Abstract
Polydatin or 3-O-β-d-resveratrol-glucopyranoside (PD), a stilbenoid component of Polygonum cuspicadum (Polygonaceae), has a variety of biological roles. In traditional Chinese medicine, P. cuspicadum extracts are used for the treatment of infections, inflammation, and cardiovascular disorders. Polydatin possesses a broad range of biological activities including antioxidant, anti-inflammatory, anticancer, and hepatoprotective, neuroprotective, and immunostimulatory effects. Currently, a major proportion of the population is victimized with cervical lung cancer, ovarian cancer and breast cancer. PD has been recognized as a potent anticancer agent. PD could effectively inhibit the migration and proliferation of ovarian cancer cells, as well as the expression of the PI3K protein. The malignancy of lung cancer cells was reduced after PD treatments via targeting caspase 3, arresting cancer cells at the S phase and inhibiting NLRP3 inflammasome by downregulation of the NF-κB pathway. This ceases cell cycle, inhibits VEGF, and counteracts ROS in breast cancer. It also prevents cervical cancer by regulating epithelial-to-mesenchymal transition (EMT), apoptosis, and the C-Myc gene. The objective of this review is thus to unveil the polydatin anticancer potential for the treatment of various tumors, as well as to examine the mechanisms of action of this compound.
1. Introduction
Cancer, a multifactorial disease, is a rapidly growing condition in which cells grow abnormally and invade many parts of the body, showing a metastasis behavior. There are many types of cancer known so far [1]. Cancer of the breast, cervix, lungs, and ovaries are the most prevalent types of the disease. About 2.2 million new instances of lung cancer, 2.3 million new cases of breast cancer, and 0.6 million new cases of cervical cancer were detected globally in 2020. The number of new cases of ovarian cancer in 2018 was close to 0.3 million [2,3,4,5]. Totally, 10 million deaths have been estimated in 2020 by cancer and it has become the most prominent cause of mortality. Breast cancer and lung cancer are the leading types of cancer, with increased cases worldwide. Treatment strategies include anticancer drugs, chemotherapy, immunotherapy, and hormonal treatments [6]. Various types of cancers respond to conventional drug therapies such as alkylating drugs, intercalating agents, topoisomerase inhibitors, antimitotic drugs, and antimetabolites as well as kinase inhibitors, but mutations assist the cell to develop resistance. Targeted chemotherapy is effective in some malignancies, but the side effects on normal cells and its high cost have limited its use. Immunotherapy and targeted monoclonal antibodies have also been recognized as successful approaches against specific cancers, but a restricted number of cancers can be totally treated using these curative methods. Unluckily, resistance to cancer therapies, side effects, and high cost continue to be challenging and increase the rate of increased mortality [7].
On the other hand, plants have been employed since ancient times for the extraction of their valuable bioactive compounds to promote the health and well-being of people [8]. Despite the popup of synthetic therapeutic molecules, natural products are still in use for the mitigation and prevention of diseases [9]. Research is developing a means to find out phytochemicals from natural sources by utilizing different approaches [10]. Polydatin (PD), a compound belonging to the stilbene family [11,12,13], is extracted from the roots of Polygonum cuspidatum (Polygonaceae). PD is very famous in China because of its usage as a painkiller and febrifuge. The trans form of PD is well known for its high therapeutic potential [10]. The anticancer activity of PD is mediated by several mechanisms such as control of reactive oxygen species (ROS) [14] and suppression of the PI3K/AKT pathway [15]. In several investigations, PI3K/AKT inhibitors were found to improve the treatment effectiveness of 2-deoxy-D-glucose (2-DG) and PD [16,17]. Furthermore, PD has attracted a lot of attention because of its positive impact on glucose and lipid management [18]. PD has therefore enhanced 2-DG’s anticancer effects via regulating glucose metabolism, blocking the PI3K/AKT, or through other pathways. PD has been investigated to reduce the growth of HeLa cells by causing these cells to enter the S phase, promote cell death, and lower AKT, mTOR PI3K, mRNA expression levels. It was also discovered that PD may limit cervical cancer HeLa cell proliferation and induce apoptosis, and the process could be linked to blockage of the PI3K/AKT/mTOR signaling pathway and gene downstream expression [15]. The anticancer potential of PD has been observed by many researchers by using different cell lines like liver, cervical, and nasopharyngeal cancer cell lines [10]. The objective of this review is thus to unveil PD anticancer potential for the treatment of various tumors, as well as to examine the mechanisms of action of this molecule.
2. Polydatin Chemistry and Biosynthesis
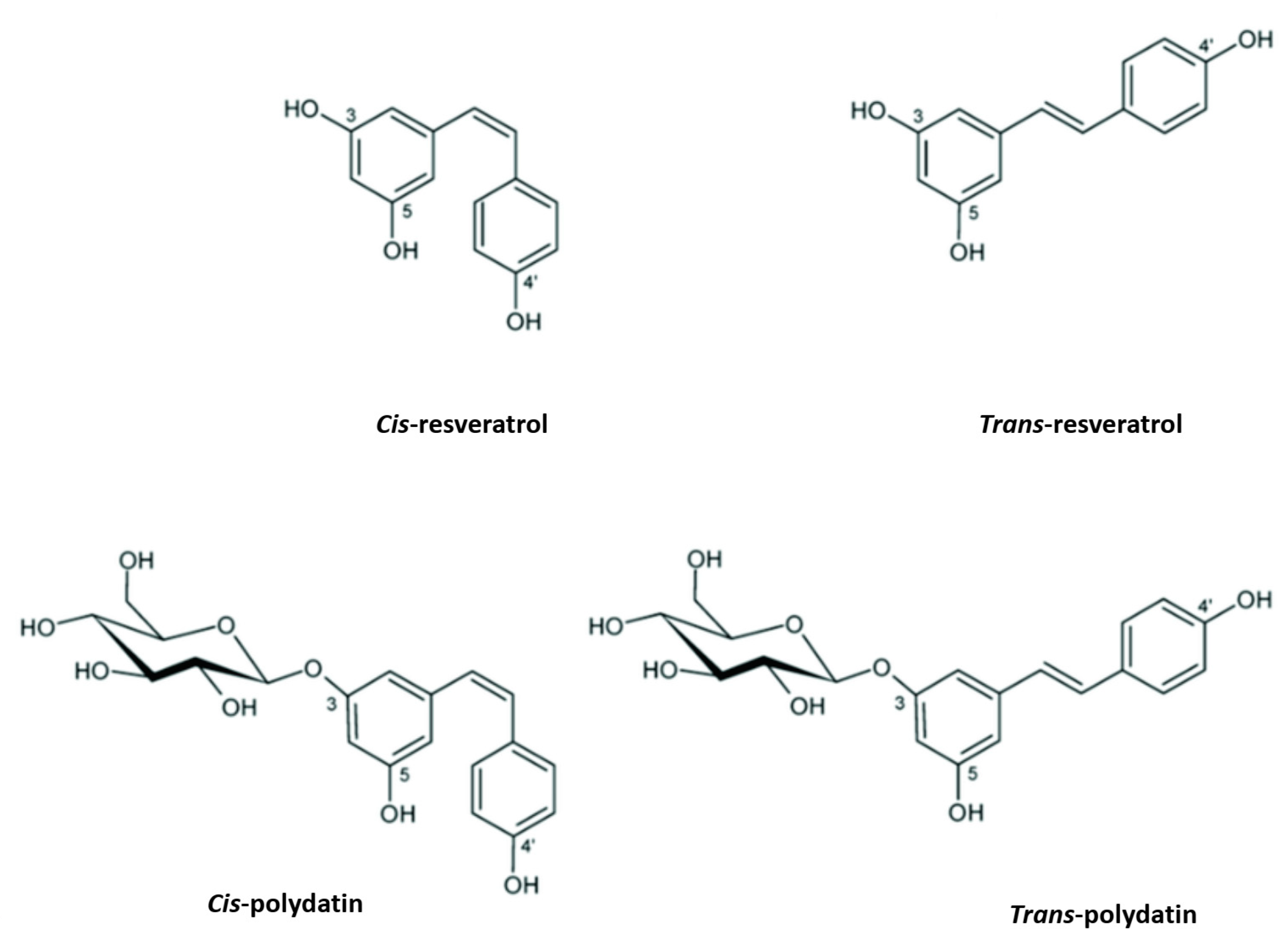
PD, also known as piceid (3-O-β-d-resveratrol glucopyranoside), (E)-polydatin, transpolydatin, (E)-piceid, is a monocrystalline substance that was first isolated from the roots and rhizome of P. cuspidatum Sieb. It is a stilbene derivative of the phytoalexin resveratrol (3,4′,5-trihydroxystilbene), in which the glucoside group linked to position C-3 replaces the hydroxyl group (Figure 1). The transisomers of stilbenes generally display a higher bioactivity than their cisisomer counterparts (Figure 1) [19]. Previously, scientists scrutinized this compound for its ability to help with heart- and liver-associated disorders [20,21].
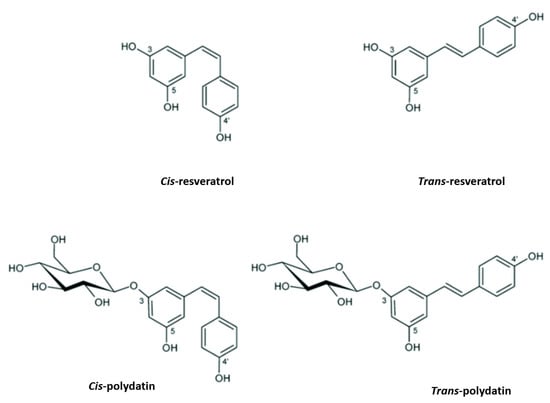
Figure 1.
Resveratrol and polydatin isomers, trans and cis.
The polyketide and phenylpropanoid routes are used to form PD. The first step in the production of PD is the deamination of phenylalanine by phenylalanine ammonia lyase (PAL), which affords cinnamic acid. Cinnamate-4-hydroxylase (C4H) subsequently hydroxylates cinnamic acid to produce p-coumaric acid. Coenzyme A (CoA) ligation then takes place through p-coumaroyl-CoA ligase activity. Finally, p-coumaroyl-CoA and three molecules of malonyl-CoA are combined together by stilbene synthase (STS) to produce resveratrol [22]. Transresveratrol can then be further metabolized to form additional stilbenoids, like polydatin, by the action of glucosyltransferases on resveratrol [23]. Interestingly, PD has also been produced on a small scale through microbial resveratrol transformation by the Bacillus cereus strain UI 1477 [24]. American pokeweed (Phytolacca americana L., Phytolaccaceae) cell suspension cultures have the ability to glucosylate transresveratrol and produce PD, as well [25]. An engineered Escherichia coli strain harboring tyrosine ammonia lyase, cinnamoyl/p-coumaroyl-coenzyme A ligase and stilbene synthase genes was used to produce PD [26].
The most common dietary sources of PD are peanuts, dairy products, chocolate, and grapes [27,28]. The greatest PD concentrations were found in cocoa powder (7.14 µg/g), followed by semisweet chocolate baking chips (2.01 µg/g), dark chocolates (1.8 µg/g), milk chocolates (0.44 µg/g), and chocolate syrups (0.35 µg/g). Nevertheless, red wine may contain as much as 29.2 mg/L of PD [19]. The highest concentration of PD in mulberry roots was 3.15 µg/g. fresh weight [29]. Since the glucoside content of PD typically exceeds that of the aglycone in red wine and other grape products, it has attracted a lot of interest, much more than resveratrol. The precise wine proportions of glycosylated to aglycone forms are affected by a variety of variables, including fermentation techniques and environmental conditions in the vineyards. Transresveratrol is found in red wine at concentrations of up to 14.3 mg/L and PD in concentrations of up to 29.2 mg/L, or about equal molar levels (60–70 µM) of the aglycone and the glucosylated form; white wine has 100 times less PD than red wine does [30]. In elaborating white wine, just the juice is fermented, whereas in making red wine, both the skins and the seeds are left on the juices until after fermentation is completed, leading to greater concentrations in the final product. Red grape skins typically have a higher PD content than white grape skins, but this can vary widely (from 50 to 200 mg/kg dry weight) across different varieties and vintages of the same variety of grape [31]. Spectrum analysis of eluting peaks from a HPLC system was used to determine the amounts of transpiceid, cispiceid, transresveratrol, and cisresveratrol in 36 different sorts of grape juices. Grape juices mostly included polydatin. The average levels of transpiceid, cispiceid, transresveratrol, and cisresveratrol in red grape juices were 3.38 mg/L, 0.79 mg/L, 0.50 mg/L, and 0.06 mg/L, respectively [32].
The Chinese resident meals guide recommends that adults consume 500 g of vegetables and 200 to 400 g of fruits daily; if we assume according to this recommendation by eating 200 g of celery, 100 g of chili pepper, 200 g of edible amaranth or leaf lettuce, 10 g of black soya beans, and one apple (200 g), then our daily PD intake would range from 100 to 1700 mg. The mulberry may increase our daily PD consumption to 3700 mg. The effective dose of PD intake can be around 2500–5000 mg/day for a 50 kg adult [33].
