Inhibition of Phenol from Entering into Condensed Freshwater by Activated Persulfate during Solar-Driven Seawater Desalination
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
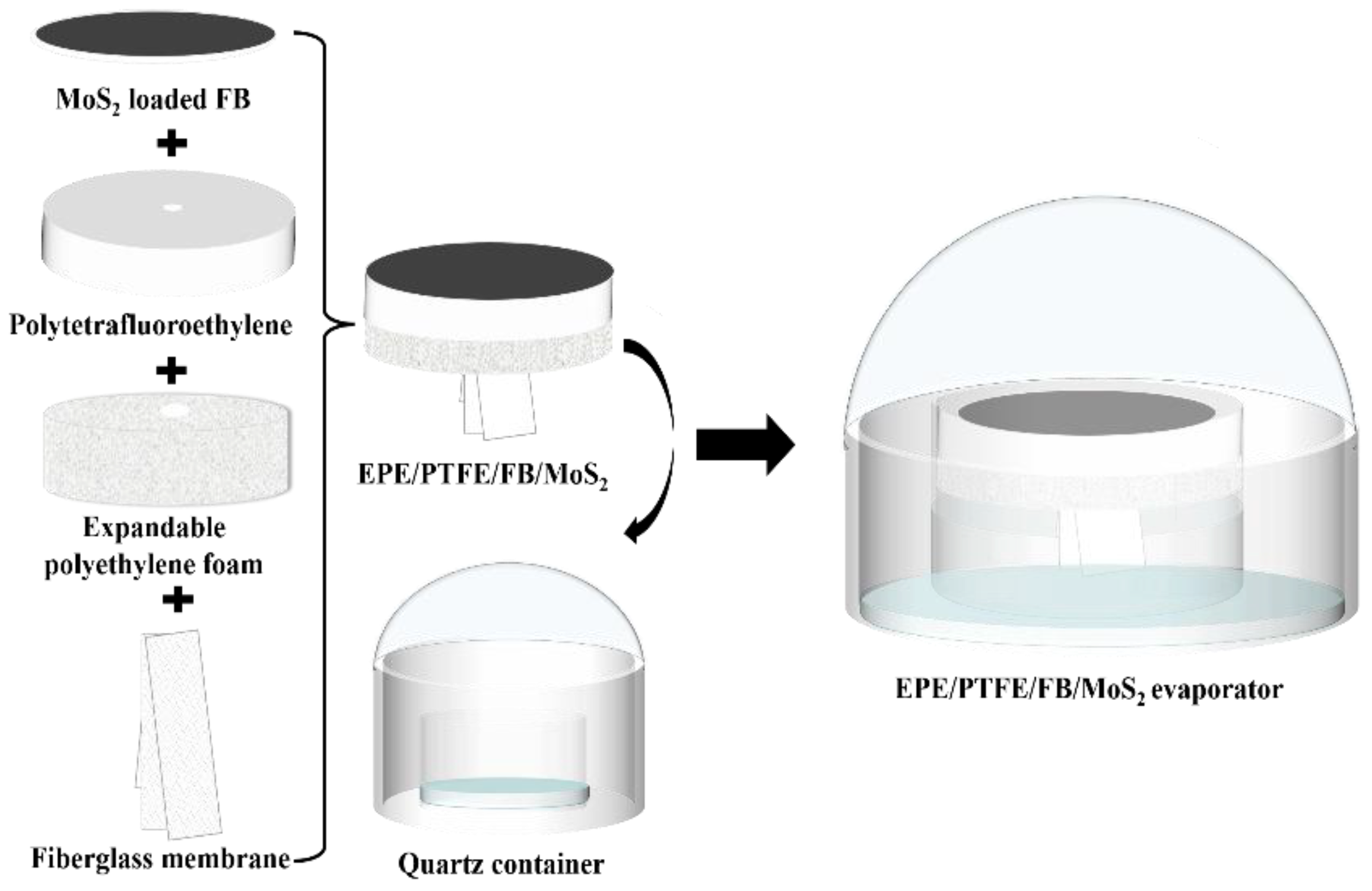
2.2. Preparation of the EPE/PTFE/FB/MoS2 Evaporator
2.3. Solar Evaporation
2.4. Solar Distillation
2.5. Total Organic Chlorine and Total Organic Bromine Detection
2.6. Distillation By-Products Detection
3. Experimental Section
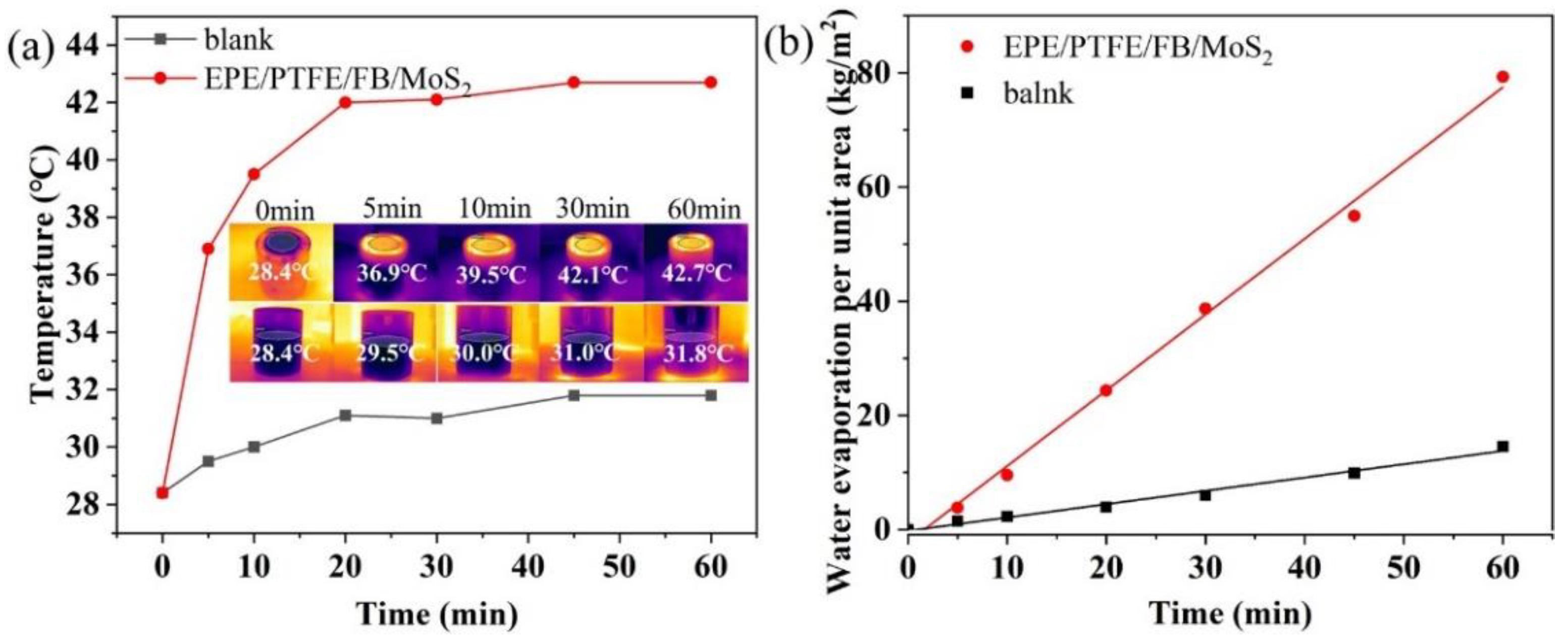
3.1. Solar Evaporation
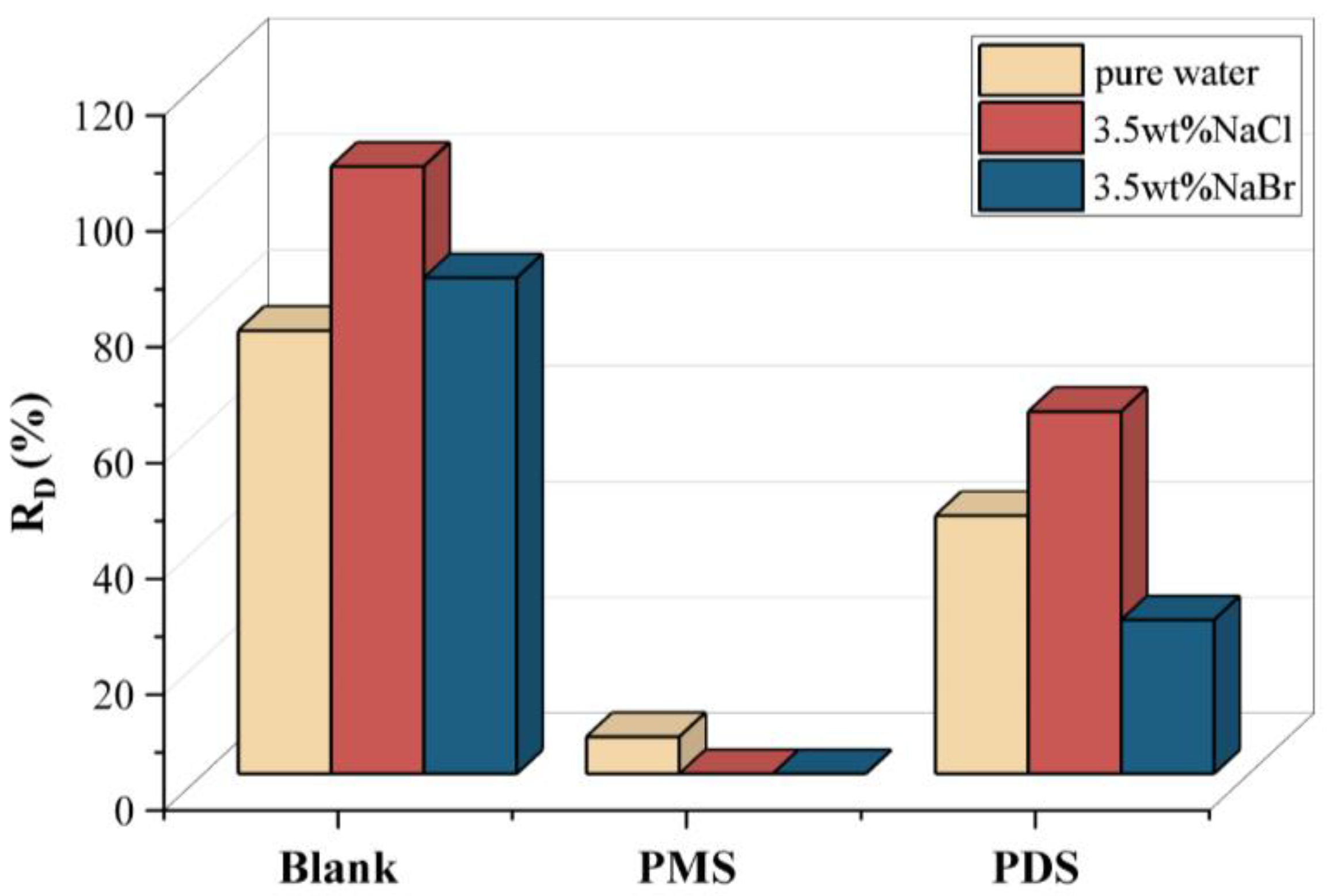
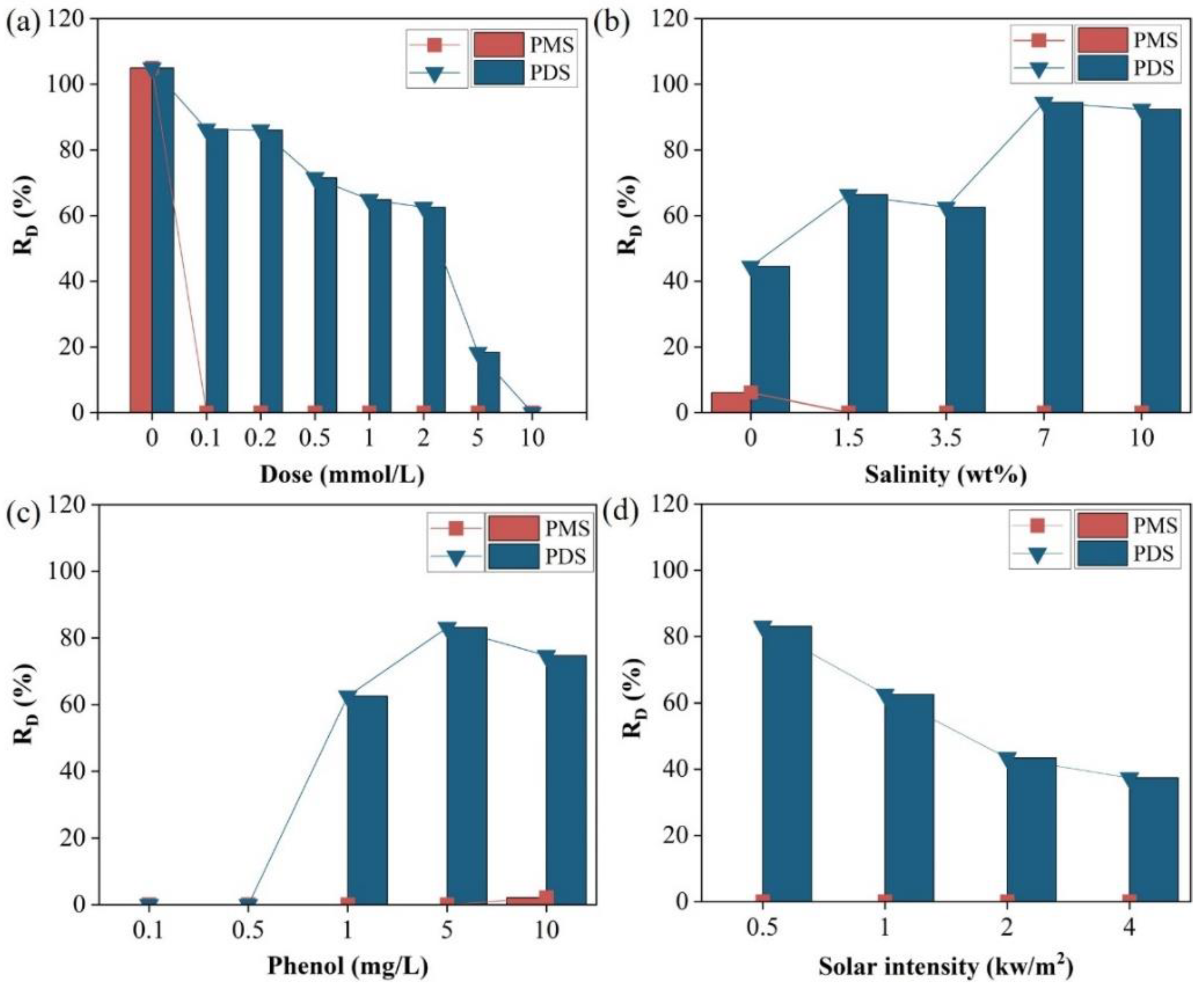
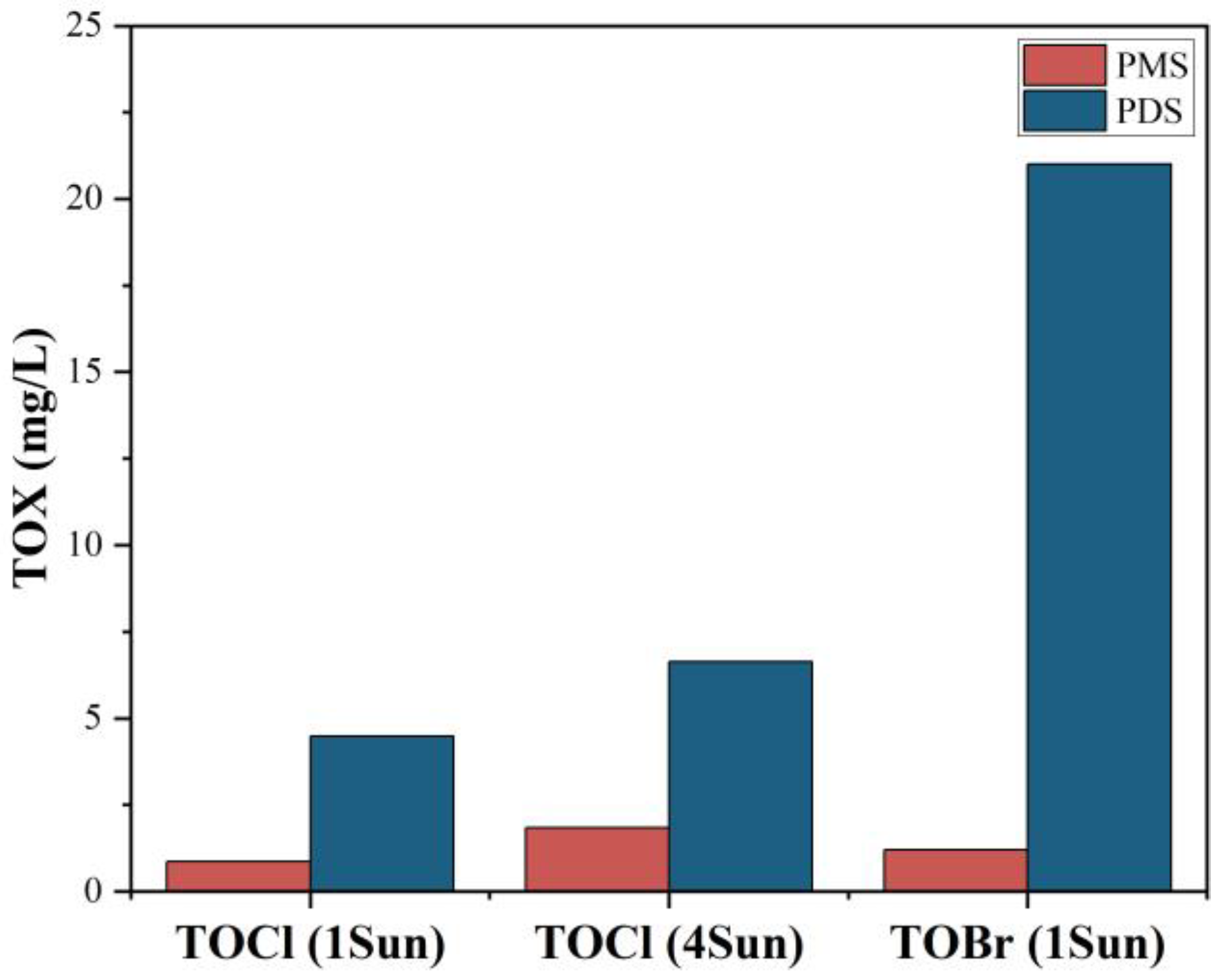
3.2. Solar Distillation by Activated Persulfate
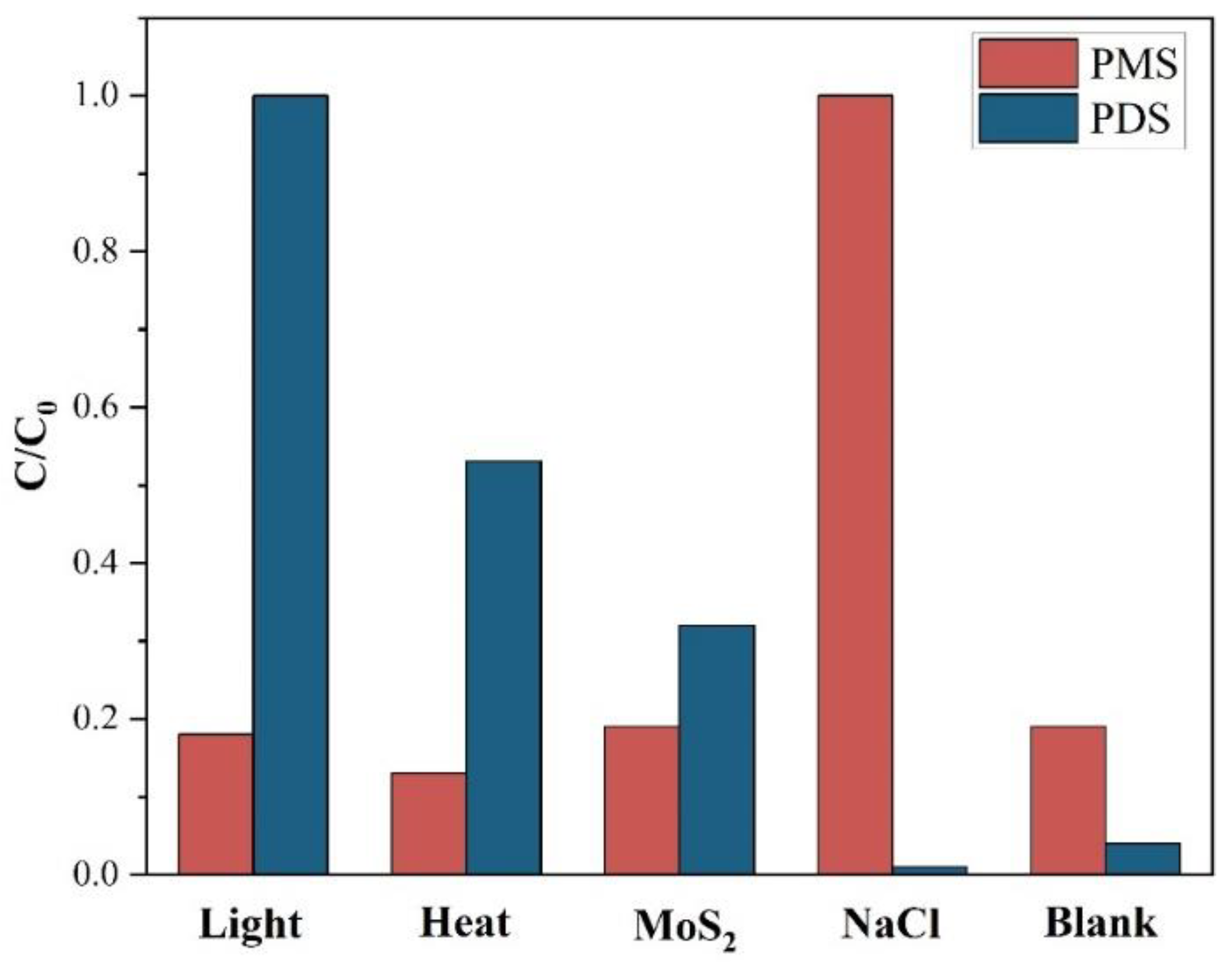
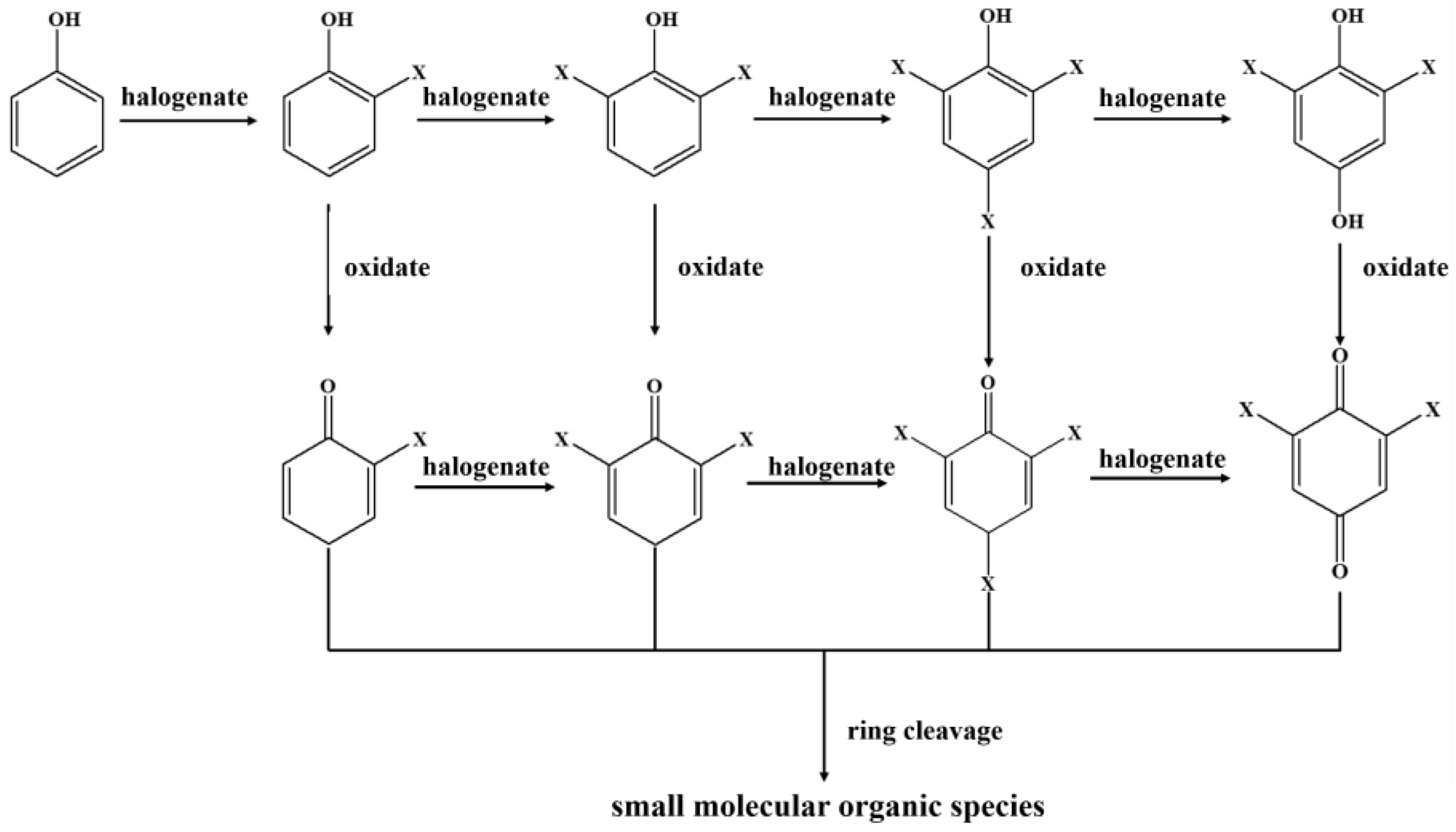
3.3. Activation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| VOCs | volatile organic compounds |
| PS | persulfate |
| PMS | peroxymonosulfate |
| PDS | peroxodisulfate |
| RD | pollutant distillation concentration ratio |
| EPE | expandable polyethylene |
| PTFE | polytetrafluoroethylene |
| FB | fiberglass membrane |
| TOCl | total organic chlorine |
References
- Zhang, T.; Li, Y.T.; Li, C.U.; Sun, S.Y. Effect of salinity on oil production: Review on low salinity waterflooding mechanisms and exploratory study on pipeline scaling. Oil Gas Sci. Technol. 2020, 75, 16. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, K.H.; Gan, Q.M.; Yu, Y.Q.; Zhang, T.Q.; Liu, X.W.; Ye, M.M.; Yin, Y.D. Interfacial solar heating by self-assembled Fe3O4@C film for steam generation. Mat. Chem. Front. 2017, 1, 2620–2626. [Google Scholar] [CrossRef]
- Hao, D.D.; Yang, Y.D.; Xu, B.; Cai, Z.S. Efficient solar water vapor generation enabled by water-absorbing polypyrrole coated cotton fabric with enhanced heat localization. Appl. Therm. Eng. 2018, 141, 406–412. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Ye, Q.X.; Liang, X.B.; Xu, J.L.; Chang, C.; Song, C.Y.; Shang, W.; Wu, J.B.; Tao, P.; Deng, T. Paper-based membranes on silicone floaters for efficient and fast solar-driven interfacial evaporation under one sun. J. Mater. Chem. A 2017, 5, 16359–16368. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Song, S.; Li, Y.; Jia, F.; Feng, T.; Hu, N. Flexible 2D@3D Janus evaporators for high-performance and continuous solar desalination. Desalination 2022, 525, 9. [Google Scholar] [CrossRef]
- Han, X.; Wang, W.P.; Zuo, K.C.; Chen, L.; Yuan, L.; Liang, J.; Li, Q.L.; Ajayan, P.M.; Zhao, Y.; Lou, J. Bio-derived ultrathin membrane for solar driven water purification. Nano Energy 2019, 60, 567–575. [Google Scholar] [CrossRef]
- Noureen, L.; Xie, Z.J.; Gao, Y.J.; Li, M.M.; Hussain, M.; Wang, K.; Zhang, L.B.; Zhu, J.T. Multifunctional Ag3PO4-rgo-coated textiles for clean water production by solar-driven evaporation, photocatalysis, and disinfection. ACS Appl. Mater. Interfaces 2020, 12, 6343–6350. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, L.; Yu, Q.; Lin, P.L.; Xu, J.; Yin, X.Z.; Chen, S.H.; Wang, H.; Wang, L.X. Flexible and highly efficient bilayer photothermal paper for water desalination and purification: Self-floating, rapid water transport, and localized heat. ACS Appl. Mater. Interfaces 2020, 12, 11204–11213. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Zhang, K.F.; Chang, S.; Fu, Q.; Sun, X.B.; Fan, Y.T.; Zhang, M.L.; Tu, X.; Qadeer, A. Occurrence and risk assessment of volatile organic compounds in multiple drinking water sources in the Yangtze River Delta region, China. Ecotox. Environ. Safe 2021, 225, 11. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, T.Q.; Kim, J.; Peng, H.; Ye, M.M.; Huang, C.H. Interfacial solar distillation for freshwater production: Fate of volatile and semivolatile organic contaminants. Environ. Sci. Technol. 2021, 55, 6248–6256. [Google Scholar] [CrossRef]
- Shi, L.; Shi, Y.; Zhuo, S.F.; Zhang, C.L.; Aldrees, Y.; Aleid, S.; Wang, P. Multi-functional 3D honeycomb ceramic plate for clean water production by heterogeneous photo-Fenton reaction and solar-driven water evaporation. Nano Energy 2019, 60, 222–230. [Google Scholar] [CrossRef]
