Resveratrol Analogues as Selective Estrogen Signaling Pathway Modulators: Structure–Activity Relationship
Abstract
1. Introduction
2. Results and Discussion
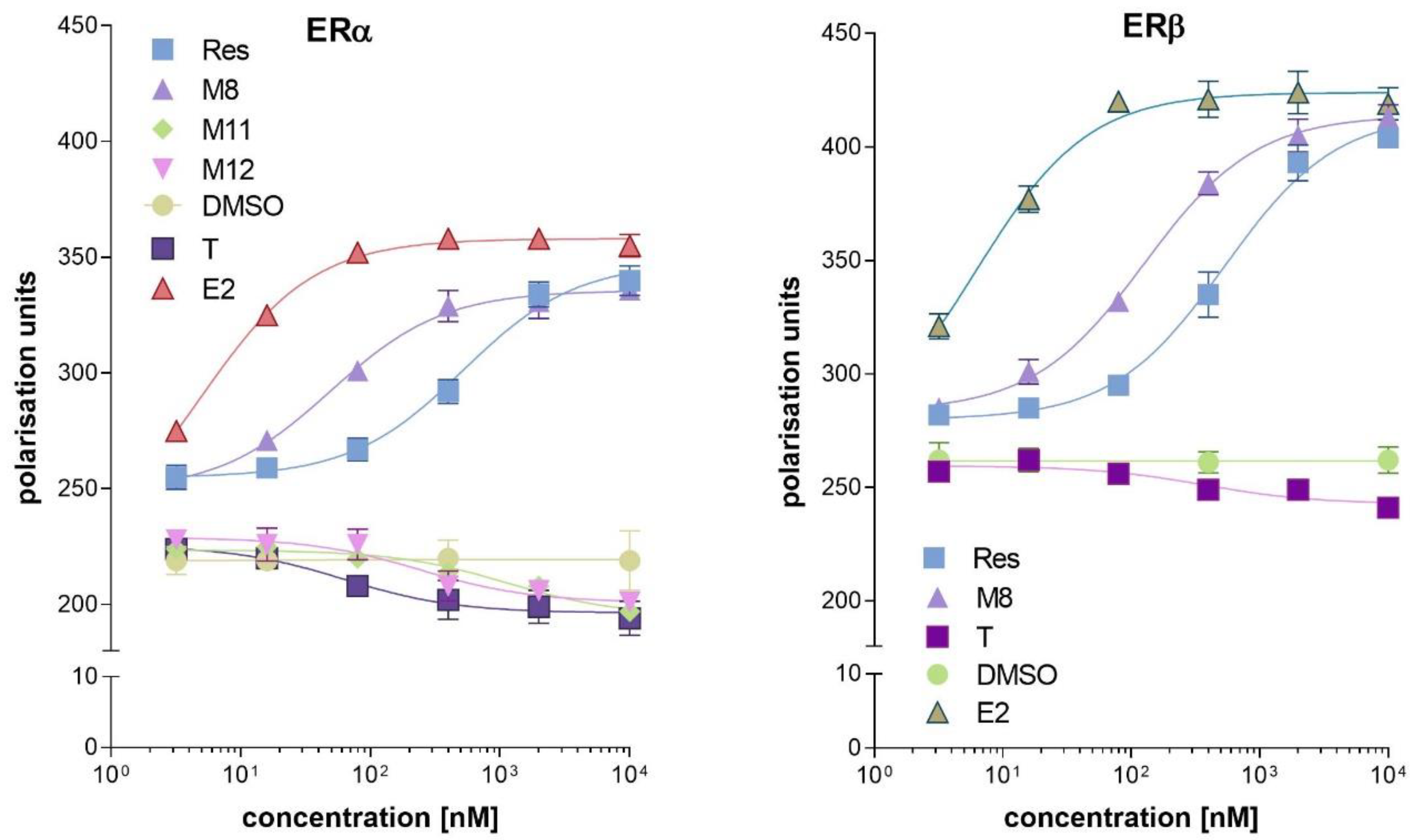
2.1. Binding Affinity of Resveratrol and Its Analogues to ERα and ERβ
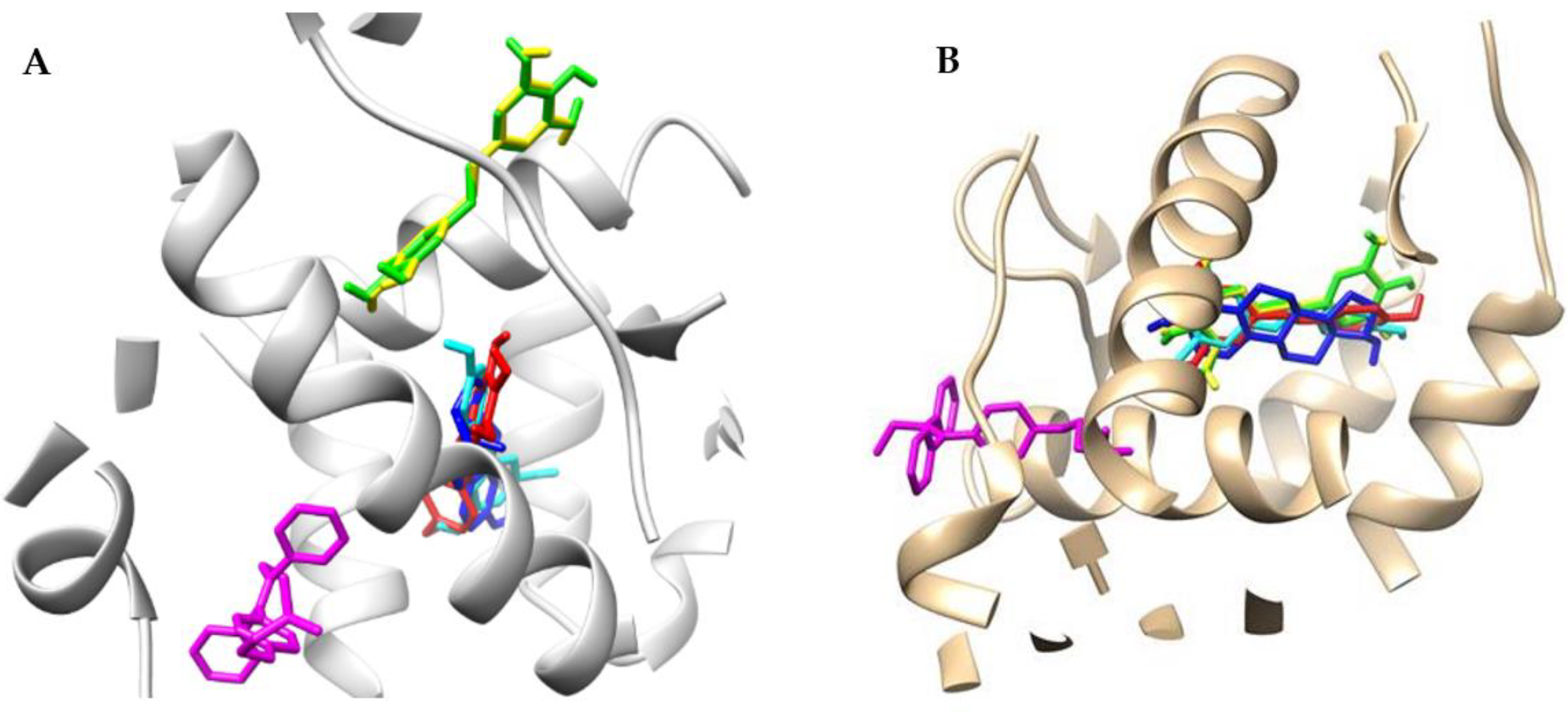
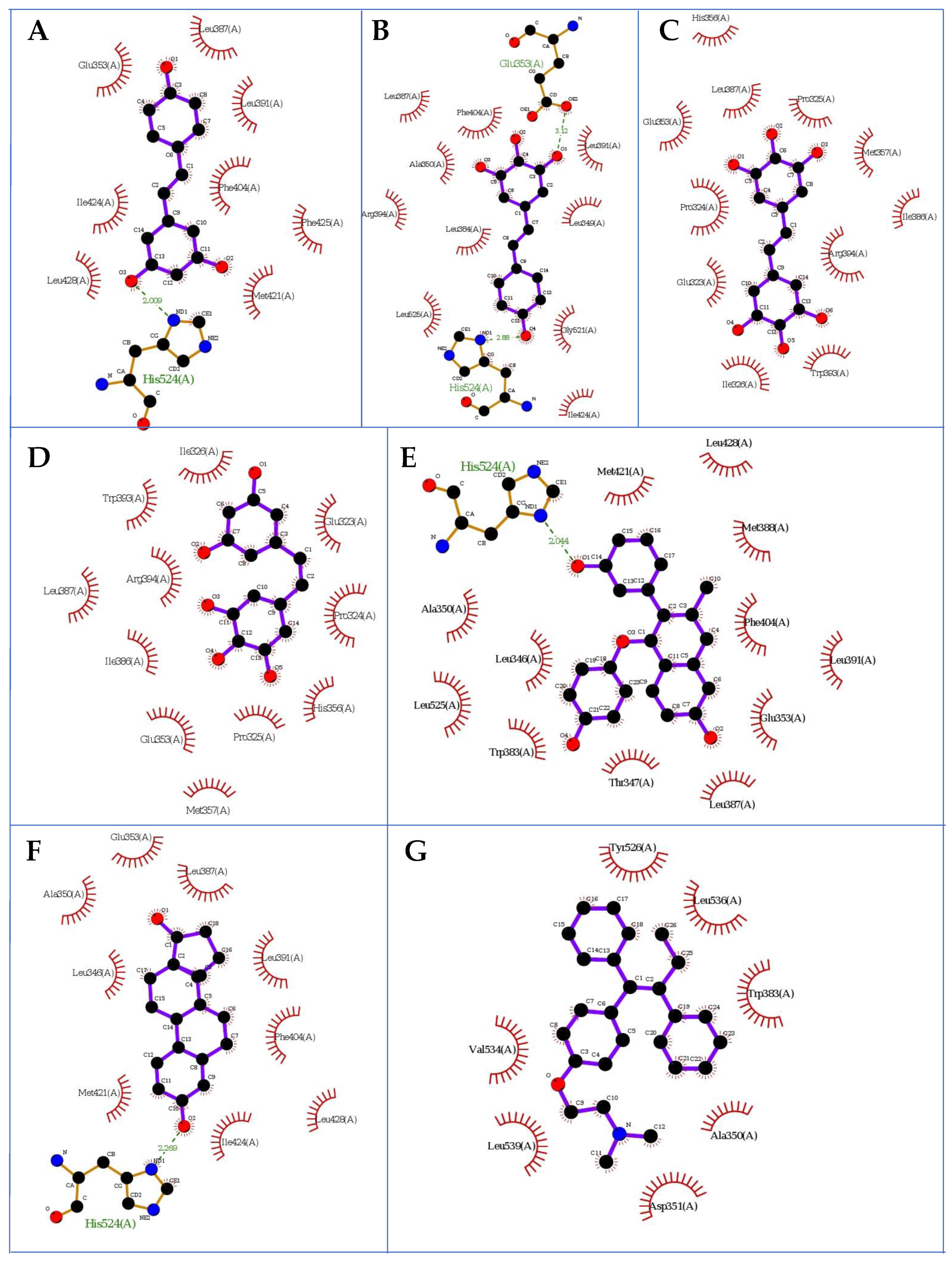
2.2. Docking Studies
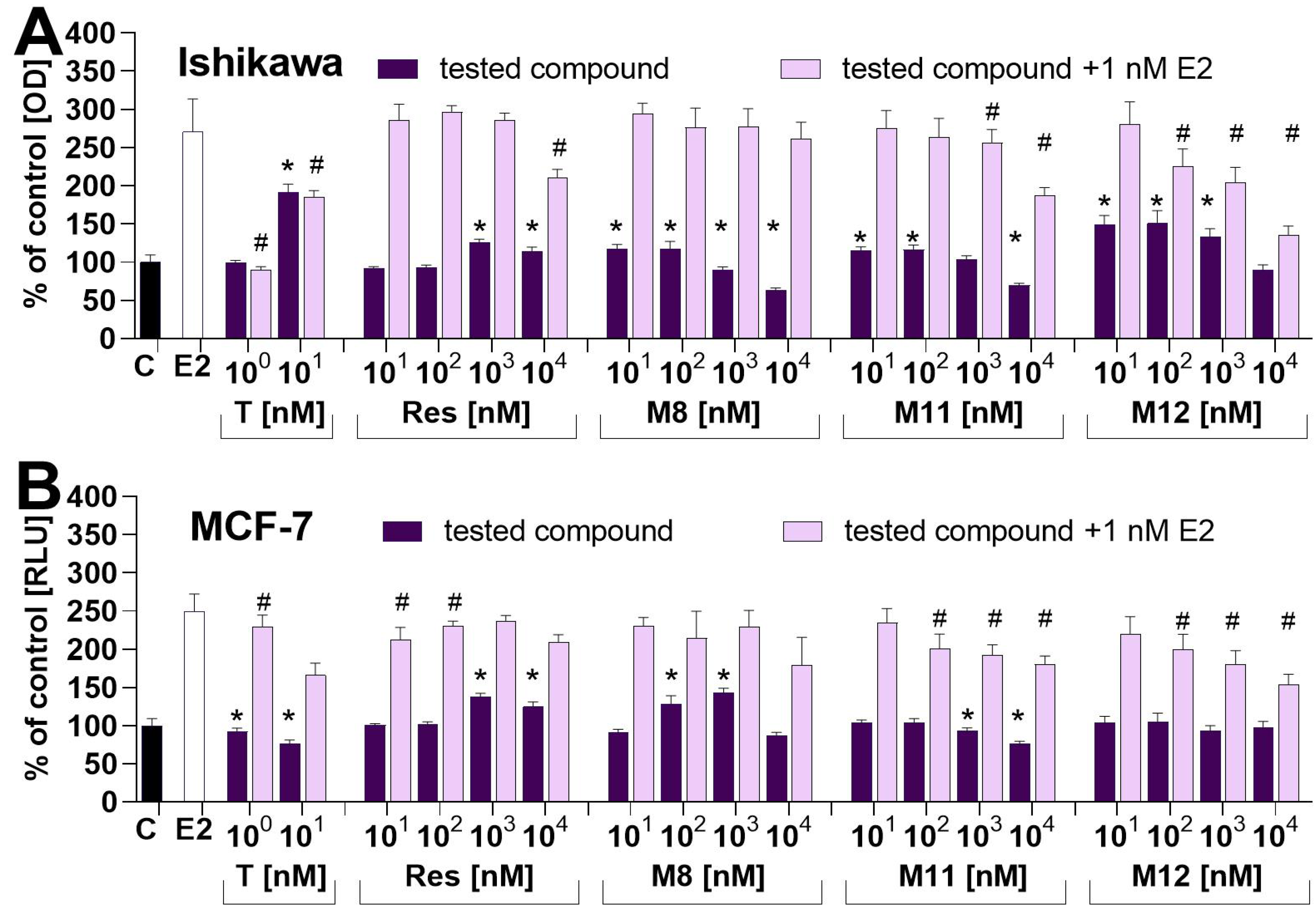
2.3. Impact on the Proliferation of Estrogen-Dependent MCF−7 and Ishikawa Cell Lines—In Vitro Study
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Ligand-Binding Studies by Fluorescence Polarization
3.3. Computational Details
3.4. Cell Culture
3.5. Ishikawa Cells Proliferation
3.6. MCF-7 Cells Proliferation
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More Than Resveratrol: New Insights into Stilbene-Based Compounds. Biomolecules 2020, 10, 1111. [Google Scholar] [CrossRef] [PubMed]
- Gille, L.; Murias, M.; Handler, N.; Erker, T.; Szekeres, T.; Nohl, H.; Jager, W. Cytotoxicity and Antioxidant-Derived Prooxidants of Hydroxystilbenes. Pharmacology 2004, 72, 152. [Google Scholar]
- Qasem, R.J. The Estrogenic Activity of Resveratrol: A Comprehensive Review of in Vitro and in Vivo Evidence and the Potential for Endocrine Disruption. Crit. Rev. Toxicol. 2020, 50, 439–462. [Google Scholar] [CrossRef] [PubMed]