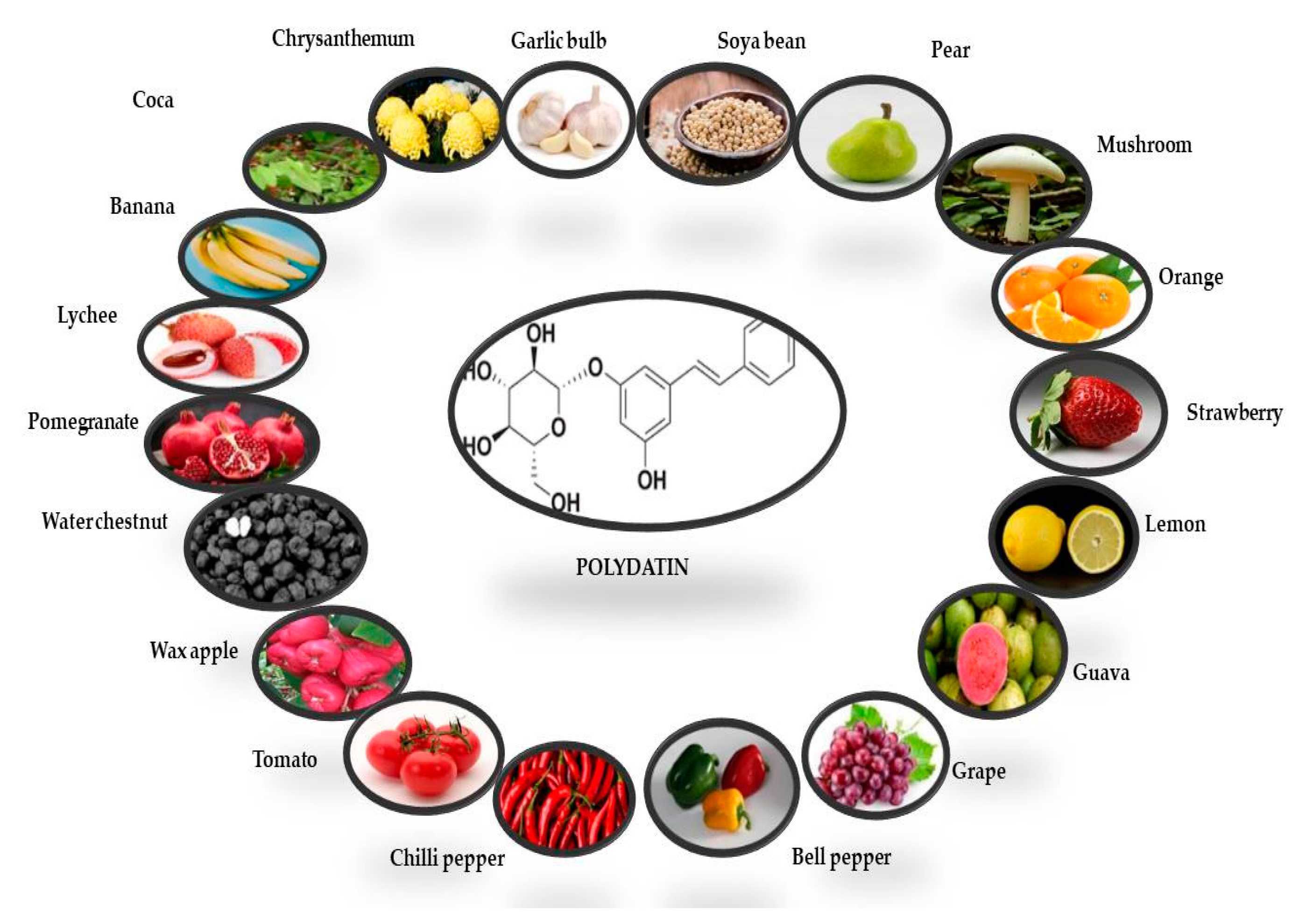
The presence of PD was first reported in the grape skin. Red, white, and grape juices are the main sources of PD, while rosé and effervescent wines mostly contain cisPD. Transresveratrol is more prevalent in grapes, berries, peanuts, and pistachios [34]. PD can also be found in a variety of fruit and vegetable foods, beer, cocoa-containing goods, and chocolate products (Figure 2) [35,36]
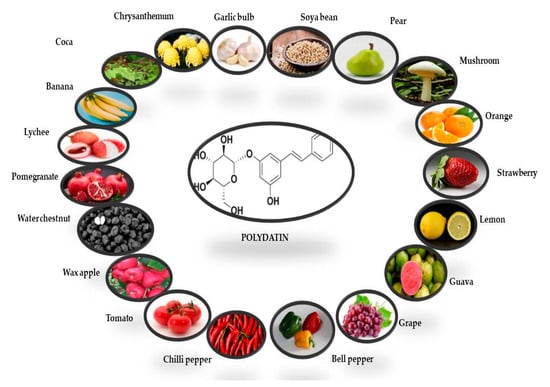
Figure 2.
Potential dietary sources of polydatin derivative (transisomer). The quantities of PD in each fruit and vegetable have been derived from Peng et al. [35].
The primary source of PD is the roots and rhizomes of Fallopia japonica, which have a long history of use in traditional Chinese and Japanese medicines such as analgesics, antipyretics, diuretics, anticancers and expectorants, as well as in the treatment of atherosclerosis [37]. However, this compound is found in a number of other taxa, including Rosa, Rumex, Picea, Malus, and species of Quercus [38]. Peng et al. [35] used chromatographic techniques to quantify polydatin in fruits and vegetables. The polydatin contents of some vegetables and fruits are summarized in Table 1.

Table 1.
Polydatin contents in vegetable and fruits samples.
3. Role of Polydatin in Cancer
PD partly exerts its anticancer activity by enhancing antioxidant activity. PD, like other polyphenols, carries out strong antioxidant activity by neutralizing ROS and boosting the body’s natural antioxidant defences. Its chemical structure of a long-conjugated system confers the compound its substantial antioxidant effects. The resistance of PD to enzymatic oxidation was found to be higher than that of resveratrol. It appears that many of the polydatin biological actions are mediated by antioxidant pathways. In vitro, PD displayed IC50 values of 87, 20, and 125 μg/mL for scavenging of the free radicals ABTS (2,2′-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid), and DPPH (2,2-Diphenyl-1-(2,4,6-trinitrophenyl)-hydrazyl), and •O2, respectively [41]. The scavenging effect of PD increases in a dose-dependent manner (0.05–2 μM) in the phenanthroline-Fe2+ system, and PD was shown to exhibit a scavenging activity of hydroxyl radicals more effective than those of resveratrol or vitamin C [42].
The earlier investigations from Yousef et al. [43] have reported that PD reduces ROS generation to protect the cell from oxidative stress. Therefore, oxidative stress was induced with H2O2 in RINm5F cells, and these latter were treated either with or without PD (20 and 40 μg/mL, 24 h), intracellular ROS being evaluated by the dichloro-fluorescein (DCF) assay. The mean fluorescence intensity (MFI) of cells treated with H2O2 was significantly higher compared to cells treated with a negative control dye, suggesting a buildup of ROS. Treatment with PD (40 g/mL) effectively mitigated the formation of ROS due to PD antioxidant properties. [43]. Yousef et al. [43] also depicted pancreatic lipid peroxidation as being significantly reduced in PD-treated diabetic rats as a consequence of an increase in the antioxidant enzymatic activity of catalase (CAT), superoxide dismutase (SOD), and GPx, following oral therapy with PD.
Otherwise, the anticancer activity of PD on tumor growth has been extensively studied in several cell culture and animal tumor models. Oncology has now been recognized as the most important area of concern in the field of cancer research [44]. Various approaches being used currently include chemotherapy, immune therapy, radiotherapy, surgery, drug combination, antibodies, and some others, all of them having their own side effects. Several researchers tried to combine different targeted cancer therapies to increase their effectiveness and more significantly hinder resistance to therapy; unfortunately, clinical trials have not shown satisfactory results [44]. PD has been recognized as a potent anticancer agent, with the ability to regulate various signaling pathways involved in the progression of several kinds of cancers [45]. The mechanisms by which PD acts in cancer include cell cycle regulation, apoptosis, autophagy, signaling pathways, epithelial-to-mesenchymal transition (EMT), inhibition of inflammation and metastasis, and regulation of enzymes related to oxidative stress [46,47,48].
4. Anticancer Activity of Polydatin on Liver, Colon, Bone, Breast, Lung, Cervical, and Ovarian Cancer Proliferation
4.1. Liver Cancer
Cancers are the top cause of death for people worldwide, especially those who are 55 and older. Chemotherapy is still the best option for many types of cancer when surgery has been exhausted. Hepatocellular carcinoma (HCC), lung cancer, and breast cancer are just a few examples of the many tumors for which promising results have been obtained from the use of natural substances such as potential medications in recent years [49,50]. Primary HCC is a prevalent secondary malignancy in patients with cirrhosis and other chronic liver disorders. Among cancer-related fatalities, HCC ranks third [51]. Unfortunately, advanced HCC cannot be effectively treated with currently available chemotherapeutic drugs [50,52]. To this end, it is important to have more potent chemicals that might lead to new therapies for treating HCC, especially in its later stages. PD exhibited considerable cytotoxicity in a concentration- and time-dependent manner against HCC (hepatocellular carcinoma) cells at 100 µM and 150 µM concentrations, inducing apoptosis and limited G2/M cell cycle arrest while phosphorylated p-signal transducer and activator of transcription 3 (STAT3), p-Janus kinase 1 and (p)-protein kinase B (AKT) were downregulated [53]. PD may also induce apoptosis by increasing the Bax/Bcl-2 ratio and lowering the Wnt/-catenin signaling in SMMC-7721 and HepG2 cells, both of which are used for the modelling of hepatocellular cancer. Cancer metastasis is thought to be facilitated in large part by the invasion and migration of cancer cells. Treatment with PD inhibited the invasion and migration of HCC cells in two different assays: one measuring invasion and the other measuring wound healing [54]. This suggests that PD may be a useful natural small molecule medication for the treatment of liver cancer at an early stage.
4.2. Colon Cancer
PD inhibited cell differentiation of CaCo-2 human colon cancer cells through inhibition of Hsp27 and vimentin expression (IC50 values of 72 and 192 μM for exponentially developing and postconfluent cells, respectively). After treatment with PD (240 μM), the cell cycle arrested at the G1 phase, coinciding with an increase in the cleaved poly-(ADP-ribose) polymerase. Both the total and phosphorylated versions of Akt were decreased though ERK1/2 phosphorylation and p21 expression, which were both enhanced in the CaCo-2 cell line [55]. The growth inhibition of Caco-2 intestinal epithelial cells exerted by PD was concentration-dependent (1–50 μM) and occurred via cell cycle arrest in the G0/G1 (10–25 μM) and apoptosis induction. Caco-2 cells treated with 50 μM PD displayed DNA fragmentation, whereas those treated with 100 μM resveratrol underwent apoptosis [56]. Use of flow cytometry and immunoblotting in the investigation by Bae et al. [57] showed that apoptosis was triggered by the disruption of calcium regulation and the expression levels of associated proteins in HT-29 and HCT116 cell lines. Both the MAPK and PI3K/AKT signaling pathways were shown to be downregulated by polydatin. It was also demonstrated that the combination of polydatin and 5-fluorouracil (5-FU) was effective in inhibiting drug resistance in 5-FU-resistant cells. Therefore, the results of this study support further research on PD in order to see whether or not it can be developed as a novel therapeutic agent for the treatment of colon cancer [57]. Polydatin significantly reduced cell growth and increased apoptosis in CRC cell lines [58]. It was shown that miR-382 specifically targets PD-L1. PD ability to upregulate miR-382 enables it to inhibit PD-L1 expression. Furthermore, PD regulates miR-382 to reduce CRC tumor development in vivo, where it suppresses tumor formation and induces death of CRC cells [58]. PD suppressed cell growth in RPMI 8226 multiple myeloma cells through the mTOR/p70s6k signaling pathway [58]. The IC50 values for PD were 131 μM and 93 μM at 24 and 48 h, respectively in RPMI 8226 cells. At a concentration of 50 μM, PD triggered apoptosis by upregulating caspase-3, caspase-9 and Bax levels and decreasing Bcl-2. The same concentrations also stimulated autophagy by increasing the expression of Beclin 1, Atg5, and LC3II. Phosphorylation of mTOR and p70s6 k was reduced [58].
4.3. Bone Cancer
The child population has a higher incidence of osteosarcoma (OS) than any other primary bone tumor [59]. There are around 3.4 new instances per 1,000,000 persons each year [60,61], with men being more affected than women (5.4 per 100,000 vs. 4.0 per 100,000). Although osteosarcoma can affect any part of the skeleton, it most commonly occurs in the long bones (90%) and the knee (50%). Researches have shown that genetic and epigenetic alterations disrupt the normal differentiation process that begins with mesenchymal stem cells, leading to the development of OS [62]. PD-induced apoptosis was triggered by the downregulation of β-catenin signaling and the upregulated expressions of Bax/Bcl-2 and caspase-3 in MG63 and 143B OS cells at dose-dependent concentrations, and a significantly reduced cell growth was observed [63]. The effect of PD on osteosarcoma cells, both before and after radiation therapy, was described [64]. In these experiments, PD was found to reduce bone cancer progression. Polydatin significantly upregulated cell cycle arrest in S-phase and elevated bone alkaline phosphatase activity in vitro. Pretreatment with PD activated the Wnt/β-catenin pathway and enhanced osteogenic marker expression as well as decreasing tumor cell survival, demonstrating a radiosensitizing effect when combined with radiation therapy for OS [64].
4.4. Breast Cancer
The class of cancer responsible for the highest mortality in women is breast cancer, (BC)which alone causes 25% of death in women as compared to other types of cancers [65]. The most common treatment used for breast cancer is chemotherapy, besides surgical and hormonal treatment [66]. Many factors including dysregulated autophagy, imbalanced apoptosis, changes in gene levels, and certain molecular signaling pathways are the leading causes of BC. These will be discussed one by one.
