptase PCR
2.11. Small Interfering RNA (siRNA) Transfection
2.12. Statistical Analysis
3. Results
3.1. DNA Breakage by Curcumin in Human Peripheral Lymphocytes
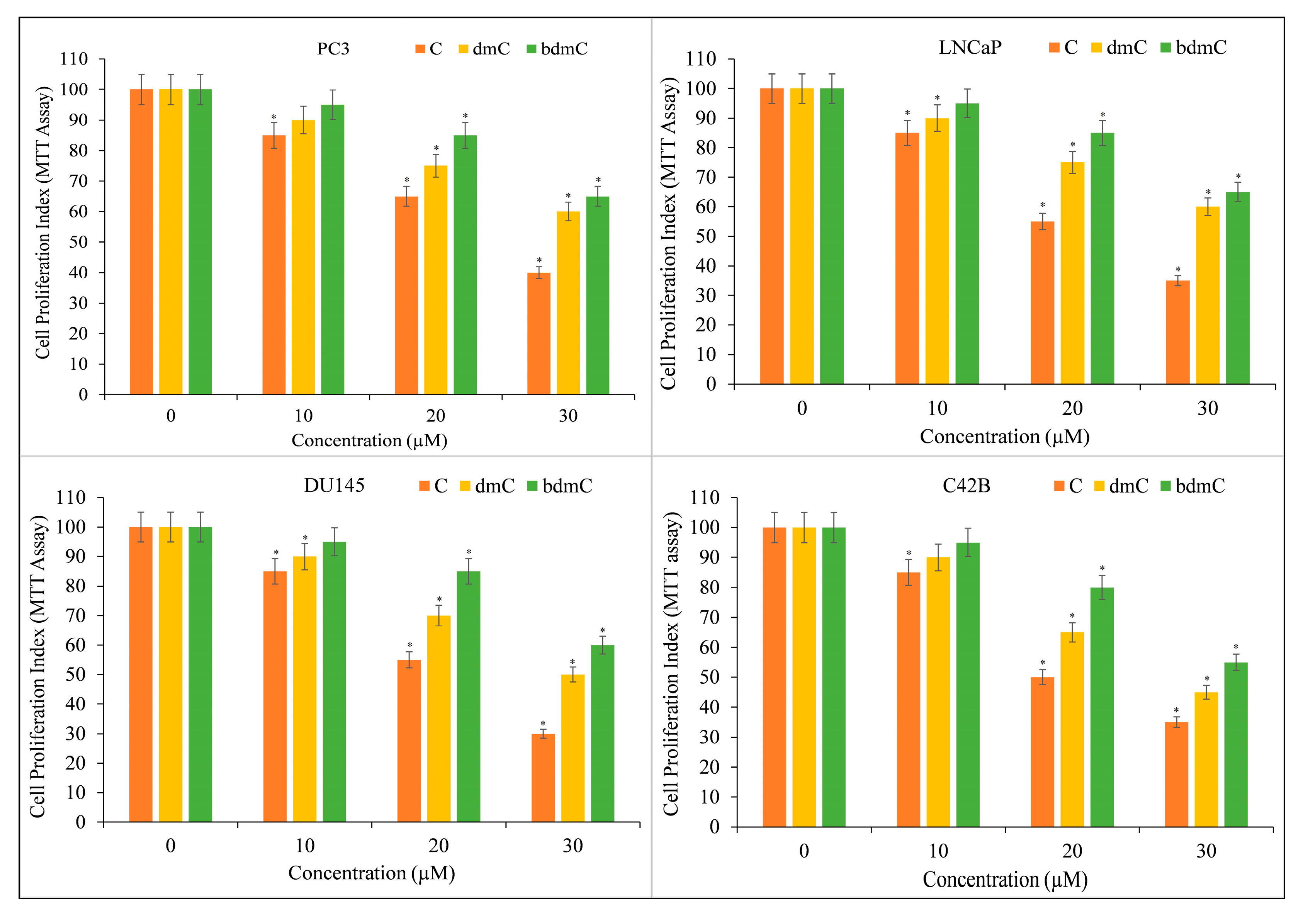
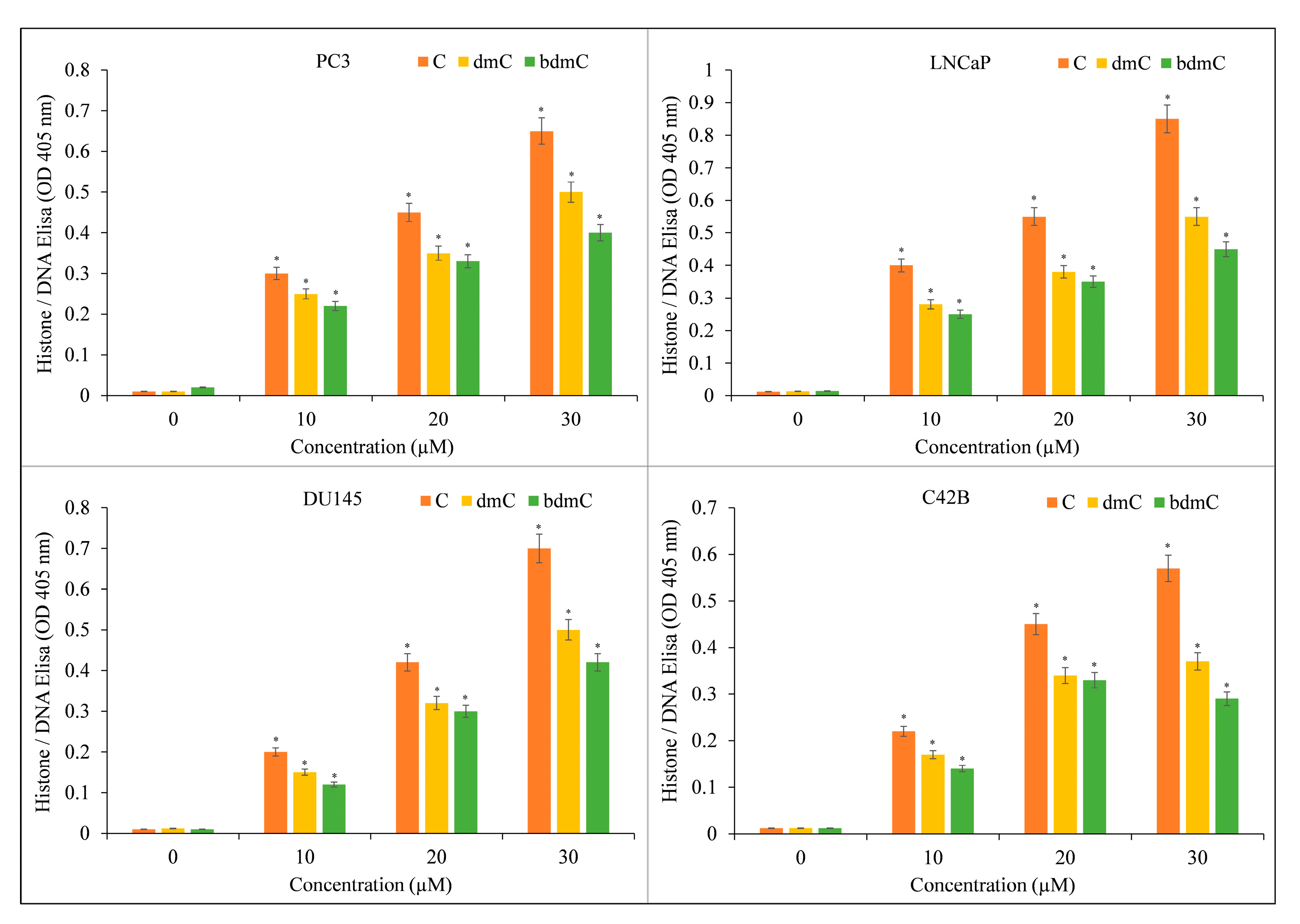
3.2. Curcumin Inhibits Growth and Induces Apoptosis in Different Types of Cancer Cells
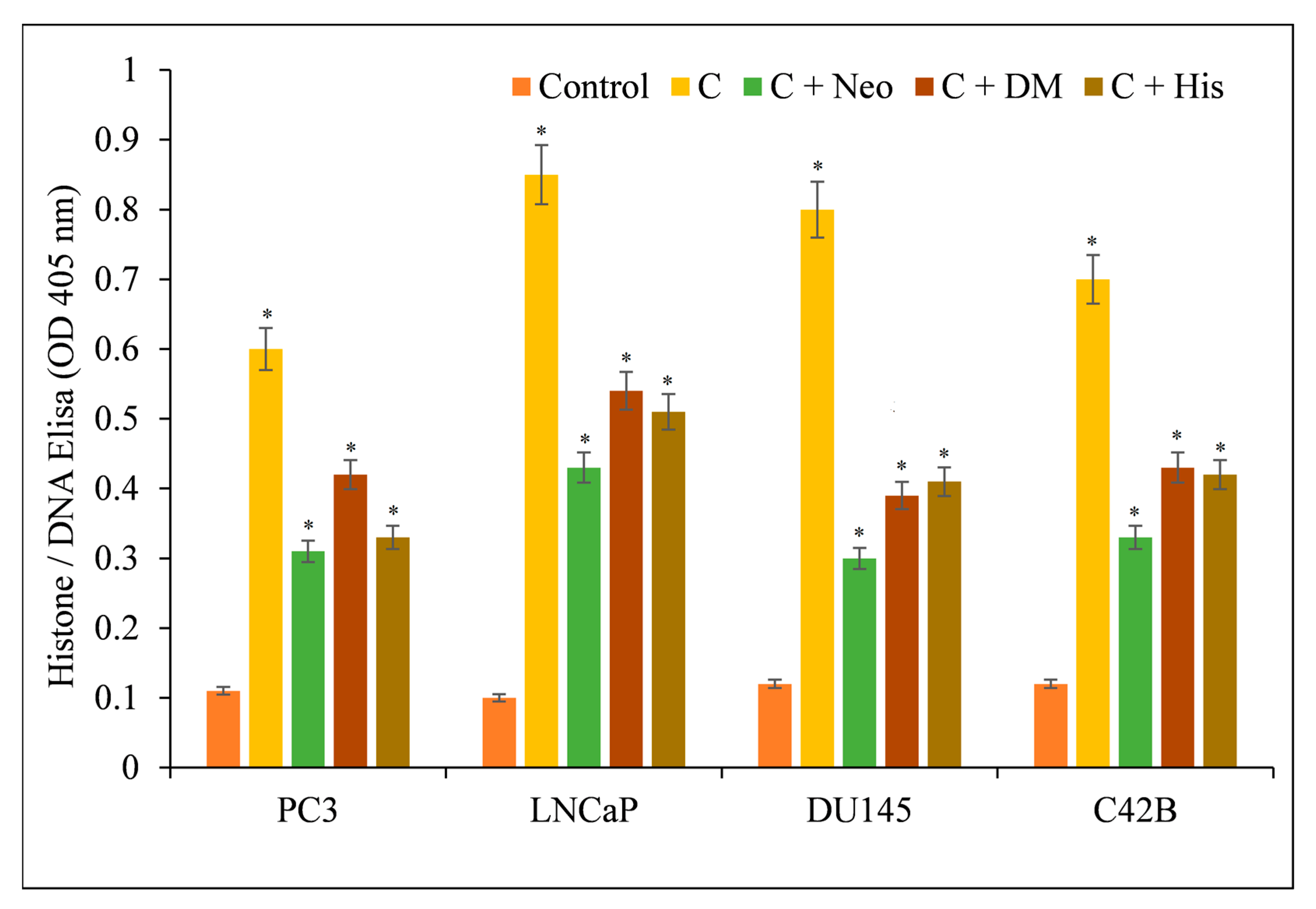
3.3. Curcumin-Induced Antiproliferation and Apoptosis in Cancer Cells Are Inhibited by a Cuprous Chelator but Not by Iron and Zinc Chelators
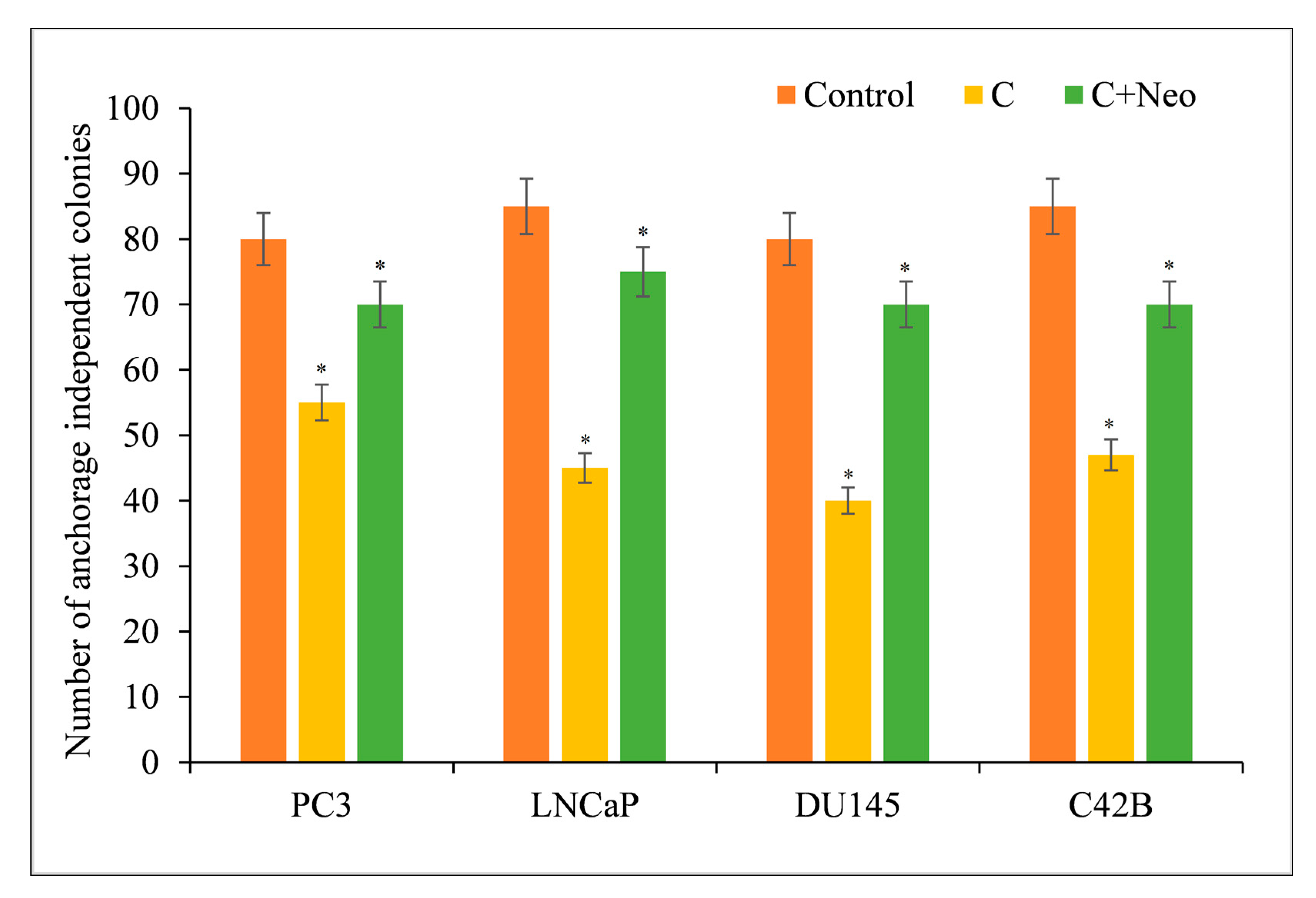
3.4. Curcumin Limits the Cancer Cell Proliferation in a Clonogenic Assay
3.5. Apoptosis of Cancer Cells Induced by Curcumin Is Mediated by ROS
3.6. Copper Chelation Reverses Curcumin-Inhibited Migration of Cancerous Cells
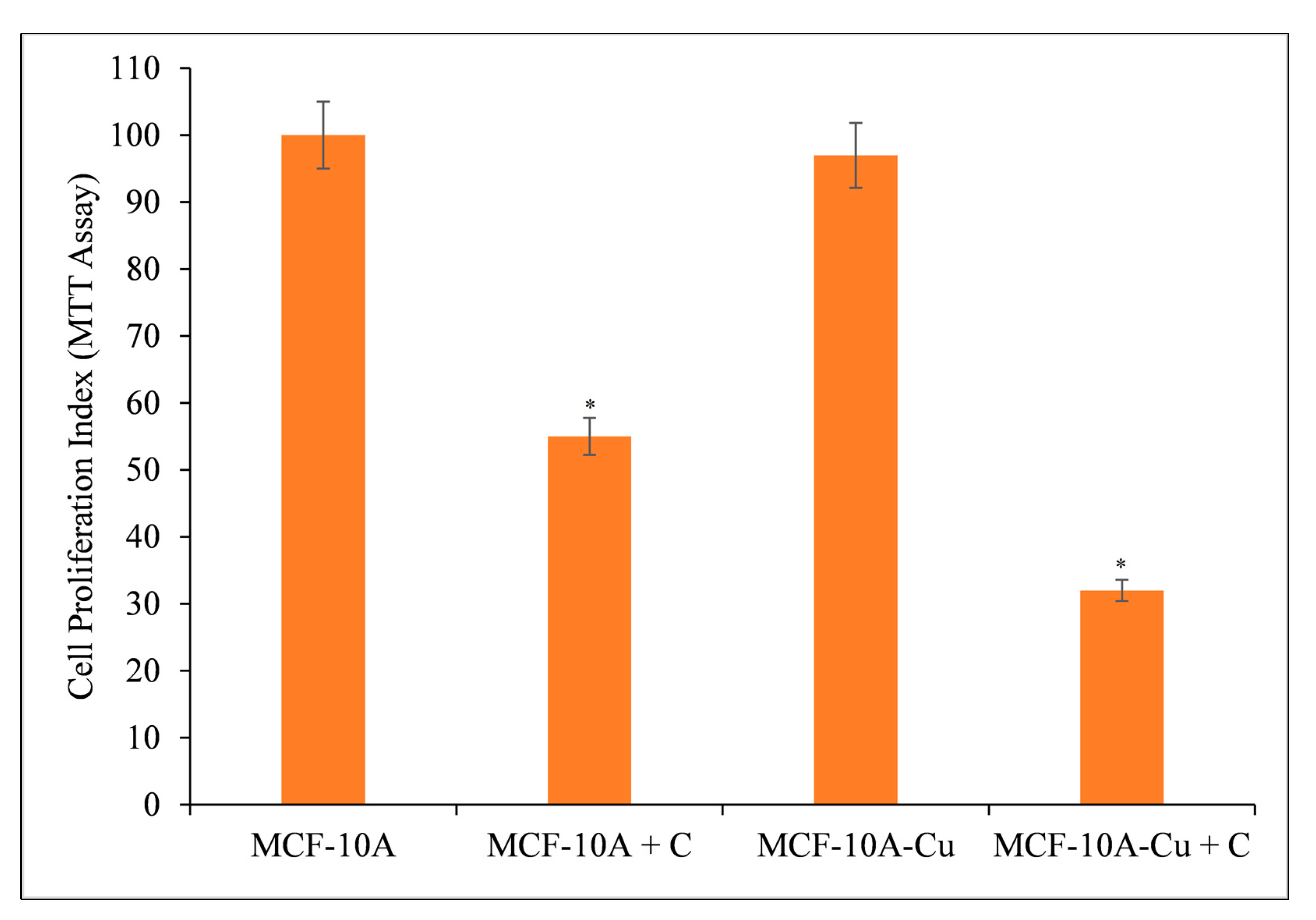
3.7. Copper Supplementation Increases the Sensitivity of Normal Breast Epithelial Cells to the Antiproliferative Effects of Curcumin
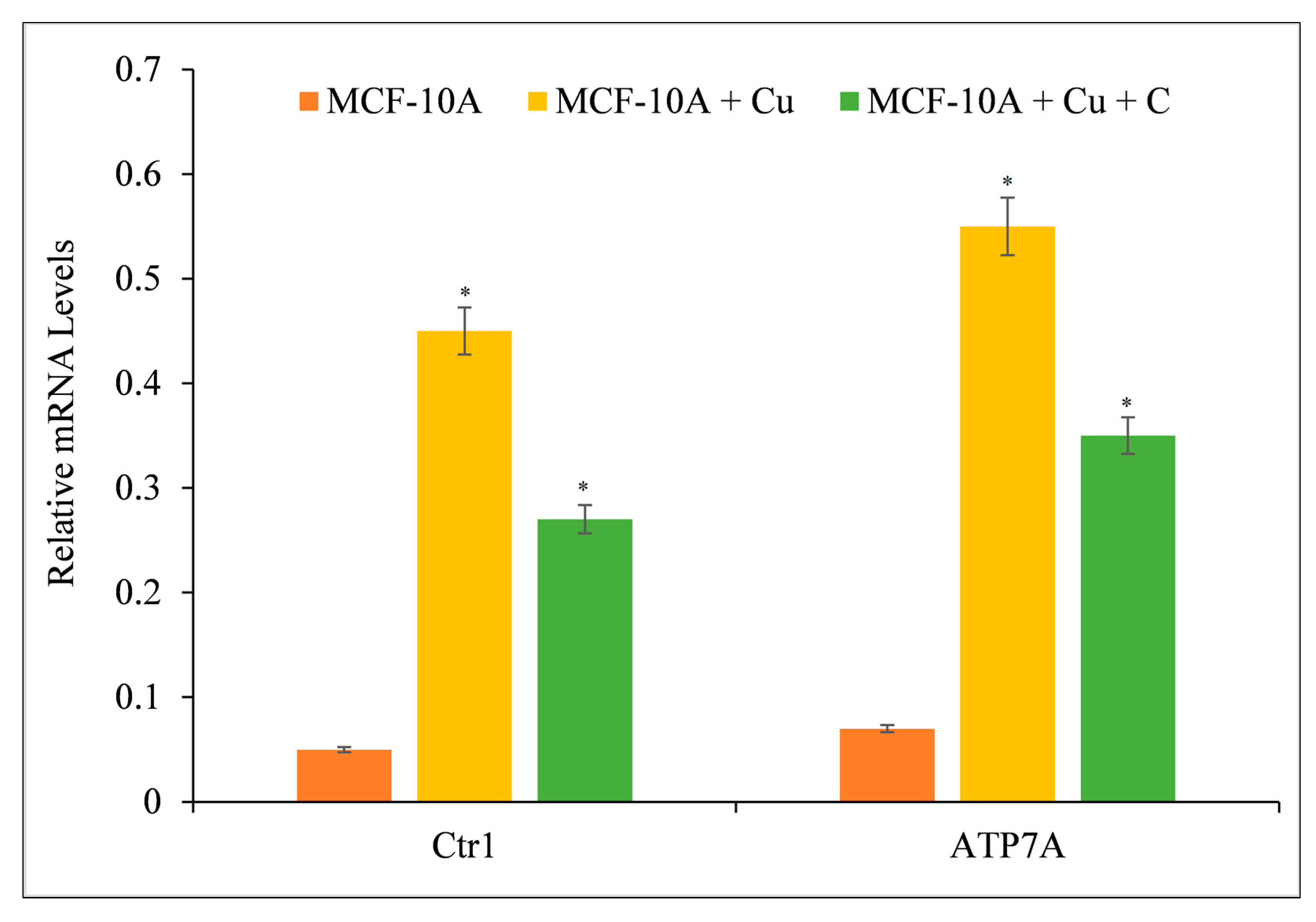
3.8. Curcumin Suppresses the Expression of Copper Transporters CTR1 and ATP7A
3.9. Targeted Silencing of CTR1 in MCF-10A Cells Cultured in Copper-Supplemented Media Reduces Curcumin’s Ability to Inhibit Cell Proliferation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Hafez Ghoran, S.; Calcaterra, A.; Abbasi, M.; Taktaz, F.; Nieselt, K.; Babaei, E. Curcumin-based nanoformulations: A promising adjuvant towards cancer treatment. Molecules 2022, 27, 5236. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-inflammatory effects of curcumin in the inflammatory diseases: Status, limitations and countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit. Complement. Med. 2017, 7, 339–346. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Druzga, A.; Katarzyna, J.; Skonieczna-Zydecka, K. Antioxidant potential of curcumin-a meta-analysis of randomized clinical trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Slavova-Kazakova, A.; Angelova, S.; Fabbri, D.; Antonietta Dettori, M.; Kancheva, V.D.; Delogu, G. Antioxidant properties of novel curcumin analogues: A combined experimental and computational study. J. Food Biochem. 2021, 45, e13584. [Google Scholar] [CrossRef]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Shankar, T.N.; Shantha, N.V.; Ramesh, H.P.; Murthy, I.A.; Murthy, V.S. Toxicity studies on turmeric (curcuma longa): Acute toxicity studies in rats, guineapigs & monkeys. Indian J. Exp. Biol. 1980, 18, 73–75. [Google Scholar]
- Soni, K.B.; Kuttan, R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J. Physiol. Pharmacol. 1992, 36, 273–275. [Google Scholar] [PubMed]
- Deshpande, S.S.; Maru, G.B. Effects of curcumin on the formation of benzo[a]pyrene derived DNA adducts in vitro. Cancer Lett. 1995, 96, 71–80. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, N.Y.; Son, J.Y.; Park, J.H.; Lee, S.H.; Kim, H.R.; Kim, B.; Kim, Y.G.; Jeong, H.G.; Lee, B.M.; et al. Curcumin ameliorates benzo[a]pyrene-induced DNA damages in stomach tissues of sprague-dawley rats. Int. J. Mol. Sci. 2019, 20, 5533. [Google Scholar] [CrossRef]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ros protection efficiency and free radical-scavenging activity of curcumin. PLoS ONE 2011, 6, e26012. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Atsumi, T.; Ishihara, M.; Kadoma, Y. Cytotoxicity, ros-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res. 2004, 24, 563–569. [Google Scholar]
- Lv, Z.D.; Liu, X.P.; Zhao, W.J.; Dong, Q.; Li, F.N.; Wang, H.B.; Kong, B. Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo. Int. J. Clin. Exp. Pathol. 2014, 7, 2818–2824. [Google Scholar]