- Murias, M.; Jäger, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, Prooxidant and Cytotoxic Activity of Hydroxylated Resveratrol Analogues: Structure-Activity Relationship. Biochem. Pharmacol. 2005, 69, 903–912. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant Properties of Resveratrol: A Structure–Activity Insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Shah, A.A.; Shah, A.; Kumar, A.; Lakra, A.; Singh, D.; Nayak, Y. Phytoestrogenic Potential of Resveratrol by Selective Activation of Estrogen Receptor-α in Osteoblast Cells. Rev. Bras. Farmacogn. 2022, 32, 248–256. [Google Scholar] [CrossRef]
- Ashby, J.; Tinwell, H.; Pennie, W.; Brooks, A.N.; Lefevre, P.A.; Beresford, N.; Sumpter, J.P. Partial and Weak Oestrogenicity of the Red Wine Constituent Resveratrol: Consideration of Its Superagonist Activity in MCF-7 Cells and Its Suggested Cardiovascular Protective Effects. J. Appl. Toxicol. 1999, 19, 39–45. [Google Scholar] [CrossRef]
- Basly, J.P.; Marre-Fournier, F.; Le Bail, J.C.; Habrioux, G.; Chulia, A.J. Estrogenic/Antiestrogenic and Scavenging Properties of (E)- and (Z)-Resveratrol. Life Sci. 2000, 66, 769–777. [Google Scholar] [CrossRef]
