Characterization and Optimization of Polymeric Bispicolamine Chelating Resin: Performance Evaluation via RSM Using Copper in Acid Liquors as a Model Substrate through Ion Exchange Method
Abstract
1. Introduction
2. Results and Discussion
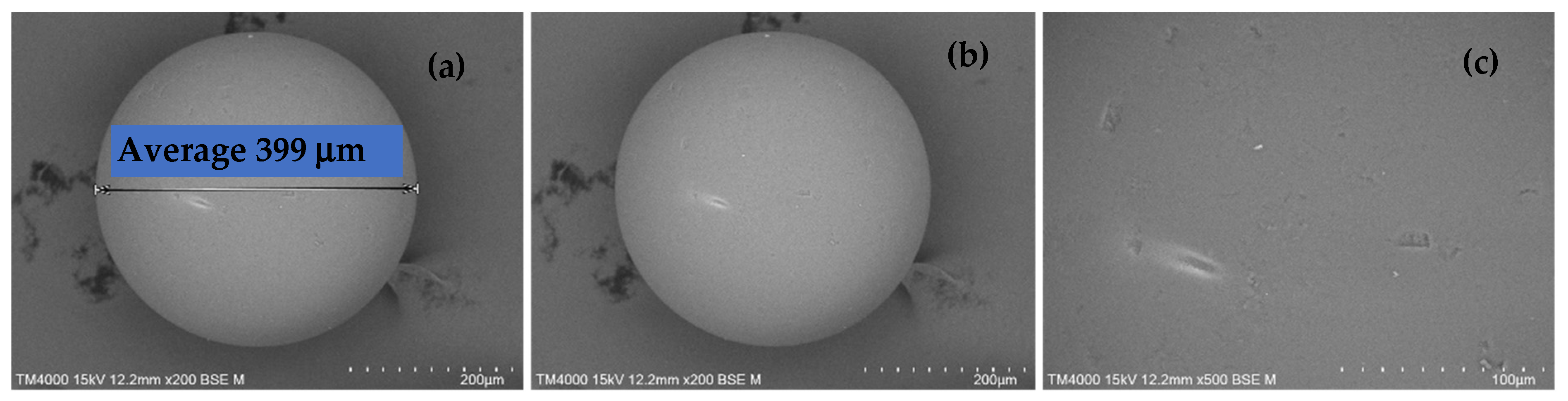
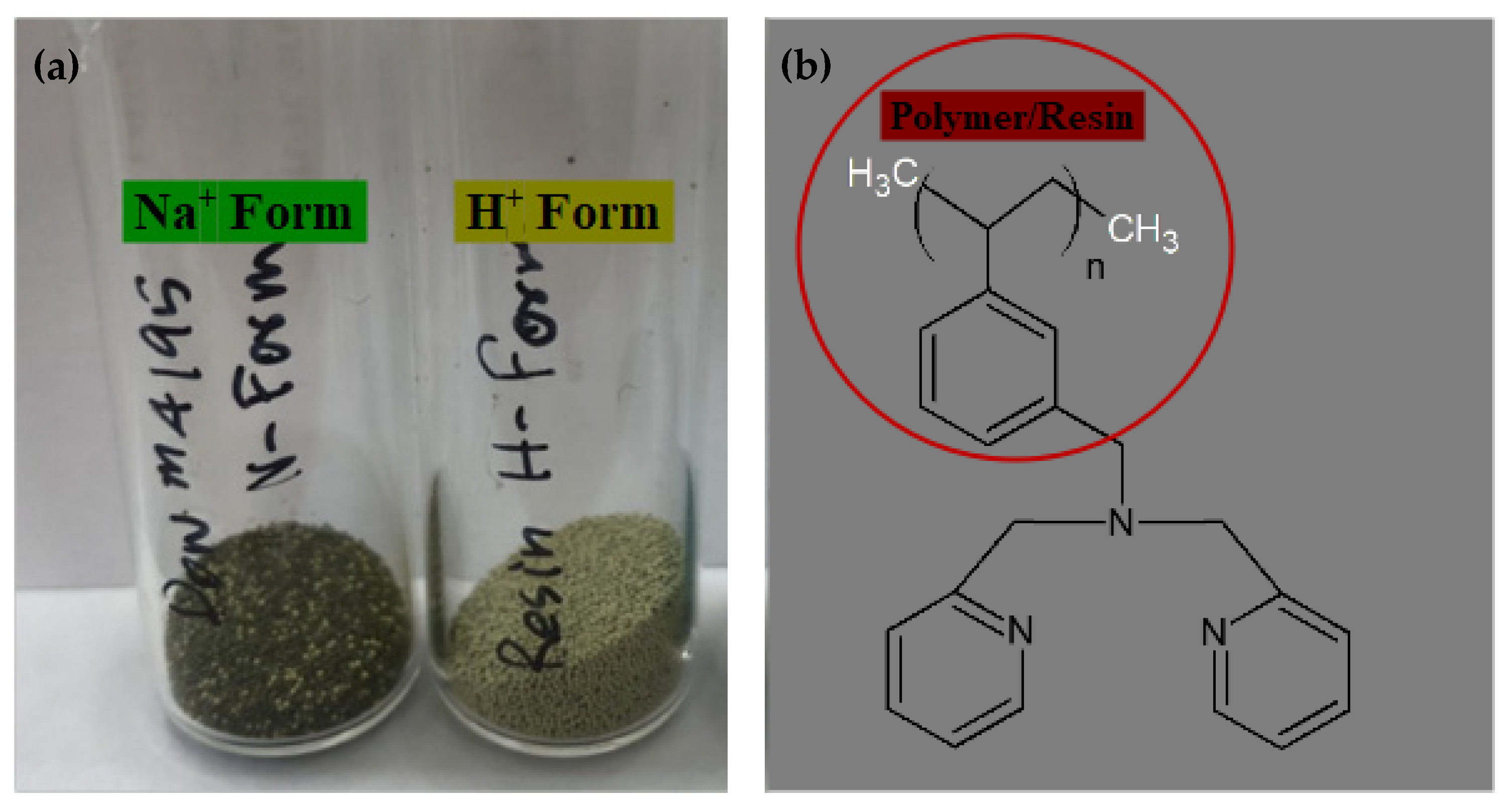
2.1. SEM Images of H+ Dowex-M4195 and Cu(II)-Loaded H+ Dowex-M4195 Chelating Resin
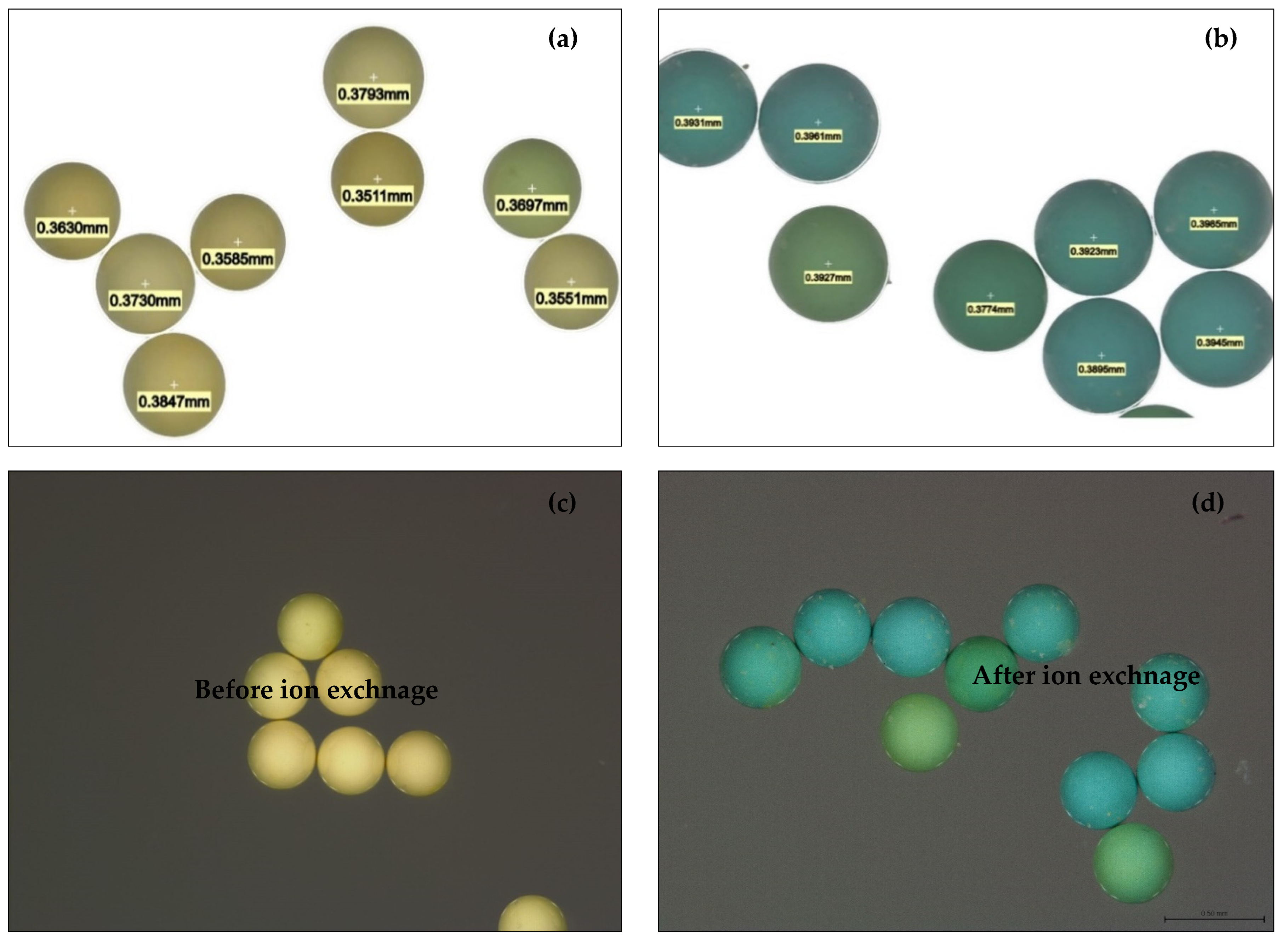
2.2. Leica Microscope Image Analyses of H+ Dowex-M4195 and Cu(II)-Loaded H+ Dowex-M4195 Chelating Resin
2.3. Elemental Analysis of Compositions of H+ Dowex-M4195 and Cu(II)-Loaded H+ Dowex-M4195 Chelating Resin
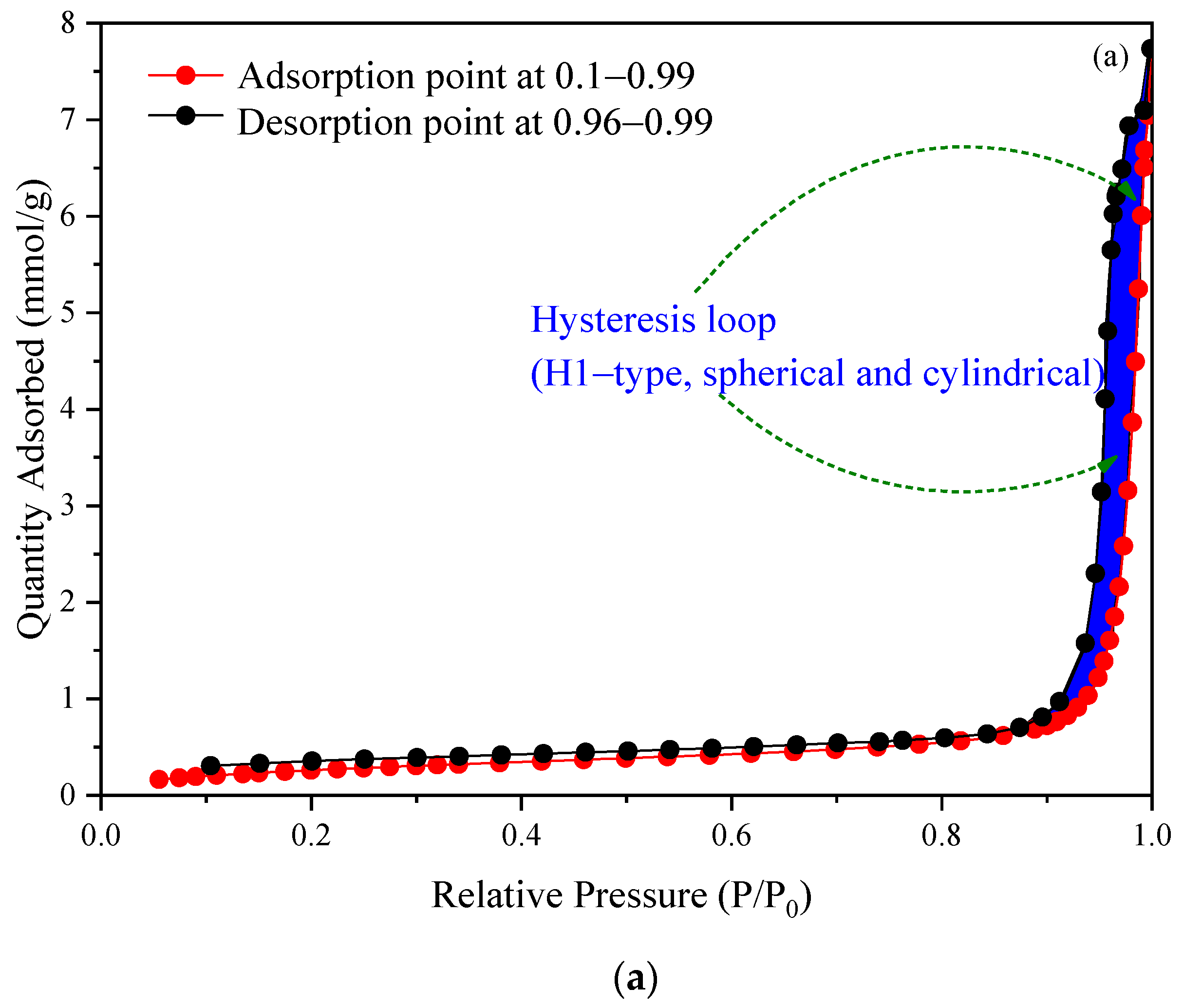
2.4. Nitrogen Adsorption–Desorption Isotherms of H+ Dowex-M4195 and Cu(II)-Loaded H+ Dowex-M4195 Chelating Resin
2.5. Physical Properties of H+ Dowex-M4195 and Cu(II)-Loaded H+ Dowex-M4195 Chelating Resin
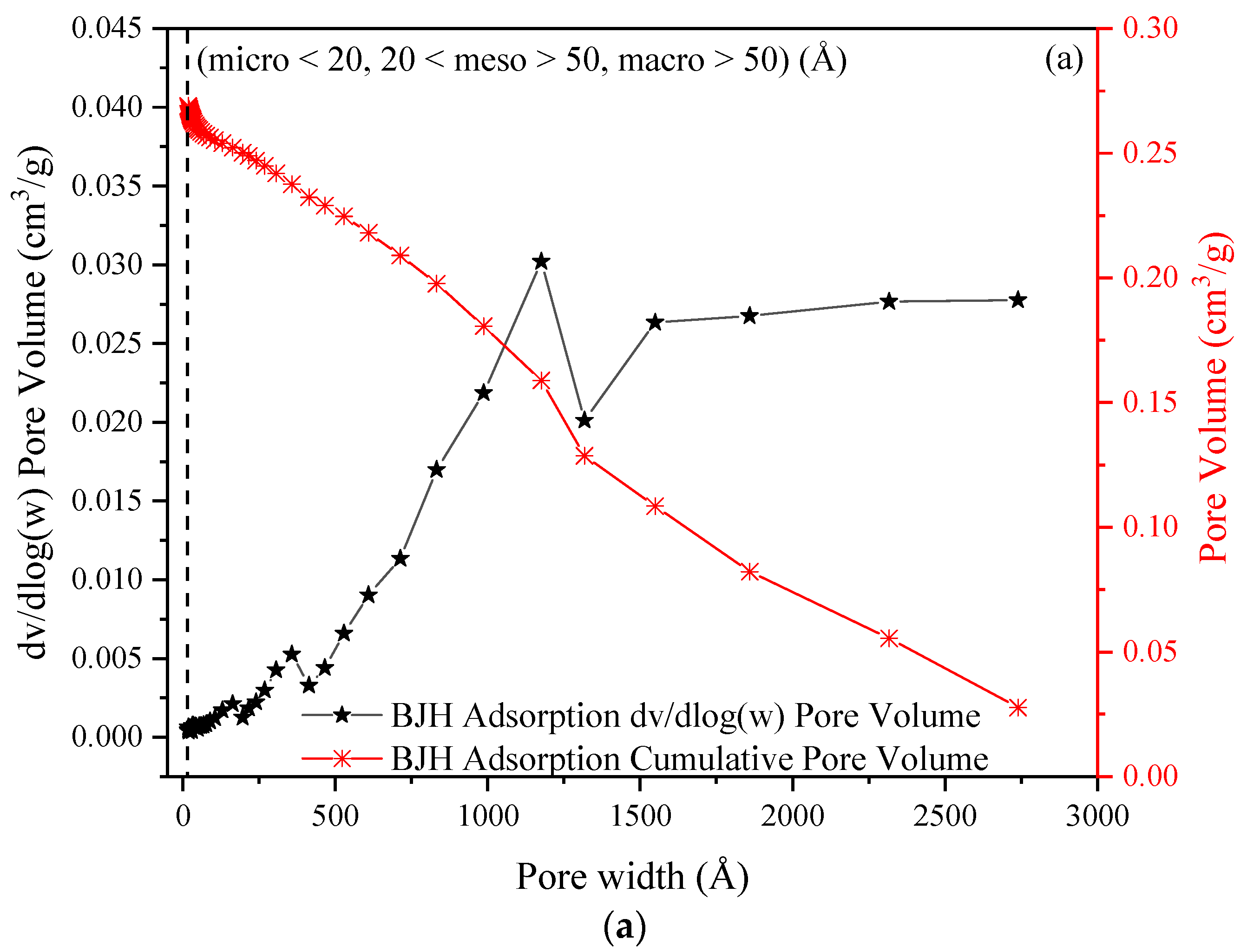
2.6. Pore Size Distribution and Pore Volume of H+ Dowex-M4195 Chelating Resin
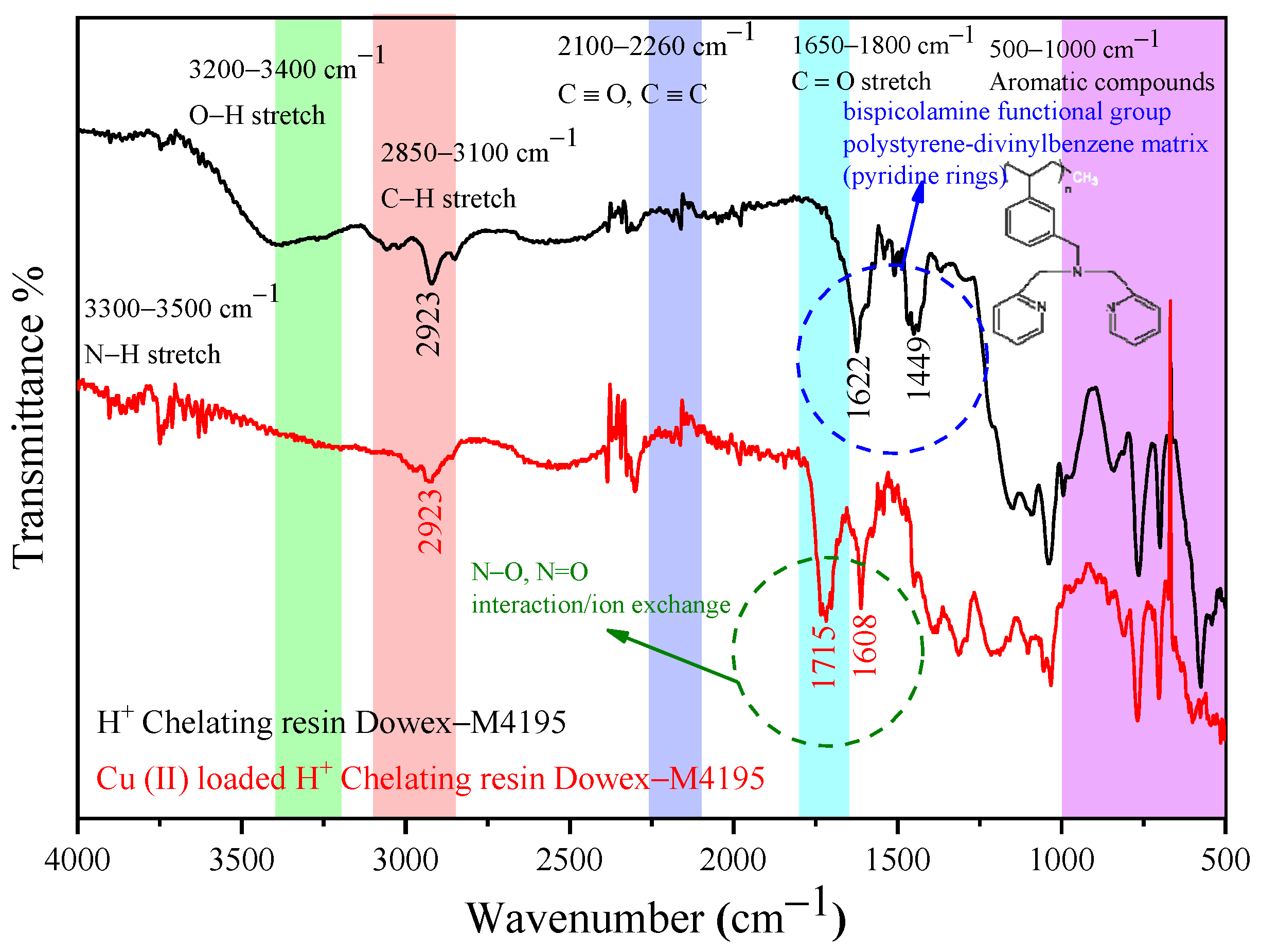
2.7. Functional Groups before and after Ion Exchange Study of the H+ Dowex-M4195 Chelating Resin
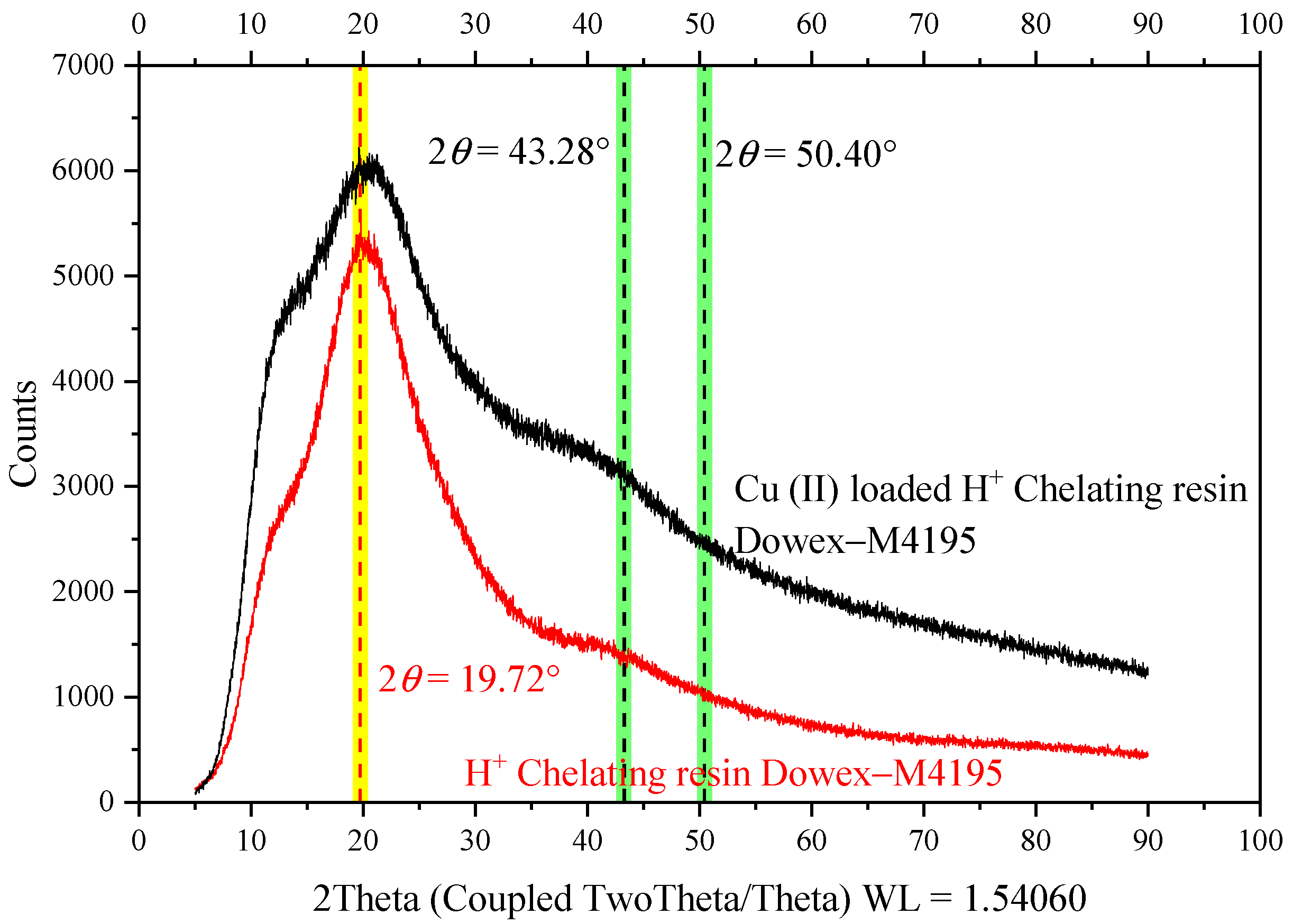
2.8. Crystal Structure before and after Ion Exchange Study of the H+ Dowex-M4195 Chelating Resin
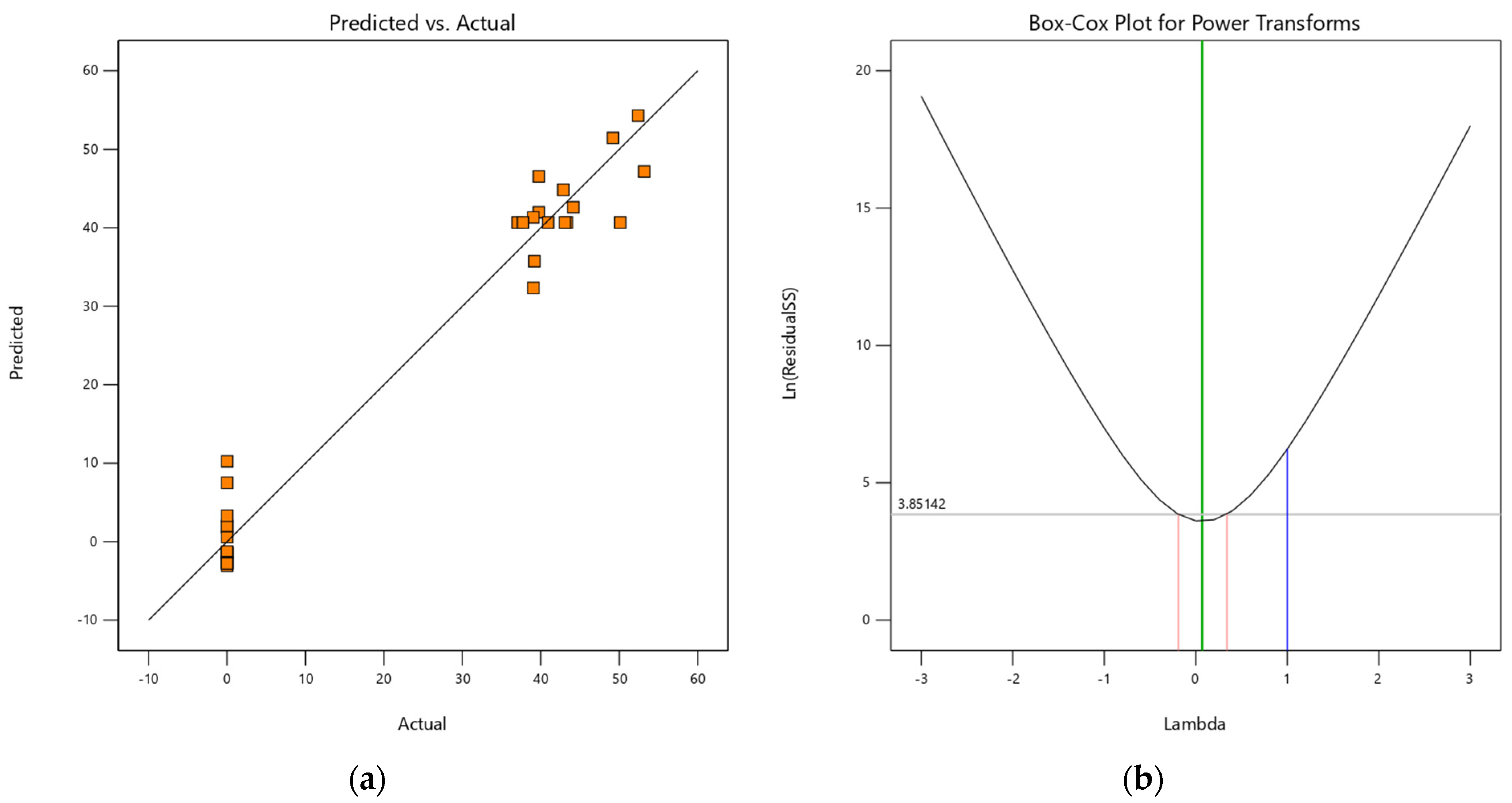
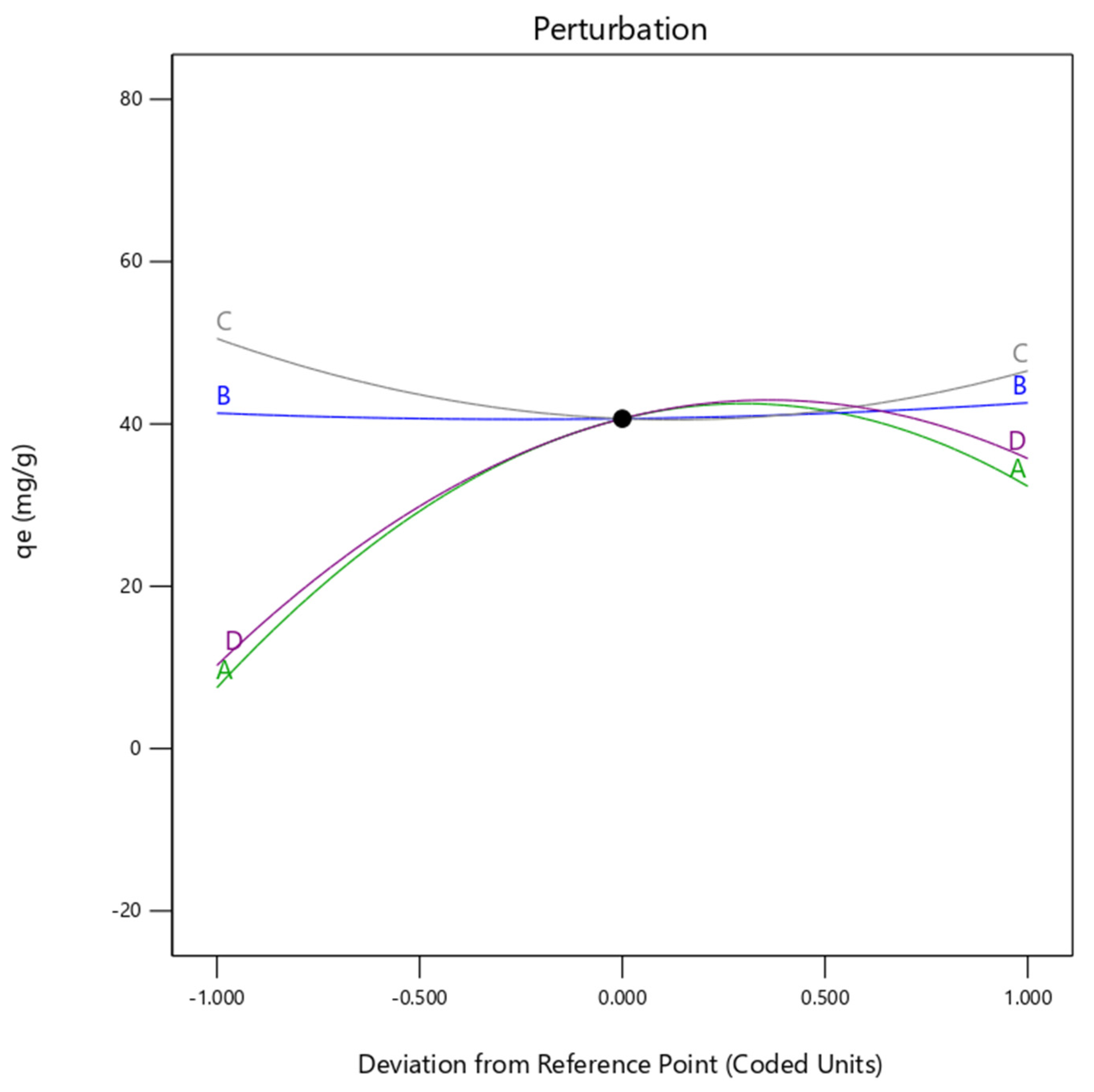
2.9. RSM and Model Fit for Cu(II) Removal onto H+ Dowex-M4195 Chelating Resin
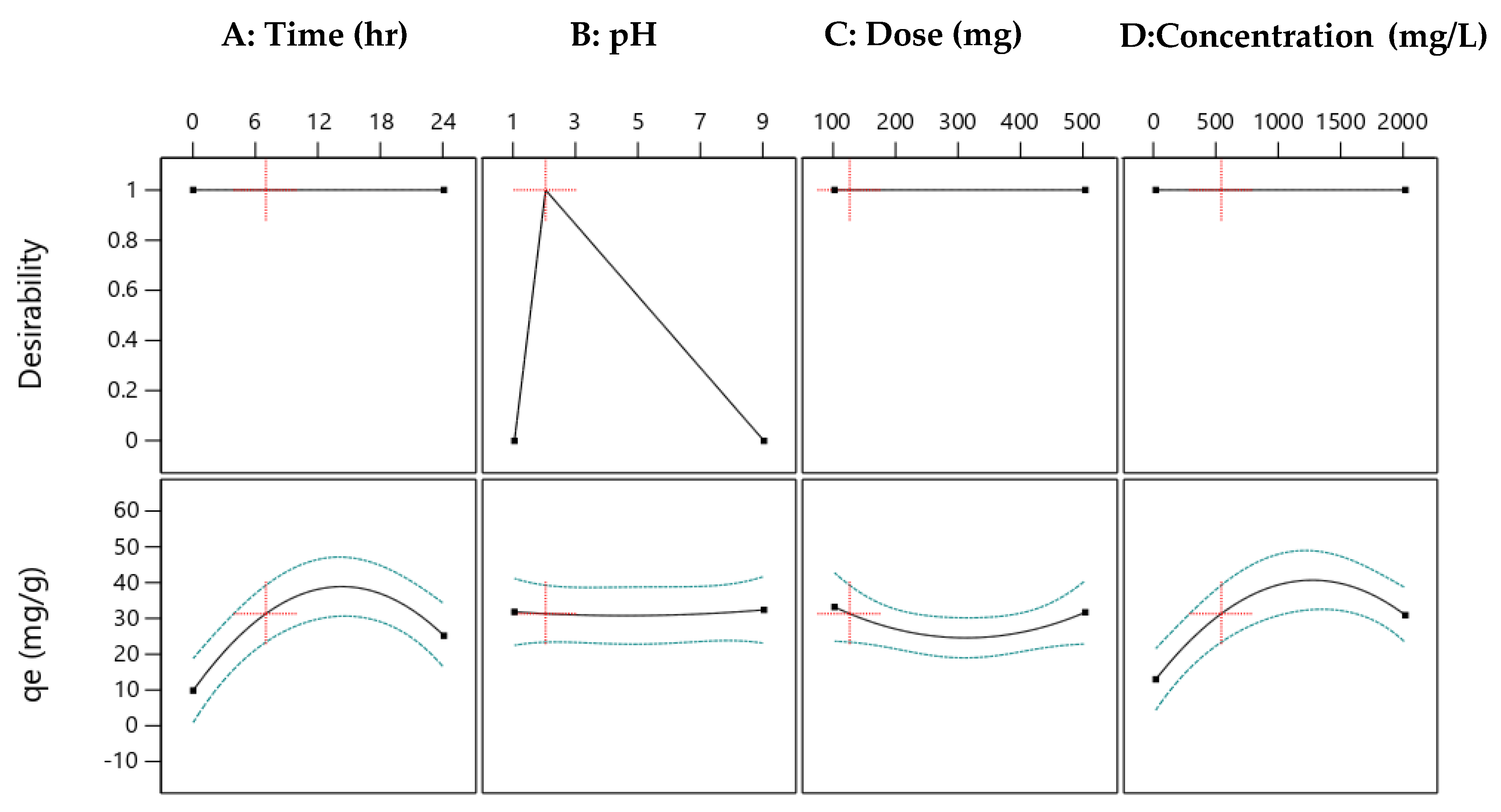
2.10. Optimum Conditions of Cu(II) Removal at a Low pH with RSM and Model Fit
3. Materials and Methods
3.1. Preparation of the Exchanger H+ Dowex-M4195 Chelating Resin
3.2. Characterization before and after Ion Exchange for H+ Dowex-M4195 Chelating Resin
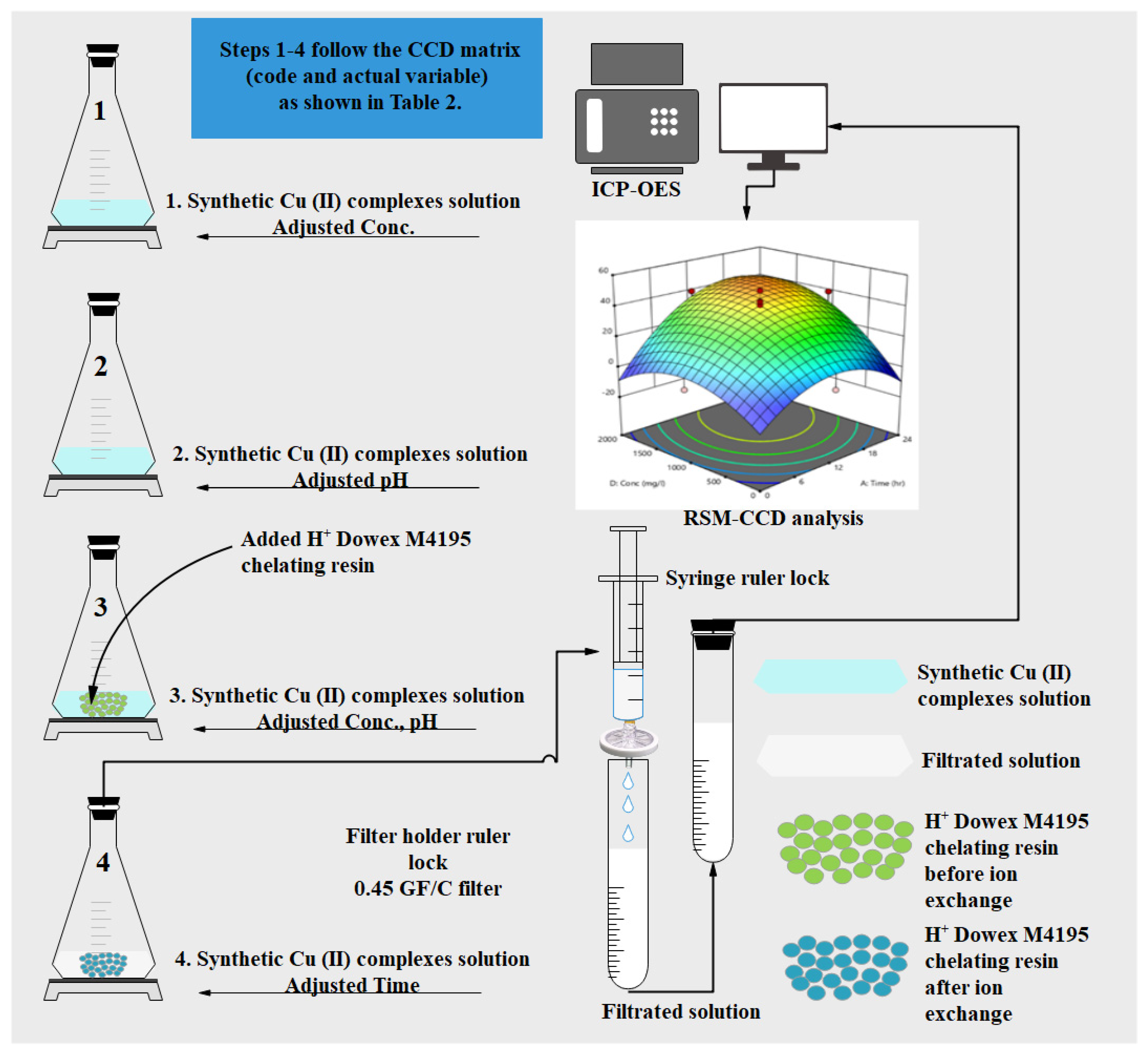
3.3. RSM Relevant Statistical Analysis for Cu(II) Removal Using H+ Dowex-M4195 Chelating Resin Adsorbent
3.4. Optimization via Batch Ion Exchange Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Botelho Junior, A.B.; Pinheiro, É.F.; Espinosa, D.C.R.; Tenório, J.A.S.; Baltazar, M.d.P.G. Adsorption of lanthanum and cerium on chelating ion exchange resins: Kinetic and thermodynamic studies. Sep. Sci. Technol. 2022, 57, 60–69. [Google Scholar] [CrossRef]
- Fatah, A.I.L.A.E.; Elashry, S.M. La (III) Separation by Tri Octyl Phosphine Oxide (Cyanex 921) Based on Amberlite Xad-4 Chelating Resin. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2793–2805. [Google Scholar] [CrossRef]
- Aleynikov, S.A.; Ponomarenko, I.V.; Sorkinova, G. Sorption of Copper Ions from the Nitric Acid Solution of Electrolytic Refining of Silver with Using Chelating Resin Axionit BPA. J. Sib. Fed. Univ. Chem. 2022, 15, 45–56. [Google Scholar] [CrossRef]
