Self-Organization of Porphyrin–POM Dyads: Nonplanar Diacids and Oxoanions in Low-Dimensional H-Bonding Networks
Abstract
1. Introduction
2. Results
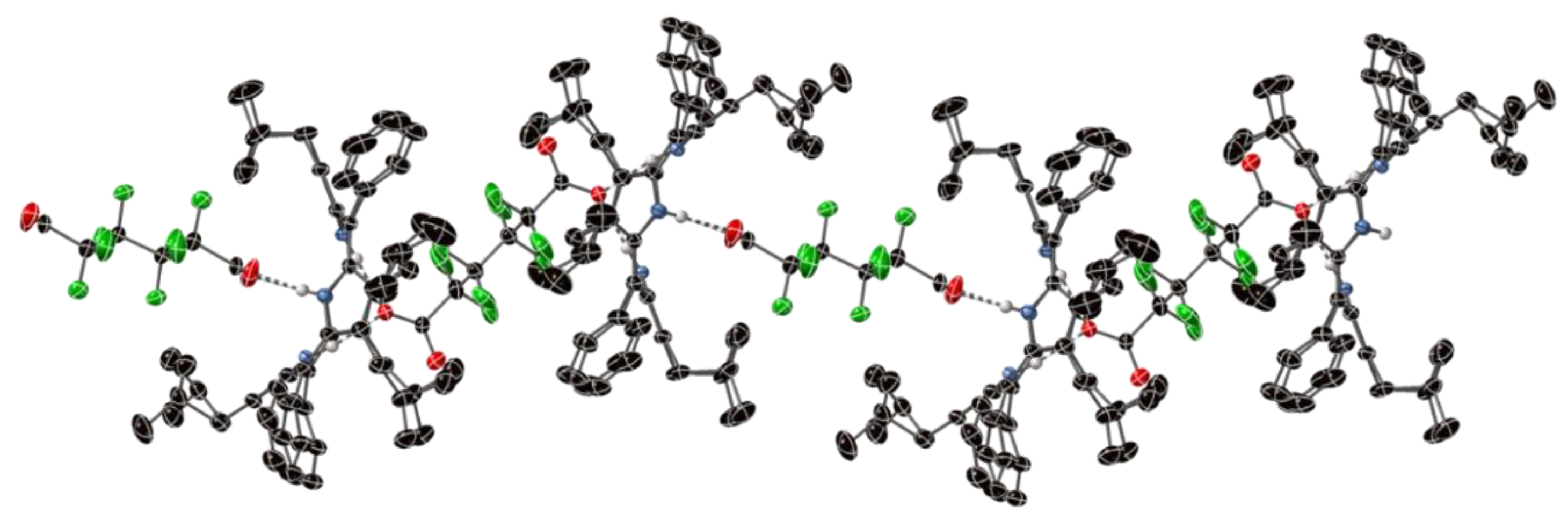
2.1. The Crystal Structure H4OiBuTPP(HSO4)2(H2O)(MeOH)3
2.2. Crystal Structures with Polyanionic Partners
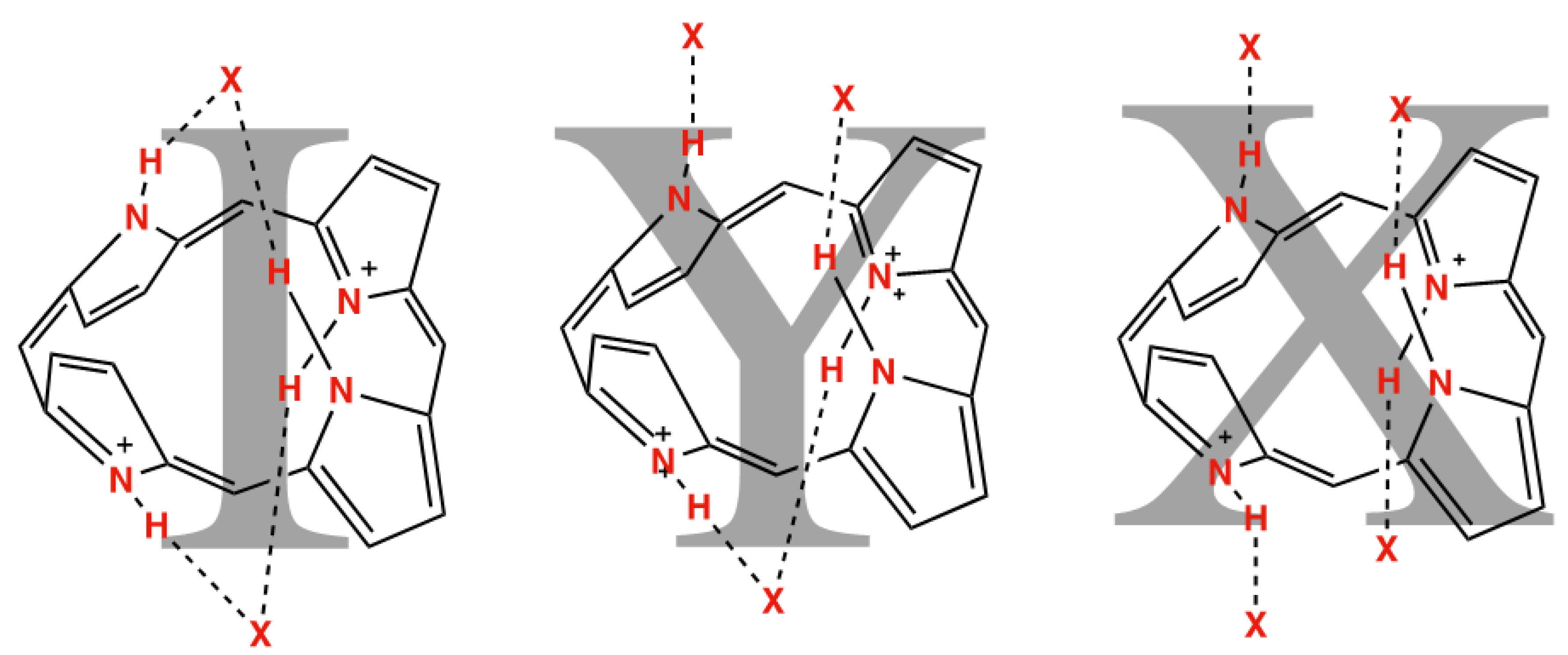
| Compound Number | Compound | N21–H⋯X (Å) | N22–H⋯X (Å) | N23–H⋯X (Å) | N24–H⋯X (Å) |
|---|---|---|---|---|---|
| 1 | [H42](HSO4)2·H2O·3(MeOH) | 2.775(6) | 2.822(6) | 2.758(9) * | 2.728(6) |
| 2 | [H42](O2C(CF2)4CO2)·1.5(CH2Cl2)·1.5(CH3OH) | 2.813(2) | 2.710(3) | 2.879(2) | 2.663(3) |
| 3 | [H42](Br)2·1.6(CH3OH) | 3.376(2) † | 3.332(2) † | =N21 | =N22 |
| 4(1) | [H42]3(MoVI12O36(PO4))2·60(CH3OH)·1.5(H2O) | 2.73(2) | 2.917(18) | 2.912(18) | 2.72(2) |
| 4(2) | 2.919(10) * | - | 2.715(19) | 2.978(17) | |
| 4(3) | 2.914(17) | 2.718(16) | - | 2.899(16) | |
| 4(4) | 2.71(2) | 2.964(18) | 2.910(13) | 2.72(2) | |
| 4(5) | 3.062(18) | 2.882(19) | 2.77(3) | 2.74(2) | |
| 4(6) | 2.80(2) | 2.76(3) | 2.75(3) | 2.812(12) | |
| 5 | [H41](O2C(C(CH2)3C)CO2)·(HO2C(C(CH2)3C)CO2H) | 2.789(6) | =N21 | =N21 | =N21 |
| 6 | [H41](HSeIVO3)2·(CH3OSeIVO2H)·3(CH3OH) | 2.799(3) | 2.812(4) | 2.819(3) | 2.768(4) |
| 7 | [H41](O2C(CH2)10CO2)·4(CH3OH)·H2O | 2.696(4) | 2.687(4) | 2.684(4) | 2.677(4) |
| 8 | [H42](O2C(CH2)CO2H)Cl0.7Br0.3·2(CH3OH) | 2.693(5) * | 3.270(4) † | 2.918(4) * | 3.220(6) † |
| 9 | [H32]2(MoVI4O12(MeO)2)·6(CH3OH) | 2.895(7) * | - | 3.176(6) | 2.94(3) * |
| 10 | [H32](O2CCH3)·0.31(H2O)·1.25(CH2Cl2)·0.69(CH3OH) | 2.816(4) * | 2.96(2) | =N21 | N24⋯H-X |
| 11 | [H42](O2CC6H4CO2H)2·2.5(CH3OH)·0.55(CH2Cl2) 0.6(H2O) | 2.624(3) | 2.890(3) | 2.837(3) | 2.824(3) |
2.3. NSD Analysis
| Compound Formula | Compound Number | |B2u(1)| (Å) | B2u(2) † (Å) | B1u(1) (Å) | A2u(1) (Å) |
|---|---|---|---|---|---|
| “Saddle” | “Ruffle” | “Dome” | |||
| [H42](HSO4)2·H2O·3(MeOH) | 1 | 3.92 | −0.68 | 0.44 | 0.04 |
| [H42](O2C(CF2)4CO2)·1.5(CH2Cl2)·1.5(CH3OH) | 2 | 3.89 | −0.65 | −0.15 | 0.14 |
| [H42](Br)2·1.6(CH3OH) | 3 | 4.08 | −0.73 | 0.25 | −0.11 |
| [H42]3(MoVI12O36(PO4))2·60(CH3OH)·1.5(H2O) | 4(1) | 3.86 | −0.64 | 0 | −0.06 |
| 4(2) | 3.95 | −0.72 | −0.41 | 0.05 | |
| 4(3) | 4.04 | −0.84 | −0.37 | 0.12 | |