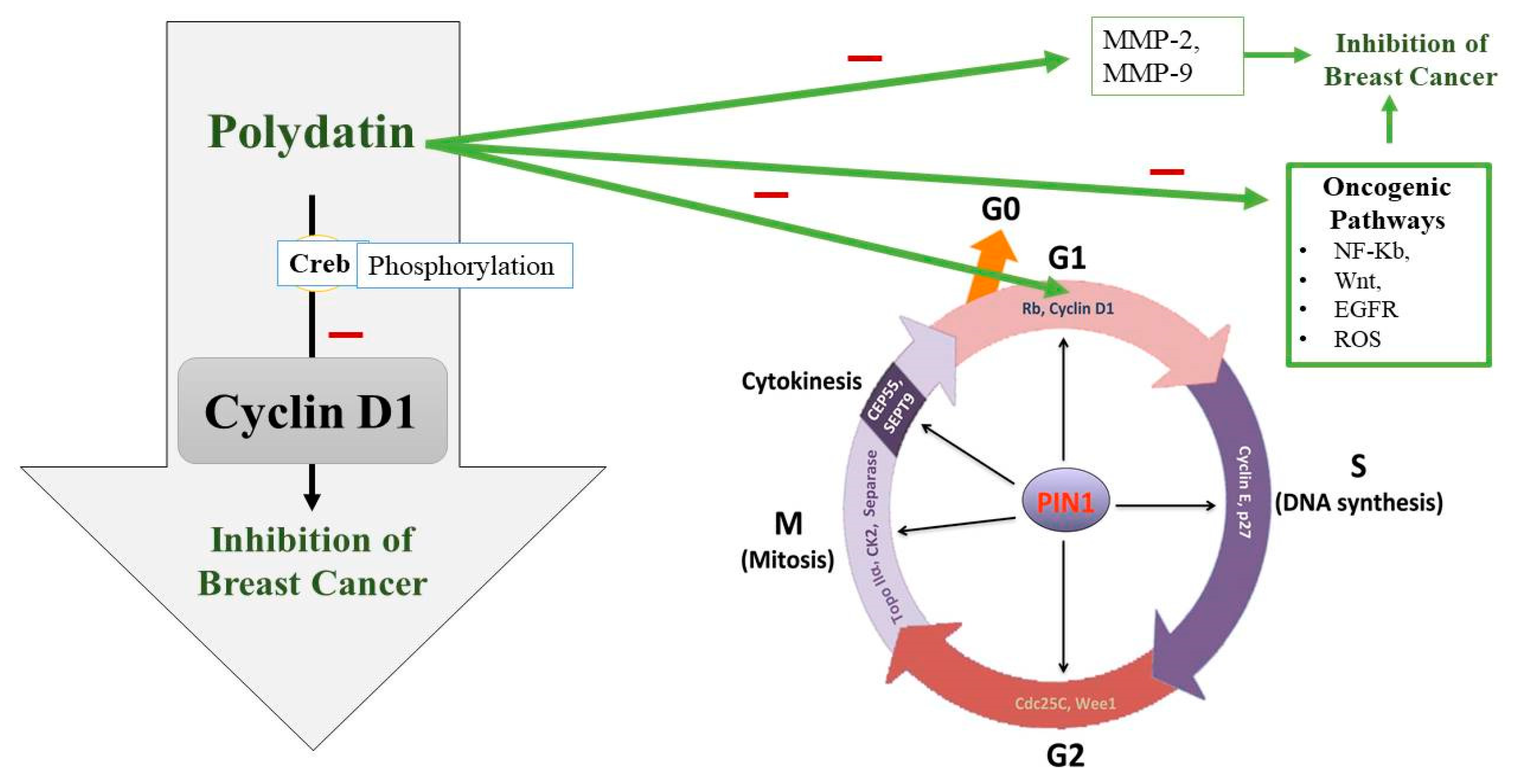
Uncontrolled cell division is also seen due to a disturbed cell cycle. The cell cycle consists of four major phases which are controlled by certain cyclin-dependent kinases (CDKs) and their partners, cyclins [67]. Sometimes, these CDKs and cyclins are upregulated or overexpressed leading to BC pathogenesis [68]. Upregulation of CDK2 and overexpression of cyclins E and B1 are observed in BC [69,70]. So, cell cycle arrest can be targeted for preventing BC progression. A transcription factor called Creb, which regulates many genes, plays a role in cell survival and multiplication [71]. Current research has also hypothesized that Creb is involved in the metastasis of cancerous cells, which means that its level is significantly increased in people suffering from BC [72]. Cyclin D1 plays a crucial role in the continuation of the cell cycle (Figure 3). To synthesize DNA, Cyclin D1 is required in a significant amount during the gap phase [73]. It is also necessary in the G2 phase for the continuation of the cell cycle [74]. It has been found that the phosphorylation process of Creb is compromised when treating BC cells with PD. So, the tumor-suppressing effect of PD appears to be due to its interference with Creb phosphorylation, which puts a lid on Cyclin D1 and thus terminates the cell cycle [75] (Figure 3).
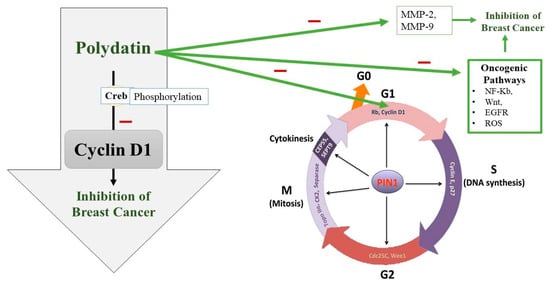
Figure 3.
Polydatin (PD) potential to inhibit the G1 phase of the cell cycle along with other oncogenic pathways. PD appears to interfere with Creb phosphorylation, which downregulates Cyclin D1 and thus terminates the cell cycle at G1 phase and in turn inhibits breast cancer growth. PIN1: peptidyl-prolyl cis/trans isomerase, CEP55: Centrosomal protein 55, CK2: casein kinase 2, Rb: the retinoblastoma protein.
Another major factor responsible for breast cancer is the matrix metalloproteinase (MMP) as it disrupts ECM. MMP is responsible for blood supply to cancerous cells, and its activity is modulated via NF-kB [76]. MMP-2 and MMP-9 are known to disturb the extracellular matrix and also play a significant role in metastasis [77]. Moreover, a direct relationship has been found between the levels of vascular endothelial growth factor (VEGF) and the development of cancer. PD is known to counteract all these factors contributing to BC [78,79], as shown in Table 2. Zhang et al. [80] investigated the anticancer activity of PD on the breast cancer cell lines 4T1 and MCF-7 and observed that, compared to the control group, PD at 100 μmol/L substantially suppressed cell growth and migration. Rising levels of the autocrine vascular VEGF are seen as a characteristic of cancer invasion in vitro. The results of Zhang et al. [80] showed that, as compared to the control, PD along with 2-Deoxy-D-glucose significantly suppressed MMP9, MMP2, and VEGF expression. Another major regulator of tumor progression is programmed cell death and apoptosis, which is utilized as a target for BC. Certain caspases like caspase-3,9 and apoptosis-related proteins such as Bcl-2 and Bax should be targeted [80,81]. Research has confirmed that by regulating proapoptotic and antiapoptotic proteins, PD causes cancer cell death [80]. Mitochondrial dysfunction and ROS production are also responsible for malignancy. ROS production is most commonly seen in triple-negative breast cancer (TNBC), thus it can be used as a target in the treatment of TNBC [82]. Certain signaling pathways which are oncogenic are upregulated by the overproduction of ROS like NF-kB, Wnt, MMPs, and EGFR [83,84,85]. ROS are involved in the upregulation of the PI3K/Akt pathway, which ultimately leads to prosurvival signaling. PD also acts as a free radical scavenger [86]. PD treatment thus balances the levels of free radicals within the body and also blocks the prosurvival signaling pathway [87,88] (Figure 4). The energy-making process of cancerous cells is aerobic glycolysis, which is required for their proliferation and migration [89]. To fulfill their energy demands, cancer cells require high levels of glucose, so glycolysis is one of the novel emerging targets of PD [90,91]. Hexokinase 2 (HK2) is an enzyme markedly expressed in cancerous cell glycolysis and which is targeted by PD [92]; PD decreases the levels of this enzyme. Uncontrolled expression of the hypoxia-inducible factor 1α (HIF1α) is also a hallmark of cancer which prevents apoptosis. HIF1α was also found to be impaired by PD [93,94,95]. In one study, the anticancer activity of PD was observed by giving it with D-glucose, using MCF-7, 4T1 cell lines. It was found that proliferation and metastasis was reduced. It was also demonstrated that its antioxidant activity was a major contributor in the treatment of BC because of its ability to reduce ROS, and also by targeting PI3K/AKt pathway that is linked with ROS production [80] (Figure 3).
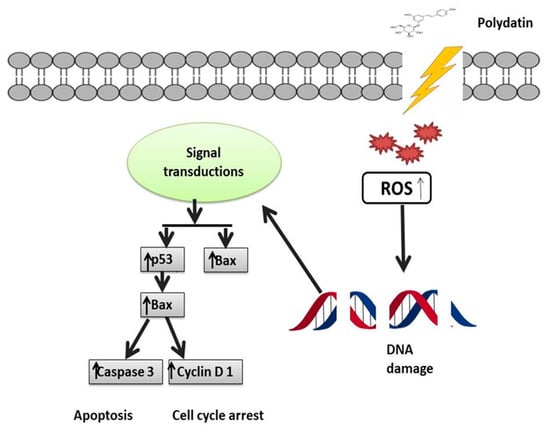
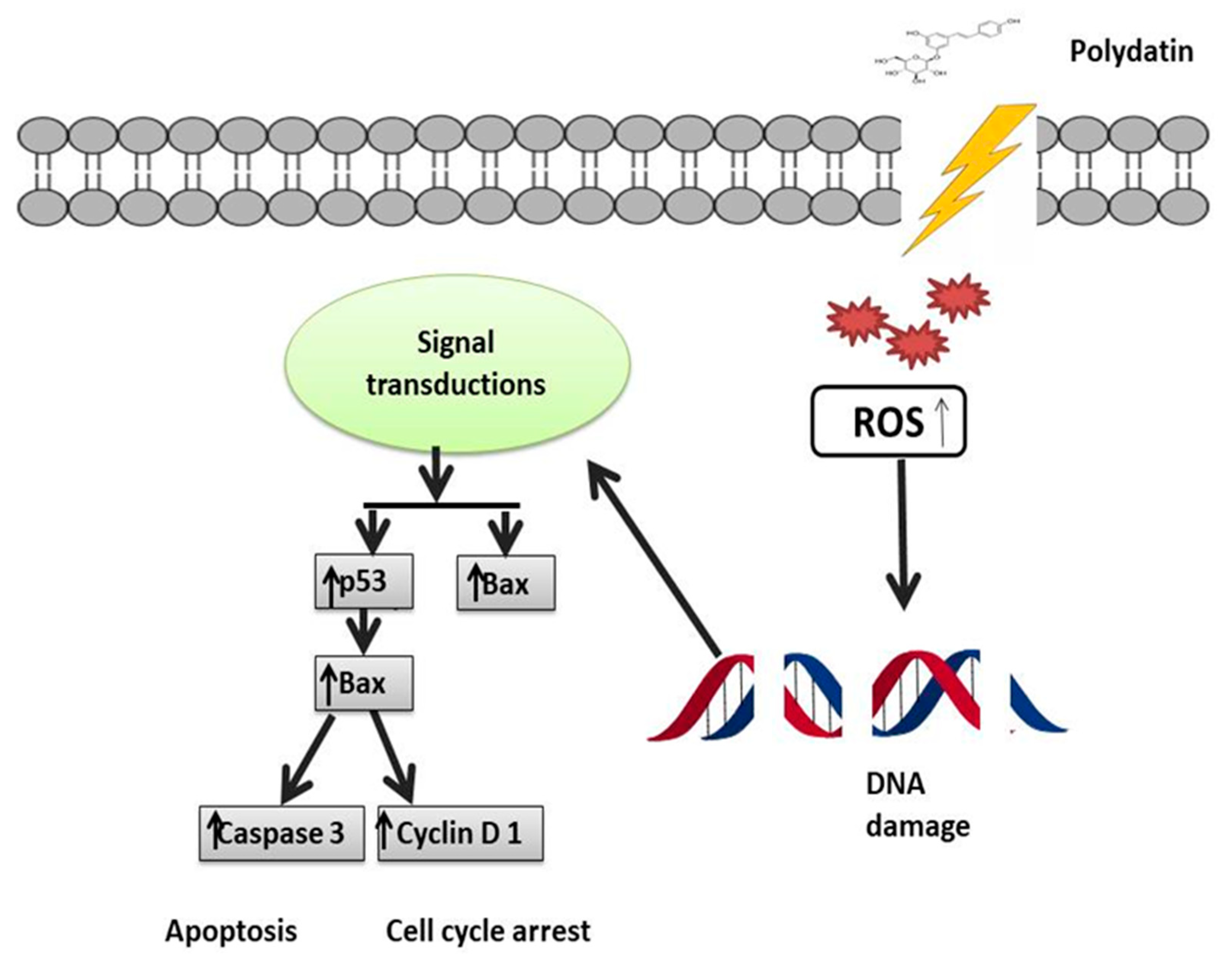
Figure 4.
Mechanistic illustration of polydatin activity in the treatment of breast cancer through p53 activation. Activation of p53 leads to activation of p21 and Bax, which in turn leads to cell cycle arrest and apoptosis. ↑ Upregulation, ↓ Downregulation.