- Vinco, J.; Junior, A.B.; Duarte, H.; Espinosa, D.; Tenorio, J. Purification of an iron contaminated vanadium solution through ion exchange resins. Miner. Eng. 2022, 176, 107337. [Google Scholar] [CrossRef]
- Elfeghe, S.; Anwar, S.; Zhang, Y. Adsorption and removal studies of cadmium ion onto sulphonic/phosphonic acid functionalization resins. Can. J. Chem. Eng. 2022, 100, 3006–3014. [Google Scholar] [CrossRef]
- De Amorim, L.H.M.; Aliprandini, P.; Botelho Junior, A.B.; Jiménez Correa, M.M.; Espinosa, D.C.R. Effect of Impurities in the Recovery of Critical Metals: The Case of Nickel Laterite in the Solvent Extraction Process. J. Sustain. Metall. 2022, 8, 501–510. [Google Scholar] [CrossRef]
- Yang, X.; Ma, N.; Jia, Y.; Huang, J.; Zhang, X. Separation and Recovery Process of Copper (II) and Nickel (II) from Wastewater Using Ion Exchange Fiber. ChemistrySelect 2021, 6, 12985–12997. [Google Scholar] [CrossRef]
- Hassan, A.U.; Sumrra, S.H. Exploring the bioactive sites of new sulfonamide metal chelates for multi-drug resistance: An experimental versus theoretical design. J. Inorg. Organomet. Polym. Mater. 2022, 32, 513–535. [Google Scholar] [CrossRef]
- Chakraborty, S.C.; Zaman, M.W.U.; Hoque, M.; Qamruzzaman, M.; Zaman, J.U.; Hossain, D.; Pramanik, B.K.; Nguyen, L.N.; Nghiem, L.D.; Mofijur, M.; et al. Metals extraction processes from electronic waste: Constraints and opportunities. Environ. Sci. Pollut. Res. 2022, 29, 32651–32669. [Google Scholar] [CrossRef]
- Suwannahong, K.; Sripirom, J.; Sirilamduan, C.; Thathong, V.; Kreetachart, T.; Panmuang, P.; Deepatana, A.; Punbut, S.; Wongcharee, S. Selective Chelating Resin for Copper Removal and Recovery in Aqueous Acidic Solution Generated from Synthetic Copper-Citrate Complexes from Bioleaching of E-waste. Adsorpt. Sci. Technol. 2022, 2022, 5009124. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D. Leaching and Selective Recovery of Cu from Printed Circuit Boards. Metals 2019, 9, 1034. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Yang, J. Leaching copper from shredded particles of waste printed circuit boards. J. Hazard. Mater. 2011, 187, 393–400. [Google Scholar] [CrossRef]
- Tong, X.-j.; Li, J.-y.; Yuan, J.-h.; Xu, R.-k. Adsorption of Cu(II) by biochars generated from three crop straws. Chem. Eng. J. 2011, 172, 828–834. [Google Scholar] [CrossRef]
- Suwannahong, K.; Wongcharee, S.; Kreetachart, T.; Sirilamduan, C.; Rioyo, J.; Wongphat, A. Evaluation of the Microsoft Excel Solver Spreadsheet-Based Program for nonlinear expressions of adsorption Isotherm models onto magnetic nanosorbent. Appl. Sci. 2021, 11, 7432. [Google Scholar] [CrossRef]