| 4(4) | 3.86 | −0.66 | 0.16 | −0.04 | |
| 4(5) | 3.93 | −0.69 | 0.15 | 0.01 | |
| 4(6) | 3.98 | −0.74 | −0.06 | 0 | |
| [H41](O2C(C(CH2)3C)CO2)·(HO2C(C(CH2)3C)CO2H) | 5 | 4.07 | −0.64 | 0 | 0 |
| [H41](HSeIVO3)2·(CH3OseIVO2H)·3(CH3OH) | 6 | 3.61 | −0.76 | −0.01 | −0.05 |
| [H41](O2C(CH2)10CO2)·4(CH3OH)·H2O | 7 | 3.94 | −0.67 | 0.18 | 0.06 |
| [H42](O2C(CH2)CO2H)Cl0.7Br0.3·2(CH3OH) | 8 | 4.08 | −0.71 | −0.23 | −0.18 |
| [H32]2(MoVI4O12(MeO)2)·6(CH3OH) | 9 | 3.87 | −0.72 | 0.11 | 0.05 |
| [H32](O2CCH3)·0.31(H2O) 1.25(CH2Cl2)·0.69(CH3OH) | 10 | 4.06 | −0.76 | −0.27 | −0.18 |
| [H42](O2CC6H4CO2H)2·2.5(CH3OH)·0.55(CH2Cl2)·0.6(H2O) | 11 | 3.97 | −0.75 | 0.02 | 0.07 |
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.2. Crystal Structure Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Senge, M.O.; Sergeeva, N.N.; Hale, K.J. Classic Highlights in Porphyrin and Porphyrinoid Total Synthesis and Biosynthesis. Chem. Soc. Rev. 2021, 50, 4730–4789. [Google Scholar] [CrossRef] [PubMed]
- Fundamentals of Porphyrin Chemistry: A 21st Century Approach; Brothers, P., Senge, M., Eds.; Wiley: Hoboken, NJ, USA, 2022; ISBN 978-1-119-12928-8. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O. Database of Tetrapyrrole Structure Determinations; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; The Porphyrin Handbook; Academic Press: San Diego, CA, USA, 2000; Volume 10, ISBN 978-0-12-393200-6. [Google Scholar]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Golubchikov, O.A.; Pukhovskaya, S.G.; Kuvshinova, E.M. Structures and Properties of Spatially Distorted Porphyrins. Russ. Chem. Rev. 2005, 74, 249–264. [Google Scholar] [CrossRef]
- Kingsbury, C.J.; Senge, M.O. The Shape of Porphyrins. Coord. Chem. Rev. 2021, 431, 213760. [Google Scholar] [CrossRef]
- Shelnutt, J.A.; Song, X.Z.; Ma, J.G.; Jia, S.L.; Jentzen, W.; Medforth, C.J. Nonplanar Porphyrins and Their Significance in Proteins. Chem. Soc. Rev. 1998, 27, 31–41. [Google Scholar] [CrossRef]
- Kielmann, M.; Senge, M.O. Molecular Engineering of Free-Base Porphyrins as Ligands—The N-H ··· X Binding Motif in Tetrapyrroles. Angew. Chem. Int. Ed. Engl. 2019, 58, 418–441. [Google Scholar] [CrossRef]
