Hierarchical Porous and Three-Dimensional MXene/SiO2 Hybrid Aerogel through a Sol-Gel Approach for Lithium–Sulfur Batteries
Abstract
1. Introduction
2. Results and Discussion
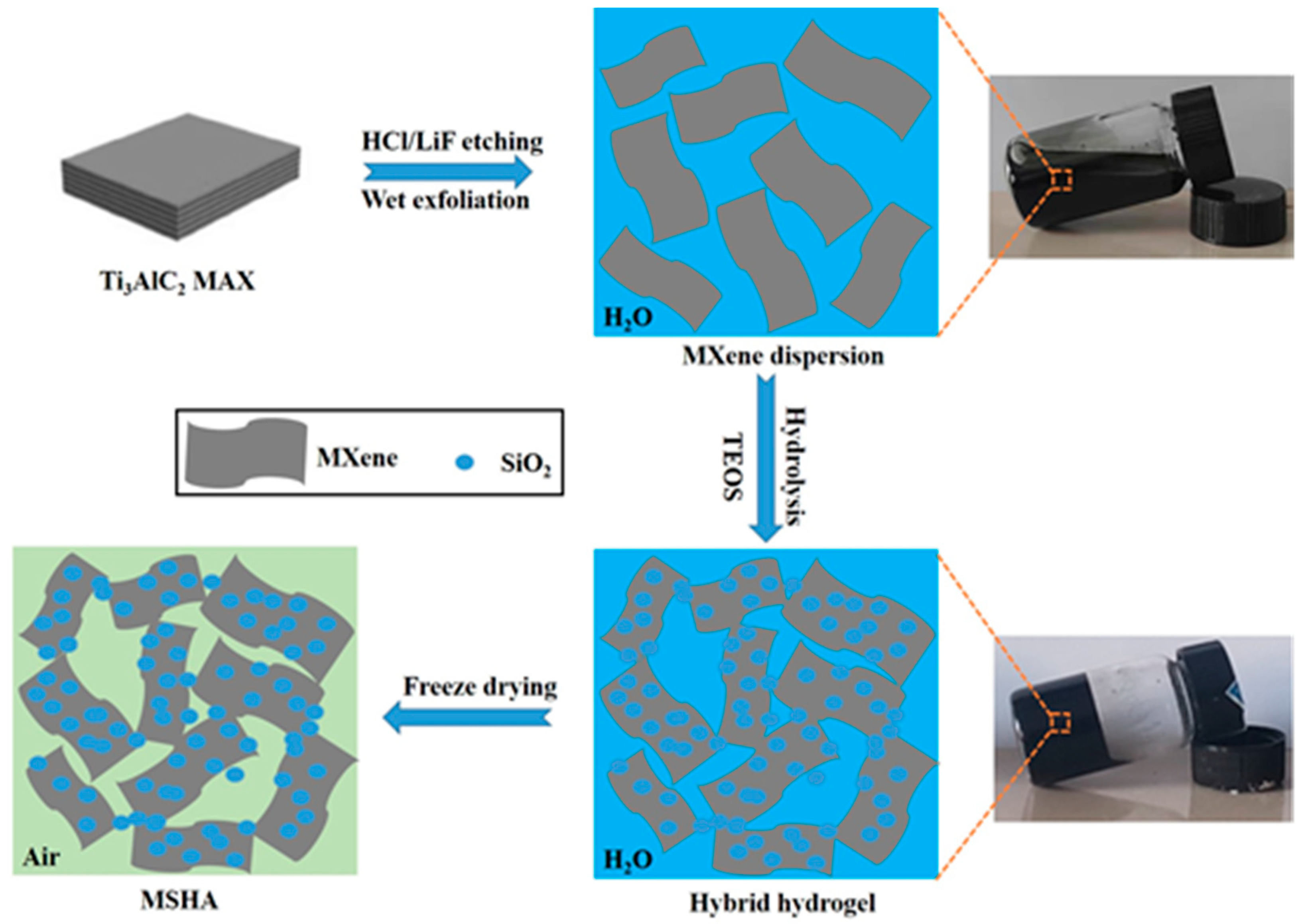
2.1. Preparation of MSHA
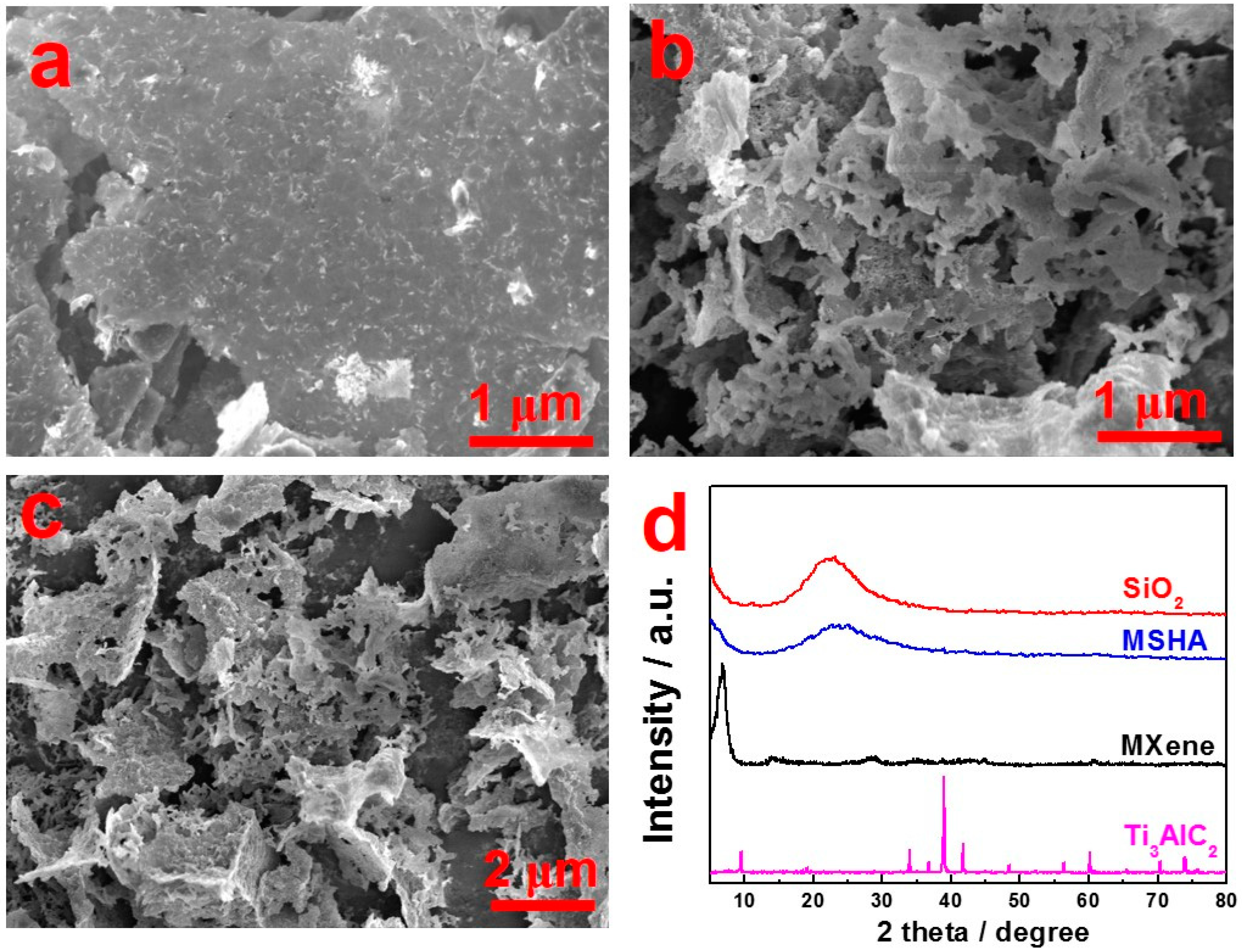
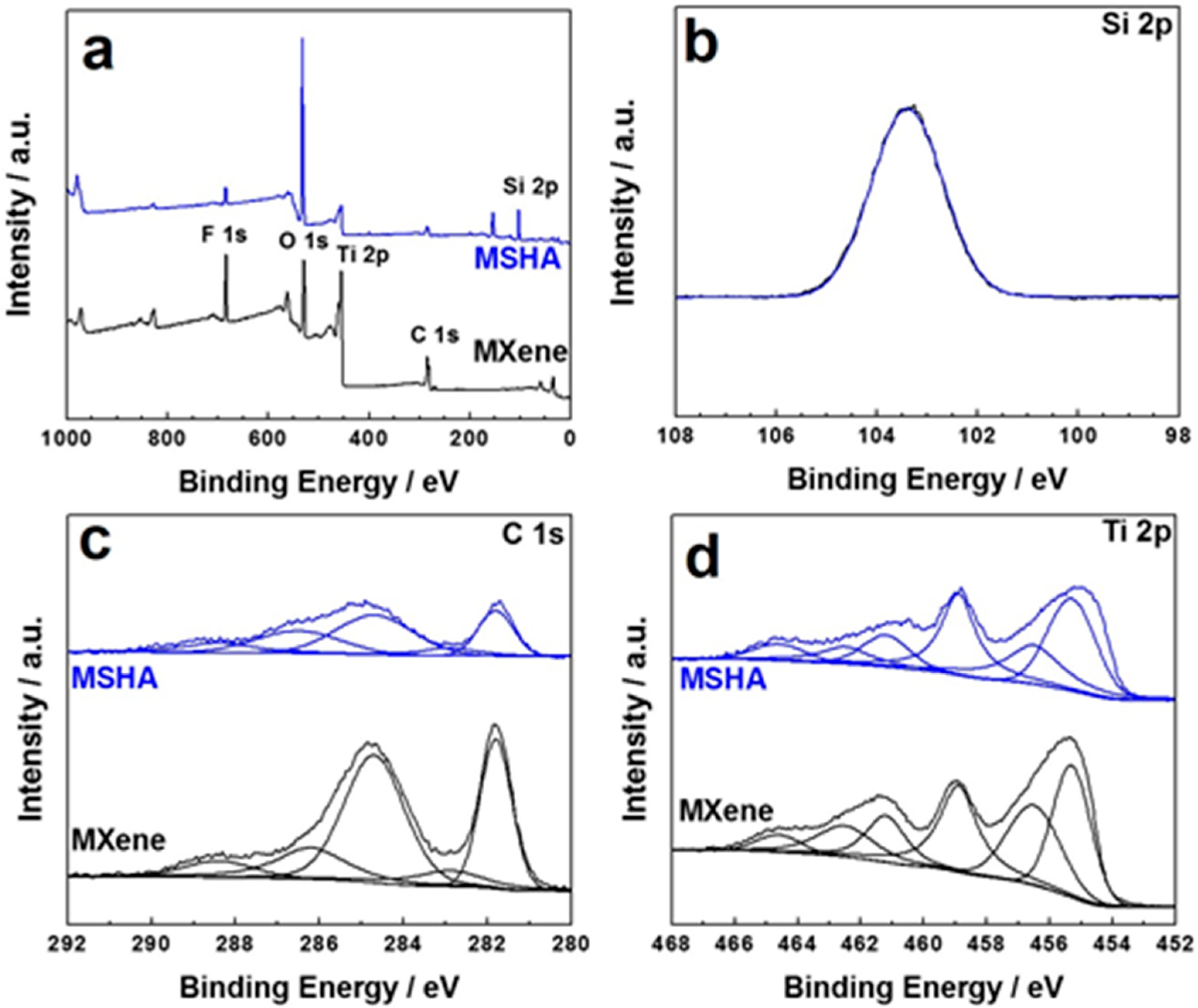
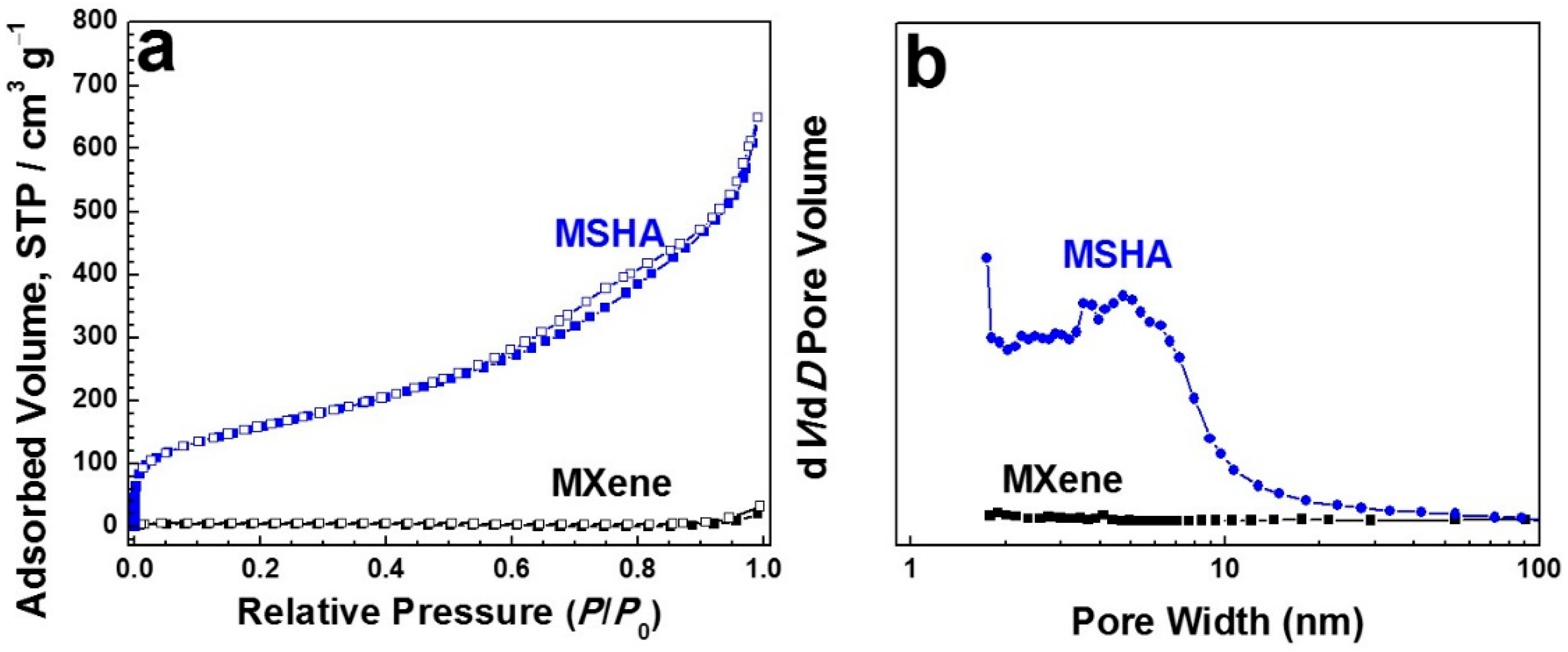
2.2. Microstructures of MSHA and Ti3C2Tx MXene
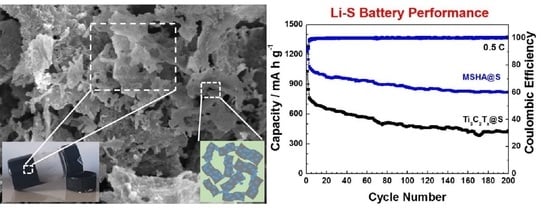
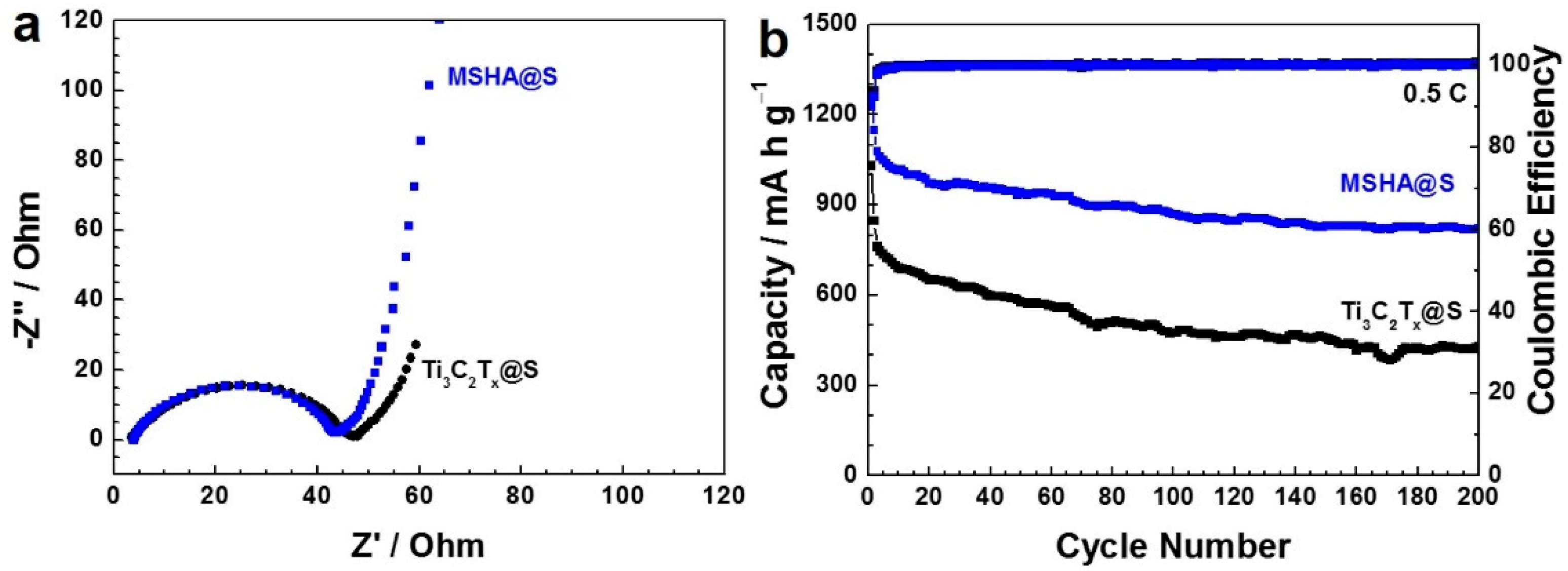
2.3. Battery Performance of MSHA as Sulfur Host
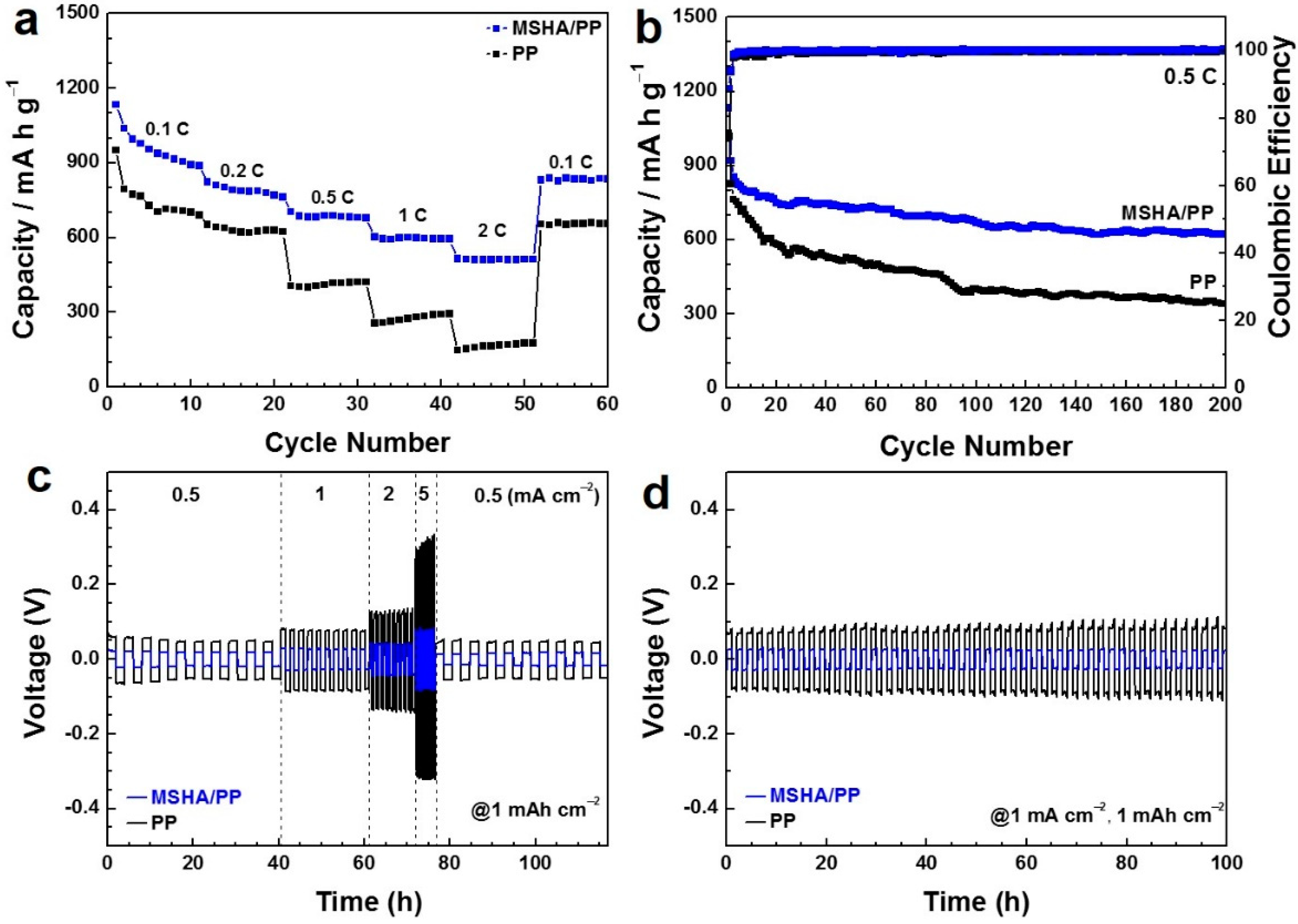
2.4. Battery Performance of Modified Separators
3. Materials and Methods
3.1. Preparation of MXene/SiO2 Hybrid Aerogel (MSHA) Material
3.2. Preparation of MSHA@S Composite
3.3. Preparation of MSHA Modified Separator
3.4. Material Characterization and Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, L.; Lei, W.; Zhang, S.; Liu, Y.; Wallace, G.G.; Chen, J. Two-dimensional transition metal dichalcogenides in supercapacitors and secondary batteries. Energy Storage Mater. 2019, 19, 408–423. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Zhou, A.; Song, H.; Cui, Z.; Du, L. Recent advances and perspectives in lithium−sulfur pouch cells. Molecules 2021, 26, 6341. [Google Scholar] [CrossRef]
- Sehk, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar]
- Zhang, Y.; Liu, Y.; Zhao, Z.; Guo, Y.; Li, Q.; Liu, P.; Hu, B.; Wang, L.; Lu, H. Horizontally aligned lamellar porous graphene/nickel composite for high volumetric capacity lithium-sulfur batteries. Appl. Surf. Sci. 2022, 586, 152805. [Google Scholar] [CrossRef]
- Zhang, N.; Li, B.; Li, S.; Yang, S. Mesoporous hybrid electrolyte for simultaneously inhibiting lithium dendrites and polysulfide shuttle in Li–S batteries. Adv. Energy Mater. 2018, 8, 1703124. [Google Scholar] [CrossRef]