- Khan, H.Y.; Zubair, H.; Faisal, M.; Ullah, M.F.; Farhan, M.; Sarkar, F.H.; Ahmad, A.; Hadi, S.M. Plant polyphenol induced cell death in human cancer cells involves mobilization of intracellular copper ions and reactive oxygen species generation: A mechanism for cancer chemopreventive action. Mol. Nutr. Food Res. 2014, 58, 437–446. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Ahmad, A.; Aatif, M.; Alam, M.W.; Hadi, S.M. Structure of Some Green Tea Catechins and the Availability of Intracellular Copper Influence Their Ability to Cause Selective Oxidative DNA Damage in Malignant Cells. Biomedicines 2022, 10, 664. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Ali, F.; Ahmad, A.; Aatif, M.; Malik, A.; Alam, M.W.; Muteeb, G.; Ahmad, S.; Noor, A.; et al. Pomegranate juice anthocyanidins induce cell death in human cancer cells by mobilizing intracellular copper ions and producing reactive oxygen species. Front. Oncol. 2022, 12, 998346. [Google Scholar] [CrossRef]
- Shamsi, F.A.; Hadi, S.M. Photoinduction of strand scission in DNA by uric acid and cu(ii). Free Radic. Biol. Med. 1995, 19, 189–196. [Google Scholar] [CrossRef]
- Arif, H.; Rehmani, N.; Farhan, M.; Ahmad, A.; Hadi, S.M. Mobilization of copper ions by flavonoids in human peripheral lymphocytes leads to oxidative DNA breakage: A structure activity study. Int. J. Mol. Sci. 2015, 16, 26754–26769. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Zafar, A.; Chibber, S.; Khan, H.Y.; Arif, H.; Hadi, S.M. Mobilization of copper ions in human peripheral lymphocytes by catechins leading to oxidative DNA breakage: A structure activity study. Arch. Biochem. Biophys. 2015, 580, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Hadi, S.M. DNA breakage by tannic acid and Cu(II): Generation of active oxygen species and biological activity of the reaction. Mutat. Res. 1994, 313, 49–55. [Google Scholar] [CrossRef]