4.5. Cervical Cancer
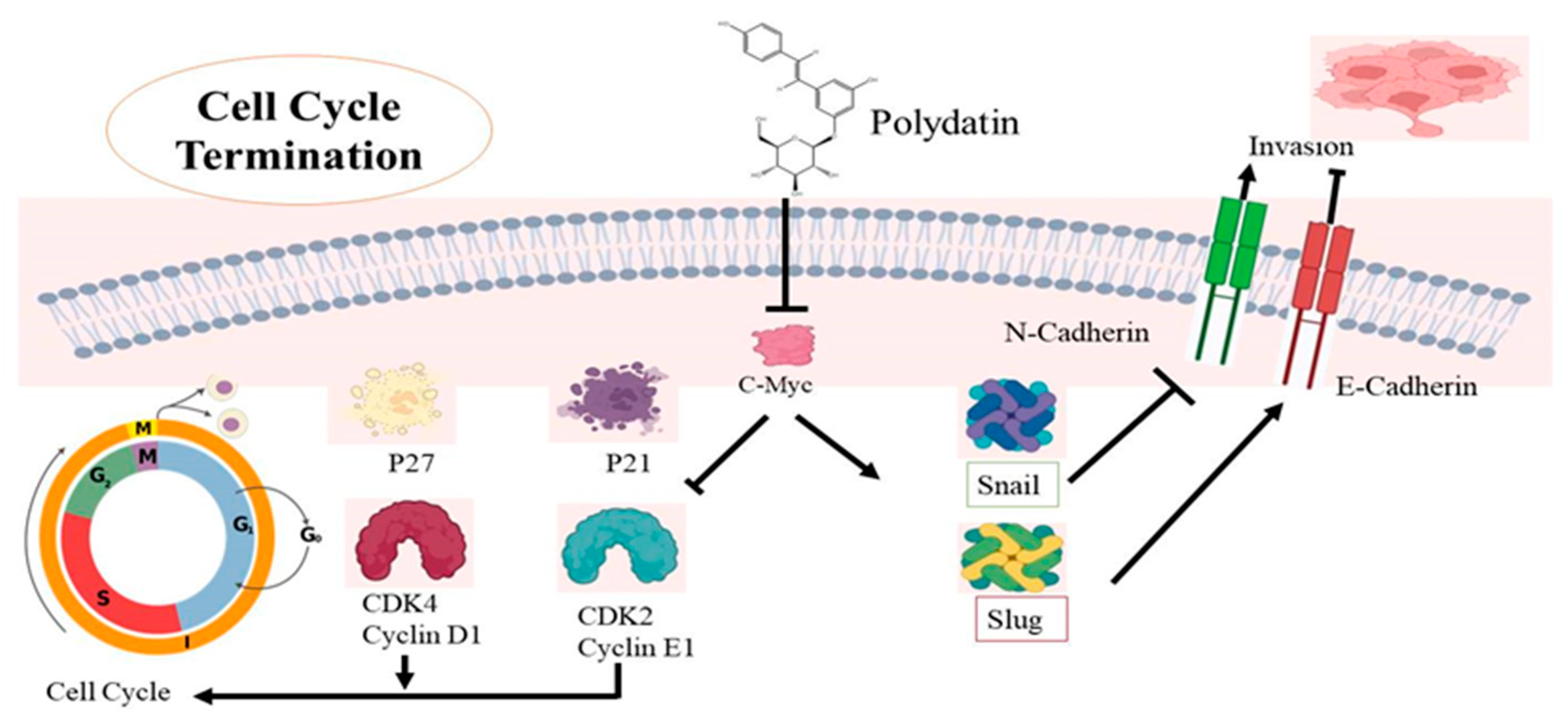
The fourth major reason for death among women is cervical cancer, which is more prevalent in developed countries [96]. Research has revealed that cervical cancer has a link to human papillomavirus (HPV). Smoking, HPV, early sexual activity, and genetic modifications lead to cervical cancer [97,98]. Undoubtedly, treatment options are available, but their outcomes are still uncertain, and there is a need to find other therapeutic alternatives [99,100,101]. PD is known to have anticancer potential and it can target some major factors involved in cervical cancer development [102,103,104]. The cell cycle is under the control of CDKs and cyclins, whose dysregulation leads to uncontrolled cellular multiplication [76]. Research has confirmed that PD causes cell cycle termination at the G0/G1 phase, upregulation of p21 and p27, and also induces repression of CDK4 and cyclin D1 [105]. One major factor affecting cell movement and invasion is EMT, which is controlled by several signaling pathways like NF-kB, MAPK, and other transcription factors [76]. During EMT, structural changes are seen in epithelial cells whose polarity is lost. Some proteins are linked with EMT, from which some are overexpressed and some are downregulated, leading to metastasis. The expression of these proteins is actually targeted by PD to prevent cell invasion, as shown in Table 2. PD causes upregulation of E-cadherin expression and downregulation of Snail and Slug expressions, thus impairing cell metastasis in cervical cancer as the switch from E-cadherin to N-cadherin, which plays a major role in the invasiveness of cancer [105,106,107]. Snail and Slug expressions also play a major role in cancer metastasis [108]. It is well-known that proto-oncogenes are involved in all the major types of cancers. The c-Myc gene is one of these specific genes which has been identified in cervical cancer. Along with cyclins and CDKs, the cell cycle is also affected by the expression of the c-Myc gene [109] so that its expression can be targeted in treating cervical cancer. C-Myc overexpression is seen as a sign of cervical cancer [110]. C-Myc gene underexpression has been reported in CaSki and C33A cells after PD exposure, suggesting its possible use for ameliorating cervical cancer treatment [111,112]. A lot of proteins regulate the cell cycle whose expression is controlled by c-Myc by altering signaling pathways [113,114]. Downregulation of the c-Myc gene by PD will impair the overexpression of these proteins. Actually, the mechanism behind the regulation of the cell cycle by c-Myc is responsible for the downregulation of the expression of both p21 and p27 [115,116]. Both are tumor-suppressant genes that arrest the cell cycle during the gap and the synthesis phases. It is known that a particular CDK interacts with a particular cyclin, allowing the continuation of the cell cycle [117]. The CDK4-Cyclin D1 interaction causes cell proliferation but p21 has the ability to stop the cell cycle by preventing this association [118] (Figure 5). The same effect is seen with p21 and p27 and CDK2-Cyclin E1 combinations, CDK2-Cyclin E1 also being a major contributor to cell cycle progress. PD was found to downregulate both p21 and p27 in cervical cancerous cells, thus stopping cell cycle progression [119]. The c-Myc gene also increases Snail and Slug expression, thus inhibiting N-cadherin, promoting E-cadherin, and leading to the prevention of the survival of cancer cells [120] (Figure 5). The anticancer potential of PD was observed on HeLa cell lines, and it was found that this stilbene decreases mRNA and protein expression levels of PI3K, AKT, mTOR, leading to apoptosis. It also causes cell death in cervical cancer by targeting the ROS/PI3K/AKT/mTOR pathway [121]. Another study was conducted by using female nude mice. PD (100 mg/kg) was given by injection and the results showed that the tumor size was small and its progression was also reduced [105] (Figure 5).
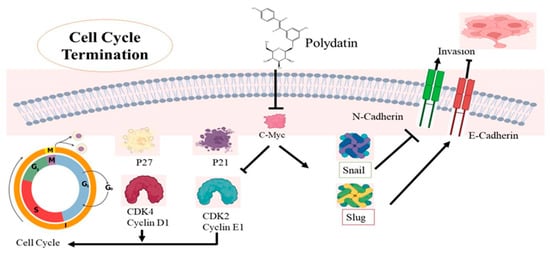
Figure 5.
The mechanistic approach of polydatin (PD) activity in terminating cell cycle in cervical cancer cells. PD causes the downregulation of the c-Myc gene, which alters two mechanisms involved in cervical cancer. PD causes upregulation of p21 and p27, and also induces repression of CDK4 and cyclin D1 as well as CDK2 and cyclin E1; their dysregulation ultimately terminates the G0/G1 phase of the cell cycle. In the second pathway, PD causes Snail and Slug expressions, leading to upregulation of E-cadherin expression and downregulation of N-cadherin, and thus impairing cell metastasis in cervical cancer.

Table 2.
Anticancer activity of polydatin on different types of cancer.
Table 2.
Anticancer activity of polydatin on different types of cancer.
| Cancer Type | Cell Line | Type of Study | Concentrations of PD | Molecular Targets | Mechanism of Action | References |
|---|---|---|---|---|---|---|
| Breast cancer | MDA-MB-231 MCF-7 | In vitro | 2, 4, 6 µM | ↑ p38 ↑ JUN ↑ ERK ↑ AKT | Promotes apoptosis by MAPK/ERK & P13K/AKT pathways | [122] |
| 4T1 MCF-7 | In vitro | 5.53 mmol/L 8.67 mmol/L | ↓ p-PI3K/PI3K ↓ p-AKT/AKT | Inhibits P13K/AKT pathways | [80] | |
| Cervical cancer | CaSki C33A | In vitro | 0.1, 10,100, 500 µM | ↑ p21 ↑ p27 ↓ Cdk4 ↓ Cdk2 Cyclin D1 ↓ Cyclin E1 | Inhibits growth promoter proteins and cell cycle arrest | [105] |
| HeLa | In vitro | 50, 100, 150 μmol/L | ↓ PI3K ↓ AKT ↓ mTOR P70S6K ↓ c-Myc | Induced apoptosis by suppression of PI3K/AKT/mTOR signaling | [15] | |
| Lung cancer | A549 NCI-H1975 | In vitro | 6 µ mol/L | ↓ Bcl 2 ↑ Bax ↑ Cyclin D1 | Cell cycle arrest and apoptotic pathway | [123] |
| A549 and H1299 cells | In vitro | ↓ NLRP3 ↓ ASC ↑ pro-caspase-1 ↑ NF-kB ↑ p56 | Promotes apoptosis and NLRP3 inflammasome inhibition by NF-kB | [20] | ||
| Ovarian cancer | OVCAR-3, A2780, and HO-8910 | In vitro | 50 μM | ↑ P13K ↑ AKT | AKT signaling | [124] |
| SKOV-3 and OVCAR-8 | In vitro | 5, 10, 50, 100 μM | ↓ Her-2 ↓ EGFR ↓ VEGF ↑ ERK ↑ PARP-1 | Down/upregulation of various cell signaling molecules | [125] | |
| Liver cancer | HCC cells | In vitro | 100 μM 150 μM | ↓G2/M Phase ↓ STAT3 ↓ AKT ↓ JAK1 | Cell cycle arrest JAK1/STAT3 and P13K/AKT signaling | [53] |
| HepG2 SMMC-7721 | In vitro | 1, 3, 10, 30, and 100 µM | ↓ β-catenin ↓ Bcl 2 ↑ Bax ↑ Caspase-3 ↑ Caspase-9 | Apoptotic pathway | [80] | |
| HepG2 | In Vitro | (10, 30, and 100 μM) | ↓ Bcl 2 ↑ Bax ↓ Wnt | Wnt signaling Apoptotic pathway | [54] | |
| Colon carcinoma | CaCo-2 | In vitro | 1–50 μM | ↓ DNA synthesis ↓G0/G1 | Cell cycle arrest | [56] |
| Caco-2 | In vitro | 100 240 μM | ↓ AKT ↑ PARP ↓ Erk-1 ↓ Erk-2 | Regulation of Akt/PKB signaling | [55] | |
| Human myeloma cells | RPMI 8226 | In vitro | 50, 100, 200 μmol/L | ↑ Caspase-3 ↑ Caspase-9 ↑ Bax ↓ mTOR/p70s6k | Apoptotic pathway | [58] |
| Osteosarcoma cells | 143B MG63 | In vitro | 10, 30, 100 μM | ↑ Caspase -3 ↓ Bcl 2 ↑ Bax ↓ β-catenin | Regulation of Apoptotic pathway | [63] |
| Lukemia cells | MOLT-4 | In vitro | 1, 4 or 20 µM | ↓ Cyclin D1 ↓ CYCLIN B1 ↓ Bcl2 | Cell cycle arrest and apoptotic pathway | [126] |
| Nasal carcinoma | CNE | In vitro | 5, 10, 20 µM | ↓ AKT ↑ Endoplasmic ↑ Reticulum stress ↑ Caspase 3 ↑ Caspase 4 ↑ Caspase 9 | Regulation of apoptotic pathway molecules | [127] |
| Laryngeal cancer | AMC-HN-8 cells | In vitro | 2, 4, 6 µM | ↓ PDGF-B ↓ Ki67 ↓ Bcl 2 ↑ Bax ↓ Akt | Regulation of apoptotic pathway and Akt signaling molecules | [128] |
NF-KB: Nuclear Factor kappa-light-chain-enhancer of activated B cells, Wnt: Wingless/Integrated, EGFR: Epidermal growth factor receptor, MMP: Matrix metalloproteinase, VEGF: Vascular endothelial growth factor, ROS: Reactive oxygen species, EMT: Epithelial-to-mesenchymal transition, Bcl-2: B-cell lymphoma 2, Bax: BCL2 associated X apoptosis regulator, NLRP3: NLR family pyrin domain containing 3, PI3K: Phosphatidylinositol 3-kinase, PD: Polydatin, Akt: Serine/threonine kinase 1, mTOR: Mammalian target of rapamycin, ERK: Extracellular signal-regulated kinase, PARP: poly adenosine diphosphate-ribose polymerase. ↑ Upregulation, ↓ Downregulation.
4.6. Lung Cancer
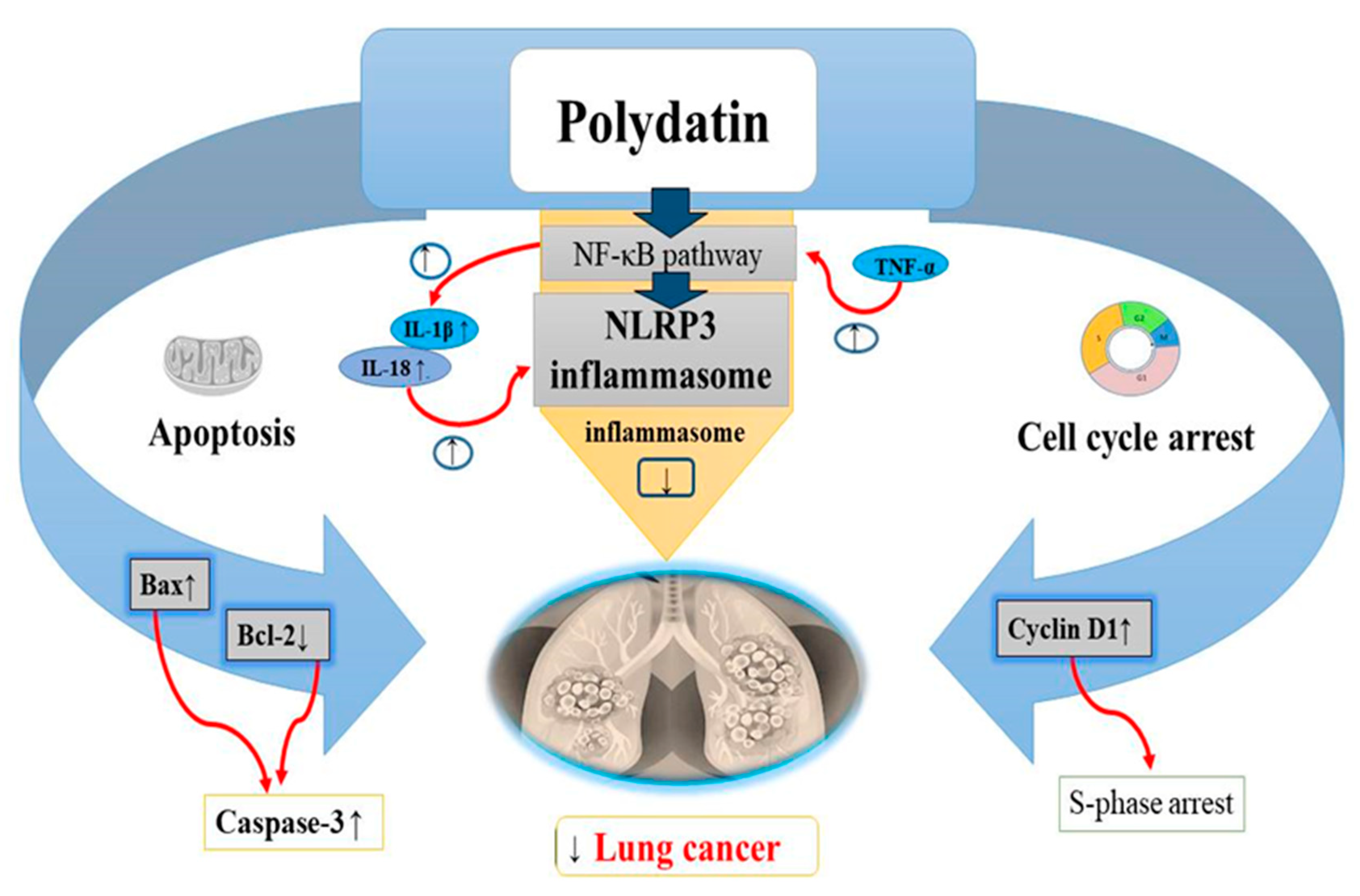
Lung cancer, a growing health problem worldwide was found to be the most common type of cancer compared to other cancers [129]. Its prevalence and increase in mortality are strongly related to the history of smoking [130]. Many treatments such as targeted chemotherapies, radiotherapies, and surgery are used to cure lung cancer but despite advancements in these therapies, lung cancer still remains antagonistic in nature with poor survival rate. Chemotherapy is used recurrently against lung cancer in progressive stages but with deleterious consequences for patients [131]. Lung cancer cells treated with doses till 6 μM PD, showed a dose-dependent reduction in Bcl-2 and cyclin D1 levels as well as an increase in Bax, leading to cell cycle arrest at the S phase. Interestingly, the human non-cancerous nasopharyngeal cell line exhibited lower cytotoxicity when exposed to PD. This was also corroborated by several researchers [132]. A recent study found that PD is advantageous for lung cancer inhibition [132]. The initiation of apoptosis in lung cancer cells is considered a good anticancer target [133,134]. In cancer cells, the antiapoptotic protein Bcl-2 is not capable of forming heterodimeric complexes with the proapoptotic protein Bax, resulting in high Bax levels. Increased Bax/Bcl-2 ratios upregulate the release of cytochrome C from mitochondria into the cytosol, leading to caspase-3 stimulation and apoptosis activation [135,136]. Research has revealed that 6 μmol/L of PD activates apoptosis in A549 lung cancer cell lines by impairing Bcl-2 levels and upregulating Bax levels [132] (Table 2). On the other hand, cell cycle arrest of the cancer cells is considered to be a potential target against cancer progression [137,138] (Figure 6). Cyclin D1 expression should be high for the normal initiation of DNA synthesis, while cyclin D1 levels should be low during the S phase [139]. Overincrease in cyclin D1 expression has been reported in many cancers including lung cancer [140,141]. In recent in vitro studies, PD has been found to hamper lung cancer cell (A549 and NCI-H1975 cells) progression by decreasing cyclin D1 levels and arresting cells at the S phase [132] (Table 2). PD also exerts some antioxidant and anti-inflammatory activities by inhibiting secretion of inflammatory oxidative factors or by increasing the scavenging of free oxygen radicals [142]. In recent studies, NLRP3 (NLR family pyrin domain containing protein 3) inflammasome has been observed to take part in inflammation related to cancer and tumor progression. Thus, suppression of the NLRP3 inflammasome might also be an effective strategy in the treatment of lung cancer [143]. NF-κB pathway has been revealed to be the significant marker in NLRP3 inflammasome activation. Increased levels of TNF-α activates the NF-κB pathway which then upregulates the IL-1β and IL-18 levels, causing the activation of NLRP3 inflammasome. PD (50 μM) has been shown to attenuate the multiplication and metastasis of human A549 and H1299 cell lines through suppression of the NLRP3 inflammasome by suppression of the NF-κB pathway [20] (Figure 6).
