WWOX Modulates ROS-Dependent Senescence in Bladder Cancer
Abstract
1. Introduction
2. Results
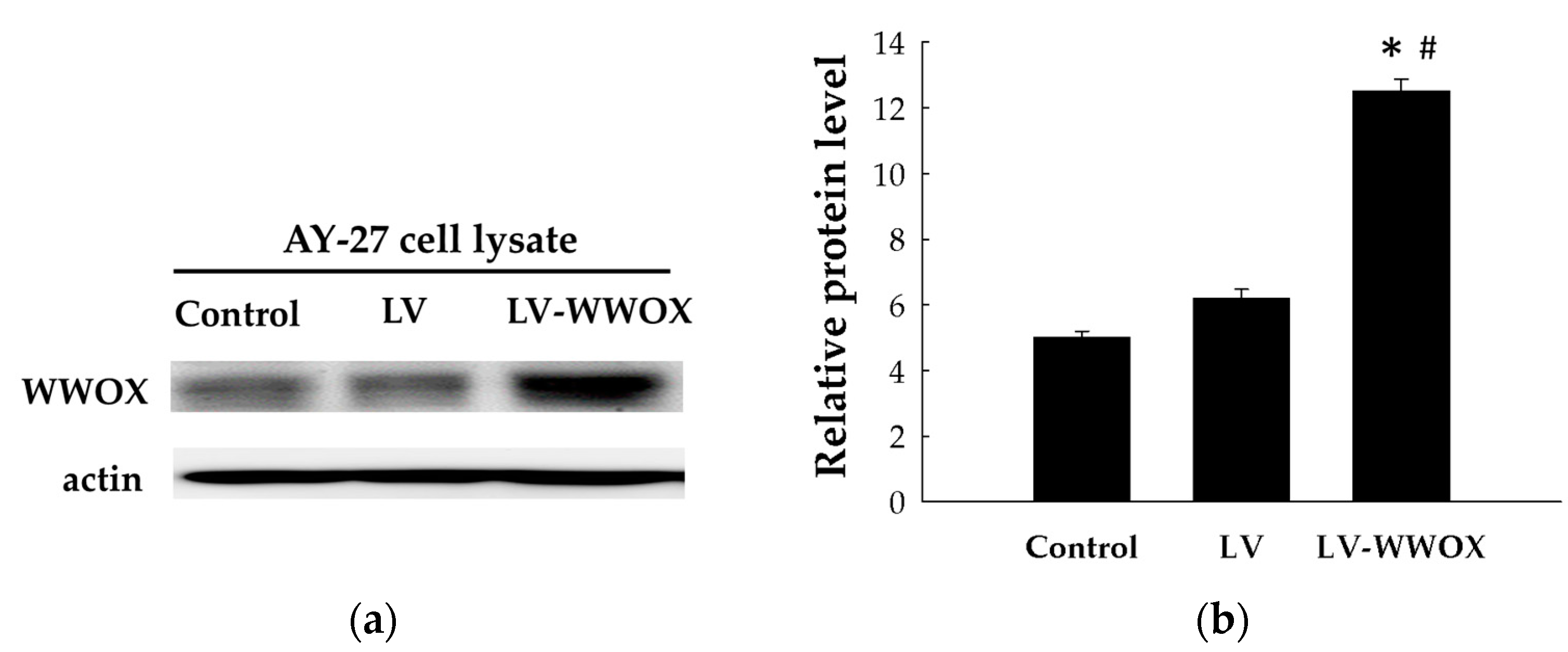
2.1. Establishment of Cell Lines Overexpressing WWOX
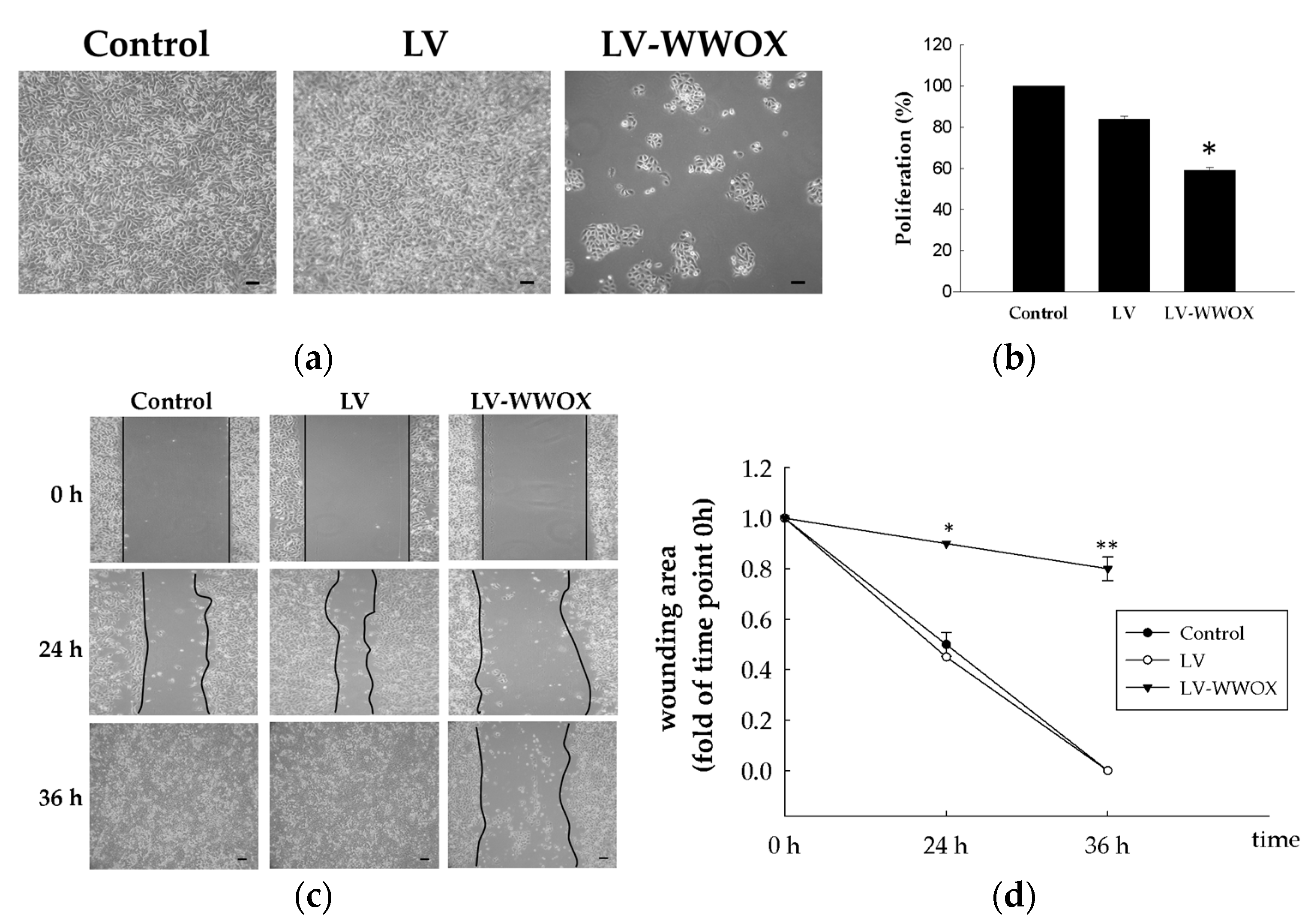
2.2. WWOX Suppressed the Proliferation and Migration of Bladder Cancer Cells
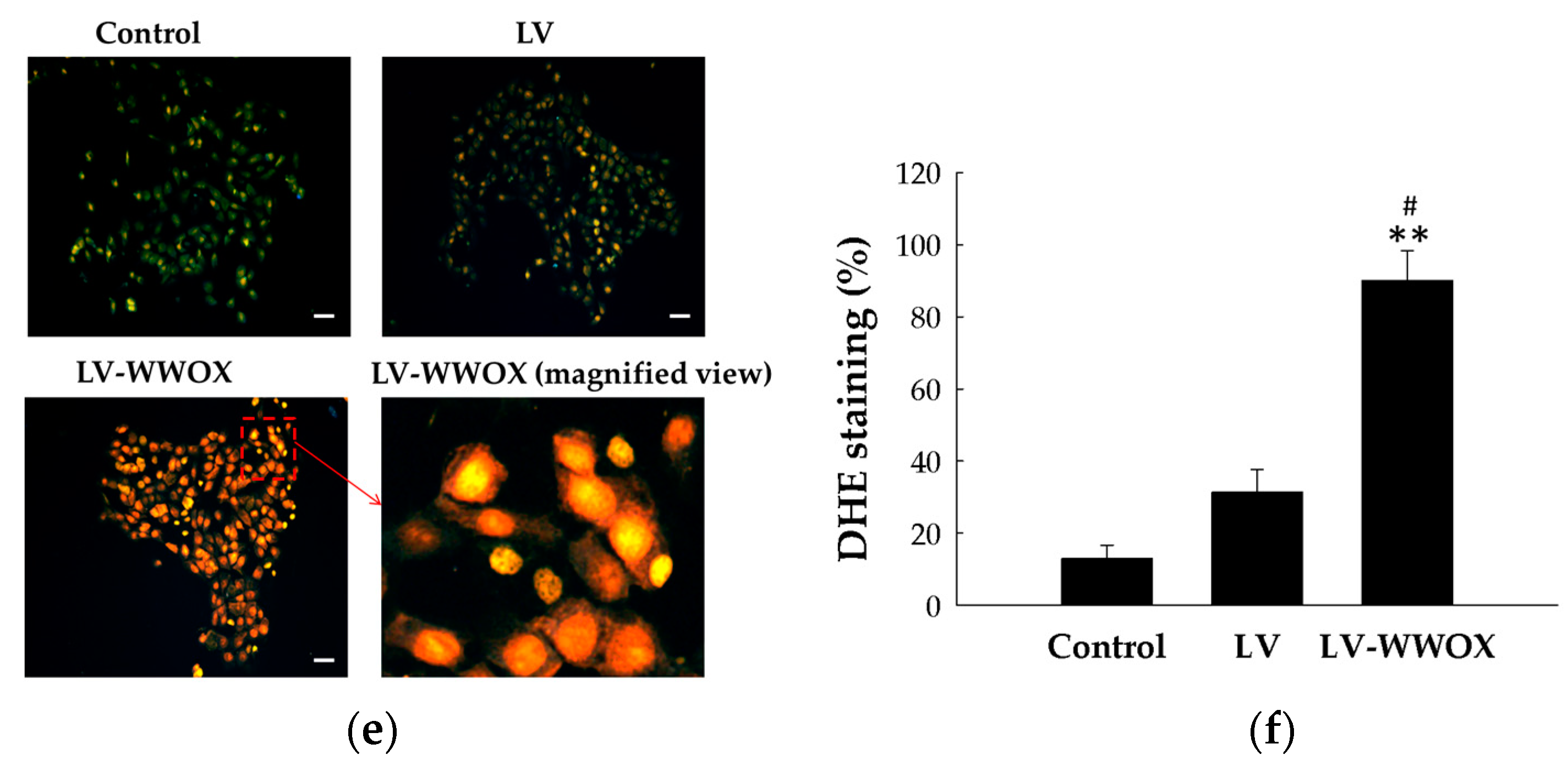
2.3. Overexpression of WWOX Increased the TNF and ROS Activity
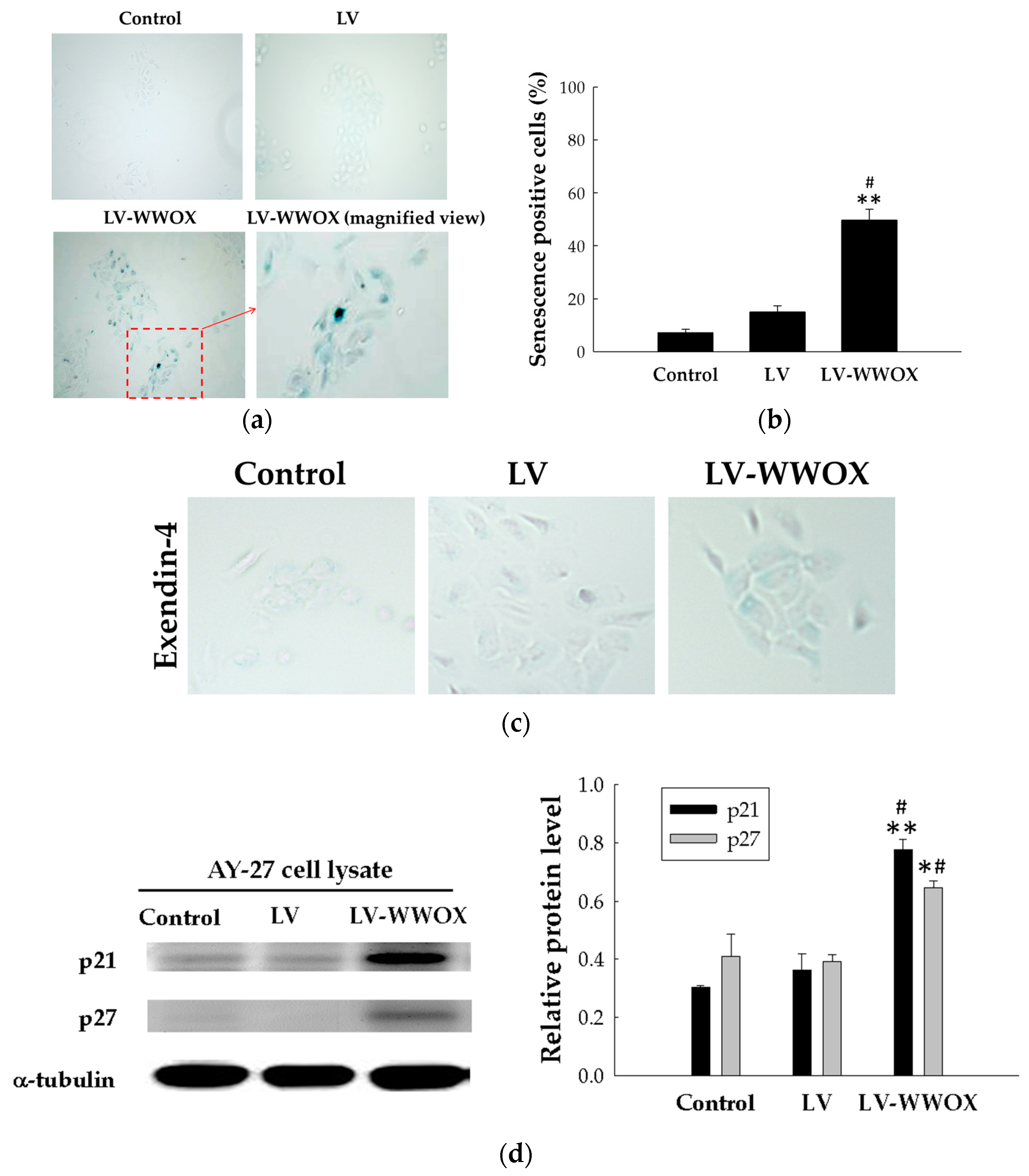
2.4. WWOX Induced Premature Senescence in the Bladder Cancer Cells
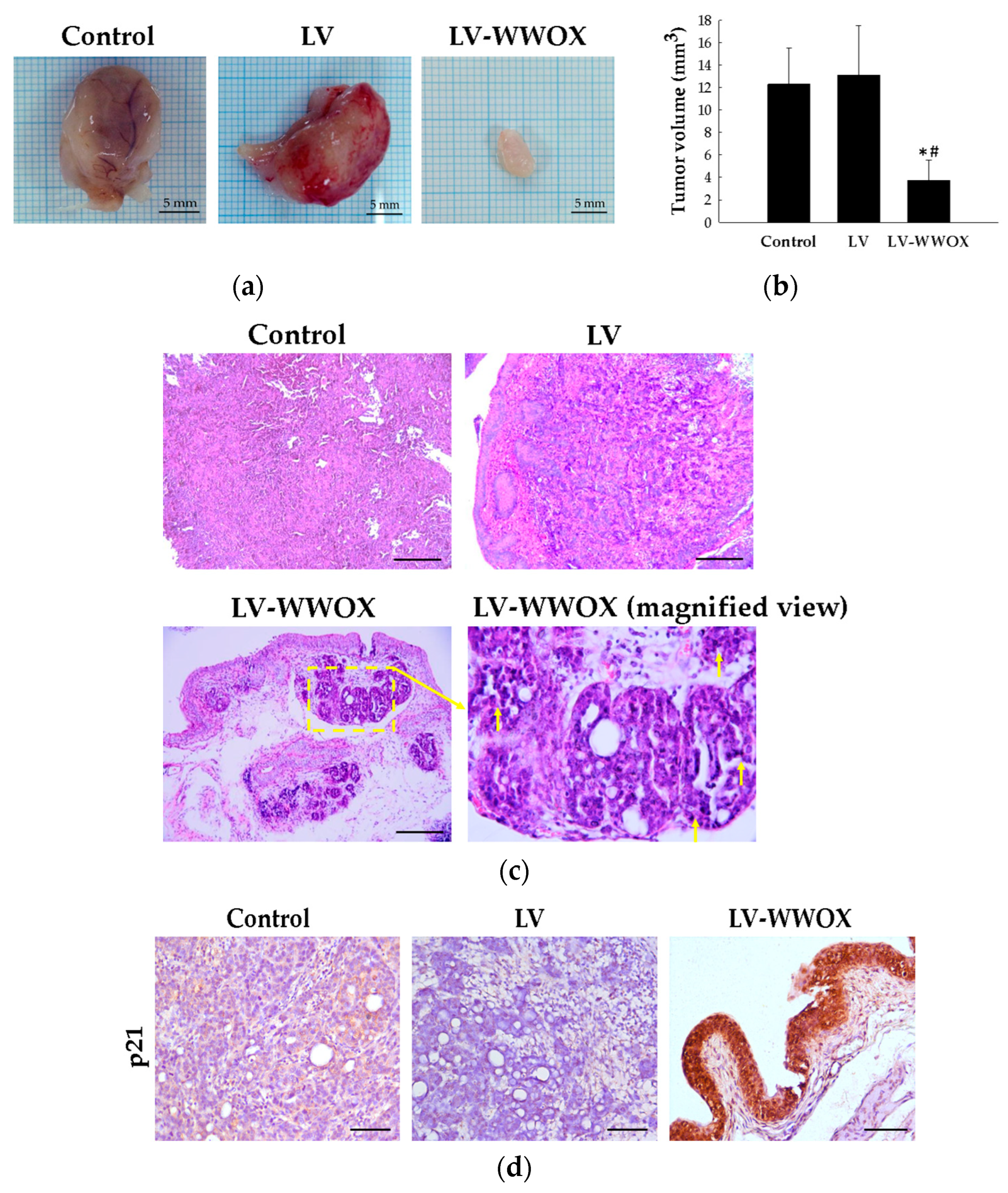
2.5. WWOX Suppressed Bladder Cancer Growth in the F344/AY-27 Rat Model
3. Discussion
4. Materials and Methods
4.1. Virus Production and Incorporation with LV-WWOX
4.2. Lentivirus Release
4.3. Cell Culture and Cytotoxic Assay
4.4. Cell Migration Test
4.5. Immunocytochemistry and Western Blotting
4.6. Measurement of Intracellular ROS
4.7. Senescence-Associated β-Galactosidase Assay (SA-β-Gal)
4.8. Ethics Statement and In Vivo Urothelium Permeability and Histologic Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Richard Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma in Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.L.; Xia, Q.D.; Lu, Y.H.; Liu, Z.; Zhou, P.; Hu, H.L.; Wang, S.G. Efficacy of intravesical therapies on the prevention of recurrence and progression of non-muscle-invasive bladder cancer: A systematic review and network meta-analysis. Cancer Med. 2020, 9, 7800–7809. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, J.; Dai, R.; Wu, S. Current status and future perspectives of immunotherapy in bladder cancer treatment. Sci. China Life Sci. 2021, 64, 512–533. [Google Scholar] [CrossRef] [PubMed]
- Pospiech, K.; Płuciennik, E.; Bednarek, A.K. WWOX Tumor Suppressor Gene in Breast Cancer, a Historical Perspective and Future Directions. Front. Oncol. 2018, 8, 345. [Google Scholar] [CrossRef]
- Lee, H.L.; Cheng, H.L.; Liu, Y.F.; Chou, M.C.; Yang, S.F.; Chou, Y.E. Functional genetic variant of WW domain-containing oxidoreductase (WWOX) gene is associated with hepatocellular carcinoma risk. PLoS ONE 2017, 12, e0176141. [Google Scholar] [CrossRef] [PubMed]
- Traczyk-Borszynska, M.; Borkowska, E.; Jablonowski, Z.; Jedrzejczyk, A.; Pietrusinski, M.; Kaluzewski, B.; Sosnowski, M.; Borowiec, M. Genetic diversity of urinary bladder cancer and the risk of recurrence based on mutation analysis. Neoplasma 2016, 63, 952–960. [Google Scholar] [CrossRef]
- O′Keefe, L.V.; Lee, C.S.; Choo, A.; Richards, R.I. Tumor Suppressor WWOX Contributes to the Elimination of Tumorigenic Cells in Drosophila melanogaster. PLoS ONE 2015, 10, e0136356. [Google Scholar] [CrossRef]
- Zhong, G.; Qin, S.; Townsend, D.; Schulte, B.A.; Tew, K.D.; Wang, G.Y. Oxidative stress induces senescence in breast cancer stem cells. Biochem. Biophys. Res. Commun. 2019, 514, 1204–1209. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Z.; Zhong, J.; Zhou, L.; Chen, J.; Zheng, L.; Li, Z.; Zhang, R.; Pan, J.; Wu, Y.; et al. Downregulation of HINFP induces senescence-associated secretory phenotype to promote metastasis in a non-cell-autonomous manner in bladder cancer. Oncogene 2022, 41, 3587–3598. [Google Scholar] [CrossRef]
- Yang, J.; Liu, M.; Hong, D.; Zeng, M.; Zhang, X. The Paradoxical Role of Cellular Senescence in Cancer. Front. Cell Dev. Biol. 2021, 9, 722205. [Google Scholar] [CrossRef]