- van Duursen, M.B.M. Modulation of Estrogen Synthesis and Metabolism by Phytoestrogens in Vitro and the Implications for Women’s Health. Toxicol. Res. 2017, 6, 772–794. [Google Scholar] [CrossRef]
- Gehm, B.D.; McAndrews, J.M.; Chien, P.Y.; Jameson, J.L. Resveratrol, a Polyphenolic Compound Found in Grapes and Wine, Is an Agonist for the Estrogen Receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 14138–14143. [Google Scholar] [CrossRef]
- Mgbonyebi, O.P.; Russo, J.; Russo, I.H. Antiproliferative Effect of Synthetic Resveratrol on Human Breast Epithelial Cells. Int. J. Oncol. 1998, 12, 865–869. [Google Scholar] [CrossRef]
- Levenson, A.S.; Gehm, B.D.; Pearce, S.T.; Horiguchi, J.; Simons, L.A.; Ward, J.E.; Jameson, J.L.; Jordan, V.C. Resveratrol Acts as an Estrogen Receptor (ER) Agonist in Breast Cancer Cells Stably Transfected with ER Alpha. Int. J. Cancer 2003, 104, 587–596. [Google Scholar] [CrossRef]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for Breast Cancer Prevention and Therapy: Preclinical Evidence and Molecular Mechanisms. Semin. Cancer Biol. 2016, 40–41, 209–232. [Google Scholar] [CrossRef]