- Pahasup-anan, T.; Kreetachat, T.; Ruengphrathuengsuka, W.; Wongcharee, S.; Usahanunth, N.; Imman, S.; Suwannahong, K. Dust Explosion Risk Assessment of Extruded Food Production Process by Fault Tree Analysis. ACS Chem. Health Saf. 2021, 29, 91–97. [Google Scholar] [CrossRef]
- Suwannahong, K.; Kreetachat, T.; Wongcharee, S. Application of photocatalytic oxidation process using modified TiO2/PBS biocomposite film for dye removal. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; p. 012013. [Google Scholar]
- Kalak, T.; Cierpiszewski, R.; Ulewicz, M. High efficiency of the removal process of Pb (II) and Cu(II) ions with the use of fly ash from incineration of sunflower and wood waste using the CFBC technology. Energies 2021, 14, 1771. [Google Scholar] [CrossRef]
- Artiningsih, A.; Zubair, H.; Imran, A.; Widodo, S. Distribution of Cu metal on the soil around the landfills of Antang, Makassar City. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; p. 012021. [Google Scholar]
- Wang, L.; Jiang, J.; Ma, J.; Pang, S.; Zhang, T. A review on advanced oxidation processes homogeneously initiated by copper (II). Chem. Eng. J. 2022, 427, 131721. [Google Scholar] [CrossRef]
- Miao, W.; Laughlin, D. Effects of Cu content and preaging on precipitation characteristics in aluminum alloy 6022. Metall. Mater. Trans. A 2000, 31, 361–371. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, S.; Albarakaty, F.M.; Kalia, A.; Hassan, M.M.; Abd-Elsalam, K.A. Biosorption and Bioleaching of Heavy Metals from Electronic Waste Varied with Microbial Genera. Sustainability 2022, 14, 935. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef]
- Chatterjee, S. Sustainable electronic waste management and recycling process. Am. J. Environ. Eng. 2012, 2, 23–33. [Google Scholar] [CrossRef]
- Saidan, M.; Brown, B.; Valix, M. Leaching of electronic waste using biometabolised acids. Chin. J. Chem. Eng. 2012, 20, 530–534. [Google Scholar] [CrossRef]
- Barragan, J.A.; Ponce de León, C.; Alemán Castro, J.R.; Peregrina-Lucano, A.; Gómez-Zamudio, F.; Larios-Durán, E.R. Copper and Antimony Recovery from Electronic Waste by Hydrometallurgical and Electrochemical Techniques. ACS Omega 2020, 5, 12355–12363. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, P.; Zhu, N.; Zhang, T.; Liu, W.; Wu, J.; Li, P. Bioleaching of copper from waste printed circuit boards by bacterial consortium enriched from acid mine drainage. J. Hazard. Mater. 2010, 184, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Valix, M. Bioleaching of electronic waste using acidophilic sulfur oxidising bacteria. J. Clean. Prod. 2014, 65, 465–472. [Google Scholar] [CrossRef]