- Peng, H.-J.; Zhang, G.; Chen, X.; Zhang, Z.-W.; Xu, W.-T.; Huang, J.-Q.; Zhang, Q. Enhanced electrochemical kinetics on conductive polar mediators for lithium–sulfur batteries. Angew. Chem. Int. Ed. 2016, 55, 12990–12995. [Google Scholar] [CrossRef]
- Yang, T.; Xia, J.; Piao, Z.; Yang, L.; Zhang, S.; Xing, Y.; Zhou, G. Graphene-based materials for flexible lithium–sulfur batteries. ACS Nano 2021, 15, 13901–13923. [Google Scholar] [CrossRef]
- Tian, J.; Xing, F.; Gao, Q. Graphene-based nanomaterials as the cathode for lithium-sulfur batteries. Molecules 2021, 26, 2507. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, W.; Feng, J.; Qin, X. A polypyrrole-coated acetylene black/sulfur composite cathode material for lithium–sulfur batteries. J. Energy Chem. 2018, 27, 813–819. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, F.; Yang, J. Core@shell sulfur@polypyrrole nanoparticles sandwiched in graphene sheets as cathode for lithium-sulfur batteries. J. Energy Chem. 2015, 24, 448–455. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Kang, F. Porous carbon nanofiber paper as an effective interlayer for high-performance lithium-sulfur batteries. Electrochim. Acta 2015, 168, 271–276. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, G.; Zhang, X.; Wang, S.; Li, C.; Wang, G. Hierarchical porous carbon derived from soybean hulls as a cathode matrix for lithium-sulfur batteries. J. Alloys Compd. 2022, 906, 164341. [Google Scholar] [CrossRef]
- Fang, R.; Chen, K.; Yin, L.; Sun, Z.; Li, F.; Cheng, H.-M. The regulating role of carbon nanotubes and graphene in lithium-ion and lithium-sulfur batteries. Adv. Mater. 2019, 31, 1800863. [Google Scholar] [CrossRef]
