The Role of Hypoxia-Inducible Factor-1 Alpha in Renal Disease
Abstract
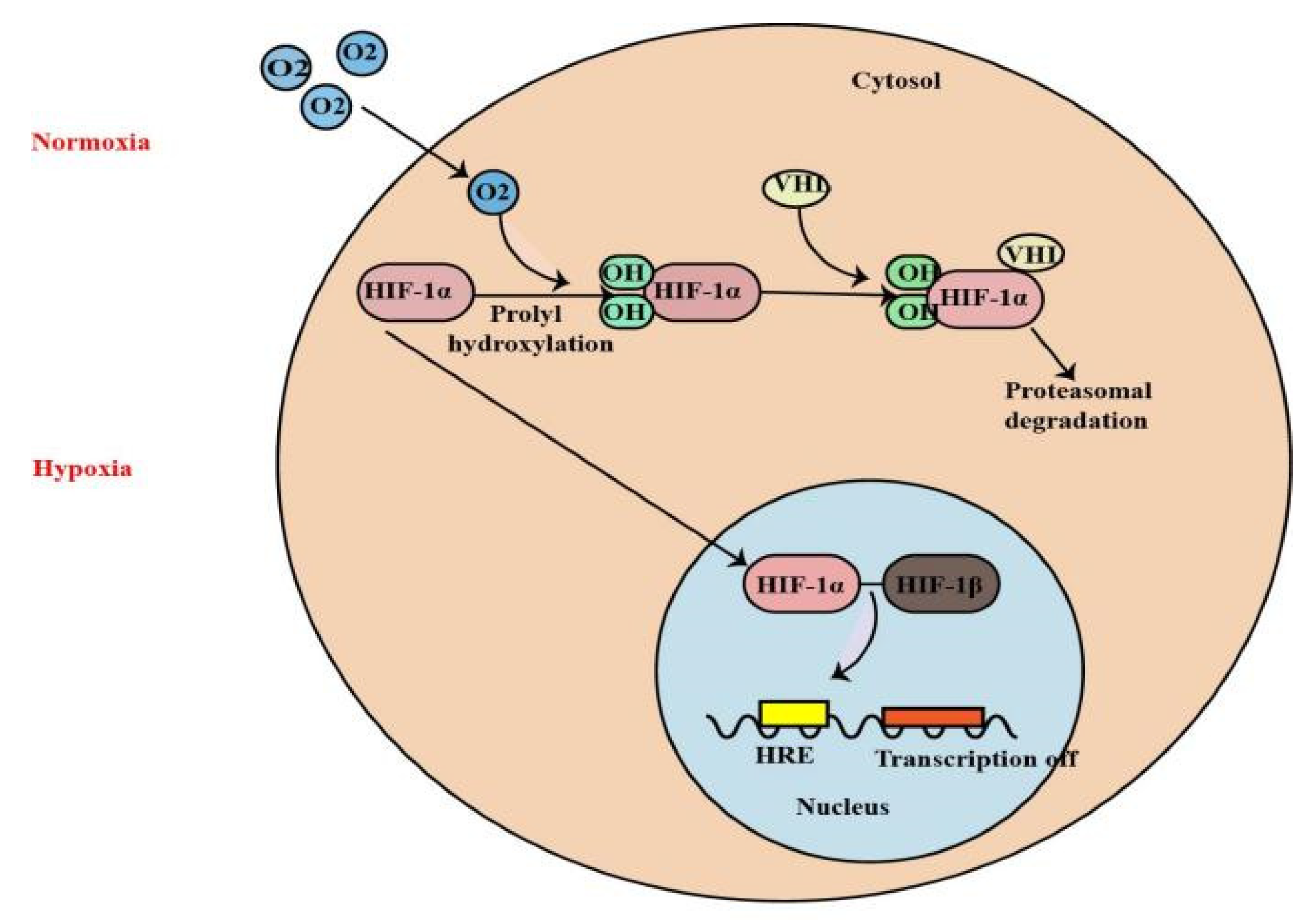
1. Introduction
2. Different Pathophysiological Mechanisms of HIF-1α in Renal Disease
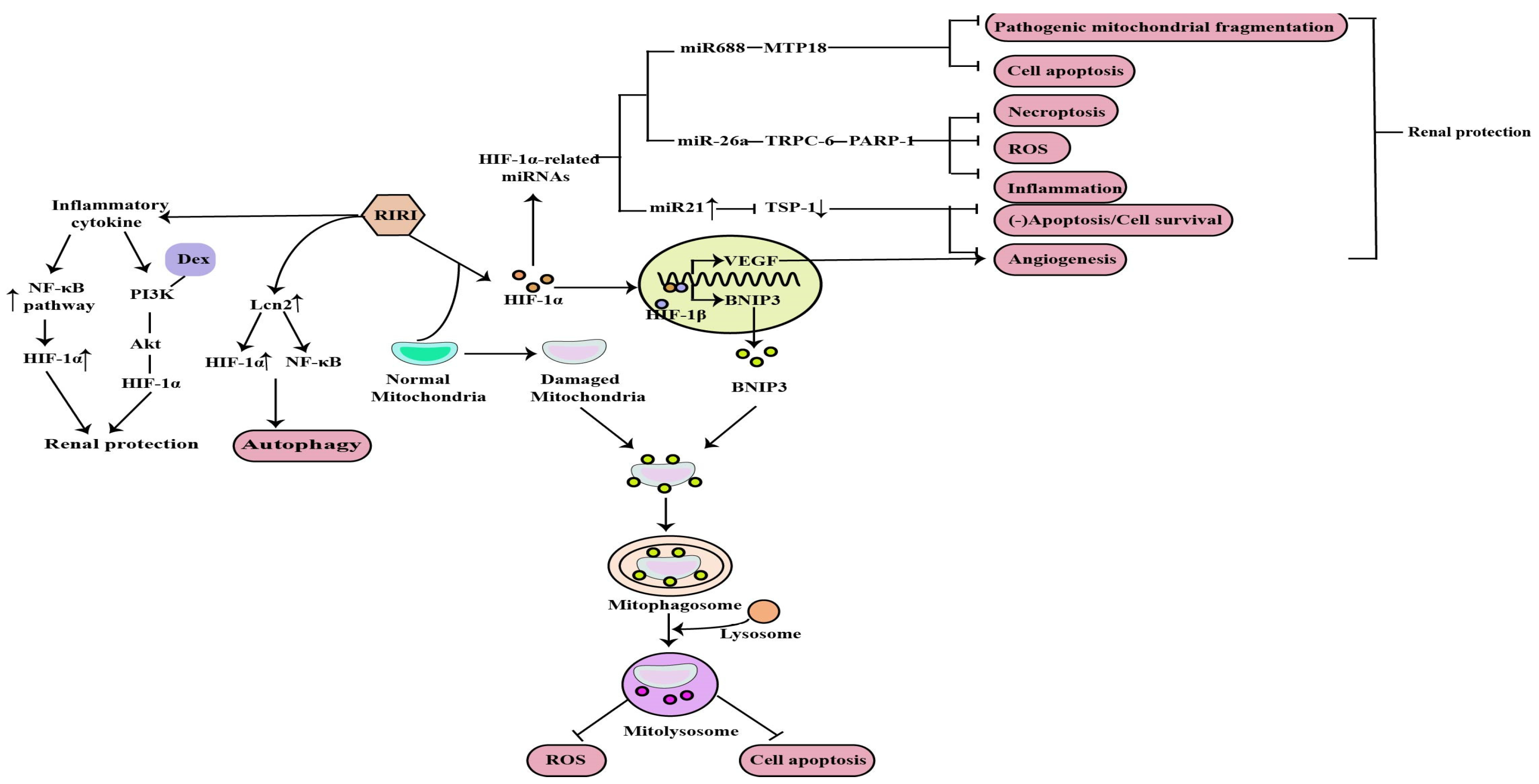
2.1. The Role of HIF-1α in RIRI
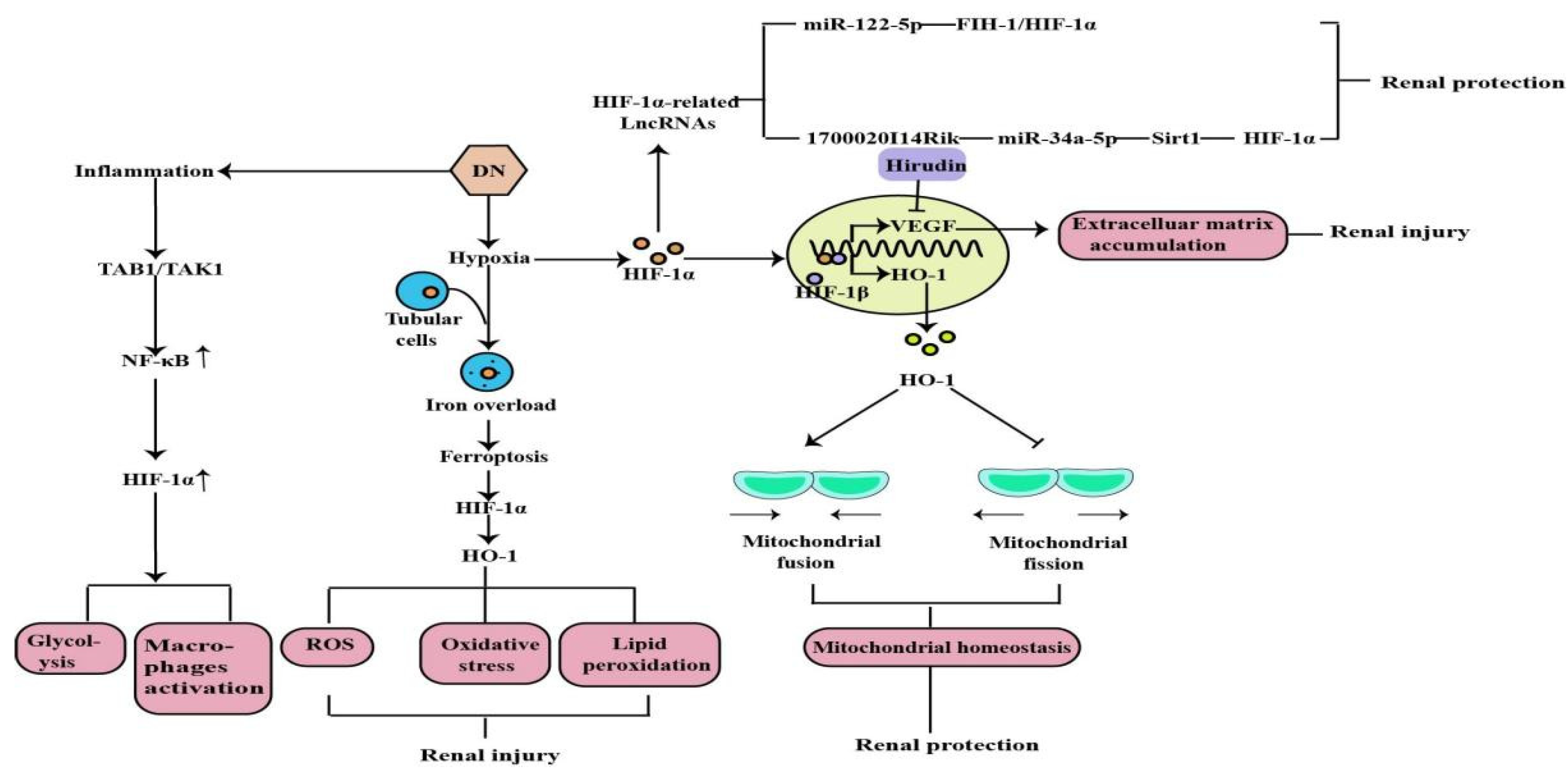
2.2. The Role of HIF-1α in Diabetic Nephropathy
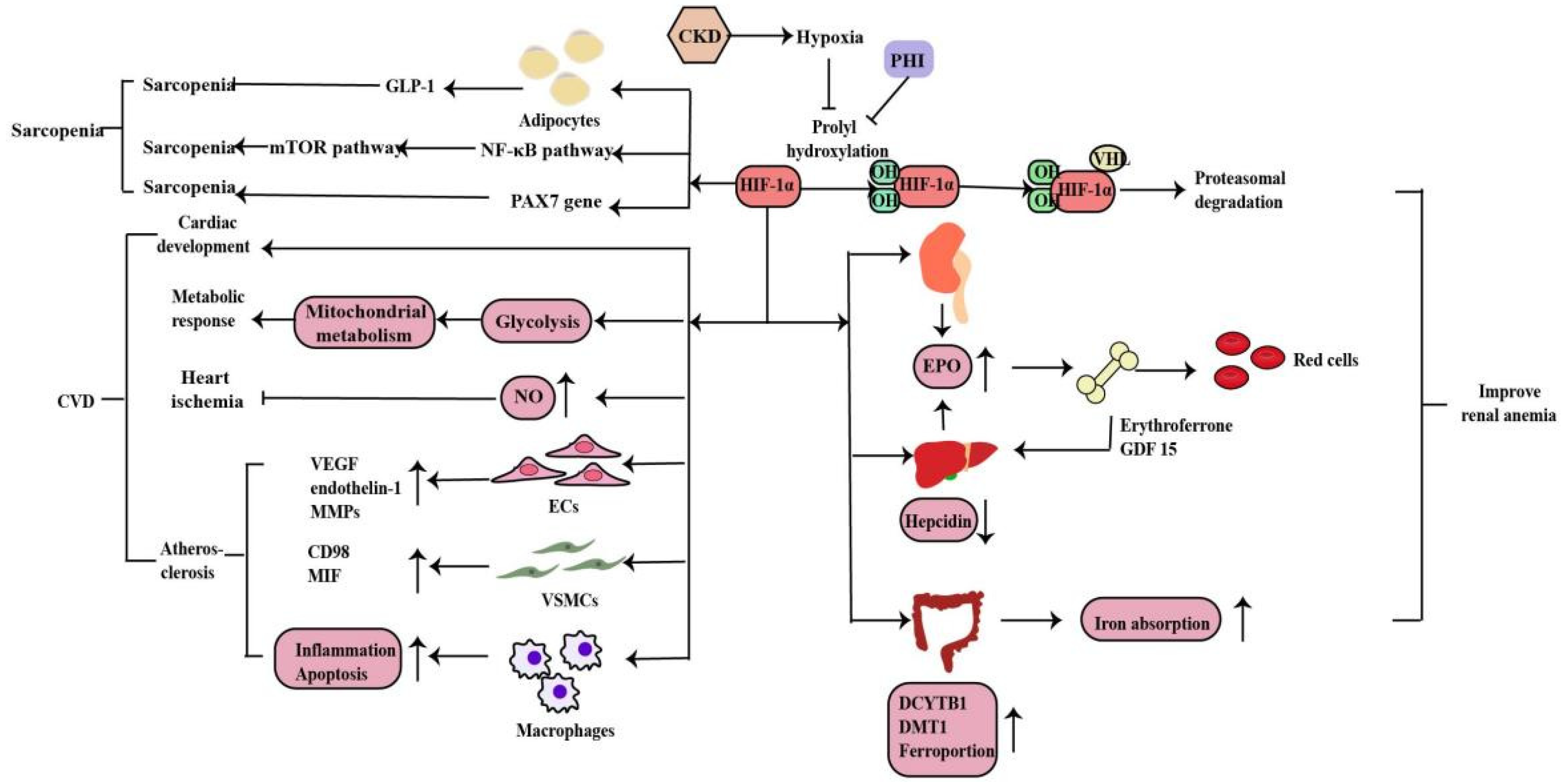
2.3. The Role of HIF-1α in Chronic Kidney Disease-Related Complications
2.3.1. Renal Anemia
2.3.2. Cardiovascular Disease
2.3.3. Sarcopenia
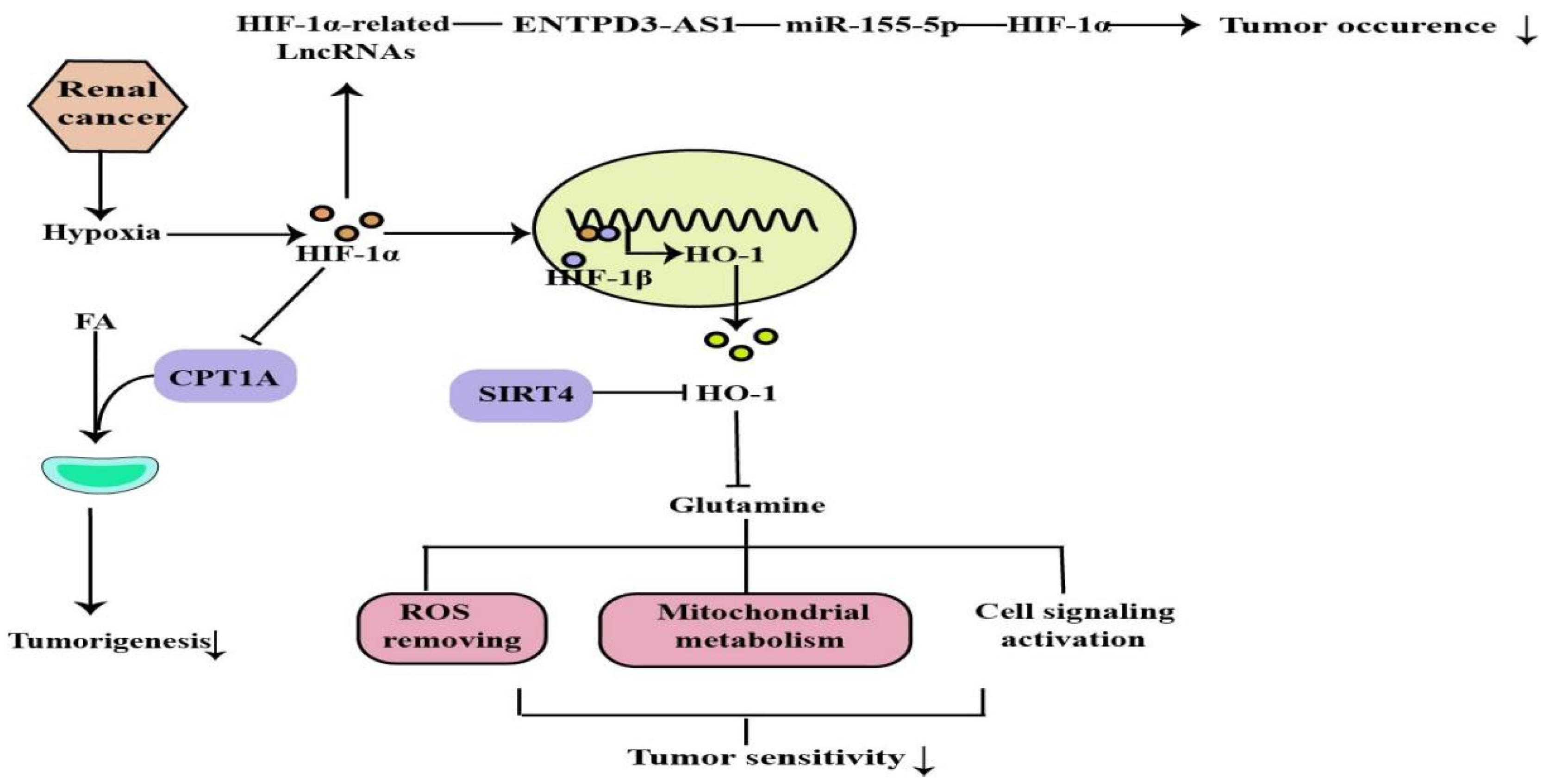
2.4. The Role of HIF-1α in Renal Cancer
2.4.1. The Tumor-Suppressor Role of HIF-1α in Renal Cancer
2.4.2. The HIF-1α/CPT1A Pathway and HIF-1α/HO-1 Pathway in Renal Cancer
2.4.3. The ENTPD3-AS1/miR-155-5p/HIF-1α Axis in Renal Cancer
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, R.G.; Smith, D.W.; Lee, C.J.; Ngo, J.P.; Gardiner, B.S. What Makes the Kidney Susceptible to Hypoxia? Anat. Rec. 2020, 303, 2544–2552. [Google Scholar] [CrossRef] [PubMed]
- Lübbers, D.W.; Baumgärtl, H. Heterogeneities and profiles of oxygen pressure in brain and kidney as examples of the pO2 distribution in the living tissue. Kidney Int. 1997, 51, 372–380. [Google Scholar] [CrossRef]
- Edwards, A.; Kurtcuoglu, V. Renal blood flow and oxygenation. Pflugers Arch. 2022, 474, 759–770. [Google Scholar] [CrossRef] [PubMed]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, H.; Harris, A.L. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018, 27, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Golinska, M.; Griffiths, J.R. HIF-1-Independent Mechanisms Regulating Metabolic Adaptation in Hypoxic Cancer Cells. Cells 2021, 10, 2371. [Google Scholar] [CrossRef]
- Bartoszewski, R.; Moszyńska, A.; Serocki, M.; Cabaj, A.; Polten, A.; Ochocka, R.; Dell’Italia, L.; Bartoszewska, S.; Króliczewski, J.; Dąbrowski, M.; et al. Primary endothelial cell-specific regulation of hypoxia-inducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia. Fed. Am. Soc. Exp. Biol. J. 2019, 33, 7929–7941. [Google Scholar] [CrossRef]
- Fandrey, J.; Gorr, T.A.; Gassmann, M. Regulating cellular oxygen sensing by hydroxylation. Cardiovasc. Res. 2006, 71, 642–651. [Google Scholar] [CrossRef]