- Davidson, C.J.; Hannigan, J.H.; Bowen, S.E. Effects of inhaled combined Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX): Toward an environmental exposure model. Environ. Toxicol. Pharmacol. 2021, 81, 14. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luo, H.; Liu, R.R.; Li, G.Y.; Yu, Y.X.; An, T.C. The exposure risk of typical VOCs to the human beings via inhalation based on the respiratory deposition rates by proton transfer reaction-time of flight-mass spectrometer. Ecotox. Environ. Safe 2020, 197, 8. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.N.; Kim, Y.J.; Ryu, J.C. Gene expression profiles of human promyelocytic leukemia cell lines exposed to volatile organic compounds. Toxicology 2010, 271, 122–130. [Google Scholar] [CrossRef]
- Williams, P.; Benton, L.; Warmerdam, J.; Sheehan, P. Comparative risk analysis of six volatile organic compounds in California drinking water. Environ. Sci. Technol. 2002, 36, 4721–4728. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Guo, Y.H.; Zhao, F.; Yu, G.H. Hydrogels as an emerging material platform for solar water purification. Acc. Chem. Res. 2019, 52, 3244–3253. [Google Scholar] [CrossRef]
- Song, C.J.; Qi, D.P.; Han, Y.; Xu, Y.; Xu, H.B.; You, S.J.; Wang, W.; Wang, C.; Wei, Y.; Ma, J. Volatile-organic-compound-intercepting solar distillation enabled by a photothermal/photocatalytic nanofibrous membrane with dual-scale pores. Environ. Sci. Technol. 2020, 54, 9025–9033. [Google Scholar] [CrossRef]
- Wen, C.Y.; Yang, J.; Guo, H.S.; Li, Q.S.; Zhang, X.Y.; Wang, X.D.; Cao, M.Y.; Zhang, L. Zwitterionic functionalized catalytic evaporator enables simultaneous solar distillation and organic pollutant degradation. Appl. Energy 2022, 321, 10. [Google Scholar] [CrossRef]
- Zuo, S.Y.; Xia, D.S.; Guan, Z.Y.; Yang, F.; Cheng, S.; Xu, H.M.; Wan, R.Z.; Li, D.Y.; Liu, M. Dual-functional CuO/CN for highly efficient solar evaporation and water purification. Sep. Purif. Technol. 2021, 254, 10. [Google Scholar] [CrossRef]