- Hubicki, Z.; Kołodyńska, D. Selective removal of heavy metal ions from waters and waste waters using ion exchange methods. Ion Exch. Technol. 2012, 7, 193–240. [Google Scholar]
- Huang, Q.; Lin, X.; Xiong, L.; Huang, C.; Zhang, H.; Luo, M.; Tian, L.; Chen, X. Equilibrium, kinetic and thermodynamic studies of acid soluble lignin adsorption from rice straw hydrolysate by a self-synthesized macro/mesoporous resin. RSC Adv. 2017, 7, 23896–23906. [Google Scholar] [CrossRef]
- Marsh, H.; Reinoso, F.R. Activated Carbon; Elsevier Science: Amsterdam, the Netherlands, 2006. [Google Scholar]
- Yang, X.; Wu, L.; Ma, L.; Li, X.; Wang, T.; Liao, S. Pd nano-particles (NPs) confined in titanate nanotubes (TNTs) for hydrogenation of cinnamaldehyde. Catal. Commun. 2015, 59, 184–188. [Google Scholar] [CrossRef]
- Kumar, K.V.; Gadipelli, S.; Wood, B.; Ramisetty, K.A.; Stewart, A.A.; Howard, C.A.; Brett, D.J.L.; Rodriguez-Reinoso, F. Characterization of the adsorption site energies and heterogeneous surfaces of porous materials. J. Mater. Chem. A 2019, 7, 10104–10137. [Google Scholar] [CrossRef]
- Muttakin, M.; Mitra, S.; Thu, K.; Ito, K.; Saha, B.B. Theoretical framework to evaluate minimum desorption temperature for IUPAC classified adsorption isotherms. Int. J. Heat Mass Transf. 2018, 122, 795–805. [Google Scholar] [CrossRef]
- Rahman, M.M.; Muttakin, M.; Pal, A.; Shafiullah, A.Z.; Saha, B.B. A Statistical Approach to Determine Optimal Models for IUPAC-Classified Adsorption Isotherms. Energies 2019, 12, 4565. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Li, X.D.; Goh, S. Specific interactions and phase behavior of ternary poly (2-vinylpyridine)/poly (N-vinyl-2-pyrrolidone)/bisphenol A blends. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1125–1134. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- McCarthy, S.A.; Davies, G.-L.; Gun’ko, Y.K. Preparation of multifunctional nanoparticles and their assemblies. Nat. Protoc. 2012, 7, 1677–1693. [Google Scholar] [CrossRef]
- Harbi, S.; Guesmi, F.; Tabassi, D.; Hannachi, C.; Hamrouni, B. Application of response surface methodology and artificial neural network: Modeling and optimization of Cr (VI) adsorption process using Dowex 1X8 anion exchange resin. Water Sci. Technol. 2016, 73, 2402–2412. [Google Scholar] [CrossRef]
- Usman, M.S.; Ibrahim, N.A.; Shameli, K.; Zainuddin, N.; Yunus, W.M.Z.W. Copper Nanoparticles Mediated by Chitosan: Synthesis and Characterization via Chemical Methods. Molecules 2012, 17, 14928–14936. [Google Scholar] [CrossRef]