- Beletskaya, I.; Tyurin, V.S.; Tsivadze, A.Y.; Guilard, R.; Stern, C. Supramolecular Chemistry of Metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, B.F.; Hoskins, B.F.; Michail, D.M.; Robson, R. Assembly of Porphyrin Building Blocks into Network Structures with Large Channels. Nature 1994, 369, 727–729. [Google Scholar] [CrossRef]
- Elliott, R.; Ryan, A.A.; Aggarwal, A.; Zhu, N.; Steuber, F.W.; Senge, M.O.; Schmitt, W. 2D Porphyrinic Metal-Organic Frameworks Featuring Rod-Shaped Secondary Building Units. Molecules 2021, 26, 2955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wasson, M.C.; Shayan, M.; Berdichevsky, E.K.; Ricardo-Noordberg, J.; Singh, Z.; Papazyan, E.K.; Castro, A.J.; Marino, P.; Ajoyan, Z.; et al. A Historical Perspective on Porphyrin-Based Metal–Organic Frameworks and Their Applications. Coord. Chem. Rev. 2021, 429, 213615. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Sanders, J.K.M. The Nature of π-π Interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Živković, J.M.; Stanković, I.M.; Ninković, D.B.; Zarić, S.D. Decisive Influence of Environment on Aromatic/Aromatic Interaction Geometries. Comparison of Aromatic/Aromatic Interactions in Crystal Structures of Small Molecules and in Protein Structures. Cryst. Growth Des. 2021, 21, 1898–1904. [Google Scholar] [CrossRef]
- Senge, M.O. Exercises in Molecular Gymnastics—Bending, Stretching and Twisting Porphyrins. Chem. Commun. 2006, 2006, 243–256. [Google Scholar] [CrossRef]
- Kingsbury, C.J.; Flanagan, K.J.; Eckhardt, H.-G.; Kielmann, M.; Senge, M.O. Weak Interactions and Conformational Changes in Core-Protonated A2- and Ax-Type Porphyrin Dications. Molecules 2020, 25, 3195. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, A.; Brancatelli, G.; Hickey, N.; Farnetti, E.; De Zorzi, R.; Bonaccorso, C.; Purrello, R.; Geremia, S. Interactions of a Water-Soluble Calix[4]Arene with Spermine: Solution and Solid-State Characterisation. Supramolec. Chem. 2016, 28, 499–505. [Google Scholar] [CrossRef]