- Schofield, C.J.; Ratcliffe, P.J. Signalling hypoxia by HIF hydroxylases. Biochem. Biophys. Res. Commun. 2005, 338, 617–626. [Google Scholar] [CrossRef]
- Yu, H.; Jin, F.; Liu, D.; Shu, G.; Wang, X.; Qi, J.; Sun, M.; Yang, P.; Jiang, S.; Ying, X.; et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics 2020, 10, 2342–2357. [Google Scholar] [CrossRef]
- Li, X.; Liao, J.; Su, X.; Li, W.; Bi, Z.; Wang, J.; Su, Q.; Huang, H.; Wei, Y.; Gao, Y.; et al. Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1. Theranostics 2020, 10, 9561–9578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Haimovich, B.; Kwon, Y.S.; Lu, T.; Fyfe-Kirschner, B.; Olweny, E.O. Unilateral Partial Nephrectomy with Warm Ischemia Results in Acute Hypoxia Inducible Factor 1-Alpha (HIF-1α) and Toll-Like Receptor 4 (TLR4) Overexpression in a Porcine Model. PLoS ONE 2016, 11, e0154708. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, A.; Wang, Z.; Sun, X.; Dong, M.; Qi, F.; Wang, L.; Zhang, Y.; Du, P. Tetramethylpyrazine alleviates acute kidney injury by inhibiting NLRP3/HIF-1α and apoptosis. Mol. Med. Rep. 2020, 22, 2655–2664. [Google Scholar] [CrossRef]
- Li, B.Y.; Liu, Y.; Li, Z.H.; An, X.L.; Xiao, S.S.; Liu, G.K.; Zhang, J. Dexmedetomidine promotes the recovery of renal function and reduces the inflammatory level in renal ischemia-reperfusion injury rats through PI3K/Akt/HIF-1α signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12400–12407. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Wang, W.; Shi, H.; Wang, J.; Liu, T. Identification of Hub Genes and Pathways in a Rat Model of Renal Ischemia-Reperfusion Injury Using Bioinformatics Analysis of the Gene Expression Omnibus (GEO) Dataset and Integration of Gene Expression Profiles. Med. Sci. Monit. 2019, 25, 8403–8411. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.L.; Zhang, Y.L.; Wen, Y.; Gao, Y.M.; Liu, B.C. Hypoxia and chronic kidney disease. EBioMedicine 2022, 77, 103942. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chiang, W.C.; Chen, Y.M. The journey from erythropoietin to 2019 Nobel Prize: Focus on hypoxia-inducible factors in the kidney. J. Formos. Med. Assoc. 2021, 120, 60–67. [Google Scholar] [CrossRef]