- Liu, H.L.; Xu, M.J.; Li, G.Y.; Zhang, W.P.; An, T.C. Solar-light-triggered regenerative adsorption removal of styrene by silver nanoparticles incorporated in metal-organic frameworks. Environ. Sci. Nano 2021, 8, 543–553. [Google Scholar] [CrossRef]
- Chen, K.Y.; Lai, W.W.P.; Wang, H.J.; Lin, C.C.; Chen, C.W.; Lin, A.Y.C. Clean water generation through a multifunctional activated carbon-TiO2 interfacial solar distillation system. RSC Adv. 2021, 11, 23036–23044. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Feng, W.H.; Liang, D.; Xu, Y.; Qiu, X.Q. Hydroxyl/amino and Fe (III) co-grafted graphite carbon nitride for photocatalytic removal of volatile organic compounds. Environ. Res. 2021, 197, 14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Xu, H.D.; Wang, L.H.; Jiang, J.; Zhang, T. Nonradical oxidation of pollutants with single-atom-Fe (III)-activated persulfate: Fe(V) being the possible intermediate oxidant. Environ. Sci. Technol. 2020, 54, 14057–14065. [Google Scholar] [CrossRef] [PubMed]
- Manos, D.; Miserli, K.; Konstantinou, I. Perovskite and spinel catalysts for sulfate radical-based advanced oxidation of organic pollutants in water and wastewater systems. Catalysts 2020, 10, 1299. [Google Scholar] [CrossRef]
- Wang, S.Z.; Wang, J.L. Degradation of carbamazepine by radiation-induced activation of peroxymonosulfate. Chem. Eng. J. 2018, 336, 595–601. [Google Scholar] [CrossRef]
- Tang, S.F.; Tang, J.C.; Yuan, D.L.; Wang, Z.T.; Zhang, Y.T.; Rao, Y.D. Elimination of humic acid in water: Comparison of UV/PDS and UV/PMS. RSC Adv. 2020, 10, 17627–17634. [Google Scholar] [CrossRef]
- Milh, H.; Cabooter, D.; Dewil, R. Role of process parameters in the degradation of sulfamethoxazole by heat-activated peroxymonosulfate oxidation: Radical identification and elucidation of the degradation mechanism. Chem. Eng. J. 2021, 422, 11. [Google Scholar] [CrossRef]