- Phul, R.; Kaur, C.; Farooq, U.; Ahmad, T. Ascorbic acid assisted synthesis, characterization and catalytic application of copper nanoparticles. Mater. Sci. Eng. Int. J. 2018, 2, 90–94. [Google Scholar]
- Kalidhasan, S.; Kumar, A.S.K.; Rajesh, V.; Rajesh, N. An efficient ultrasound assisted approach for the impregnation of room temperature ionic liquid onto Dowex 1 × 8 resin matrix and its application toward the enhanced adsorption of chromium (VI). J. Hazard. Mater. 2012, 213, 249–257. [Google Scholar] [CrossRef]
- Suwannahong, K.; Wongcharee, S.; Rioyo, J.; Sirilamduan, C.; Kreetachart, T. Insight into molecular weight cut off characteristics and reduction of melanoidin using microporous and mesoporous adsorbent. Eng. Appl. Sci. Res. 2021, 49, 47–57. [Google Scholar]
- Wongcharee, S.; Aravinthan, V. Application of mesoporous magnetic nanosorbent developed from macadamia nut shell residues for the removal of recalcitrant melanoidin and its fractions. Sep. Sci. Technol. 2020, 55, 1636–1649. [Google Scholar] [CrossRef]
- Suwannahong, K.; Wongcharee, S.; Kreanuarte, J.; Kreetachat, T. Pre-treatment of acetic acid from food processing wastewater using response surface methodology via Fenton oxidation process for sustainable water reuse. J. Sustain. Dev. Energy Water Environ. Syst. 2021, 9, 1080363. [Google Scholar] [CrossRef]
- Florence, T.M.; Batley, G. Trace metals species in sea-water—I: Removal of trace metals from sea-water by a chelating resin. Talanta 1976, 23, 179–186. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Hubicki, Z.; Pasieczna-Patkowska, S. FT-IR/PAS studies of Cu(II)–EDTA complexes sorption on the chelating ion exchangers. Acta Phys. Pol. A 2009, 116, 340–343. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Ling, P.; Jing, X.; Li, C.; Li, A.; You, X. Interaction mechanism of aqueous heavy metals onto a newly synthesized IDA-chelating resin: Isotherms, thermodynamics and kinetics. Chem. Eng. J. 2011, 173, 106–114. [Google Scholar] [CrossRef]
- Brandl, H.; Bosshard, R.; Wegmann, M. Computer-munching microbes: Metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy 2001, 59, 319–326. [Google Scholar] [CrossRef]
- Brandl, H.; Faramarzi, M.A. Microbe-metal-interactions for the biotechnological treatment of metal-containing solid waste. China Particuology 2006, 4, 93–97. [Google Scholar] [CrossRef]
- Faraji, F.; Golmohammadzadeh, R.; Rashchi, F.; Alimardani, N. Fungal bioleaching of WPCBs using Aspergillus niger: Observation, optimization and kinetics. J. Environ. Manag. 2018, 217, 775–787. [Google Scholar] [CrossRef]

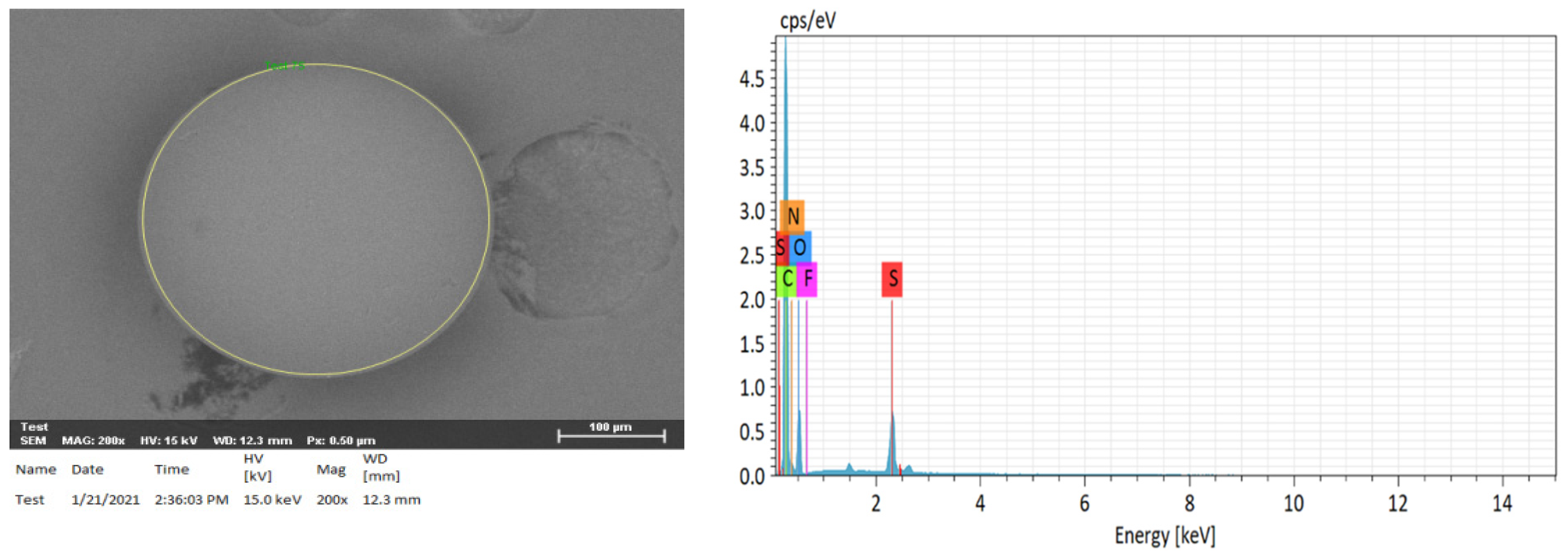
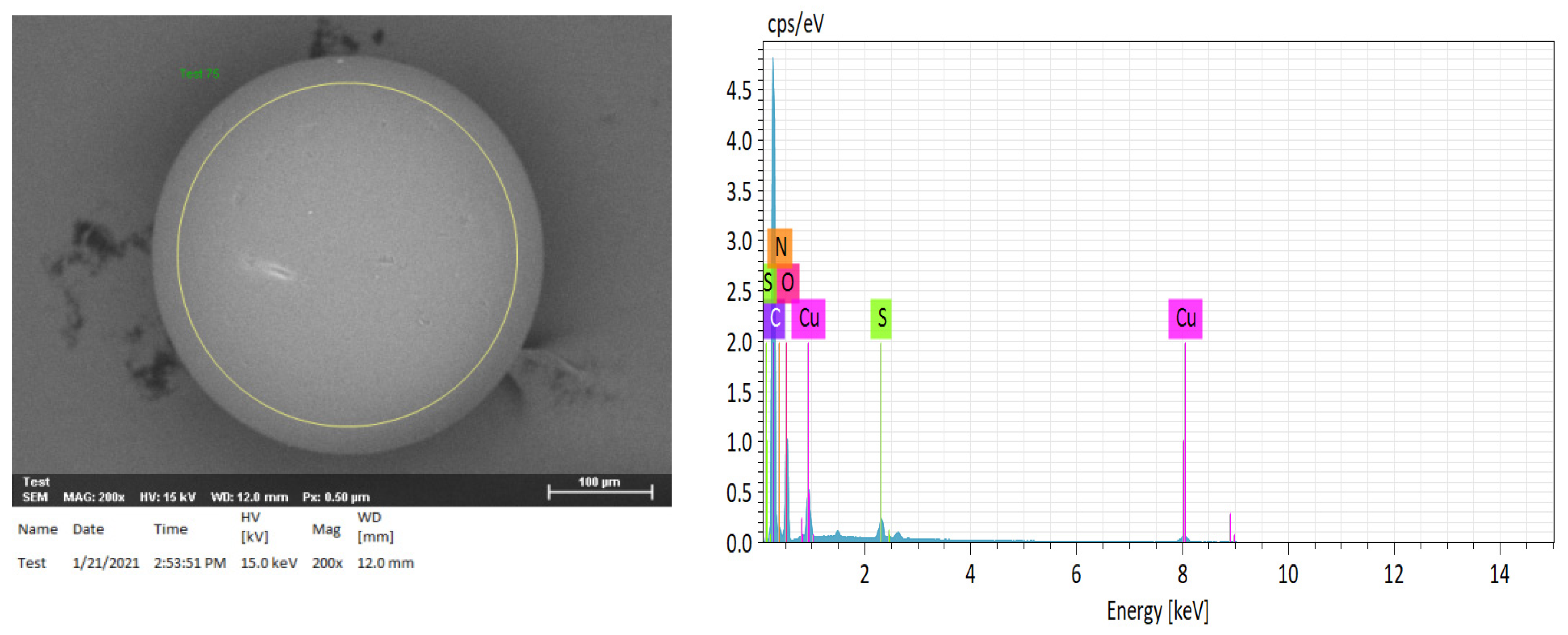