- Chakraborty, S.; Levenson, A.S.; Biswas, P.K. Structural Insights into Resveratrol’s Antagonist and Partial Agonist Actions on Estrogen Receptor Alpha. BMC Struct. Biol. 2013, 13, 27. [Google Scholar] [CrossRef]
- Hsieh, C.-J.; Hsu, Y.-L.; Huang, Y.-F.; Tsai, E.-M. Molecular Mechanisms of Anticancer Effects of Phytoestrogens in Breast Cancer. Curr. Protein Pept. Sci. 2018, 19, 323–332. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol Commonly Displays Hormesis: Occurrence and Biomedical Significance. Hum. Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.-Å. Estrogen Receptor Alpha and Beta in Health and Disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen Receptor Signaling Mechanisms. In Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 116, pp. 135–170. ISBN 978-0-12-815561-5. [Google Scholar]
- Yu, K.; Huang, Z.-Y.; Xu, X.-L.; Li, J.; Fu, X.-W.; Deng, S.-L. Estrogen Receptor Function: Impact on the Human Endometrium. Front. Endocrinol. 2022, 13, 827724. [Google Scholar] [CrossRef]
- Yu, P.; Wang, Y.; Li, C.; Lv, L.; Wang, J. Protective Effects of Downregulating Estrogen Receptor Alpha Expression in Cervical Cancer. Anticancer. Agents Med. Chem. 2018, 18, 1975–1982. [Google Scholar] [CrossRef]