- Chen, R.; Wang, X.; Gan, Q.M.; Zhang, T.Q.; Zhu, K.H.; Ye, M.M. A bifunctional MoS2-based solar evaporator for both efficient water evaporation and clean freshwater collection. J. Mater. Chem. A 2019, 7, 11177–11185. [Google Scholar] [CrossRef]
- Wang, X.; Gan, M.; Chen, R.; Peng, H.; Zhang, T.Q.; Ye, M.M. Water Delivery Channel Design in Solar Evaporator for Efficient and Durable Water Evaporation with Salt Rejection. ACS Sustain. Chem. Eng. 2020, 8, 7753–7761. [Google Scholar] [CrossRef]
- Xiao, Y.Y.; Li, C.X.; Zhou, X.J.; Tao, N.Y.; Ye, M.M. Removal of typical volatile organic compounds in condensed freshwater by activated persulfate during interfacial solar distillation. ACS ES&T Water 2021, 1, 2423–2430. [Google Scholar]
- Oyekunle, D.T.; Cai, J.Y.; Gendy, E.A.; Chen, Z.Q. Impact of chloride ions on activated persulfates based advanced oxidation process (AOPs): A mini review. Chemosphere 2021, 280, 13. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wan, A.M. Effect of ionic strength on Henry’s constants of volatile organic compounds. Chemosphere 1998, 36, 2731–2740. [Google Scholar] [CrossRef]
- Guo, Y.G.; Zhou, J.; Lou, X.Y.; Liu, R.L.; Xiao, D.X.; Fang, C.L.; Wang, Z.H.; Liu, J.S. Enhanced degradation of tetrabromobisphenol A in water by a UV/base/persulfate system: Kinetics and intermediates. Chem. Eng. J. 2014, 254, 538–544. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Liang, H.; Li, G.B.; Xu, D.L.; Yan, Z.S.; Chen, R.; Zhao, J.; Tang, X.B. A solar photo-thermochemical hybrid system using peroxydisulfate for organic matters removal and improving ultrafiltration membrane performance in surface water treatment. Water Res. 2021, 188, 13. [Google Scholar] [CrossRef]
- Ma, J.; Li, H.Y.; Yang, Y.Q.; Li, X.N. Influence of water matrix species on persulfate oxidation of phenol: Reaction kinetics and formation of undesired degradation byproducts. Water Sci. Technol. 2018, 2017, 340–350. [Google Scholar] [CrossRef]
- Liu, K.; Lu, J.H.; Ji, Y.F. Formation of brominated disinfection by-products and bromate in cobalt catalyzed peroxymonosulfate oxidation of phenol. Water Res. 2015, 84, 1–7. [Google Scholar] [CrossRef]