| Element | H+ Dowex-M4195 (wt %) | Cu(II)-Loaded H+ Dowex-M4195 (wt %) |
|---|---|---|
| Carbon (C) | 64.50 | 58.51 |
| Oxygen (O) | 20.26 | 24.82 |
| Nitrogen (N) | 11.77 | 11.00 |
| Fluorine (F) | 0.38 | ND |
| Sulfur (S) | 3.01 | 1.01 |
| Copper (Cu) | ND | 4.66 |
| Method | Physical Properties | H+ Dowex-M4195 | Cu(II)-Loaded H+ Dowex-M4195 |
|---|---|---|---|
| BET | Specific surface area (m2/g) | 26.5060 | 21.7810 |
| Total pore volume (cm3/g) | 0.2892 | 0.2687 | |
| Micropore volume (cm3/g) | LD | LD | |
| Mesopore volume (cm3/g) | LD | LD | |
| Macropore volume (cm3/g) | 0.2892 | 0.2687 | |
| Average pore diameter Å (angstrom) | 493.6370 | 436.5590 | |
| BJH | Specific surface area (m2/g) | 28.2635 | 24.2043 |
| Total pore volume (cm3/g) | 0.2690 | 0.2545 | |
| Micropore volume (cm3/g) | LD | LD | |
| Mesopore volume (cm3/g) | LD | LD | |
| Macropore volume (cm3/g) | 0.2690 | 0.2545 | |
| Average pore diameter Å (angstrom) | 380.7060 | 420.6690 |
| Source | Sequential p-Value | Lack of Fit p-Value | Standard Derivative | R-Squared | Adjusted R-Squared | Predicted R-Squared | PRESS | |
|---|---|---|---|---|---|---|---|---|
| Linear | 0.0055 | 0.0023 | 18.06 | 0.4316 | 0.3407 | 0.1624 | 12010.91 | |
| 2FI | 0.3545 | 0.0023 | 17.66 | 0.5866 | 0.3690 | −0.3035 | 18690.54 | |
| Quadratic | <0.0001 | 0.2775 | 5.82 | 0.9645 | 0.9314 | 0.8553 | 2075.51 | Suggested |
| Cubic | 0.1563 | 0.9968 | 4.33 | 0.9921 | 0.9620 | - | * | Aliased |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 13,830.46 | 14 | 987.89 | 29.13 | <0.0001 | significant |
| x1-Time | 2770.33 | 1 | 2770.33 | 81.69 | <0.0001 | |
| x2-pH | 7.24 | 1 | 7.24 | 0.2134 | 0.6508 | |
| x3-Dose | 69.06 | 1 | 69.06 | 2.04 | 0.1741 | |
| x4-Conc. | 3011.38 | 1 | 3011.38 | 88.79 | <0.0001 | |
| x1·x2 | 2.50 | 1 | 2.50 | 0.0736 | 0.7899 | |
| x1·x3 | 22.47 | 1 | 22.47 | 0.6625 | 0.4284 | |
| x1·x4 | 2121.52 | 1 | 2121.52 | 62.56 | <0.0001 | |
| x2·x3 | 0.0004 | 1 | 0.0004 | 0.0000 | 0.9973 | |
| x2·x4 | 2.50 | 1 | 2.50 | 0.0736 | 0.7899 | |
| x3·x4 | 40.30 | 1 | 40.30 | 1.19 | 0.2929 | |
| x12 | 1188.83 | 1 | 1188.83 | 35.05 | <0.0001 | |
| x22 | 4.81 | 1 | 4.81 | 0.1417 | 0.7118 | |
| x32 | 120.71 | 1 | 120.71 | 3.56 | 0.0787 | |
| x42 | 756.00 | 1 | 756.00 | 22.29 | 0.0003 | |
| Residual | 508.72 | 15 | 33.91 | |||
| Lack of Fit | 395.98 | 10 | 39.60 | 1.76 | 0.2775 | not significant |
| Pure Error | 112.73 | 5 | 22.55 | |||
| Cor Total | 14,339.18 | 29 |
| Run | Code Variable | Actual Variable | Responses qe (mg/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x4 | x1 | x2 | x3 | x4 | Observed | Predicted | Residual | ||
| Value | Value | Value | ||||||||||