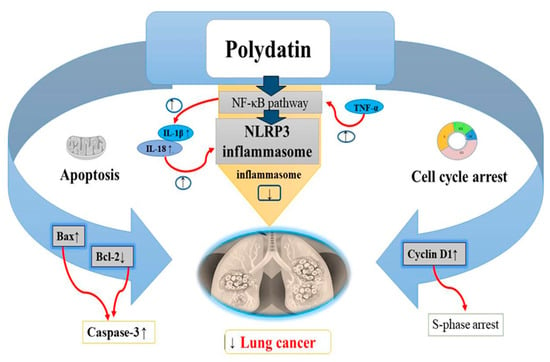
Figure 6.
Anticancer effects of polydatin on lung cancer via the apoptotic pathway by increasing BAX levels, decreasing Bcl-2 levels and increasing caspase-3 levels, induction of cell cycle arrest at S phase by decreasing the cyclin D1 levels, and inhibition of the NLRP3 inflammasome by suppressing the NF-kB pathway in tumor cells. Upregulation ↑ Downregulation ↓.
4.7. Ovarian Cancer
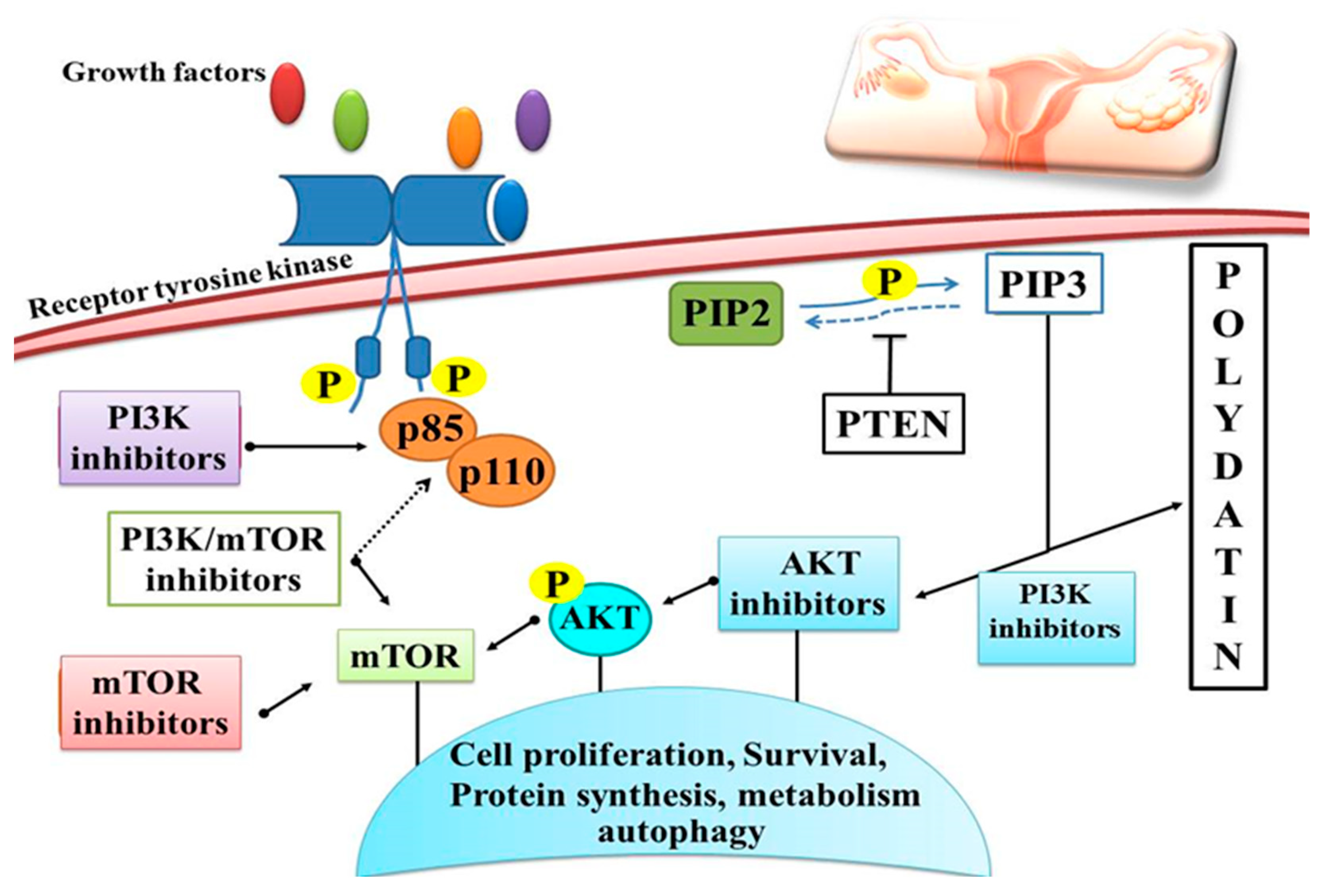
Ovarian cancer is the most frequent cause of death among women within gynecological cancers worldwide [144]. Researchers have discovered in recent years that Chinese medicine displays a significant anticancer activity with fewer side effects as compared to synthetic drugs. PD ought to enhance ovarian cancer cell susceptibility to radiations, limit cell growth, and promote apoptosis. It has been found that PD has the potential to facilitate cancer cell apoptosis [145] (Table 2). PI3K signaling controls cell growth, death, and survival [146]. PD triggered apoptosis in cancer cells, namely ovarian cancer cells, and protected against inflammatory damage through the phosphoinositide, 3-kinase/protein kinase B/mammalian target of rapamycin (mTOR) pathway [75,147]. PD was able to successfully limit the growth of the ovarian cancer cell lines OVCAR-3, A2780 and HO-8910. There was a decrease in proliferation, migration and invasion after treatment with PD in the cancer cell lines OVCAR-3, A2780, and HO-8910 [124]. In addition, PD inhibited PI3K, which in turn increased extracellular signaling and regulated ERK phosphorylation, thus inhibiting cancer cell growth [124] (Figure 7). The anticancer effect of PD was demonstrated by the downregulation of tumor suppressor genes via inhibition of the PI3K/Akt signaling and upregulation of bone morphogenetic protein 7 (BMP7) [127]. Inhibiting the proliferation, migration, and invasion of ovarian cancer cells is one of the main PD’s effects [148]. PD prevents ovarian cancer cell proliferation, migration and invasion by inhibiting the expression of the PI3K protein, which is the cornerstone of ovarian cancer treatment. By decreasing EGFR phosphorylation and production of ERK and VEGF, PD inhibited the cellular aggregation of ovarian cancer cell lines in three dimensions. At concentrations of 5–100 μM, PD was shown to suppress growth of the ovarian cancer cell lines SKOV-3 and OVCAR-8 by decreasing EGFR phosphorylation levels, which in turn increases the likelihood of the cells committing suicide. [149]. Earlier investigations utilising polydatin have suggested that this compound suppresses PI3K protein expression and blocks growth, migration and invasion of OVCAR-3, A2780 and HO-8910 cells. It appears that PI3K is the target of PD, since increasing PI3K protein expression greatly attenuates the inhibitory impact of PD on proliferation, migration and invasiveness of OVCAR-3, A2780 and HO-8910 cell lines. Experimental evidence supports the possible use of PD in the treatment of ovarian cancers because of its ability to suppress the growth, migration, and invasion of these cell lines by downregulating PI3K protein expression [124].
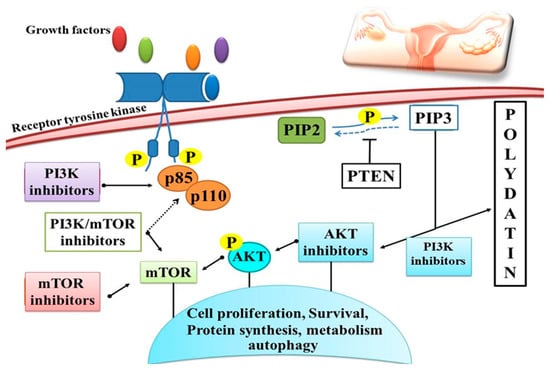
Figure 7.
Schematic overview of polydatin activity on the PI3K/AKT/mTOR signaling pathway with different strategies for inhibition. PD induces apoptosis in cancer cells through the PI3K/Akt/mTOR signaling pathway and protects against inflammatory damage, as well as inhibiting cell proliferation, survival and protein synthesis through protein phosphorylation.
PD inhibited OVCAR-8 and SKOV-3 cell growth in a dose-dependent manner. A growth rate decrease was achieved by triggering apoptosis through the cleavage of poly(ADP-ribose) polymerase (PARP-1) at PD concentrations of 50 and 100 µM. In the SKOV-3 line, PD inhibited Her-2 and EGFR phosphorylation and Erk expression, as well as the VEGF, when used at greater dosages, and stimulated Erk activation in the OVCAR-8 cell line. Results of this investigation showed that PD has the potential to block the formation of 3D cell aggregates in ovarian cancer cell lines by influencing a variety of signaling molecules. However, more experiments for the in vivo testing of resveratrol and PD are needed to determine their potential therapeutic values [125]. Several other cancer cell lines were shown to be significantly inhibited by PD (Table 2).
5. Underlying Polydatin Anticancer Mechanisms of Action
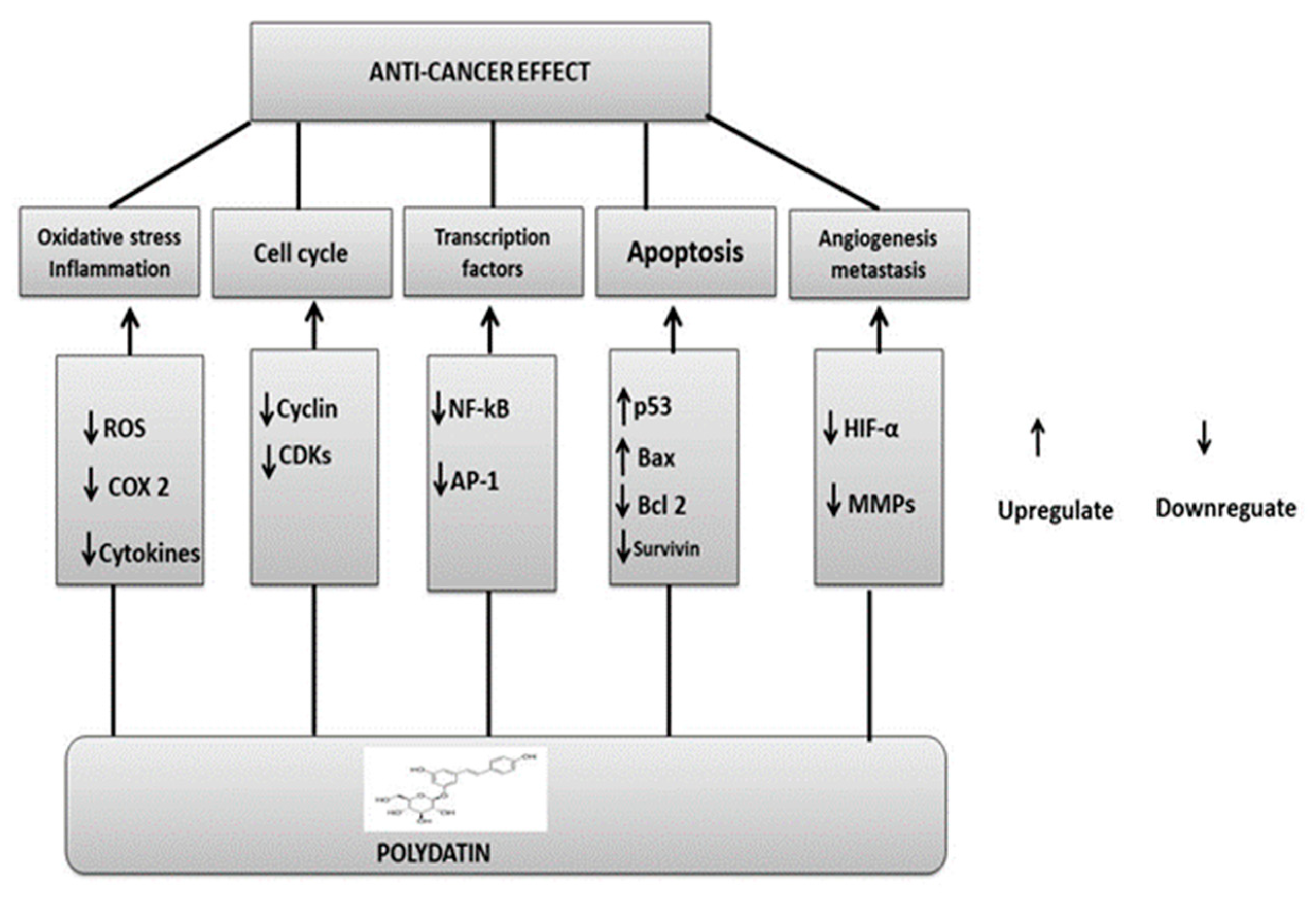
PD has been studied extensively for its potential as a chemopreventive agent and chemotherapeutic treatment for halting or reversing carcinogenesis at multiple stages. PD, like other phytochemicals, can act as a suppressive agent on multiple impaired signaling pathways; as such, it has been classified as a functionally pleiotropic agent, capable of expressing its activity on multiple targets in cancer cells while causing only mild side effects in healthy cells. Important cellular changes include increased oxidative stress, overproduction of growth-regulatory hormones, accelerated transition of cells through cell cycle checkpoints, abnormal cell proliferation, genome instability, abnormal response to signals or other stimulators of programmed cell death, uncontrolled neoangiogenesis, and altered host immune responses. In addition, oxidative stress-related damage (including DNA damage, protein oxidation, and lipid peroxidation) is mitigated by antioxidant, anti-inflammatory, and immunomodulatory activities, which also boost immune oncosurveillance [150]. PD inhibits the monooxygenase cytochrome P450 isoenzyme CYP1 A1, the enzyme deputed to the liver metabolism of xenobiotics, and hence it can also function as a cancer-blocking agent by preventing the transformation of procarcinogens into carcinogens [151].