- Ahsan, H.; Hadi, S.M. Strand scission in DNA induced by curcumin in the presence of Cu(II). Cancer Lett. 1998, 124, 23–30. [Google Scholar] [CrossRef]
- Kagawa, T.F.; Geierstanger, B.H.; Wang, A.H.; Ho, P.S. Covalent modification of guanine bases in double-stranded DNA. The 1.2-a z-DNA structure of d(cgcgcg) in the presence of cucl2. J. Biol. Chem. 1991, 266, 20175–20184. [Google Scholar] [CrossRef]
- Geierstanger, B.H.; Kagawa, T.F.; Chen, S.L.; Quigley, G.J.; Ho, P.S. Base-specific binding of copper(ii) to z-DNA. The 1.3-a single crystal structure of d(m5cguam5cg) in the presence of cucl2. J. Biol. Chem. 1991, 266, 20185–20191. [Google Scholar] [CrossRef]
- Kaiafa, G.D.; Saouli, Z.; Diamantidis, M.D.; Kontoninas, Z.; Voulgaridou, V.; Raptaki, M.; Arampatzi, S.; Chatzidimitriou, M.; Perifanis, V. Copper levels in patients with hematological malignancies. Eur. J. Intern. Med. 2012, 23, 738–741. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef]
- Rizvi, A.; Furkan, M.; Naseem, I. Physiological serum copper concentrations found in malignancies cause unfolding induced aggregation of human serum albumin in vitro. Arch. Biochem. Biophys. 2017, 636, 71–78. [Google Scholar] [CrossRef]
- Gali, H.U.; Perchellet, E.M.; Klish, D.S.; Johnson, J.M.; Perchellet, J.P. Hydrolyzable tannins: Potent inhibitors of hydroperoxide production and tumor promotion in mouse skin treated with 12-O-tetradecanoylphorbol-13-acetate in vivo. Int J Cancer. 1992, 51, 425–432. [Google Scholar] [CrossRef]