- Jiang, N.; Zhao, H.; Han, Y.; Li, L.; Xiong, S.; Zeng, L.; Xiao, Y.; Wei, L.; Xiong, X.; Gao, P.; et al. HIF-1α ameliorates tubular injury in diabetic nephropathy via HO-1-mediated control of mitochondrial dynamics. Cell Prolif. 2020, 53, e12909. [Google Scholar] [CrossRef]
- Voit, R.A.; Sankaran, V.G. Stabilizing HIF to Ameliorate Anemia. Cell 2020, 180, 6. [Google Scholar] [CrossRef]
- Knutson, A.K.; Williams, A.L.; Boisvert, W.A.; Shohet, R.V. HIF in the heart: Development, metabolism, ischemia, and atherosclerosis. J. Clin. Investig. 2021, 131, e137557. [Google Scholar] [CrossRef]
- Lee, J.W.; Ko, J.; Ju, C.; Eltzschig, H.K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Yang, J.Y.; Park, C.H.; Son, K.H.; Byun, K. Dieckol Reduces Muscle Atrophy by Modulating Angiotensin Type II Type 1 Receptor and NADPH Oxidase in Spontaneously Hypertensive Rats. Antioxidants 2021, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.Y.; Li, Z.L.; Guo, Y.D.; Gao, H.C.; Gu, L.H.; Le, K.; Xie, C.M.; Wang, B.; Zhang, Z.J. Hypoxia-inducible factor-prolyl hydroxylase inhibitor ameliorates myopathy in a mouse model of chronic kidney disease. Am. J. Physiol. Renal Physiol. 2019, 317, F1265–F1273. [Google Scholar] [CrossRef] [PubMed]
- Albadari, N.; Deng, S.; Li, W. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert Opin. Drug Discov. 2019, 14, 667–682. [Google Scholar] [CrossRef]
- Hoefflin, R.; Harlander, S.; Schäfer, S.; Metzger, P.; Kuo, F.; Schönenberger, D.; Adlesic, M.; Peighambari, A.; Seidel, P.; Chen, C.Y.; et al. HIF-1α and HIF-2α differently regulate tumour development and inflammation of clear cell renal cell carcinoma in mice. Nat. Commun. 2020, 11, 4111. [Google Scholar] [CrossRef]
- Signore, P.E.; Guo, G.; Wei, Z.; Zhang, W.; Lin, A.; Del Balzo, U. A small-molecule inhibitor of hypoxia-inducible factor prolyl hydroxylase improves obesity, nephropathy and cardiomyopathy in obese ZSF1 rats. PLoS ONE 2021, 16, e0255022. [Google Scholar] [CrossRef]
- Fu, Z.J.; Wang, Z.Y.; Xu, L.; Chen, X.H.; Li, X.X.; Liao, W.T.; Ma, H.K.; Jiang, M.D.; Xu, T.T.; Xu, J.; et al. HIF-1α-BNIP3-mediated mitophagy in tubular cells protects against renal ischemia/reperfusion injury. Redox Biol. 2020, 36, 101671. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, Q.; Lv, X. MicroRNA-668-3p Protects Against Oxygen-Glucose Deprivation in a Rat H9c2 Cardiomyocyte Model of Ischemia-Reperfusion Injury by Targeting the Stromal Cell-Derived Factor-1 (SDF-1)/CXCR4 Signaling Pathway. Med. Sci. Monit. 2020, 26, e919601. [Google Scholar] [CrossRef]