| 1 | −1 | −1 | 1 | 1 | 0 | 1 | 500 | 2000 | 0.00 | −2.70 | 2.70 | |
| 2 | −1 | −1 | 1 | −1 | 0 | 1 | 500 | 0 | 0.00 | −1.25 | 1.25 | |
| 3 | 0 | 0 | −1 | 1 | 12 | 5 | 100 | 2000 | 53.20 | 47.18 | 6.02 | |
| 4 | 0 | 0 | 0 | 1 | 12 | 5 | 300 | 2000 | 39.20 | 35.75 | 3.45 | |
| 5 | 1 | 0 | 0 | 0 | 24 | 5 | 300 | 1000 | 39.07 | 32.33 | 6.74 | |
| 6 | 1 | 1 | −1 | −1 | 24 | 9 | 100 | 0 | 0.00 | 1.86 | −1.86 | |
| 7 | 0 | 0 | 0 | −1 | 12 | 5 | 300 | 0 | 0.00 | 10.25 | −10.25 | |
| 8 | 0 | 0 | 0 | 0 | 12 | 5 | 300 | 1000 | 40.93 | 40.65 | 0.28 | |
| 9 | 0 | 0 | 0 | 0 | 12 | 5 | 300 | 1000 | 37.07 | 40.65 | −3.59 | |
| 10 | 0 | 0 | 0 | 0 | 12 | 5 | 300 | 1000 | 37.73 | 40.65 | −2.92 | |
| 11 | −1 | 1 | 1 | −1 | 0 | 9 | 500 | 0 | 0.00 | −1.57 | 1.57 | |
| 12 | 1 | −1 | −1 | −1 | 24 | 1 | 100 | 0 | 0.00 | 0.58 | −0.58 | |
| 13 | −1 | 0 | 0 | 0 | 0 | 5 | 300 | 1000 | 0.00 | 7.51 | −7.51 | |
| 14 | −1 | −1 | −1 | 1 | 0 | 1 | 100 | 2000 | 0.00 | 2.02 | −2.02 | |
| 15 | −1 | 1 | −1 | 1 | 0 | 9 | 100 | 2000 | 0.00 | 3.30 | −3.30 | |
| 16 | 1 | 1 | 1 | −1 | 24 | 9 | 500 | 0 | 0.00 | −1.37 | 1.37 | |
| 17 | −1 | 1 | −1 | −1 | 0 | 9 | 100 | 0 | 0.00 | −3.08 | 3.08 | |
| 18 | 1 | 1 | −1 | 1 | 24 | 9 | 100 | 2000 | 52.40 | 54.30 | −1.90 | |
| 19 | 1 | −1 | −1 | 1 | 24 | 1 | 100 | 2000 | 69.20 | 51.44 | −2.24 | |
| 20 | −1 | 1 | 1 | 1 | 0 | 9 | 500 | 2000 | 0.00 | −1.44 | 1.44 | |
| 21 | 0 | 0 | 0 | 0 | 12 | 5 | 300 | 1000 | 50.13 | 40.65 | 9.48 | |
| 22 | 1 | −1 | 1 | 1 | 24 | 1 | 500 | 2000 | 79.76 | 41.98 | −2.22 | |
| 23 | 1 | 1 | 1 | 1 | 24 | 9 | 500 | 2000 | 42.88 | 44.82 | −1.94 | |
| 24 | 0 | 0 | 0 | 0 | 12 | 5 | 300 | 1000 | 43.07 | 40.65 | 2.41 | |
| 25 | 0 | 0 | 0 | 0 | 12 | 5 | 300 | 1000 | 43.33 | 40.65 | 2.68 | |
| 26 | 1 | −1 | 1 | −1 | 24 | 1 | 500 | 0 | 0.00 | −2.63 | 2.63 | |
| 27 | 0 | −1 | 0 | 0 | 12 | 1 | 300 | 1000 | 67.04 | 41.34 | −2.30 | |
| 28 | 0 | 1 | 0 | 0 | 12 | 9 | 300 | 1000 | 44.13 | 42.61 | 1.53 | |
| 29 | −1 | −1 | −1 | −1 | 0 | 1 | 100 | 0 | 0.00 | −2.78 | 2.78 | |
| 30 | 0 | 0 | 1 | 0 | 12 | 5 | 500 | 1000 | 39.76 | 46.55 | −6.79 | |
| Factor/Name | Unit | Levels (Coding Actual) | ||
|---|---|---|---|---|
| −1 (Low Level) | 0 (Central Level) | 1 (High Level) | ||
| x1, Time | h | 0 | 12 | 24 |
| x2, pH | - | 1 | 5 | 9 |
| x3, Dose | mg | 100 | 300 | 500 |
| x4, Conc. | ppm | 0 | 1000 | 2000 |
| Run | Code Variable | Actual Variable | ||||||
|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x4 | x1 | x2 | x3 | x4 | |
| 1 | −1 | −1 | 1 | 1 | 0 | 1 | 500 | 2000 |
| 2 | −1 | −1 | 1 | −1 | 0 | 1 | 500 | 0 |
| 3 | 0 | 0 | −1 | 1 | 12 | 5 | 100 | 2000 |