- Hadi, S.M.; Asad, S.F.; Singh, S.; Ahmad, A. Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life 2000, 50, 167–171. [Google Scholar]
- Inoue, M.; Suzuki, R.; Koide, T.; Sakaguchi, N.; Ogihara, Y.; Yabu, Y. Antioxidant, gallic acid induces apoptosis in HL 60 r cells. Biochem Biophys Res Commun. 1994, 204, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chen, L.G.; Lee, L.T.; Yang, L.L. Effects of 6-gingerol, an antioxidant from ginger, on inducing apoptosis in human leukemic hl-60 cells. In Vivo 2003, 17, 641–645. [Google Scholar] [PubMed]
- Hadi, S.M.; Bhat, S.H.; Azmi, A.S.; Hanif, S.; Shamim, U.; Ullah, M.F. Oxidative breakage of cellular DNA by plant polyphenols: A putative mechanism for anticancer properties. Semin. Cancer Biol. 2007, 17, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Pool-Zobel, B.L.; Guigas, C.; Klein, R.; Neudecker, C.; Renner, H.W.; Schmezer, P. Assessment of genotoxic effects by lindane. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1993, 31, 271–283. [Google Scholar] [CrossRef]
- Ullah, M.F.; Shamim, U.; Hanif, S.; Azmi, A.S.; Hadi, S.M. Cellular DNA breakage by soy isoflavone genistein and its methylated structural analogue biochanin A. Mol. Nutr. Food Res. 2009, 53, 1376–1385. [Google Scholar] [CrossRef]
- Zubair, H.; Khan, H.Y.; Ullah, M.F.; Ahmad, A.; Wu, D.; Hadi, S.M. Apogossypolone, derivative of gossypol, mobilizes endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage. Eur. J. Pharm. Sci. 2012, 47, 280–286. [Google Scholar] [CrossRef]
- Gao, C.; Zhu, L.; Zhu, F.; Sun, J.; Zhu, Z. Effects of different sources of copper on ctr1, atp7a, atp7b, mt and dmt1 protein and gene expression in caco-2 cells. J. Trace Elem. Med. Biol. 2014, 28, 344–350. [Google Scholar] [CrossRef]