- Chun, N.; Coca, S.G.; He, J.C. A protective role for microRNA-688 in acute kidney injury. J. Clin. Investig. 2018, 128, 5216–5218. [Google Scholar] [CrossRef]
- Li, Z.L.; Ji, J.L.; Wen, Y.; Cao, J.Y.; Kharbuja, N.; Ni, W.J.; Yin, D.; Feng, S.T.; Liu, H.; Lv, L.L.; et al. HIF-1α is transcriptionally regulated by NF-κB in acute kidney injury. Am. J. Physiol. Renal Physiol. 2021, 321, F225–F235. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, X.; Pang, Y.; Zhang, Z. Lipocalin-2 protects against renal ischemia/reperfusion injury in mice through autophagy activation mediated by HIF1α and NF-κb crosstalk. Biomed. Pharmacother. 2018, 108, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Jaberi, S.A.; Cohen, A.; D’Souza, C.; Abdulrazzaq, Y.M.; Ojha, S.; Bastaki, S.; Adeghate, E.A. Lipocalin-2: Structure, function, distribution and role in metabolic disorders. Biomed. Pharmacother. 2021, 142, 112002. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Mei, M.; Pu, Y.; Zhang, H.; Liu, H.; Tang, M.; Pan, Q.; He, Y.; Wu, X.; Zhao, H. Necrostatin-1 Attenuates Renal Ischemia and Reperfusion Injury via Meditation of HIF-1α/mir-26a/TRPC6/PARP1 Signaling. Mol. Ther. Nucleic Acids 2019, 17, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Xu, Z.; Chen, L.; Wei, Q.; Huang, Z.; Liu, G.; Li, W.; Wang, J.; Tang, Q.; Pu, J. Long non-coding RNA PAARH promotes hepatocellular carcinoma progression and angiogenesis via upregulating HOTTIP and activating HIF-1α/VEGF signaling. Cell Death Dis. 2022, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, D.; Ding, W.; Zhang, T.; Ma, X.; Zou, J. MiRNA-21- HIF-1α-VEGF Axis is Associated with Myopic Choroidal Neovascularization in Guinea Pigs. Ophthalmic. Res. 2022, 65, 493–505. [Google Scholar] [CrossRef]
- Xu, X.; Song, N.; Zhang, X.; Jiao, X.; Hu, J.; Liang, M.; Teng, J.; Ding, X. Renal Protection Mediated by Hypoxia Inducible Factor-1α Depends on Proangiogenesis Function of miR-21 by Targeting Thrombospondin 1. Transplantation 2017, 101, 1811–1819. [Google Scholar] [CrossRef]