- Seh, Z.W.; Li, W.; Cha, J.J.; Zheng, G.; Yang, Y.; McDowell, M.T.; Hsu, P.-C.; Cui, Y. Sulphur-TiO2 yolk-shell nanoarchitecture with internal void space for long-cycle lithium-sulphur batteries. Nat. Commun. 2013, 4, 1331. [Google Scholar] [CrossRef]
- Dong, W.; Meng, L.; Hong, X.; Liu, S.; Shen, D.; Xia, Y.; Yang, S. MnO2/rGO/CNTs framework as a sulfur host for high-performance Li-S batteries. Molecules 2020, 25, 1989. [Google Scholar] [CrossRef]
- Lee, S.-K.; Kim, H.; Bang, S.; Myung, S.-T.; Sun, Y.-K. WO3 nanowire/carbon nanotube interlayer as a chemical adsorption mediator for high-performance lithium-sulfur batteries. Molecules 2021, 26, 377. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Yen, Y.-J.; Tseng, Y.-H.; Chung, S.-H. Module-designed carbon-coated separators for high-loading, high-sulfur-utilization cathodes in lithium–sulfur batteries. Molecules 2022, 27, 228. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Liu, H.; Guo, Z. A strategy for configuration of an integrated flexible sulfur cathode for high-performance lithium-sulfur batteries. Angew. Chem. Int. Ed. 2016, 55, 3992–3996. [Google Scholar] [CrossRef]
- Wu, P.; Tan, L.; Wang, X.-D.; Liao, P.; Liu, Z.; Hou, P.-P.; Zhou, Q.-Y.; Jin, X.-J.; Li, M.-C.; Shao, X.-R.; et al. Porous 3D nitrogen-doped rGO/Co-Ni-S composite modified separator for high-capacity and stable lithium-sulfur batteries. Mater. Res. Bull. 2022, 145, 111550. [Google Scholar] [CrossRef]
- Balach, J.; Singh, H.K.; Gomoll, S.; Jaumann, T.; Klose, M.; Oswald, S.; Richter, M.; Eckert, J.; Giebeler, L. Synergistically enhanced polysulfide chemisorption using a flexible hybrid separator with N and S dual-doped mesoporous carbon coating for advanced lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2016, 8, 14586–14595. [Google Scholar] [CrossRef]