One of the primary functions of phytochemicals is to inhibit growth-signaling activity. A transmembrane tyrosine kinase is activated by ligands; the epidermal growth factor (EGF), and its related receptor (EGF-R) represent two of the resveratrol’s primary targets. Overexpression of EGF-R is a hallmark of malignant tumors with aggressive characteristics because it stimulates cell growth and proliferation [152]. Resveratrol, acetyl-resveratrol and polydatin showed dose-dependent antigrowth activities against 3D cell aggregates of EGF-R/Her-2-positive and -negative ovarian cancer cell lines [125]. The phosphorylation of Her-2 and EGF-R, as well as the expression of extracellular-signal-regulated kinases (ERK) and VEGF, were all significantly reduced when resveratrol, PD and acetyl-resveratrol were tested at high concentrations on the positive ovarian cell line [125].
PD and its analogues displayed an interactive effect with TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) and triggered apoptosis (programmed cell death). Particularly resistant to TRAIL are androgen-dependent LNCaP cells in prostate cancer; however, PD downregulated the PI3K/AKT pathway to make these cells more responsive to TRAIL-mediated apoptosis. Treatment of LNCaP cells with PD induced ROS production, mitochondrial membrane potential decreases, and translocation of the Bcl2-like protein 4, also known as Bcl-2-associated X protein (Bax), and p53 tumor suppressor protein. Proteins such as cytochrome c CASP-3 and CASP-9, apoptosis-inducing factor (AIF), the second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI (Smac/DIABLO), and protein high-temperature requirement serine protease A2 (HtrA2), also known as Omi, are among the proapoptotic proteins released by mitochondria in relation to PD analogues [153] (Figure 8).
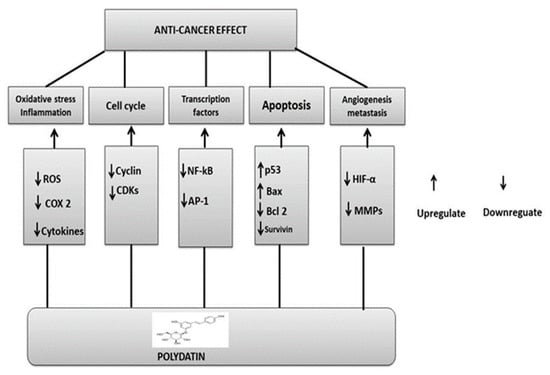
Figure 8.
Polydatin anticancer mechanisms: upregulation or downregulation of various pathways.
6. Concluding Remarks
Currently, phytomedicine is gaining the attention of researchers and nutritionists due to its diversified pharmacological activities. The data collected showed the anticancer activities of PD. So, it would be valuable to investigate the in-depth mechanisms of PD activities. In recent years, PD has gained attention as a promising anticancer drug due to its potential to modulate many signaling pathways associated with cancer development. PD’s anticancer effects arise from the fact that it boosts antioxidant activity. Due to its long-conjugated chemical structure, this molecule displays powerful antioxidant properties. PD may trigger apoptosis by raising the Bax/Bcl-2 ratio and decreasing Wnt/catenin signaling to kill cancer cells. It also targets cell cycle arrest to inhibit the development of BC. Among the several oncogenic pathways involved in BC the cell cycle arrest is linked to PD’s apparent interference with Creb phosphorylation, which in turn downregulates Cyclin D1. PD therapy also restores the body’s natural equilibrium of free radicals. Tumor size and rate of cancer progression were both significantly decreased after intravenous administration of PD. Furthermore, it was shown that PI3K levels are decreased in PD-treated patients. PD also protects against inflammatory damage and inhibits cell proliferation, survival, and protein synthesis by targeting the PI3K/Akt/mTOR signaling pathway, which is activated in cancer cells. Liver xenobiotic metabolism is mostly carried out by the monooxygenase cytochrome P450 isoenzyme CYP1 A1, which is inhibited by PD. As a blocking agent, it can stop procarcinogens from becoming carcinogenic. In this review, we have thus emphasized the anticancer activity of PD and its underlying pharmacological modes of action. The anticancer effect of PD should also be investigated through thorough preclinical trials. There is thus a need for carefully monitored human studies to determine its therapeutic efficacy.
Author Contributions
Conceptualization: M.A.S., H.K. and P.J.; Methodology: M.S.A., A.H., H.I.F., A.R., M.H. and R.Y. Project administration: M.S.A. and P.J.; Supervision: M.S.A. and P.J.; Writing—original draft: M.A.S., A.H., H.I.F., A.R., M.H., R.Y. and P.J.; Data curation: A.H., A.R., M.H., U.S., M.S.A. and G.H.; Investigation: H.I.F., T.A.S.B., R.Y., U.S., S.I., G.H. and H.K.; Writing—review & editing: T.A.S.B., U.S., S.I., Z.K., G.H., I.A. and H.K.; Formal analysis: T.A.S.B. and M.S.A. Validation: T.A.S.B., U.S. and M.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- National Cancer Institute. What Is Cancer. Available online: https://www.cancer.gov/about-cancer/understanding/what-is-cancer (accessed on 5 May 2021).
- WHO Cancer Prevalence. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer#:~:text=In%202020%2C%20there%20were%202.3,the%20world's%20most%20prevalent%20cancer (accessed on 26 March 2021).
- WHO, Cervical Cancer Prevalence. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 22 February 2022).
- Medscape, Non Small Lung Cancer. Available online: https://emedicine.medscape.com/article/279960-overview (accessed on 19 August 2022).
- SEER Cancer Stat Facts: Ovarian Cancer. National Cancer Institute. Bethesda, MD, USA. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 3 August 2022).
- Breast Cancer: Types of Treatment. Available online: https://www.cancer.net/cancer-types/breast-cancer/types-treatment (accessed on 19 October 2021).
- Meegan, M.J.; O’Boyle, N.M. Special Issue “Anticancer Drugs”. Pharmaceuticals 2019, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, A.; Javeed, M.; Shah, M.A.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Riaz, A.; Sadiqa, A.; Zara, R.; Zafar, S. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ. 2020, 722, 137907. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.-H.; Peng, C.; Zhang, H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm Biol 2013, 51, 1347–1354. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clément, C.; Courot, E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. Biofactors 2010, 36, 331–341. [Google Scholar] [CrossRef]
- Jeandet, P.; Vannozzi, A.; Sobarzo-Sánchez, E.; Uddin, M.S.; Bru, R.; Martínez-Márquez, A.; Clément, C.; Cordelier, S.; Manayi, A.; Nabavi, S.F. Phytostilbenes as agrochemicals: Biosynthesis, bioactivity, metabolic engineering and biotechnology. Nat. Prod. Rep. 2021, 38, 1282–1329. [Google Scholar] [CrossRef]
- Jeandet, P.; Sobarzo-Sánchez, E.; Uddin, M.S.; Bru, R.; Clément, C.; Jacquard, C.; Nabavi, S.F.; Khayatkashani, M.; Batiha, G.E.-S.; Khan, H. Resveratrol and cyclodextrins, an easy alliance: Applications in nanomedicine, green chemistry and biotechnology. Biotechnol Adv. 2021, 53, 107844. [Google Scholar] [CrossRef]
- Quan, Z.; Gu, J.; Dong, P.; Lu, J.; Wu, X.; Wu, W.; Fei, X.; Li, S.; Wang, Y.; Wang, J. Reactive oxygen species-mediated endoplasmic reticulum stress and mitochondrial dysfunction contribute to cirsimaritin-induced apoptosis in human gallbladder carcinoma GBC-SD cells. Cancer Lett. 2010, 295, 252–259. [Google Scholar] [CrossRef]
- Pan, J.-H.; Wang, H.-B.; Du, X.-F.; Liu, J.-Y.; Zhang, D.-J. Polydatin induces human cervical cancer cell apoptosis via PI3K/AKT/mTOR signaling pathway. Zhongguo Zhongyao Zazhi—China J. Chin. Mater. Med. 2017, 42, 2345–2349. [Google Scholar]
- DeSalvo, J.; Kuznetsov, J.N.; Du, J.; Leclerc, G.M.; Leclerc, G.J.; Lampidis, T.J.; Barredo, J.C. Inhibition of Akt potentiates 2-DG–Induced apoptosis via downregulation of UPR in acute lymphoblastic leukemia. Mol. Cancer Res. 2012, 10, 969–978. [Google Scholar] [CrossRef]
- Estañ, M.C.; Calviño, E.; de Blas, E.; del Carmen Boyano-Adánez, M.; Mena, M.L.; Gómez-Gómez, M.; Rial, E.; Aller, P. 2-Deoxy-D-glucose cooperates with arsenic trioxide to induce apoptosis in leukemia cells: Involvement of IGF-1R-regulated Akt/mTOR, MEK/ERK and LKB-1/AMPK signaling pathways. Biochem. Pharmacol. 2012, 84, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Peng, J.; Huang, K.; Huang, J.; Shen, X.; Liu, P.; Huang, H. Polydatin ameliorates experimental diabetes-induced fibronectin through inhibiting the activation of NF-κB signaling pathway in rat glomerular mesangial cells. Mol. Cell. Endocrinol. 2012, 362, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Mikulski, D.; Molski, M. Quantitative structure–antioxidant activity relationship of trans-resveratrol oligomers, trans-4, 4′-dihydroxystilbene dimer, trans-resveratrol-3-O-glucuronide, glucosides: Trans-piceid, cis-piceid, trans-astringin and trans-resveratrol-4′-O-β-D-glucopyranoside. Eur. J. Med. Chem. 2010, 45, 2366–2380. [Google Scholar]
- Zou, J.; Yang, Y.; Yang, Y.; Liu, X. Polydatin suppresses proliferation and metastasis of non-small cell lung cancer cells by inhibiting NLRP3 inflammasome activation via NF-κB pathway. Biomed. Pharm. 2018, 108, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.-W.; Wu, J.-Z.; Jia, M.; Du, J.; Zhang, H.; Qin, L.-P. Effects of polydatin from Polygonum cuspidatum on lipid profile in hyperlipidemic rabbits. Biomed. Pharm. 2009, 63, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Lee, J.K.; Kang, S.-Y.; Pandey, R.P.; Sohng, J.-K.; Ahn, J.S.; Hong, Y.-S. Construction of artificial biosynthetic pathways for resveratrol glucoside derivatives. J. Microbiol. Biotechnol. 2014, 24, 614–618. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Grigorchuk, V.P.; Ogneva, Z.V.; Suprun, A.; Dubrovina, A. Stilbene biosynthesis in the needles of spruce Picea jezoensis. Phytochemistry 2016, 131, 57–67. [Google Scholar] [CrossRef]
- Cichewicz, R.H.; Kouzi, S.A. Biotransformation of resveratrol to piceid by Bacillus cereus. J. Nat. Prod. 1998, 61, 1313–1314. [Google Scholar] [CrossRef]
- Ozaki, S.-i.; Imai, H.; Iwakiri, T.; Sato, T.; Shimoda, K.; Nakayama, T.; Hamada, H. Regioselective glucosidation of trans-resveratrol in Escherichia coli expressing glucosyltransferase from Phytolacca americana. Biotechnol. Lett. 2012, 34, 475–481. [Google Scholar] [CrossRef]
- Choi, O.; Wu, C.-Z.; Kang, S.Y.; Ahn, J.S.; Uhm, T.-B.; Hong, Y.-S. Biosynthesis of plant-specific phenylpropanoids by construction of an artificial biosynthetic pathway in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2011, 38, 1657–1665. [Google Scholar] [CrossRef]