- Sugahara, M.; Tanaka, S.; Tanaka, T.; Saito, H.; Ishimoto, Y.; Wakashima, T.; Ueda, M.; Fukui, K.; Shimizu, A.; Inagi, R.; et al. Prolyl Hydroxylase Domain Inhibitor Protects against Metabolic Disorders and Associated Kidney Disease in Obese Type 2 Diabetic Mice. J. Am. Soc. Nephrol. 2020, 31, 560–577. [Google Scholar] [CrossRef]
- Yamazaki, T.; Mimura, I.; Tanaka, T.; Nangaku, M. Treatment of Diabetic Kidney Disease: Current and Future. Diabetes Metab. J. 2021, 45, 11–26. [Google Scholar] [CrossRef]
- Rosenberger, C.; Khamaisi, M.; Abassi, Z.; Shilo, V.; Weksler-Zangen, S.; Goldfarb, M.; Shina, A.; Zibertrest, F.; Eckardt, K.U.; Rosen, S.; et al. Adaptation to hypoxia in the diabetic rat kidney. Kidney Int. 2008, 73, 34–42. [Google Scholar] [CrossRef]
- Chiang, S.K.; Chen, S.E.; Chang, L.C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2018, 20, 39. [Google Scholar] [CrossRef]
- Song, L.; Wang, K.; Yin, J.; Yang, Y.; Li, B.; Zhang, D.; Wang, H.; Wang, W.; Zhan, W.; Guo, C.; et al. Traditional Chinese Medicine Fufang-Zhenzhu-Tiaozhi capsule prevents renal injury in diabetic minipigs with coronary heart disease. Chin. Med. 2022, 17, 102. [Google Scholar] [CrossRef]
- Sun, H.K.; Lee, Y.M.; Han, K.H.; Kim, H.S.; Ahn, S.H.; Han, S.Y. Phosphodiesterase inhibitor improves renal tubulointerstitial hypoxia of the diabetic rat kidney. Korean J. Intern. Med. 2012, 27, 163–170. [Google Scholar] [CrossRef]
- Feng, X.; Wang, S.; Sun, Z.; Dong, H.; Yu, H.; Huang, M.; Gao, X. Ferroptosis Enhanced Diabetic Renal Tubular Injury via HIF-1α/HO-1 Pathway in db/db Mice. Front. Endocrinol. 2021, 12, 626390. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Qiu, X.; He, L.; Liu, L. MicroRNA-122-5p ameliorates tubular injury in diabetic nephropathy via FIH-1/HIF-1α pathway. Renal Fail. 2022, 44, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, Y.; Zhou, H. The potential role of lncRNAs in diabetes and diabetic microvascular complications. Endocr. J. 2020, 67, 659–668. [Google Scholar] [CrossRef]
- Li, A.; Peng, R.; Sun, Y.; Liu, H.; Peng, H.; Zhang, Z. LincRNA 1700020I14Rik alleviates cell proliferation and fibrosis in diabetic nephropathy via miR-34a-5p/Sirt1/HIF-1α signaling. Cell Death Dis. 2018, 9, 461. [Google Scholar] [CrossRef]