- O′Keefe, L.V.; Colella, A.; Dayan, S.; Chen, Q.; Choo, A.; Jacob, R.; Price, G.; Venter, D.; Richards, R.I. Drosophila orthologue of WWOX, the chromosomal fragile site FRA16D tumour suppressor gene, functions in aerobic metabolism and regulates reactive oxygen species. Hum. Mol. Genet. 2011, 20, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.D. Healing and hurting: Molecular mechanisms, functions, and pathologies of cellular senescence. Mol. Cell. 2009, 36, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Płuciennik, E.; Nowakowska, M.; Stępien, A.; Wołkowicz, M.; Stawińsk, A.; Różański, W.; Lipiński, M.; Bednarek, A.K. Alternating expression levels of WWOX tumor suppressor and cancer-related genes in patientswith bladder cancer. Oncol. Lett. 2014, 8, 2291–2297. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zu, C.; Qin, G.; Yang, C.; Liu, N.; He, A.; Zhang, M.; Zheng, X. Low dose Emodin induces tumor senescence for boosting breast cancer chemotherapy via silencing NRARP. Biochem. Biophys. Res. Commun. 2018, 505, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sun, L.; Mu, Z.; Huang, Y.; Fu, C.; Hu, B. Ectopic WWOX Expression Inhibits Growth of 5637 Bladder Cancer Cell In Vitro and In Vivo. Cell Biochem. Biophys. 