- Xu, X.-L.; Huang, Z.-Y.; Yu, K.; Li, J.; Fu, X.-W.; Deng, S.-L. Estrogen Biosynthesis and Signal Transduction in Ovarian Disease. Front. Endocrinol. 2022, 13, 827032. [Google Scholar] [CrossRef]
- Haldosén, L.-A.; Zhao, C.; Dahlman-Wright, K. Estrogen Receptor Beta in Breast Cancer. Mol. Cell. Endocrinol. 2014, 382, 665–672. [Google Scholar] [CrossRef]
- Leygue, E.; Murphy, L.C. A Bi-Faceted Role of Estrogen Receptor β in Breast Cancer. Endocr. -Relat. Cancer 2013, 20, R127–R139. [Google Scholar] [CrossRef]
- De Amicis, F.; Chimento, A.; Montalto, F.; Casaburi, I.; Sirianni, R.; Pezzi, V. Steroid Receptor Signallings as Targets for Resveratrol Actions in Breast and Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1087. [Google Scholar] [CrossRef]
- Fang, J.; Akwabi-Ameyaw, A.; Britton, J.E.; Katamreddy, S.R.; Navas, F.; Miller, A.B.; Williams, S.P.; Gray, D.W.; Orband-Miller, L.A.; Shearin, J.; et al. Synthesis of 3-Alkyl Naphthalenes as Novel Estrogen Receptor Ligands. Bioorganic Med. Chem. Lett. 2008, 18, 5075–5077. [Google Scholar] [CrossRef]
- RCSB Protein Data Bank (RCSB PDB). RCSB PDB-3DT3: Human Estrogen Receptor Alpha LBD with GW368. Available online: https://www.rcsb.org/structure/3dt3 (accessed on 11 July 2022).