- Zeng, H.; Qi, X.; Xu, X.; Wu, Y. TAB1 regulates glycolysis and activation of macrophages in diabetic nephropathy. Inflamm. Res. 2020, 69, 1215–1234. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhang, Y.; Shi, X.; Peng, Z.; Xing, Y.; Jiarui, H. Hirudin Reduces the Expression of Markers of the Extracellular Matrix in Renal Tubular Epithelial Cells in a Rat Model of Diabetic Kidney Disease Through the Hypoxia-Inducible Factor-1α (HIF-1α)/Vascular Endothelial Growth Factor (VEGF) Signaling Pathway. Med. Sci. Monit. 2020, 26, e921894. [Google Scholar] [CrossRef]
- Rosenberger, C.; Mandriota, S.; Jürgensen, J.S.; Wiesener, M.S.; Hörstrup, J.H.; Frei, U.; Ratcliffe, P.J.; Maxwell, P.H.; Bachmann, S.; Eckardt, K.U. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J. Am. Soc. Nephrol. 2002, 13, 1721–1732. [Google Scholar] [CrossRef]
- Koury, M.J.; Haase, V.H. Anaemia in kidney disease: Harnessing hypoxia responses for therapy. Nat. Rev. Nephrol. 2015, 11, 394–410. [Google Scholar] [CrossRef]
- Li, Z.L.; Tu, Y.; Liu, B.C. Treatment of Renal Anemia with Roxadustat: Advantages and Achievement. Kidney Dis. 2020, 6, 65–73. [Google Scholar] [CrossRef]
- Iyer, N.V.; Kotch, L.E.; Agani, F.; Leung, S.W.; Laughner, E.; Wenger, R.H.; Gassmann, M.; Gearhart, J.D.; Lawler, A.M.; Yu, A.Y.; et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998, 12, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Kihira, Y.; Miyake, M.; Hirata, M.; Hoshina, Y.; Kato, K.; Shirakawa, H.; Sakaue, H.; Yamano, N.; Izawa-Ishizawa, Y.; Ishizawa, K.; et al. Deletion of hypoxia-inducible factor-1α in adipocytes enhances glucagon-like peptide-1 secretion and reduces adipose tissue inflammation. PLoS ONE 2014, 9, e93856. [Google Scholar] [CrossRef]
- Remels, A.H.; Gosker, H.R.; Verhees, K.J.; Langen, R.C.; Schols, A.M. TNF-α-induced NF-κB activation stimulates skeletal muscle glycolytic metabolism through activation of HIF-1α. Endocrinology 2015, 156, 1770–1781. [Google Scholar] [CrossRef]
- D’Hulst, G.; Jamart, C.; Van Thienen, R.; Hespel, P.; Francaux, M.; Deldicque, L. Effect of acute environmental hypoxia on protein metabolism in human skeletal muscle. Acta Physiol. 2013, 208, 251–264. [Google Scholar] [CrossRef]