- Xu, G.; Yan, Q.B.; Wang, S.; Kushima, A.; Bai, P.; Liu, K.; Zhang, X.; Tang, Z.; Li, J. A thin multifunctional coating on a separator improves the cyclability and safety of lithium sulfur batteries. Chem. Sci. 2017, 8, 6619–6625. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Liu, X.; Zhu, K.; Wu, S.; Zhou, H. Metal–organic framework-based separator for lithium–sulfur batteries. Nat. Energy 2016, 1, 16094. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Zhuang, T.-Z.; Zhang, Q.; Peng, H.-J.; Chen, C.-M.; Wei, F. Permselective graphene oxide membrane for highly stable and anti-self-discharge lithium–sulfur batteries. ACS Nano 2015, 9, 3002–3011. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, Y.; Pasta, M.; Zhou, G.; Li, Y.; Liu, W.; Xiong, F.; Cui, Y. Entrapment of polysulfides by a black-phosphorus-modified separator for lithium-sulfur batteries. Adv. Mater. 2016, 28, 9797–9803. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhao, M.; Anasori, B.; Maleski, K.; Ren, C.E.; Li, J.; Byles, B.W.; Pomerantseva, E.; Wang, G.; Gogotsi, Y. Porous heterostructured MXene/carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices. Nano Energy 2016, 26, 513–523. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Tang, H.; Li, W.; Pan, L.; Tu, K.; Du, F.; Qiu, T.; Yang, J.; Cullen, C.P.; McEvoy, N.; Zhang, C. A robust, freestanding MXene-sulfur conductive paper for long-lifetime Li-S batteries. Adv. Funct. Mater. 2019, 29, 1901907. [Google Scholar] [CrossRef]
- Xiong, C.; Zhu, G.Y.; Jiang, H.R.; Chen, Q.; Zhao, T.S. Achieving multiplexed functionality in a hierarchical MXene-based sulfur host for high-rate, high-loading lithium-sulfur batteries. Energy Storage Mater. 2020, 33, 147–157. [Google Scholar] [CrossRef]