- Regev-Shoshani, G.; Shoseyov, O.; Bilkis, I.; Kerem, Z. Glycosylation of resveratrol protects it from enzymic oxidation. Biochem. J. 2003, 374, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Jiang, K.; Wu, H.; Qiu, C.; Deng, G.; Peng, X. Polydatin reduces Staphylococcus aureus lipoteichoic acid-induced injury by attenuating reactive oxygen species generation and TLR 2-NF κB signalling. J. Cell. Mol. Med. 2017, 21, 2796–2808. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, X.; Liu, G.; Li, J.; Zhang, J.; Cao, Y.; Miao, J. Antioxidant activity and mechanism of resveratrol and polydatin isolated from mulberry (Morus alba L.). Molecules 2021, 26, 7574. [Google Scholar] [CrossRef]
- Galeano-Díaz, T.; Durán-Merás, I.; Airado-Rodríguez, D. Isocratic chromatography of resveratrol and piceid after previous generation of fluorescent photoproducts: Wine analysis without sample preparation. J. Sep. Sci. 2007, 30, 3110–3119. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Romero-Perez, A.I.; Ibern-Gomez, M.; Lamuela-Raventos, R.M.; de La Torre-Boronat, M.C. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food Chem. 1999, 47, 1533–1536. [Google Scholar] [CrossRef]
- Peng, X.L.; Qu, W.; Wang, L.Z.; Huang, B.Q.; Ying, C.J.; Sun, X.F.; Hao, L.P. Resveratrol ameliorates high glucose and high-fat/sucrose diet-induced vascular hyperpermeability involving Cav-1/eNOS regulation. PLoS ONE 2014, 9, e113716. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Andres-Lacueva, C.; Lamuela-Raventós, R.M.; Berenguer, T.; Jakszyn, P.; Martínez, C.; Sanchez, M.J.; Navarro, C.; Chirlaque, M.D.; Tormo, M.-J. Concentrations of resveratrol and derivatives in foods and estimation of dietary intake in a Spanish population: European Prospective Investigation into Cancer and Nutrition (EPIC)-Spain cohort. Br. J. Nutr. 2008, 100, 188–196. [Google Scholar] [CrossRef]
- Peng, X.L.; Xu, J.; Sun, X.F.; Ying, C.J.; Hao, L.P. Analysis of trans-resveratrol and trans-piceid in vegetable foods using high-performance liquid chromatography. Int. J. Food Sci. Nutr. 2015, 66, 729–735. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Rotchés-Ribalta, M.; Zamora-Ros, R.; Llorach, R.; Lamuela-Raventós, R.M.; Estruch, R.; Andrés-Lacueva, C. Determination of resveratrol and piceid in beer matrices by solid-phase extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 698–705. [Google Scholar] [CrossRef]
- Jensen, J.S.; Wertz, C.F.; O’Neill, V.A. Preformulation stability of trans-resveratrol and trans-resveratrol glucoside (piceid). J. Agric. Food Chem. 2010, 58, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Şöhretoğlu, D.; Baran, M.Y.; Arroo, R.; Kuruüzüm-Uz, A. Recent advances in chemistry, therapeutic properties and sources of polydatin. Phytochem. Rev. 2018, 17, 973–1005. [Google Scholar] [CrossRef]
- Ibern-Gomez, M.; Roig-Perez, S.; Lamuela-Raventos, R.M.; de la Torre-Boronat, M.C. Resveratrol and piceid levels in natural and blended peanut butters. J. Agric. Food Chem. 2000, 48, 6352–6354. [Google Scholar] [CrossRef] [PubMed]
- Hurst, W.J.; Glinski, J.A.; Miller, K.B.; Apgar, J.; Davey, M.H.; Stuart, D.A. Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products. J. Agric. Food Chem. 2008, 56, 8374–8378. [Google Scholar] [CrossRef]
- Xu, L.-Q.; Xie, Y.-L.; Gui, S.-H.; Zhang, X.; Mo, Z.-Z.; Sun, C.-Y.; Li, C.-L.; Luo, D.-D.; Zhang, Z.-B.; Su, Z.-R. Polydatin attenuates d-galactose-induced liver and brain damage through its anti-oxidative, anti-inflammatory and anti-apoptotic effects in mice. Food Funct. 2016, 7, 4545–4555. [Google Scholar] [CrossRef]
- Su, D.; Cheng, Y.; Liu, M.; Liu, D.; Cui, H.; Zhang, B.; Zhou, S.; Yang, T.; Mei, Q. Comparision of piceid and resveratrol in antioxidation and antiproliferation activities in vitro. PLoS ONE 2013, 8, e54505. [Google Scholar] [CrossRef]
- Yousef, A.I.; Shawki, H.H.; El-Shahawy, A.A.; El-Twab, S.M.A.; Abdel-Moneim, A.; Oishi, H. Polydatin mitigates pancreatic β-cell damage through its antioxidant activity. Biomed. Pharmacother. 2021, 133, 111027. [Google Scholar] [CrossRef]
- Aggarwal, S. Targeted cancer therapies. Nat. Rev. Drug Discov. 2010, 9, 427. [Google Scholar] [CrossRef]
- Zhang, H.; Park, S.; Huang, H.; Kim, E.; Yi, J.; Choi, S.-K.; Ryoo, Z.; Kim, M. Anticancer effects and potential mechanisms of ginsenoside Rh2 in various cancer types. Oncol. Rep. 2021, 45, 1–10. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Mao, Z.; Yu, D.; Gao, C. Toxicity of ZnO nanoparticles to macrophages due to cell uptake and intracellular release of zinc ions. J. Nanosci. Nanotechnol. 2014, 14, 5688–5696. [Google Scholar] [CrossRef]
- Hong, C.; Wang, D.; Liang, J.; Guo, Y.; Zhu, Y.; Xia, J.; Qin, J.; Zhan, H.; Wang, J. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics 2019, 9, 4437. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wan, J.Y.; Zeng, J.; Huang, W.H.; Sava-Segal, C.; Li, L.; Niu, X.; Wang, Q.; Wang, C.Z.; Yuan, C.S. Effects of compound K, an enteric microbiome metabolite of ginseng, in the treatment of inflammation associated colon cancer. Oncol. Lett. 2018, 15, 8339–8348. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-S.; Jia, M.; Chen, L.; Zhu, B.; Dong, H.-X.; Si, J.-P.; Peng, W.; Han, T. Cytotoxic and antifungal constituents isolated from the metabolites of endophytic fungus DO14 from Dendrobium officinale. Molecules 2015, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Trautwein, C.; Friedman, S.L.; Schuppan, D.; Pinzani, M. Hepatic fibrosis: Concept to treatment. J. Hepatol. 2015, 62, S15–S24. [Google Scholar] [CrossRef]
- Nakamoto, Y. Promising new strategies for hepatocellular carcinoma. Hepatol. Res. 2017, 47, 251–265. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, Y.; Dong, T.; Yue, M.; Zhang, Y.; An, T.; Zhang, J.; Liu, P.; Yang, X. Polydatin inhibits hepatocellular carcinoma via the AKT/STAT3-FOXO1 signaling pathway. Oncol. Lett. 2019, 17, 4505–4513. [Google Scholar] [CrossRef]
- Jiao, Y.; Wu, Y.; Du, D. Polydatin inhibits cell proliferation, invasion and migration, and induces cell apoptosis in hepatocellular carcinoma. Braz. J. Med. Biol. Res. 2018, 51. [Google Scholar] [CrossRef]
- De Maria, S.; Scognamiglio, I.; Lombardi, A.; Amodio, N.; Caraglia, M.; Cartenì, M.; Ravagnan, G.; Stiuso, P. Polydatin, a natural precursor of resveratrol, induces cell cycle arrest and differentiation of human colorectal Caco-2 cell. J. Transl. Med. 2013, 11, 1–11. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Quifer-Rada, P.; Lamuela-Raventos, R.M.; Moreno, J.J. Piceid presents antiproliferative effects in intestinal epithelial Caco-2 cells, effects unrelated to resveratrol release. Food Funct. 2014, 5, 2137–2144. [Google Scholar] [CrossRef]
- Bae, H.; Lee, W.; Song, J.; Hong, T.; Kim, M.H.; Ham, J.; Song, G.; Lim, W. Polydatin Counteracts 5-Fluorouracil Resistance by Enhancing Apoptosis via Calcium Influx in Colon Cancer. Antioxidants 2021, 10, 1477. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhao, S. Polydatin regulates proliferation, apoptosis and autophagy in multiple myeloma cells through mTOR/p70s6k pathway. Onco Targets. 2017, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Taran, S.J.; Taran, R.; Malipatil, N.B. Pediatric osteosarcoma: An updated review. Indian J. Med. Paediatr. Oncol. 2017, 38, 33–43. [Google Scholar] [CrossRef]
- Misaghi, A.; Goldin, A.; Awad, M.; Kulidjian, A.A. Osteosarcoma: A comprehensive review. Sicot-J 2018, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, W.; He, J.; Jiang, L.; Li, X. Osteopontin as a biomarker for osteosarcoma therapy and prognosis. Oncol. Lett. 2019, 17, 2592–2598. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, C.P.; Kukekov, V.G.; Reith, J.D.; Tchigrinova, O.; Suslov, O.N.; Scott, E.W.; Ghivizzani, S.C.; Ignatova, T.N.; Steindler, D.A. Stem-like cells in bone sarcomas: Implications for tumorigenesis. Neoplasia 2005, 7, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Kuang, G.; Jiang, W.; Jiang, R.; Jiang, D. Polydatin promotes apoptosis through upregulation the ratio of Bax/Bcl-2 and inhibits proliferation by attenuating the β-catenin signaling in human osteosarcoma cells. Am. J. Transl. Res. 2016, 8, 922. [Google Scholar]
- Luce, A.; Lama, S.; Millan, P.C.; Itro, A.; Sangiovanni, A.; Caputo, C.; Ferranti, P.; Cappabianca, S.; Caraglia, M.; Stiuso, P. Polydatin Induces Differentiation and Radiation Sensitivity in Human Osteosarcoma Cells and Parallel Secretion through Lipid Metabolite Secretion. Oxid. Med. Cell. Longev. 2021, 2021, 3337013. [Google Scholar] [CrossRef]
- United States Cancer Statistics (USCS). Prevention, Global Cancer Statistics. 2012. 2019. Available online: https://www.cdc.gov/cancer/uscs/index.htm (accessed on 3 August 2022).
- Ginsburg, O.; Yip, C.H.; Brooks, A.; Cabanes, A.; Caleffi, M.; Dunstan Yataco, J.A.; Gyawali, B.; McCormack, V.; McLaughlin de Anderson, M.; Mehrotra, R. Breast cancer early detection: A phased approach to implementation. Cancer 2020, 126, 2379–2393. [Google Scholar] [CrossRef]
- Schwartz, G.K.; Shah, M.A. Targeting the cell cycle: A new approach to cancer therapy. J. Clin. Oncol. 2005, 23, 9408–9421. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Cao, J.; Lin, W.; Chen, H.; Xiong, X.; Ao, H.; Yu, M.; Lin, J.; Cui, Q. The roles of cyclin-dependent kinases in cell-cycle progression and therapeutic strategies in human breast cancer. Int. J. Mol. Sci. 2020, 21, 1960. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.P.; Zhu, Y.-L.; Ratner, E.S. Targeting cyclin-dependent kinases for treatment of gynecologic cancers. Front. Oncol. 2018, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.-H.; Wei, M.-F.; Lee, Y.-H.; Yang, W.-C.; Yang, S.-Y.; Lin, J.-C.; Huang, C.-S. MAP3K1 Expression Is Associated with Progression and Poor Prognosis of Hormone Receptor-Positive, HER2-Negative Early-Stage Breast Cancer. 2020. Available online: https://assets.researchsquare.com/files/rs-53956/v1/20512c99-6481-480b-9d9e-37881a993bb9.pdf?c=1631849978 (accessed on 3 August 2022).