2015, 73, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Bajpai, P.; Siddiqui, M.H.; Sayyed, U.; Tiwari, R.; Shekh, R.; Mishra, K.; Kapoor, V.K. Elucidation of the Chemopreventive Role of Stigmasterol Against Jab1 in Gall Bladder Carcinoma. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 826–837. [Google Scholar] [CrossRef]
- Pandey, P.; Siddiqui, M.H.; Behari, A.; Kapoor, V.K.; Mishra, K.; Sayyed, U.; Tiwari, R.K.; Shekh, R.; Bajpai, P. Jab1-siRNA Induces Cell Growth Inhibition and Cell Cycle Arrest in Gall Bladder Cancer Cells via Targeting Jab1 Signalosome. Anticancer. Agents Med. Chem. 2019, 19, 2019–2033. [Google Scholar] [CrossRef]
- Lee, C.S.; Choo, A.; Dayan, S.; Richards, R.I.; O’Keefe, L.V. Molecular Biology of the WWOX Gene That Spans Chromosomal Fragile Site FRA16D. Cells 2021, 10, 1637. [Google Scholar] [CrossRef]
- Gao, G.; Smith, D.I. WWOX, large common fragile site genes, and cancer. Exp. Biol. Med. 2015, 240, 285–295. [Google Scholar] [CrossRef]