| 4 | 0 | 0 | 0 | 1 | 12 | 5 | 300 | 2000 |
| 5 | 1 | 0 | 0 | 0 | 24 | 5 | 300 | 1000 |
| 6 | 1 | 1 | −1 | −1 | 24 | 9 | 100 | 0 |
| 7 | 0 | 0 | 0 | −1 | 12 | 5 | 300 | 0 |
| 8 | 0 | 0 | 0 | 0 | 12 | 5 | 300 | 1000 |
| 9 | 0 | 0 | 0 | 0 | 12 | 5 | 300 | 1000 |
| 10 | 0 | 0 | 0 | 0 | 12 | 5 | 300 | 1000 |
| 11 | −1 | 1 | 1 | −1 | 0 | 9 | 500 | 0 |
| 12 | 1 | −1 | −1 | −1 | 24 | 1 | 100 | 0 |
| 13 | −1 | 0 | 0 | 0 | 0 | 5 | 300 | 1000 |
| 14 | −1 | −1 | −1 | 1 | 0 | 1 | 100 | 2000 |
| 15 | −1 | 1 | −1 | 1 | 0 | 9 | 100 | 2000 |
| 16 | 1 | 1 | 1 | −1 | 24 | 9 | 500 | 0 |
| 17 | −1 | 1 | −1 | −1 | 0 | 9 | 100 | 0 |
| 18 | 1 | 1 | −1 | 1 | 24 | 9 | 100 | 2000 |
| 19 | 1 | −1 | −1 | 1 | 24 | 1 | 100 | 2000 |
| 20 | −1 | 1 | 1 | 1 | 0 | 9 | 500 | 2000 |
| 21 | 0 | 0 | 0 | 0 | 12 | 5 | 300 | 1000 |
| 22 | 1 | −1 | 1 | 1 | 24 | 1 | 500 | 2000 |
| 23 | 1 | 1 | 1 | 1 | 24 | 9 | 500 | 2000 |
| 24 | 0 | 0 | 0 | 0 | 12 | 5 | 300 | 1000 |
| 25 | 0 | 0 | 0 | 0 | 12 | 5 | 300 | 1000 |
| 26 | 1 | −1 | 1 | −1 | 24 | 1 | 500 | 0 |
| 27 | 0 | −1 | 0 | 0 | 12 | 1 | 300 | 1000 |
| 28 | 0 | 1 | 0 | 0 | 12 | 9 | 300 | 1000 |
| 29 | −1 | −1 | −1 | −1 | 0 | 1 | 100 | 0 |
| 30 | 0 | 0 | 1 | 0 | 12 | 5 | 500 | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwannahong, K.; Sirilamduan, C.; Deepatana, A.; Kreetachat, T.; Wongcharee, S. Characterization and Optimization of Polymeric Bispicolamine Chelating Resin: Performance Evaluation via RSM Using Copper in Acid Liquors as a Model Substrate through Ion Exchange Method. Molecules 2022, 27, 7210. https://doi.org/10.3390/molecules27217210
Suwannahong K, Sirilamduan C, Deepatana A, Kreetachat T, Wongcharee S. Characterization and Optimization of Polymeric Bispicolamine Chelating Resin: Performance Evaluation via RSM Using Copper in Acid Liquors as a Model Substrate through Ion Exchange Method. Molecules. 2022; 27(21):7210. https://doi.org/10.3390/molecules27217210
Chicago/Turabian StyleSuwannahong, Kowit, Chadrudee Sirilamduan, Anat Deepatana, Torpong Kreetachat, and Surachai Wongcharee. 2022. "Characterization and Optimization of Polymeric Bispicolamine Chelating Resin: Performance Evaluation via RSM Using Copper in Acid Liquors as a Model Substrate through Ion Exchange Method" Molecules 27, no. 21: 7210. https://doi.org/10.3390/molecules27217210
APA StyleSuwannahong, K., Sirilamduan, C., Deepatana, A., Kreetachat, T., & Wongcharee, S. (2022). Characterization and Optimization of Polymeric Bispicolamine Chelating Resin: Performance Evaluation via RSM Using Copper in Acid Liquors as a Model Substrate through Ion Exchange Method. Molecules, 27(21), 7210. https://doi.org/10.3390/molecules27217210