- Ahmad, A.; Maitah, M.Y.; Ginnebaugh, K.R.; Li, Y.; Bao, B.; Gadgeel, S.M.; Sarkar, F.H. Inhibition of hedgehog signaling sensitizes nsclc cells to standard therapies through modulation of emt-regulating mirnas. J. Hematol. Oncol. 2013, 6, 77. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Bryan, S.E. Metal Ions in Biological Systems; Marcel Dekker: New York, NY, USA, 1979. [Google Scholar]
- Farhan, M.; Khan, H.Y.; Oves, M.; Al-Harrasi, A.; Rehmani, N.; Arif, H.; Hadi, S.M.; Ahmad, A. Cancer therapy by catechins involves redox cycling of copper ions and generation of reactive oxygen species. Toxins 2016, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Farhan, M.; Naseem, I.; Hadi, S.M. Calcitriol-copper interaction leads to non enzymatic, reactive oxygen species mediated DNA breakage and modulation of cellular redox scavengers in hepatocellular carcinoma. Apoptosis 2016, 21, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; DeNicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S.; et al. Connecting copper and cancer: From transition metal signalling to metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, V.; Jasmer-McDonald, K.; Zhu, S.; Martin, A.L.; Gudekar, N.; Khan, A.; Ladomersky, E.; Singh, K.; Weisman, G.A.; Petris, M.J. Atp7a delivers copper to the lysyl oxidase family of enzymes and promotes tumorigenesis and metastasis. Proc. Natl. Acad. Sci. USA 2019, 116, 6836–6841. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Ramesh, V.; Nagaraja, V.; Parish, J.H.; Hadi, S.M. Mode of binding of quercetin to DNA. Mutagenesis 1994, 9, 193–197. [Google Scholar] [CrossRef]
- Singh, S.; Asad, S.F.; Ahmad, A.; Khan, N.U.; Hadi, S.M. Oxidative DNA damage by capsaicin and dihydrocapsaicin in the presence of cu(ii). Cancer Lett. 2001, 169, 139–146. [Google Scholar] [CrossRef]