- Neta, P.; Grodkowski, J.; Ross, A.B. Rate constants for reactions of aliphatic carbon-centered radicals in aqueous solution. J. Phys. Chem. Ref. Data 1996, 25, 709–1050. [Google Scholar] [CrossRef]
- Buxton, G.V.; Bydder, M.; Salmon, G.A. The reactivity of chlorine atoms in aqueous solution—Part II. The equilibrium SO4−+Cl−reversible arrow Cl−+SO42. PCCP Phys. Chem. Chem. Phys. 1999, 1, 269–273. [Google Scholar] [CrossRef]
- Abusallout, I.; Rahman, S.; Hua, G.H. Effect of temperature and pH on dehalogenation of total organic chlorine, bromine and iodine in drinking water. Chemosphere 2017, 187, 11–18. [Google Scholar] [CrossRef]
- Kristiana, I.; McDonald, S.; Joll, C.A. The forest or the trees: A critical review on the analysis of total organic halogen (TOX) in drinking waters and its utility as a water quality parameter. Environ. Sci. Water Res. Technol. 2020, 6, 2313–2330. [Google Scholar] [CrossRef]
- Fischer, A.; Henderson, G.N. Ipso chlorination of 4-alkylphenols-formation of 4-alkyl-4-chlorocyclohexa-2,5-dienones. Can. J. Chem. Rev. Can. Chim. 1979, 57, 552–557. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhou, X.; Chen, G.B.; Wang, F.; Mao, J.L.; Long, Y.; Sun, H.X.; Zhu, Z.Q.; Liang, W.D.; Li, A. Evaporation efficiency monitoring device based on biomass photothermal material for salt-resistant solar-driven interfacial evaporation. Sol. Energy Mater. Sol. Cells 2021, 222, 9. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, H.B.; Zhu, Z.G.; Hou, H.Q.; Zuo, J.L.; Cui, F.Y.; Liu, D.M.; Wang, W. A mechanically durable, sustained corrosion-resistant photothermal nanofiber membrane for highly efficient solar distillation. J. Mater. Chem. A 2019, 7, 22296–22306. [Google Scholar] [CrossRef]
- Chen, Z.C.; Li, A.; Chen, X.M. Porous graphene/polyimide membrane with a three-dimensional architecture for rapid and efficient solar desalination via interfacial evaporation. ACS Sustain. Chem. Eng. 2020, 8, 13850–13858. [Google Scholar] [CrossRef]