- De Zorzi, R.; Guidolin, N.; Randaccio, L.; Purrello, R.; Geremia, S. Nanoporous Crystals of Calixarene/Porphyrin Supramolecular Complex Functionalized by Diffusion and Coordination of Metal Ions. J. Am. Chem. Soc. 2009, 131, 2487–2489. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, M.; Sortino, G.; Randazzo, R.; Pisagatti, I.; Notti, A.; Fragalà, M.E.; Parisi, M.F.; D’Urso, A.; Purrello, R. Long-Range Chiral Induction by a Fully Noncovalent Approach in Supramolecular Porphyrin–Calixarene Assemblies. Chem. Eur. J. 2020, 26, 3515–3518. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, A.; Marino, N.; Gaeta, M.; Rizzo, M.S.; Cristaldi, D.A.; Fragalà, M.E.; Pappalardo, S.; Gattuso, G.; Notti, A.; Parisi, M.F.; et al. Porphyrin Stacks as an Efficient Molecular Glue to Induce Chirality in Hetero-Component Calixarene–Porphyrin Assemblies. New J. Chem. 2017, 41, 8078–8083. [Google Scholar] [CrossRef]
- Ribo, J.M.; Rubires, R.; El-Hachemi, Z.; Farrera, J.-A.; Campos, L.; Pakhomov, G.L.; Vendrell, M. Self-Assembly to Ordered Films of the Homoassociate Solutions of the Tetrasodium Salt of 5,10,15,20-Tetrakis(4-Sulfonatophenyl) Porphyrin Dihydrochloride. Mater. Sci. Eng. C 2000, 11, 107–115. [Google Scholar] [CrossRef]
- Borovkov, V.V.; Fujii, I.; Muranaka, A.; Hembury, G.A.; Tanaka, T.; Ceulemans, A.; Kobayashi, N.; Inoue, Y. Rationalization of Supramolecular Chirality in a Bisporphyrin System. Angew. Chem. Int. Ed. 2004, 43, 5481–5485. [Google Scholar] [CrossRef] [PubMed]
- Charalambidis, G.; Georgilis, E.; Panda, M.K.; Anson, C.E.; Powell, A.K.; Doyle, S.; Moss, D.; Jochum, T.; Horton, P.N.; Coles, S.J.; et al. A Switchable Self-Assembling and Disassembling Chiral System Based on a Porphyrin-Substituted Phenylalanine–Phenylalanine Motif. Nat. Commun. 2016, 7, 12657. [Google Scholar] [CrossRef] [PubMed]
- Drain, C.M.; Varotto, A.; Radivojevic, I. Self-Organized Porphyrinic Materials. Chem. Rev. 2009, 109, 1630–1658. [Google Scholar] [CrossRef]
- Goldberg, I. Crystal Engineering of Nanoporous Architectures and Chiral Porphyrin Assemblies. Cryst. Eng. Comm. 2008, 10, 637. [Google Scholar] [CrossRef]
- Borovkov, V. Supramolecular Chirality in Porphyrin Chemistry. Symmetry 2014, 6, 256–294. [Google Scholar] [CrossRef]
- Kojima, T.; Honda, T.; Ohkubo, K.; Shiro, M.; Kusukawa, T.; Fukuda, T.; Kobayashi, N.; Fukuzumi, S. A Discrete Supramolecular Conglomerate Composed of Two Saddle-Distorted Zinc(II)-Phthalocyanine Complexes and a Doubly Protonated Porphyrin with Saddle Distortion Undergoing Efficient Photoinduced Electron Transfer. Angew. Chem. Int. Ed. 2008, 47, 6712–6716. [Google Scholar] [CrossRef]