- Ahmad, A.; Farhan Asad, S.; Singh, S.; Hadi, S.M. DNA breakage by resveratrol and cu(ii): Reaction mechanism and bacteriophage inactivation. Cancer Lett. 2000, 154, 29–37. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: Implications for anticancer properties. Toxicol. In Vitro 2004, 18, 555–561. [Google Scholar] [CrossRef]
- Ahsan, H.; Parveen, N.; Khan, N.U.; Hadi, S.M. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem.-Biol. Interact. 1999, 121, 161–175. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper that cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Naseem, I.; Hadi, S.M.; Ahmad, A. Targeting increased copper levels in diethylnitrosamine induced hepatocellular carcinoma cells in rats by epigallocatechin-3-gallate. Tumour Biol. 2015, 36, 8861–8867. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ji, C.; Mayfield, J.E.; Goel, A.; Xiao, J.; Dixon, J.E.; Guo, X. Ancient drug curcumin impedes 26s proteasome activity by direct inhibition of dual-specificity tyrosine-regulated kinase 2. Proc. Natl. Acad. Sci. USA 2018, 115, 8155–8160. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Rizvi, A. Understanding the prooxidant action of plant polyphenols in the cellular microenvironment of malignant cells: Role of copper and therapeutic implications. Front. Pharmacol. 2022, 13, 929853. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| CTR1 | GCT GGA AGA AGG CAG TGG TA | AAA GAG GAG CAA GAA GGG ATG |
| ATP7A | ACG AAT GAG CCG TTG GTA GTA | CCT CCT TGT CTT GAA CTG GTG |
| GADPH | TGG GTG TGA ACC ATG AGA AGT | TGA GTC CTT CCA CGA TAC CAA |
| Cancer Cell Line | Dose | Apoptosis (Folds) | Effect of Scavengers |
|---|---|---|---|
| PC3 | Untreated | - | - |