- Chen, S.J.; Huang, S.S.; Chang, N. Role of WWOX and NF-κB in lung cancer progression. Transl. Respir. Med. 2013, 1, 15. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, C.; Sun, A.; Qiao, L.; Zhang, X.; Huang, J.; Sun, X.; Yang, X.; Sun, S. Hydrogen alleviates cellular senescence via regulation of ROS/p53/p21 pathway in bone marrow-derived mesenchymal stem cells in vivo. Biomed. Pharmacother. 2018, 106, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Boehm, B.E.; Svatek, R.S. Novel therapeutic approaches for recurrent nonmuscle invasive bladder cancer. Urol. Clin. N. Am. 2015, 42, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Wu, Y.T.; Lin, K.J.; Yu, T.J.; Kuo, Y.L.; Chang, L.C. A Hydrogel-Based Epirubicin Delivery System for Intravesical Chemotherapy. Molecules 2016, 21, 712. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Chang, L.C.; Lin, K.J.; Yu, T.J.; Tsai, C.C.; Wang, H.K.; Tsai, T.R. Preparation and characterization of gelatin-based mucoadhesive nanocomposites as intravesical gene delivery scaffolds. Biomed. Res. Int. 2014, 2014, 473823. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Yue, P.Y.K.; Leung, E.P.Y.; Mak, N.K.; Wong, R.N.S. A simplified method for quantifying cell migration/wound healing in 96-well plates. J. Biomol. Screen. 2010, 15, 427–433. [Google Scholar] [CrossRef]