- Ishizuka, T.; Ohkawa, S.; Ochiai, H.; Hashimoto, M.; Ohkubo, K.; Kotani, H.; Sadakane, M.; Fukuzumi, S.; Kojima, T. A Supramolecular Photocatalyst Composed of a Polyoxometalate and a Photosensitizing Water-Soluble Porphyrin Diacid for the Oxidation of Organic Substrates in Water. Green Chem. 2018, 20, 1975–1980. [Google Scholar] [CrossRef]
- Fischer, H.; Orth, H. Die Chemie Des Pyrrols; Akademische Verlagsgesellschaft: Leipzig, Germany, 1937. [Google Scholar]
- Stone, A.; Fleischer, E.B. The Molecular and Crystal Structure of Porphyrin Diacids. J. Am. Chem. Soc. 1968, 90, 2735–2748. [Google Scholar] [CrossRef]
- Senge, M.O.; Kalisch, W.W. Structure and Conformation of Tetra-Meso-, Octa-β-, and Dodecasubstituted 22,24-Dihydroporphyrins (Porphyrin Dications). Z. Naturforsch. B 1999, 54, 943–959. [Google Scholar] [CrossRef]
- Senge, M.O.; Forsyth, T.P.; Nguyen, L.T.; Smith, K.M. Sterically Strained Porphyrins—Influence of Core Protonation and Peripheral Substitution on the Conformation of Tetra-Meso-, Octa-β-, and Dodeca-Substituted Porphyrin Dications. Angew. Chem. Int. Ed. Engl. 1995, 33, 2485–2487. [Google Scholar] [CrossRef]
- Roucan, M.; Kielmann, M.; Connon, S.J.; Bernhard, S.S.R.; Senge, M.O. Conformational Control of Nonplanar Free Base Porphyrins: Towards Bifunctional Catalysts of Tunable Basicity. Chem. Commun. 2017, 54, 26–29. [Google Scholar] [CrossRef]
- Chen, W.-T. Crystal Structure and Photophysical Properties of a Novel Polyoxomolybdate Porphyrin. Acta Crystallogr. C Struct. Chem. 2020, 76, 1062–1067. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Yamashita, K.; Tani, Y.; Aoyama, T.; Ogawa, T. Structure Determination and Negative Differential Resistance of Tetraarylporphyrin/Polyoxometalate 2:1 Complexes. J. Mater. Chem. C 2020, 8, 14423–14430. [Google Scholar] [CrossRef]
- Lamare, R.; Ruppert, R.; Boudon, C.; Ruhlmann, L.; Weiss, J. Porphyrins and Polyoxometalate Scaffolds. Chem. Eur. J. 2021, 27, 16071–16081. [Google Scholar] [CrossRef] [PubMed]