- Mayr, B.; Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001, 2, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, A.; Fernando, H.; Watkins, G.; Mansel, R.E.; Jiang, W.G. Expression of transcription factor CREB1 in human breast cancer and its correlation with prognosis. Oncol. Rep. 2007, 18, 953–958. [Google Scholar] [CrossRef]
- Zhang, X.; Odom, D.T.; Koo, S.-H.; Conkright, M.D.; Canettieri, G.; Best, J.; Chen, H.; Jenner, R.; Herbolsheimer, E.; Jacobsen, E. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc. Natl. Acad. Sci. 2005, 102, 4459–4464. [Google Scholar] [CrossRef]
- Alao, J.P. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol. Cancer 2007, 6, 1–16. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef]
- Jin, Y.; Huynh, D.T.N.; Nguyen, T.L.L.; Jeon, H.; Heo, K.S. Therapeutic effects of ginsenosides on breast cancer growth and metastasis. Arch. Pharmacal Res. 2020, 43, 773–787. [Google Scholar] [CrossRef]
- Orgaz, J.L.; Pandya, P.; Dalmeida, R.; Karagiannis, P.; Sanchez-Laorden, B.; Viros, A.; Albrengues, J.; Nestle, F.O.; Ridley, A.J.; Gaggioli, C. Diverse matrix metalloproteinase functions regulate cancer amoeboid migration. Nat. Commun. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Chabottaux, V.; Noel, A. Breast cancer progression: Insights into multifaceted matrix metalloproteinases. Clin. Exp. Metastasis 2007, 24, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.V.; Chang, J.P.; Parachoniak, C.A.; Pandika, M.M.; Aghi, M.K.; Meyronet, D.; Isachenko, N.; Fouse, S.D.; Phillips, J.J.; Cheresh, D.A. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 2012, 22, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhu, X.; Wu, H.; Jiang, K.; Zhao, G.; Shaukat, A.; Deng, G.; Qiu, C. Targeting the ROS/PI3K/AKT/HIF-1alpha/HK2 axis of breast cancer cells: Combined administration of Polydatin and 2-Deoxy-d-glucose. J. Cell. Mol. Med. 2019, 23, 3711–3723. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Song, D.Y.; Hwang, J.J.; Park, H.J.; Lee, J.S.; Song, S.Y.; Jeong, S.-Y.; Choi, E.K. Induction of p53-mediated senescence is essential for the eventual anticancer therapeutic effect of RH1. Arch. Pharm Res. 2019, 42, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Salinas, F.L.; Delgado-Magallón, A.; Montes-Alvarado, J.B.; Ramírez-Ramírez, D.; Flores-Alonso, J.C.; Cortés-Hernández, P.; Reyes-Leyva, J.; Herrera-Camacho, I.; Anaya-Ruiz, M.; Pelayo, R. Breast cancer subtypes present a differential production of reactive oxygen species (ROS) and susceptibility to antioxidant treatment. Front. Oncol. 2019, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- de Sá Junior, P.L.; Câmara, D.A.D.; Porcacchia, A.S.; Fonseca, P.M.M.; Jorge, S.D.; Araldi, R.P.; Ferreira, A.K. The roles of ROS in cancer heterogeneity and therapy. Oxidative Med. Cell. Longev. 2017, 2017, 2467940. [Google Scholar] [CrossRef]
- Kubli, S.P.; Bassi, C.; Roux, C.; Wakeham, A.; Göbl, C.; Zhou, W.; Jafari, S.M.; Snow, B.; Jones, L.; Palomero, L. AhR controls redox homeostasis and shapes the tumor microenvironment in BRCA1-associated breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 3604–3613. [Google Scholar] [CrossRef]
- Han, B.; Liu, W.; Li, J.; Wang, J.; Zhao, D.; Xu, R.; Lin, Z. Catalytic hydrodechlorination of triclosan using a new class of anion-exchange-resin supported palladium catalysts. Water Res. 2017, 120, 199–210. [Google Scholar] [CrossRef]
- Chen, L.; Lan, Z.; Lin, Q.; Mi, X.; He, Y.; Wei, L.; Lin, Y.; Zhang, Y.; Deng, X. Polydatin ameliorates renal injury by attenuating oxidative stress-related inflammatory responses in fructose-induced urate nephropathic mice. Food Chem. Toxicol. 2013, 52, 28–35. [Google Scholar] [CrossRef]
- Checker, R.; Gambhir, L.; Sharma, D.; Kumar, M.; Sandur, S.K. Plumbagin induces apoptosis in lymphoma cells via oxidative stress mediated glutathionylation and inhibition of mitogen-activated protein kinase phosphatases (MKP1/2). Cancer Lett. 2015, 357, 265–278. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Dwarakanath, B. Cytotoxicity, radiosensitization, and chemosensitization of tumor cells by 2-deoxy-D-glucose in vitro. J. Cancer Res. Ther. 2009, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, J.; Wang, F.; Hu, J.; Wang, S.; Sun, Y. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014, 355, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Agnihotri, S.; Micallef, J.; Mukherjee, J.; Sabha, N.; Cairns, R.; Hawkins, C.; Guha, A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med. 2011, 208, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Shafaee, A.; Dastyar, D.Z.; Islamian, J.P.; Hatamian, M. Inhibition of tumor energy pathways for targeted esophagus cancer therapy. Metabolism 2015, 64, 1193–1198. [Google Scholar] [CrossRef]
- Palazon, A.; Tyrakis, P.A.; Macias, D.; Veliça, P.; Rundqvist, H.; Fitzpatrick, S.; Vojnovic, N.; Phan, A.T.; Loman, N.; Hedenfalk, I. An HIF-1α/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell 2017, 32, 669–683.e5. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Dai, W.; Zhang, Q.; Feng, J.; Wu, L.; Liu, T.; Yu, Q.; Xu, S.; Wang, W. Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. Br. J. Cancer 2017, 117, 1518–1528. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA A Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Vaccarella, S.; Laversanne, M.; Ferlay, J.; Bray, F. Cervical cancer in a frica, L atin a merica and the C aribbean and a sia: Regional inequalities and changing trends. Int. J. Can. 2017, 141, 1997–2001. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ma, D. The precision prevention and therapy of HPV-related cervical cancer: New concepts and clinical implications. Can. Med. 2018, 7, 5217–5236. [Google Scholar] [CrossRef] [PubMed]
- Den Boon, J.A.; Pyeon, D.; Wang, S.S.; Horswill, M.; Schiffman, M.; Sherman, M.; Zuna, R.E.; Wang, Z.; Hewitt, S.M.; Pearson, R. Molecular transitions from papillomavirus infection to cervical precancer and cancer: Role of stromal estrogen receptor signaling. Proc. Natl. Acad. Sci. USA 2015, 112, E3255–E3264. [Google Scholar] [CrossRef] [PubMed]
- Marquina, G.; Manzano, A.; Casado, A. Targeted agents in cervical cancer: Beyond bevacizumab. Curr. Oncol. Rep. 2018, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Lowe, G.; Roberts, C.M.; Finlay, J.; Han, E.S.; Glackin, C.A.; Dellinger, T.H. Pterostilbene suppresses ovarian cancer growth via induction of apoptosis and blockade of cell cycle progression involving inhibition of the STAT3 pathway. Int J. Mol. Sci. 2018, 19, 1983. [Google Scholar] [CrossRef]
- Yu, H.; Pan, C.; Zhao, S.; Wang, Z.; Zhang, H.; Wu, W. Resveratrol inhibits tumor necrosis factor-α-mediated matrix metalloproteinase-9 expression and invasion of human hepatocellular carcinoma cells. Biomed. Pharmacother. 2008, 62, 366–372. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Y.; Di, S.; Feng, X.; Liu, D.; Jiang, S.; Hu, W.; Qin, Z.; Li, Y.; Lv, J. Pterostilbene exerts anticancer activity on non-small-cell lung cancer via activating endoplasmic reticulum stress. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Bai, L.; Ma, Y.; Wang, X.; Feng, Q.; Zhang, Z.; Wang, S.; Zhang, H.; Lu, X.; Xu, Y.; Zhao, E. Polydatin inhibits cell viability, migration, and invasion through suppressing the c-Myc expression in human cervical cancer. Front. Cell Dev. Biol. 2021, 9, 587218. [Google Scholar] [CrossRef]
- Nishioka, R.; Itoh, S.; Gui, T.; Gai, Z.; Oikawa, K.; Kawai, M.; Tani, M.; Yamaue, H.; Muragaki, Y. SNAIL induces epithelial-to-mesenchymal transition in a human pancreatic cancer cell line (BxPC3) and promotes distant metastasis and invasiveness in vivo. Exp. Mol. Pathol. 2010, 89, 149–157. [Google Scholar] [CrossRef]
- Cercelaru, L.; Stepan, A.E.; Mărgăritescu, C.; Osman, A.; Popa, I.-C.; Florescu, M.M.; Simionescu, C.E.; Mărgăritescu, O.C. E-cadherin, β-catenin and Snail immunoexpression in laryngeal squamous cell carcinoma. Rom. J. Morphol. Embryol. 2017, 58, 761–766. [Google Scholar]
- Dhasarathy, A.; Phadke, D.; Mav, D.; Shah, R.R.; Wade, P.A. The transcription factors Snail and Slug activate the transforming growth factor-beta signaling pathway in breast cancer. PLoS ONE 2011, 6, e26514. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Guo, W.-J.; Yao, R.-X. Advances research on C-MYC proto-oncogene in multiple myeloma-review. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2016, 24, 1248–1251. [Google Scholar] [PubMed]
- Wu, S.-H.; Zeng, X.-F.; Wang, P.; Zhou, Y.; Lin, W. The Expression and Significance of c-myc and bcat1 in Cervical Cancer. Djournal Sichuan Univ. 2018, 49, 725–730. [Google Scholar]
- Gao, K.; Eurasian, M.; Zhang, J.; Wei, Y.; Zheng, Q.; Ye, H.; Li, L. Can genomic amplification of human telomerase gene and C-MYC in liquid-based cytological specimens be used as a method for opportunistic cervical cancer screening? Gynecol. Obstet. Investig. 2015, 80, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Ding, J.; Xia, Y.; Liu, M.; Ye, B.; Choi, J.-H.; Yan, C.; Dong, Z.; Huang, S.; Zha, Y. KDM4C and ATF4 cooperate in transcriptional control of amino acid metabolism. Cell Rep. 2016, 14, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.G.; Cho, H.; Herzka, T.; Watrud, K.; DeMarco, D.V.; Wang, V.M.; Senturk, S.; Fellmann, C.; Ding, D.; Beinortas, T. MYC drives Pten/Trp53-deficient proliferation and metastasis due to IL6 secretion and AKT suppression via PHLPP2. Cancer Discov. 2015, 5, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, H.; Qing, G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal. Transduct. Target. Ther. 2018, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, E.A.; Lee, C.; Sutherland, R.L. Progestins both stimulate and inhibit breast cancer cell cycle progression while increasing expression of transforming growth factor alpha, epidermal growth factor receptor, c-fos, and c-myc genes. Mol. Cell. Biol. 1991, 11, 5032–5043. [Google Scholar]
- García-Gutiérrez, L.; Delgado, M.D.; León, J. MYC oncogene contributions to release of cell cycle brakes. Genes 2019, 10, 244. [Google Scholar] [CrossRef]
- Finn, R.S.; Aleshin, A.; Slamon, D.J. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016, 18, 1–11. [Google Scholar] [CrossRef]
- Thomasova, D.; Anders, H.-J. Cell cycle control in the kidney. Nephrol. Dial. Transplant. 2015, 30, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. Dna Repair 2016, 42, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Aleem, E.; Kiyokawa, H.; Kaldis, P. Cdc2–cyclin E complexes regulate the G1/S phase transition. Nat. Cell Biol. 2005, 7, 831–836. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Lin, C.-W.; Wang, P.-H.; Hsin, M.-C.; Yang, S.-F. The potential of Chinese herbal medicines in the treatment of cervical cancer. Integr. Cancer Ther. 2019, 18, 1534735419861693. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tao, J.; Zhong, F.; Jiao, Y.; Xu, J.; Shen, Q.; Wang, H.; Fan, S.; Zhang, Y. Polydatin down-regulates the phosphorylation level of Creb and induces apoptosis in human breast cancer cell. PLoS ONE 2017, 12, e0176501. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, Z.; Meng, Q.; Jiao, Y.; Xu, J.; Fan, S. Polydatin inhibits growth of lung cancer cells by inducing apoptosis and causing cell cycle arrest. Oncol Lett 2014, 7, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Effects of polydatin on the proliferation, migration, and invasion of ovarian cancer. Biocell 2019, 43, 313. [Google Scholar] [CrossRef]
- Hogg, S.J.; Chitcholtan, K.; Hassan, W.; Sykes, P.H.; Garrill, A. Resveratrol, acetyl-resveratrol, and polydatin exhibit antigrowth activity against 3D cell aggregates of the SKOV-3 and OVCAR-8 ovarian cancer cell lines. Obs. Gynecol. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Cao, W.J.; Wu, K.; Wang, C.; Wan, D.M. Polydatin-induced cell apoptosis and cell cycle arrest are potentiated by Janus kinase 2 inhibition in leukemia cells. Mol. Med. Rep. 2016, 13, 3297–3302. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, S.; Zhang, Y.; Wu, J.; Peng, H.; Fan, J.; Liao, J. Reactive oxygen species-mediated endoplasmic reticulum stress and mitochondrial dysfunction contribute to polydatin-induced apoptosis in human nasopharyngeal carcinoma CNE cells. J. Cell. Biochem. 2011, 112, 3695–3703. [Google Scholar] [CrossRef]
- Li, H.; Shi, B.; Li, Y.; Yin, F. Polydatin inhibits cell proliferation and induces apoptosis in laryngeal cancer and HeLa cells via suppression of the PDGF/AKT signaling pathway. J. Biochem. Mol. Toxicol. 2017, 31, e21900. [Google Scholar] [CrossRef] [PubMed]
- Spiro, S.G.; Tanner, N.T.; Silvestri, G.A.; Janes, S.M.; Lim, E.; Vansteenkiste, J.F.; Pirker, R. Lung cancer: Progress in diagnosis, staging and therapy. Respirology 2010, 15, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Lemjabbar-Alaoui, H.; Hassan, O.U.; Yang, Y.-W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta (Bba)—Rev. Cancer 2015, 1856, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Duan, H.; Liu, Q.; Yagasaki, K.; Zhang, G. Inhibitory effects of norcantharidin against human lung cancer cell growth and migration. Cytotechnology 2010, 62, 349–355. [Google Scholar] [CrossRef]
- Jiang, T.; Tian, F.; Zheng, H.; Whitman, S.A.; Lin, Y.; Zhang, Z.; Zhang, N.; Zhang, D.D. Nrf2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-κB-mediated inflammatory response. Kidney Int. 2014, 85, 333–343. [Google Scholar] [CrossRef]
- Neto, C.C.; Amoroso, J.W.; Liberty, A.M. Anticancer activities of cranberry phytochemicals: An update. Mol. Nutr. Food Res. 2008, 52, S18–S27. [Google Scholar] [CrossRef]
- Verma, N.; Tiku, A.B. Polydatin-induced direct and bystander effects in a549 lung cancer cell line. Nutr. Cancer 2022, 74, 237–249. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.-I.; Jones, D.P.; Wang, X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef]
- Kluck, R.M.; Bossy-Wetzel, E.; Green, D.R.; Newmeyer, D.D. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 1997, 275, 1132–1136. [Google Scholar] [CrossRef]
- Graña, X.; Reddy, E.P. Cell cycle control in mammalian cells: Role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 1995, 11, 211–220. [Google Scholar]
- Pavletich, N.P. Mechanisms of cyclin-dependent kinase regulation: Structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J. Mol. Biol. 1999, 287, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Hitomi, M.; Stacey, D.W. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, J.J.; Ebus, M.E.; Koster, J.; van Sluis, P.; van Noesel, C.J.; Versteeg, R.; Caron, H.N. Cyclin D1 and CDK4 activity contribute to the undifferentiated phenotype in neuroblastoma. Cancer Res. 2008, 68, 2599–2609. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Peters, G. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv. Cancer Res. 1996, 68, 67–108. [Google Scholar]
- Liu, Y.-L.; Chen, B.-Y.; Nie, J.; Zhao, G.-H.; Zhuo, J.-Y.; Yuan, J.; Li, Y.-C.; Wang, L.-L.; Chen, Z.-W. Polydatin prevents bleomycin-induced pulmonary fibrosis by inhibiting the TGF-β/Smad/ERK signaling pathway. Exp. Ther. Med. 2020, 20, 1. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, H.; Zeng, X.; Liu, W.; Wang, Z.; Yan, X.; Wang, H.; Xie, W. Activation of NLRP3 inflammasome enhances the proliferation and migration of A549 lung cancer cells. Oncol. Rep. 2016, 35, 2053–2064. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.-M.; Krapcho, M.; Garshell, J.; Neyman, N.; Altekruse, S.; Kosary, C.; Yu, M.; Ruhl, J.; Tatalovich, Z. SEER cancer statistics review, 1975–2010. Natl. Cancer Inst. 2014. [Google Scholar]
- Bast, R.C.; Hennessy, B.; Mills, G.B. The biology of ovarian cancer: New opportunities for translation. Nat. Rev. Cancer 2009, 9, 415–428. [Google Scholar] [CrossRef]
- Cheaib, B.; Auguste, A.; Leary, A. The PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities and challenges. Chin. J. Cancer 2015, 34, 4–16. [Google Scholar] [CrossRef]
- Leary, A.; Auclin, E.; Pautier, P.; Lhommé, C. The PI3K/Akt/mTOR pathway in ovarian cancer: Biological rationale and therapeutic opportunities. Ovarian Cancer—A Clin. Transl. Update 2013, 275–302. [Google Scholar]
- Mabuchi, S.; Kuroda, H.; Takahashi, R.; Sasano, T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol. Oncol. 2015, 137, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Wu, H.; Jiang, Y.; Xiao, X.; Song, D.; Xu, N.; Ma, X.; Zeng, J.; Guo, Y. Old dog, new tricks: Polydatin as a multitarget agent for current diseases. Phytother. Res. 2022, 36, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer molecular mechanisms of resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Uemura, Y.; Kobayashi, M.; Taguchi, H. Combined effects of resveratrol and paclitaxel on lung cancer cells. Anticancer Res. 2003, 23, 4039–4046. [Google Scholar]
- Shankar, S.; Chen, Q.; Siddiqui, I.; Sarva, K.; Srivastava, R.K. Sensitization of TRAIL-resistant LNCaP cells by resveratrol (3,4′,5-tri-hydroxystilbene): Molecular mechanisms and therapeutic potential. J. Mol. Signal. 2007, 2, 1–17. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).