- Kou, W.; Li, X.; Liu, Y.; Zhang, X.; Yang, S.; Jiang, X.; He, G.; Dai, Y.; Zheng, W.; Yu, G. Triple-layered carbon-SiO2 composite membrane for high energy density and long cycling Li−S batteries. ACS Nano 2019, 13, 5900–5909. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Zhang, S.; Jia, W.; Wang, X.; Guo, Y.; Jia, D.; Wang, L. Decoration of silica nanoparticles on polypropylene separator for lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 7499–7504. [Google Scholar] [CrossRef]
- Lin, L.-W.; Qi, M.; Bai, Z.-T.; Yan, S.-X.; Sui, Z.-Y.; Han, B.-H.; Liu, Y.-W. Crumpled nitrogen-doped aerogels derived from MXene and pyrrole-formaldehyde as modified separators for stable lithium-sulfur batteries. Appl. Surf. Sci. 2021, 555, 149717. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, X.; Chen, Y.; Liu, Y.; Yang, R.; Sui, G. Hierarchically structured cellulose aerogels with interconnected MXene networks and their enhanced microwave absorption properties. J. Mater. Chem. C 2018, 6, 8679–8687. [Google Scholar] [CrossRef]
- Hui, X.; Zhao, R.; Zhang, P.; Li, C.; Wang, C.; Yin, L. Low-temperature reduction strategy synthesized Si/Ti3C2 MXene composite anodes for high-performance Li-ion batteries. Adv. Energy Mater. 2019, 9, 1901065. [Google Scholar] [CrossRef]
- Sui, Z.; Zhang, X.; Lei, Y.; Luo, Y. Easy and green synthesis of reduced graphite oxide-based hydrogels. Carbon 2011, 49, 4314–4321. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, L.; Tang, H.; Du, F.; Guo, Y.; Qiu, T.; Yang, J. Synthesis of two-dimensional Ti3C2Tx MXene using HCl plus LiF etchant: Enhanced exfoliation and delamination. J. Alloys Compd. 2017, 695, 818–826. [Google Scholar] [CrossRef]