| Curcumin (25 µM) | 2.54 | - | |
| Thiourea | 1.58 | 37.79 | |
| Catalase | 2.03 | 20.07 | |
| SOD | 1.77 | 30.31 | |
| LNCaP | Untreated | - | - |
| Curcumin (25 µM) | 3.67 | - | |
| Thiourea | 1.84 | 49.86 | |
| Catalase | 2.01 | 45.23 | |
| SOD | 1.94 | 47.13 | |
| DU145 | Untreated | - | - |
| Curcumin (25 µM) | 3.46 | - | |
| Thiourea | 1.78 | 48.55 | |
| Catalase | 2.13 | 38.43 | |
| SOD | 1.97 | 43.06 | |
| C42B | Untreated | - | - |
| Curcumin (25 µM) | 3.10 | - | |
| Thiourea | 2.17 | 30.00 | |
| Catalase | 2.69 | 13.22 | |
| SOD | 2.51 | 19.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhasawi, M.A.I.; Aatif, M.; Muteeb, G.; Alam, M.W.; Oirdi, M.E.; Farhan, M. Curcumin and Its Derivatives Induce Apoptosis in Human Cancer Cells by Mobilizing and Redox Cycling Genomic Copper Ions. Molecules 2022, 27, 7410. https://doi.org/10.3390/molecules27217410
Alhasawi MAI, Aatif M, Muteeb G, Alam MW, Oirdi ME, Farhan M. Curcumin and Its Derivatives Induce Apoptosis in Human Cancer Cells by Mobilizing and Redox Cycling Genomic Copper Ions. Molecules. 2022; 27(21):7410. https://doi.org/10.3390/molecules27217410
Chicago/Turabian StyleAlhasawi, Mohammed Ahmed Ismail, Mohammad Aatif, Ghazala Muteeb, Mir Waqas Alam, Mohamed El Oirdi, and Mohd Farhan. 2022. "Curcumin and Its Derivatives Induce Apoptosis in Human Cancer Cells by Mobilizing and Redox Cycling Genomic Copper Ions" Molecules 27, no. 21: 7410. https://doi.org/10.3390/molecules27217410
APA StyleAlhasawi, M. A. I., Aatif, M., Muteeb, G., Alam, M. W., Oirdi, M. E., & Farhan, M. (2022). Curcumin and Its Derivatives Induce Apoptosis in Human Cancer Cells by Mobilizing and Redox Cycling Genomic Copper Ions. Molecules, 27(21), 7410. https://doi.org/10.3390/molecules27217410