- Padmasekar, M.; Lingwal, N.; Samikannu, B.; Chen, C.; Sauer, H.; Linn, T. Exendin-4 protects hypoxic islets from oxidative stress and improves islet transplantation outcome. Endocrinology 2013, 154, 1424–1433. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-W.; Chen, P.-H.; Yu, T.-J.; Lin, K.-J.; Chang, L.-C. WWOX Modulates ROS-Dependent Senescence in Bladder Cancer. Molecules 2022, 27, 7388. https://doi.org/10.3390/molecules27217388
Liu C-W, Chen P-H, Yu T-J, Lin K-J, Chang L-C. WWOX Modulates ROS-Dependent Senescence in Bladder Cancer. Molecules. 2022; 27(21):7388. https://doi.org/10.3390/molecules27217388
Chicago/Turabian StyleLiu, Ching-Wen, Po-Hen Chen, Tsan-Jung Yu, Kai-Jen Lin, and Li-Ching Chang. 2022. "WWOX Modulates ROS-Dependent Senescence in Bladder Cancer" Molecules 27, no. 21: 7388. https://doi.org/10.3390/molecules27217388
APA StyleLiu, C.-W., Chen, P.-H., Yu, T.-J., Lin, K.-J., & Chang, L.-C. (2022). WWOX Modulates ROS-Dependent Senescence in Bladder Cancer. Molecules, 27(21), 7388. https://doi.org/10.3390/molecules27217388