- Ma, J.; Cheng, Y.; Wang, L.; Dai, X.; Yu, F. Free-standing Ti3C2Tx MXene film as binder-free electrode in capacitive deionization with an ultrahigh desalination capacity. Chem. Eng. J. 2020, 384, 123329. [Google Scholar] [CrossRef]
- Mu, G.; Mu, D.; Wu, B.; Ma, C.; Bi, J.; Zhang, L.; Yang, H.; Wu, F. Microsphere-like SiO2/MXene hybrid material enabling high performance anode for lithium ion batteries. Small 2020, 16, 1905430. [Google Scholar] [CrossRef]
- Zeng, F.; Sui, Z.; Liu, S.; Liang, H.; Zhan, H.; Han, B. Nitrogen-doped carbon aerogels with high surface area for supercapacitors and gas adsorption. Mater. Today Commun. 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Bao, W.; Liu, L.; Wang, C.; Choi, S.; Wang, D.; Wang, G. Facile synthesis of crumpled nitrogen-doped MXene nanosheets as a new sulfur host for lithium–sulfur batteries. Adv. Energy Mater. 2018, 8, 1702485. [Google Scholar] [CrossRef]
- Xue, W.; Yan, Q.-B.; Xu, G.; Suo, L.; Chen, Y.; Wang, C.; Wang, C.-A.; Li, J. Double-oxide sulfur host for advanced lithium-sulfur batteries. Nano Energy 2017, 38, 12–18. [Google Scholar] [CrossRef]
- Liang, Y.; Xia, T.; Chang, Z.; Xie, W.; Li, Y.; Li, C.; Fan, R.; Wang, W.; Sui, Z.; Chen, Q. Boric acid functionalized triazine-based covalent organic frameworks with dual-function for selective adsorption and lithium-sulfur battery cathode. Chem. Eng. J. 2022, 437, 135314. [Google Scholar] [CrossRef]
- Liu, X.; Xia, M.; Zhao, Y.; Xia, T.; Li, Y.; Xiao, J.; Sui, Z.; Chen, Q. Cationic covalent organic framework via cycloaddition reactions as sulfur-loaded matrix for lithium-sulfur batteries. Mater. Today Chem. 2022, 23, 100664. [Google Scholar]