- Cirillo, F.; Mangiavini, L.; La Rocca, P.; Piccoli, M.; Ghiroldi, A.; Rota, P.; Tarantino, A.; Canciani, B.; Coviello, S.; Messina, C.; et al. Human Sarcopenic Myoblasts Can Be Rescued by Pharmacological Reactivation of HIF-1α. Int. J. Mol. Sci. 2022, 23, 7114. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Aljahdali, I.A.M.; Zhang, R.; Nastiuk, K.L.; Krolewski, J.J.; Ling, X. Kidney cancer biomarkers and targets for therapeutics: Survivin (BIRC5), XIAP, MCL-1, HIF1α, HIF2α, NRF2, MDM2, MDM4, p53, KRAS and AKT in renal cell carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 254. [Google Scholar] [CrossRef]
- Xiao, Y.; Thakkar, K.N.; Zhao, H.; Broughton, J.; Li, Y.; Seoane, J.A.; Diep, A.N.; Metzner, T.J.; von Eyben, R.; Dill, D.L.; et al. The m(6)A RNA demethylase FTO is a HIF-independent synthetic lethal partner with the VHL tumor suppressor. Proc. Natl. Acad. Sci. USA 2020, 117, 21441–21449. [Google Scholar] [CrossRef]
- Ullah, A.; Leong, S.W.; Wang, J.; Wu, Q.; Ghauri, M.A.; Sarwar, A.; Su, Q.; Zhang, Y. Cephalomannine inhibits hypoxia-induced cellular function via the suppression of APEX1/HIF-1α interaction in lung cancer. Cell Death Dis. 2021, 12, 490. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, N.; Nawaz, T.; Aziz, T. Molecular mechanisms of Sanguinarine in cancer prevention and treatment. Anti-Cancer Agents Med. Chem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Elvidge, G.P.; Glenny, L.; Appelhoff, R.J.; Ratcliffe, P.J.; Ragoussis, J.; Gleadle, J.M. Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: The role of HIF-1alpha, HIF-2alpha, and other pathways. J. Biol. Chem. 2006, 281, 15215–15226. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, C.; Zaborowska, Z.; Kurreck, J.; Erdmann, V.A.; Frei, U.; Wiesener, M.; Eckardt, K.U. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: Erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J. 2004, 18, 1462–1464. [Google Scholar] [CrossRef]
- Monzon, F.A.; Alvarez, K.; Peterson, L.; Truong, L.; Amato, R.J.; Hernandez-McClain, J.; Tannir, N.; Parwani, A.V.; Jonasch, E. Chromosome 14q loss defines a molecular subtype of clear-cell renal cell carcinoma associated with poor prognosis. Mod. Pathol. 