- Zhu, B.; Kou, H.; Liu, Z.X.; Wang, Z.J.; Macharia, D.K.; Zhu, M.F.; Wu, B.H.; Liu, X.G.; Chen, Z.G. Flexible and washable CNT-embedded pan nonwoven fabrics for solar-enabled evaporation and desalination of seawater. ACS Appl. Mater. Interfaces 2019, 11, 35005–35014. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, J.X.; Liu, D.Q.; Xu, H.B.; Cui, F.Y.; Wang, W. Origami system for efficient solar driven distillation in emergency water supply. Chem. Eng. J. 2019, 356, 869–876. [Google Scholar] [CrossRef]
- Qiao, P.Z.; Wu, J.X.; Li, H.Z.; Xu, Y.C.; Ren, L.; Lin, K.; Zhou, W. Plasmon ag-promoted solar-thermal conversion on floating carbon cloth for seawater desalination and sewage disposal. ACS Appl. Mater. Interfaces 2019, 11, 7066–7073. [Google Scholar] [CrossRef]
- Shang, M.Y.; Xu, S.H.; Li, J.L.; Sun, H.J.; Peng, J.; Wang, S.; Zhang, M. CuS hollow nanospheres/cellulose composite film as a recyclable interfacial photothermal evaporator for solar steam generation. Energy Technol. 2022, 10, 9. [Google Scholar] [CrossRef]
- Mnoyan, A.; Choi, M.; Kim, D.H.; Ku, B.J.; Kim, H.; Lee, K.J.; Yasin, A.S.; Nam, S.; Lee, K. Cheap, facile, and upscalable activated carbon-based photothermal layers for solar steam generation. RSC Adv. 2020, 10, 42432–42440. [Google Scholar] [CrossRef]
- Wu, S.H.; Gong, B.Y.; Yang, H.C.; Tian, Y.K.; Xu, C.X.; Guo, X.Z.; Xiong, G.P.; Luo, T.F.; Yan, J.H.; Cen, K.F.; et al. Plasma-made graphene nanostructures with molecularly dispersedF and Na sites for solar desalination of oil-contaminated seawater with complete in-water and in-air oil rejection. ACS Appl. Mater. Interfaces 2020, 12, 38512–38521. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.L.; Ding, H.R.; Sharshir, S.W.; Li, X.J.; Liu, H.C.; Ma, D.K.; Wu, L.R.; Zang, J.F.; Liu, H.; Yu, W.; et al. Low-cost high-efficiency solar steam generator by combining thin film evaporation and heat localization: Both experimental and theoretical study. Appl. Therm. Eng. 2018, 143, 1079–1084. [Google Scholar] [CrossRef]