- Liang, Y.; Xia, M.; Zhao, Y.; Wang, D.; Li, Y.; Sui, Z.; Xiao, J.; Chen, Q. Functionalized triazine-based covalent organic frameworks containing quinoline via aza-Diels-Alder reaction for enhanced lithium-sulfur batteries performance. J. Colloid Interf. Sci. 2022, 608, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-Y.; Sui, Z.-Y.; Zhao, F.-L.; Sun, Y.-N.; Wang, H.-Y.; Han, B.-H. A nanostructured porous carbon/MoO2 composite with efficient catalysis in polysulfide conversion for lithium–sulfur batteries. Nanotechnology 2020, 31, 315601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-Y.; Sui, Z.-Y.; Amin, K.; Lin, L.-W.; Wang, H.-Y.; Han, B.-H. Investigating the electrocatalysis of a Ti3C2/carbon hybrid in polysulfide conversion of lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2020, 12, 13904–13913. [Google Scholar] [CrossRef]
- Abbas, S.A.; Ibrahem, M.A.; Hu, L.H.; Lin, C.N.; Fang, J.; Boopathi, K.M.; Wang, P.; Li, L.J.; Chu, C.W. Bifunctional separator as a polysulfide mediator for highly stable Li-S batteries. J. Mater. Chem. A 2016, 4, 9661–9669. [Google Scholar] [CrossRef]
- Campbell, B.; Bell, J.; Bay, H.; Favors, Z.; Ionescu, R.; Ozkan, C.S.; Ozkan, M. SiO2-coated sulfur particles with mildly reduced graphene oxide as a cathode material for lithium–sulfur batteries. Nanoscale 2015, 7, 7051–7055. [Google Scholar] [CrossRef]
- Rajkumar, P.; Diwakar, K.; Subadevi, R.; Gnanamuthu, R.M.; Wang, F.; Liu, W.; Sivakumar, M. Graphene sheet-encased silica/sulfur composite cathode for improved cyclability of lithium-sulfur batteries. J. Solid State Electr. 2021, 25, 939–948. [Google Scholar] [CrossRef]
- Wu, H.; Tang, Q.; Fan, H.; Liu, Z.; Hu, A.; Zhang, S.; Deng, W.; Chen, X. Dual-confined and hierarchical-porous graphene/C/SiO2 hollow microspheres through spray drying approach for lithium-sulfur batteries. Electrochim. Acta 2017, 255, 179–186. [Google Scholar] [CrossRef]