- Aoki, E.; Suzuki, W.; Kotani, H.; Ishizuka, T.; Sakai, H.; Hasobe, T.; Kojima, T. Efficient Photocatalytic Proton-Coupled Electron-Transfer Reduction of O 2 Using a Saddle-Distorted Porphyrin as a Photocatalyst. Chem. Commun. 2019, 55, 4925–4928. [Google Scholar] [CrossRef] [PubMed]
- Kupitz, C.; Basu, S.; Grotjohann, I.; Fromme, R.; Zatsepin, N.A.; Rendek, K.N.; Hunter, M.S.; Shoeman, R.L.; White, T.A.; Wang, D.; et al. Serial Time-Resolved Crystallography of Photosystem II Using a Femtosecond X-ray Laser. Nature 2014, 513, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Norvaiša, K.; Flanagan, K.J.; Gibbons, D.; Senge, M.O. Conformational Re-engineering of Porphyrins as Receptors with Switchable N−H⋅⋅⋅X-Type Binding Modes. Angew. Chem. Int. Ed. Engl. 2019, 58, 16553–16557. [Google Scholar] [CrossRef] [PubMed]
- Norvaiša, K.; O’Brien, J.E.; Gibbons, D.J.; Senge, M.O. Elucidating Atropisomerism in Nonplanar Porphyrins with Tunable Supramolecular Complexes. Chem. Eur. J. 2021, 27, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.F. Three-Dimensional Nets and Polyhedra; Wiley Monographs in Crystallography; Wiley: New York, NY, USA, 1977; ISBN 0-471-02151-2. [Google Scholar]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. Engl. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Grover, N.; Flanagan, K.J.; Trujillo, C.; Kingsbury, C.J.; Senge, M.O. An Insight into Non-Covalent Interactions on the Bicyclo[1.1.1]Pentane Scaffold. Eur. J. Org. Chem. 2021, 2021, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Barkigia, K.M.; Fajer, J.; Berber, M.D.; Smith, K.M. A Solvate of the Diacetate Salt of the Octaethyltetraphenylporphyrin Dication, H4OETPP2+.2CH3COO−.3CH3COOH.CH2Cl2. Acta Crystallogr. C Cryst. Struct. Commun. 1995, 51, 511–515. [Google Scholar] [CrossRef]
- Kielmann, M.; Grover, N.; Kalisch, W.W.; Senge, M.O. Incremental Introduction of Organocatalytic Activity into Conformationally Engineered Porphyrins. Eur. J. Org. Chem. 2019, 2019, 2448–2452. [Google Scholar] [CrossRef]
- Collman, J.P.; Brauman, J.I.; Collins, T.J.; Iverson, B.; Sessler, J.L.; Morris, R.M.; Gibson, Q.H. The ‘Pocket’ Porphyrins: Hemoprotein Models with Lowered CO Affinities. Inorg. Chim. Acta 1983, 79, 101–102. [Google Scholar] [CrossRef]