- Xue, G.B.; Liu, K.; Chen, Q.; Yang, P.H.; Li, J.; Ding, T.P.; Duan, J.J.; Qi, B.; Zhou, J. Robust and Low-Cost Flame-Treated Wood for High-Performance Solar Steam Generation. ACS Appl. Mater. Interfaces 2017, 9, 15052–15057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Peng, X.; Feng, N.; Yan, L.K.; Liu, Q.Q.; Zhang, D.; Gu, J.C.; Wang, W.Q.; Chen, T. Converting pomelo peel into eco-friendly and low-consumption photothermic biomass sponge toward multifunctioal solar-to-heat conversion. ACS Sustain. Chem. Eng. 2020, 8, 5328–5337. [Google Scholar] [CrossRef]
- Ni, F.; Xiao, P.; Qiu, N.X.; Zhang, C.; Liang, Y.; Gu, J.C.; Xia, J.Y.; Zeng, Z.X.; Wang, L.P.; Xue, Q.J.; et al. Collective behaviors mediated multifunctional black sand aggregate towards environmentally adaptive solar-to-thermal purified water harvesting. Nano Energy 2020, 68, 10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Tao, N.; Jin, W.; Wang, X.; Zhang, T.; Ye, M. Inhibition of Phenol from Entering into Condensed Freshwater by Activated Persulfate during Solar-Driven Seawater Desalination. Molecules 2022, 27, 7160. https://doi.org/10.3390/molecules27217160
Zhou X, Tao N, Jin W, Wang X, Zhang T, Ye M. Inhibition of Phenol from Entering into Condensed Freshwater by Activated Persulfate during Solar-Driven Seawater Desalination. Molecules. 2022; 27(21):7160. https://doi.org/10.3390/molecules27217160
Chicago/Turabian StyleZhou, Xiaojiao, Ningyao Tao, Wen Jin, Xingyuan Wang, Tuqiao Zhang, and Miaomiao Ye. 2022. "Inhibition of Phenol from Entering into Condensed Freshwater by Activated Persulfate during Solar-Driven Seawater Desalination" Molecules 27, no. 21: 7160. https://doi.org/10.3390/molecules27217160
APA StyleZhou, X., Tao, N., Jin, W., Wang, X., Zhang, T., & Ye, M. (2022). Inhibition of Phenol from Entering into Condensed Freshwater by Activated Persulfate during Solar-Driven Seawater Desalination. Molecules, 27(21), 7160. https://doi.org/10.3390/molecules27217160