- Xu, J.; An, S.; Song, X.; Cao, Y.; Wang, N.; Qiu, X.; Zhang, Y.; Chen, J.; Duan, X.; Huang, J.; et al. Towards high performance Li–S batteries via sulfonate-rich COF-modified separator. Adv. Mater. 2021, 33, 2105178. [Google Scholar] [CrossRef]
- Yin, L.; Xu, G.; Nie, P.; Dou, H.; Zhang, X. MXene debris modified eggshell membrane as separator for high-performance lithium-sulfur batteries. Chem. Eng. J. 2018, 352, 695–703. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Pei, Z.; Sui, Z.; Liang, Y.; Xu, X.; Li, Y.; Li, Y.; Qiu, J.; Chen, Q. Hierarchical Porous and Three-Dimensional MXene/SiO2 Hybrid Aerogel through a Sol-Gel Approach for Lithium–Sulfur Batteries. Molecules 2022, 27, 7073. https://doi.org/10.3390/molecules27207073
Zhou J, Pei Z, Sui Z, Liang Y, Xu X, Li Y, Li Y, Qiu J, Chen Q. Hierarchical Porous and Three-Dimensional MXene/SiO2 Hybrid Aerogel through a Sol-Gel Approach for Lithium–Sulfur Batteries. Molecules. 2022; 27(20):7073. https://doi.org/10.3390/molecules27207073
Chicago/Turabian StyleZhou, Jianping, Ziyuan Pei, Zhuyin Sui, Ying Liang, Xiufeng Xu, Yongpeng Li, Yulin Li, Jingyi Qiu, and Qi Chen. 2022. "Hierarchical Porous and Three-Dimensional MXene/SiO2 Hybrid Aerogel through a Sol-Gel Approach for Lithium–Sulfur Batteries" Molecules 27, no. 20: 7073. https://doi.org/10.3390/molecules27207073
APA StyleZhou, J., Pei, Z., Sui, Z., Liang, Y., Xu, X., Li, Y., Li, Y., Qiu, J., & Chen, Q. (2022). Hierarchical Porous and Three-Dimensional MXene/SiO2 Hybrid Aerogel through a Sol-Gel Approach for Lithium–Sulfur Batteries. Molecules, 27(20), 7073. https://doi.org/10.3390/molecules27207073