- Shi, Z.; Zhou, Y.; Zhang, L.; Mu, C.; Ren, H.; Hassan, D.U.; Yang, D.; Asif, H.M. New Supramolecular Compounds Based on Porphyrin and Polyoxometalate: Synthesis, Characterization and Nonlinear Optical and Optical Limiting Properties. RSC Adv. 2014, 4, 50277–50284. [Google Scholar] [CrossRef]
- Cao, N.; Riss, A.; Corral-Rascon, E.; Meindl, A.; Auwärter, W.; Senge, M.O.; Ebrahimi, M.; Barth, J.V. Surface-Confined Formation of Conjugated Porphyrin-Based Nanostructures on Ag(111). Nanoscale 2021, 13, 19884–19889. [Google Scholar] [CrossRef]
- Káfuňková, E.; Lang, K.; Kubát, P.; Klementová, M.; Mosinger, J.; Šlouf, M.; Troutier-Thuilliez, A.-L.; Leroux, F.; Verney, V.; Taviot-Guého, C. Porphyrin-Layered Double Hydroxide/Polymer Composites as Novel Ecological Photoactive Surfaces. J. Mater. Chem. 2010, 20, 9423–9432. [Google Scholar] [CrossRef]
- Lackinger, M.; Janson, M.S.; Ho, W. Localized Interaction of Single Porphyrin Molecules with Oxygen Vacancies on TiO 2 (110). J. Chem. Phys. 2012, 137, 234707. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamamoto, A.; Furuta, S.; Horie, M.; Kodama, M.; Sato, W.; Akiba, K.; Tsuzuki, S.; Uchimaru, T.; Hashizume, D.; et al. Synthesis and Structure of 16 π Octaalkyltetraphenylporphyrins. J. Am. Chem. Soc. 2005, 127, 14540–14541. [Google Scholar] [CrossRef]
- Barkigia, K.M.; Fajer, J.; Renner, M.W.; Dolores Berber, M.; Medforth, C.J.; Smith, K.M. Nonplanar Porphyrins. X-Ray Structures of (2,3,7,8,12,13,17,18-Octaethyl-and-Octamethyl-5,10,15,20-Tetraphenylporphinato)Zinc(II). J. Am. Chem. Soc. 1990, 112, 8851–8857. [Google Scholar] [CrossRef]
- Hope, H. X-Ray Crystallography: A Fast, First-Resort Analytical Tool; Progress in Inorganic Chemistry; John Wiley & Sons, Inc.: New York, NY, USA, 1994; Volume 41, ISBN 978-0-470-16642-0. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. PLATON SQUEEZE: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Crystallogr. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Woods, J.F.; Gallego, L.; Pfister, P.; Maaloum, M.; Vargas Jentzsch, A.; Rickhaus, M. Shape-Assisted Self-Assembly. Nat. Commun. 2022, 13, 3681. [Google Scholar] [CrossRef] [PubMed]

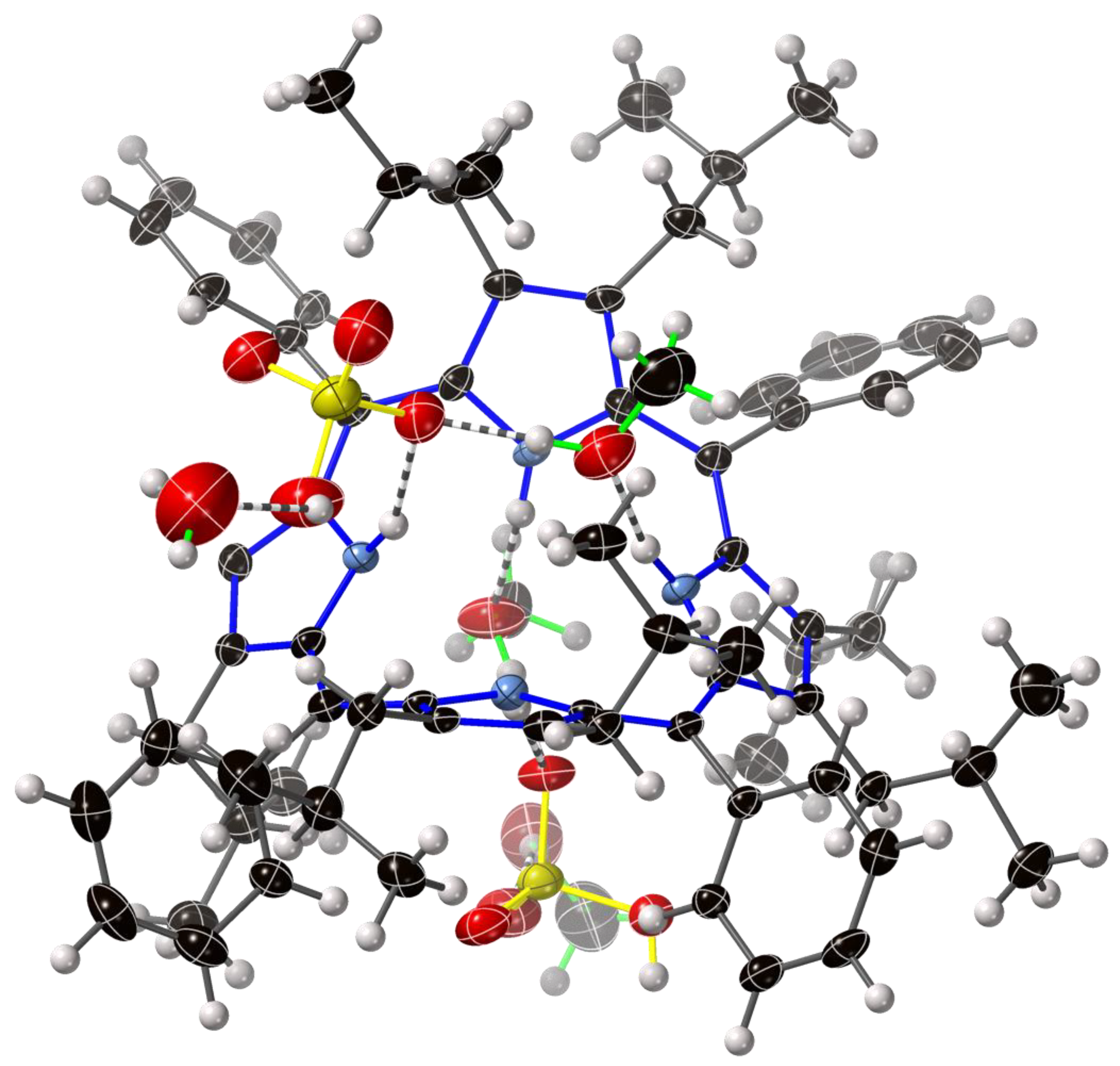
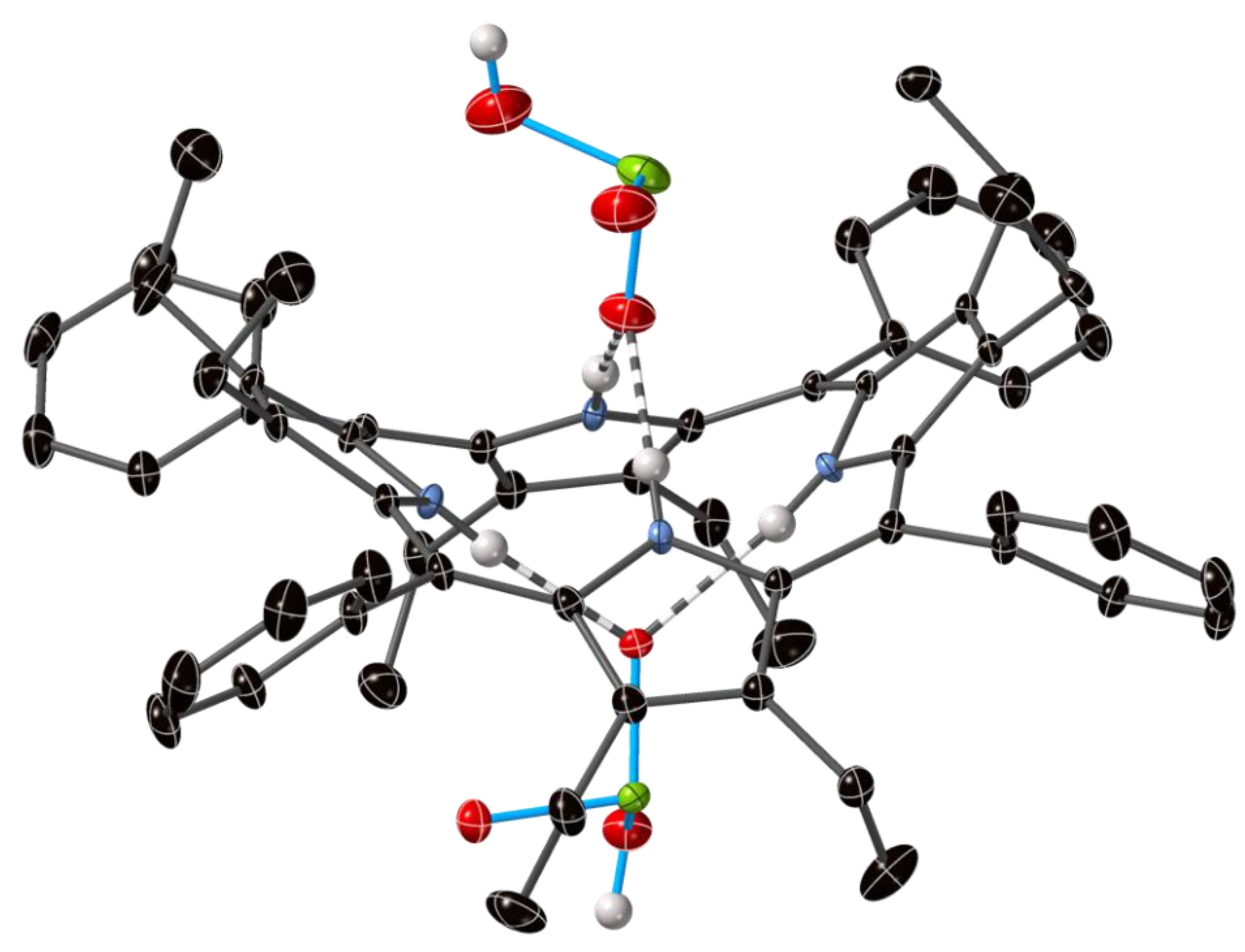