2011, 24, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Ferro, M.; Loizzo, D.; Bianchi, C.; Terracciano, D.; Cantiello, F.; Bell, L.N.; Battaglia, S.; Porta, C.; Gernone, A.; et al. Integration of Lipidomics and Transcriptomics Reveals Reprogramming of the Lipid Metabolism and Composition in Clear Cell Renal Cell Carcinoma. Metabolites 2020, 10, 509. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Venneti, S.; Nagrath, D. Glutaminolysis: A Hallmark of Cancer Metabolism. Annu. Rev. Biomed. Eng. 2017, 19, 163–194. [Google Scholar] [CrossRef]
- Loboda, A.; Jozkowicz, A.; Dulak, J. HO-1/CO system in tumor growth, angiogenesis and metabolism—Targeting HO-1 as an anti-tumor therapy. Vascul. Pharmacol. 2015, 74, 11–22. [Google Scholar] [CrossRef]
- Tong, Y.; Kai, J.; Wang, S.; Yu, Y.; Xie, S.; Zheng, H.; Wang, Y.; Liu, Y.; Zhu, K.; Guan, X.; et al. VHL regulates the sensitivity of clear cell renal cell carcinoma to SIRT4-mediated metabolic stress via HIF-1α/HO-1 pathway. Cell Death Dis. 2021, 12, 621. [Google Scholar] [CrossRef]
- Larkin, J.; Goh, X.Y.; Vetter, M.; Pickering, L.; Swanton, C. Epigenetic regulation in RCC: Opportunities for therapeutic intervention? Nat. Rev. Urol. 2012, 9, 147–155. [Google Scholar] [CrossRef]
- Cookson, W.; Liang, L.; Abecasis, G.; Moffatt, M.; Lathrop, M. Mapping complex disease traits with global gene expression. Nat. Rev. Genet. 2009, 10, 184–194. [Google Scholar] [CrossRef]
- Wang, J.; Zou, Y.; Du, B.; Li, W.; Yu, G.; Li, L.; Zhou, L.; Gu, X.; Song, S.; Liu, Y.; et al. SNP-mediated lncRNA-ENTPD3-AS1 upregulation suppresses renal cell carcinoma via miR-155/HIF-1α signaling. Cell Death Dis. 2021, 12, 672. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Li, Y.; Xiong, J. The Role of Hypoxia-Inducible Factor-1 Alpha in Renal Disease. Molecules 2022, 27, 7318. https://doi.org/10.3390/molecules27217318
Liu H, Li Y, Xiong J. The Role of Hypoxia-Inducible Factor-1 Alpha in Renal Disease. Molecules. 2022; 27(21):7318. https://doi.org/10.3390/molecules27217318
Chicago/Turabian StyleLiu, Huixia, Yujuan Li, and Jing Xiong. 2022. "The Role of Hypoxia-Inducible Factor-1 Alpha in Renal Disease" Molecules 27, no. 21: 7318. https://doi.org/10.3390/molecules27217318
APA StyleLiu, H., Li, Y., & Xiong, J. (2022). The Role of Hypoxia-Inducible Factor-1 Alpha in Renal Disease. Molecules, 27(21), 7318. https://doi.org/10.3390/molecules27217318