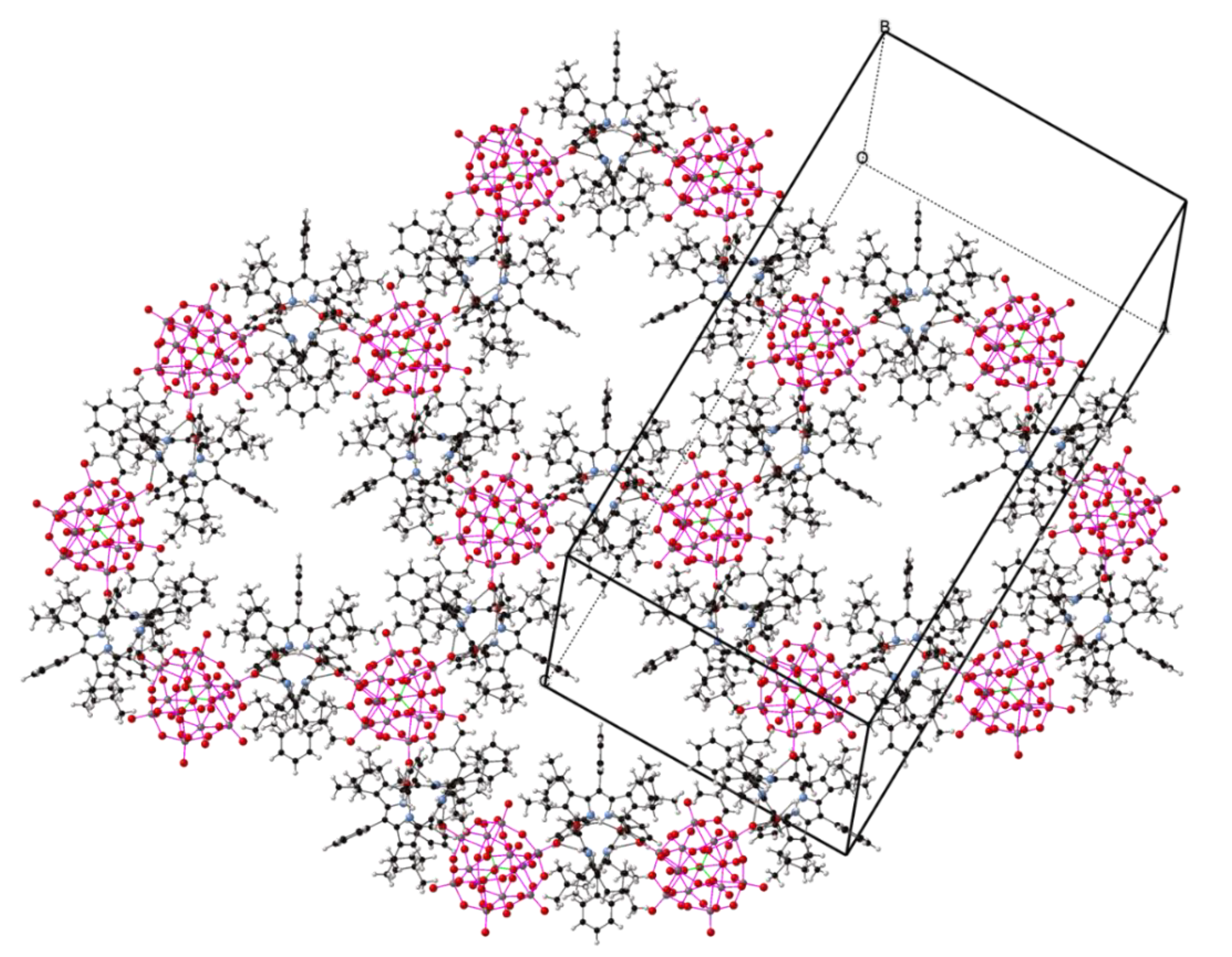
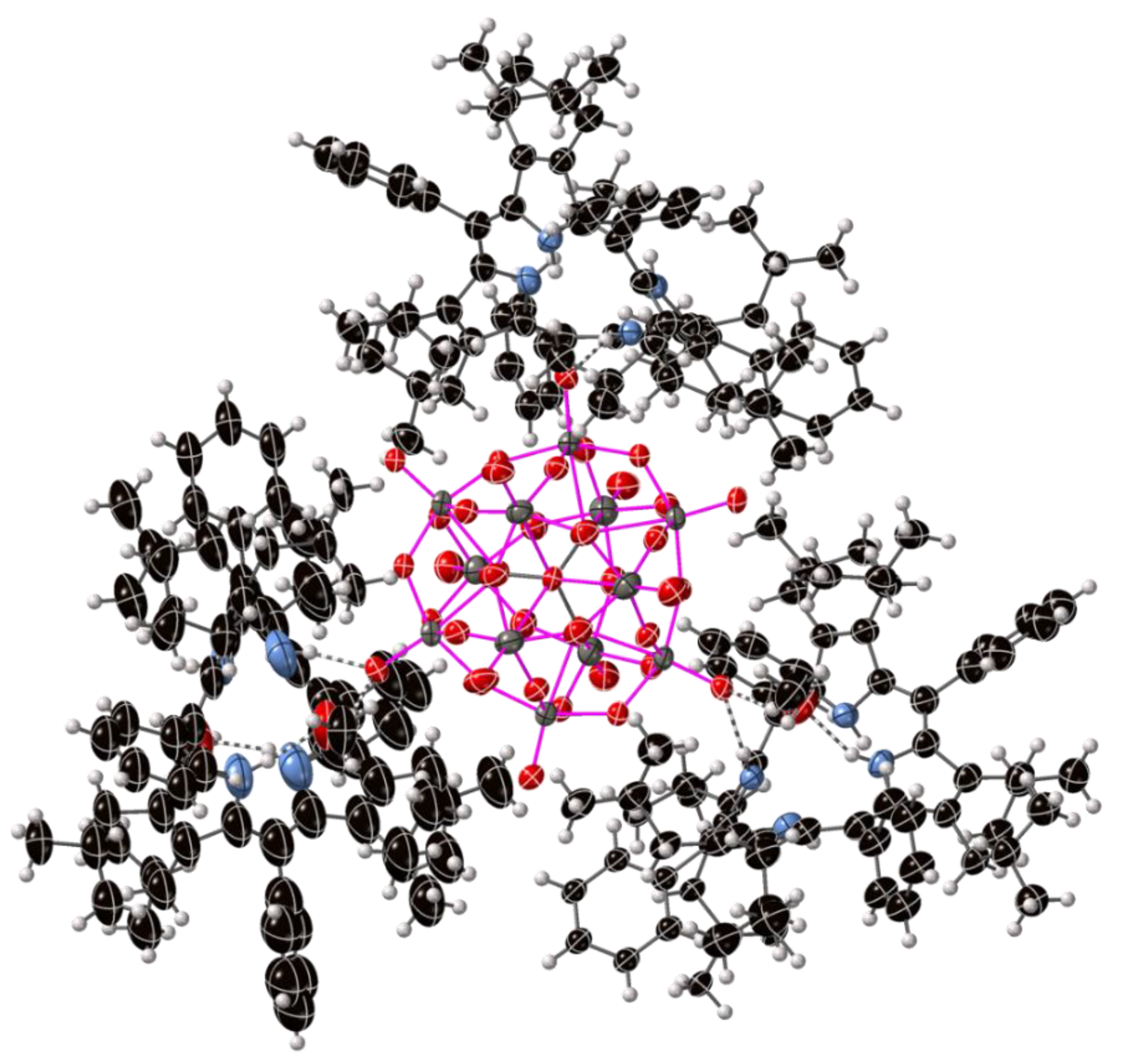

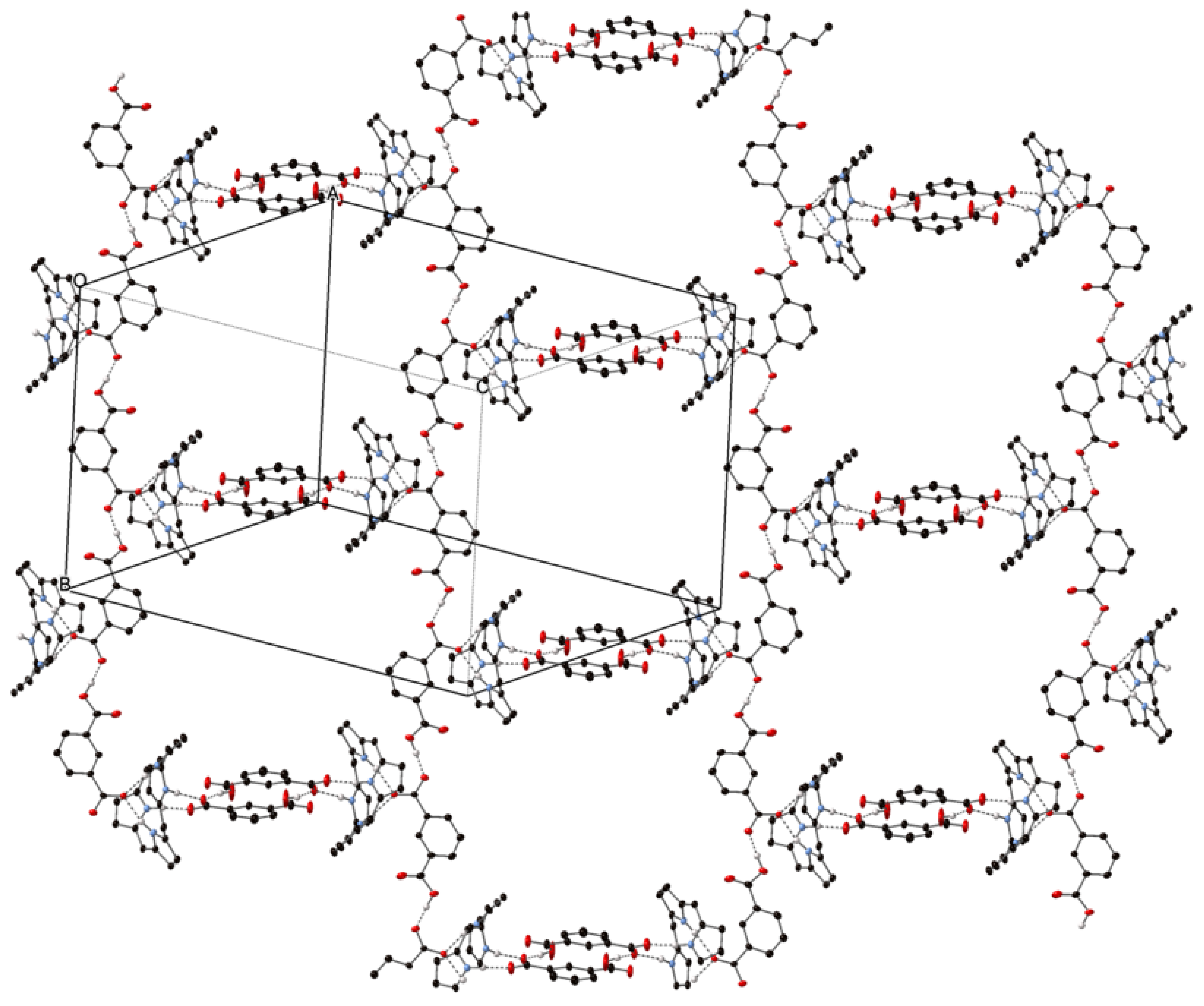
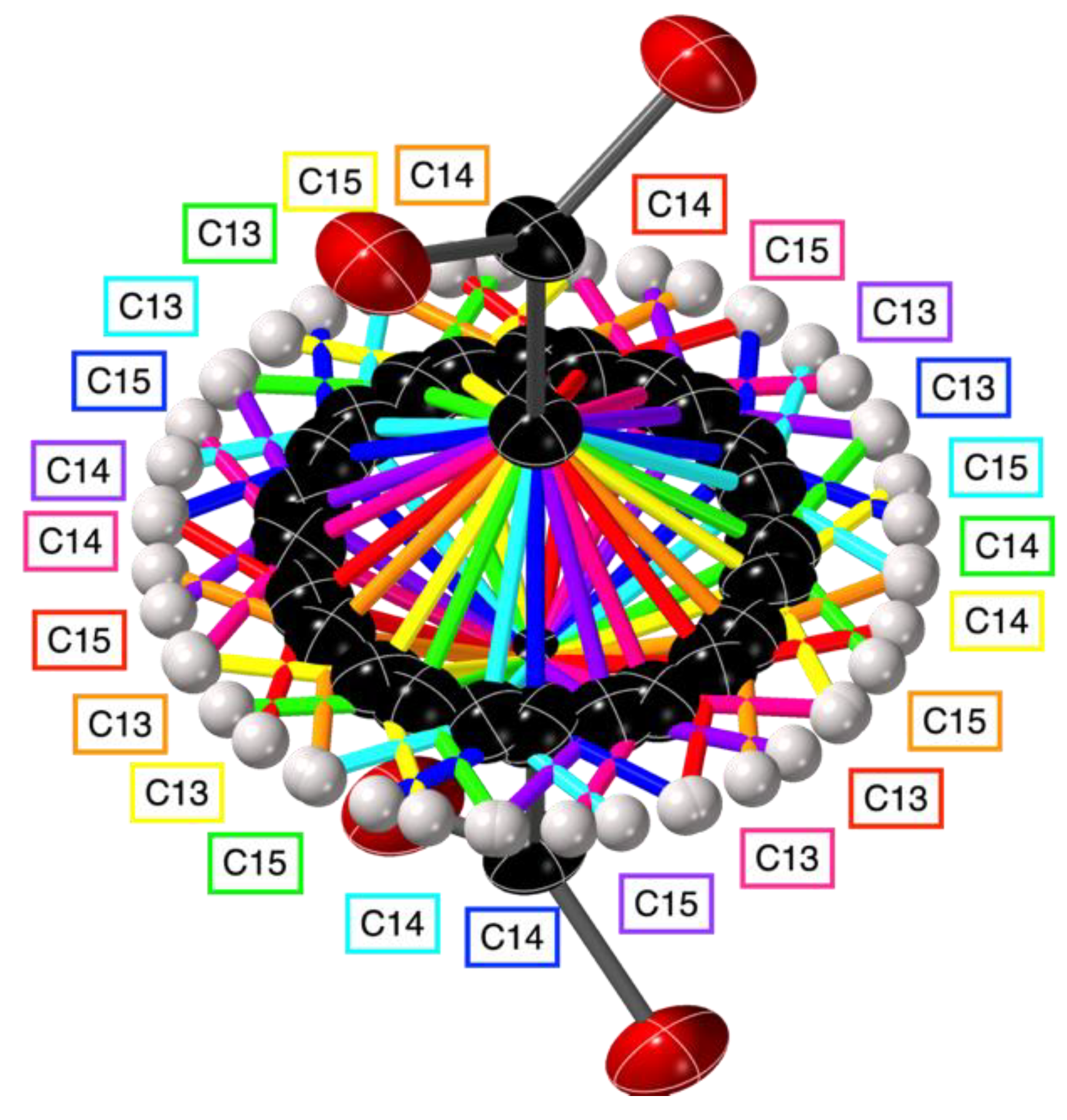

| Compound Number | Compound | Porphyrin | Anion | Ratio P:A |
|---|---|---|---|---|
| 1 | [H42](HSO4)2·H2O·3(MeOH) | H4OiBuTPP | HSO4 | 1:2 |
| 2 | [H42]O2C(CF2)4CO2·1.5(CH2Cl2)·1.5(CH3OH) | H4OiBuTPP | Octafluorohexane-1,6-dicarboxylate | 1:1 |
| 3 | [H42]Br2·1.6(CH3OH) | H4OiBuTPP | Br | 1:2 |
| 4 | [H42]3(MoVI12O36PO4)2·60(CH3OH)·1.5(H2O) | H4OiBuTPP | Mo12O36(PO4) | 3:2 |
| 5 | [H41]O2C(C(CH2)3C)CO2·HO2C(C(CH2)3C)CO2H | H4OETPP | Bicyclo[1.1.1]pentane-1,3-dicarboxylate | 1:1 § |
| 6 | [H41](HSeIVO3)2·CH3OSeIVO2H·3(CH3OH) | H4OETPP | HSeO3 | 1:2 |
| 7 | [H41]O2C(CH2)10CO2·4(CH3OH)·H2O | H4OETPP | Dodecane-1,12-dicarboxylate | 1:1 |
| 8 | [H42](O2C(CH2)CO2H)Cl0.7Br0.3·2(CH3OH) | H4OiBuTPP | Malonate, (0.3Br/0.7Cl) | 1:1:1 |
| 9 | [H32]2MoVI4O12(MeO)2·6(CH3OH) | H3OiBuTPP | MoVI4O12(MeO)2 | 2:1 |
| 10 | [H32]O2CCH3·0.31(H2O) 1.25(CH2Cl2)·0.69(CH3OH) | H3OiBuTPP | Acetate | 1:1 |
| 11 | [H42](O2CC6H4CO2H)2·2.5(CH3OH)·0.55(CH2Cl2) 0.6(H2O) | H4OiBuTPP | Hydrogen phthalate | 1:2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kingsbury, C.J.; Kielmann, M.; Twamley, B.; Senge, M.O. Self-Organization of Porphyrin–POM Dyads: Nonplanar Diacids and Oxoanions in Low-Dimensional H-Bonding Networks. Molecules 2022, 27, 7060. https://doi.org/10.3390/molecules27207060
Kingsbury CJ, Kielmann M, Twamley B, Senge MO. Self-Organization of Porphyrin–POM Dyads: Nonplanar Diacids and Oxoanions in Low-Dimensional H-Bonding Networks. Molecules. 2022; 27(20):7060. https://doi.org/10.3390/molecules27207060
Chicago/Turabian StyleKingsbury, Christopher J., Marc Kielmann, Brendan Twamley, and Mathias O. Senge. 2022. "Self-Organization of Porphyrin–POM Dyads: Nonplanar Diacids and Oxoanions in Low-Dimensional H-Bonding Networks" Molecules 27, no. 20: 7060. https://doi.org/10.3390/molecules27207060
APA StyleKingsbury, C. J., Kielmann, M., Twamley, B., & Senge, M. O. (2022). Self-Organization of Porphyrin–POM Dyads: Nonplanar Diacids and Oxoanions in Low-Dimensional H-Bonding Networks. Molecules, 27(20), 7060. https://doi.org/10.3390/molecules27207060