- Kucinska, M.; Giron, M.-D.; Piotrowska, H.; Lisiak, N.; Granig, W.H.; Lopez-Jaramillo, F.-J.; Salto, R.; Murias, M.; Erker, T. Novel Promising Estrogenic Receptor Modulators: Cytotoxic and Estrogenic Activity of Benzanilides and Dithiobenzanilides. PLoS ONE 2016, 11, e0145615. [Google Scholar] [CrossRef]
- Yu, E.; Xu, Y.; Shi, Y.; Yu, Q.; Liu, J.; Xu, L. Discovery of Novel Natural Compound Inhibitors Targeting Estrogen Receptor α by an Integrated Virtual Screening Strategy. J. Mol. Model 2019, 25, 278. [Google Scholar] [CrossRef]
- Czaja, K.; Kujawski, J.; Śliwa, P.; Kurczab, R.; Kujawski, R.; Stodolna, A.; Myślińska, A.; Bernard, M.K. Theoretical Investigations on Interactions of Arylsulphonyl Indazole Derivatives as Potential Ligands of VEGFR2 Kinase. Int. J. Mol. Sci. 2020, 21, 4793. [Google Scholar] [CrossRef]
- Pratama, M.R.F.; Poerwono, H.; Siswandono, S. Design and Molecular Docking of Novel 5-O-Benzoylpinostrobin Derivatives as Anti-Breast Cancer. Thai J. Pharm. Sci. 2020, 43, 201–212. [Google Scholar]
- McCullough, C.; Neumann, T.S.; Gone, J.R.; He, Z.; Herrild, C.; Wondergem (nee Lukesh), J.; Pandey, R.K.; Donaldson, W.A.; Sem, D.S. Probing the Human Estrogen Receptor-α Binding Requirements for Phenolic Mono- and Di-Hydroxyl Compounds: A Combined Synthesis, Binding and Docking Study. Bioorganic Med. Chem. 2014, 22, 303–310. [Google Scholar] [CrossRef][Green Version]
- Amaya, S.C.; Savaris, R.F.; Filipovic, C.J.; Wise, J.D.; Hestermann, E.; Young, S.L.; Lessey, B.A. Resveratrol and Endometrium: A Closer Look at an Active Ingredient of Red Wine Using In Vivo and In Vitro Models. Reprod. Sci. 2014, 21, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.H.; O’Donnell, A.L.; Mohamed, S.; Mousa, S.; Dandona, P. Overexpression of Estrogen Receptor-α in the Endometrial Carcinoma Cell Line Ishikawa: Inhibition of Growth and Angiogenic Factors. Gynecol. Oncol. 2004, 95, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.P.; Pezzuto, J.M. Resveratrol Exhibits Cytostatic and Antiestrogenic Properties with Human Endometrial Adenocarcinoma (Ishikawa) Cells. Cancer Res. 2001, 61, 6137–6144. [Google Scholar] [PubMed]
- Wober, J.; Weißwange, I.; Vollmer, G. Stimulation of Alkaline Phosphatase Activity in Ishikawa Cells Induced by Various Phytoestrogens and Synthetic Estrogens. J. Steroid Biochem. Mol. Biol. 2002, 83, 227–233. [Google Scholar] [CrossRef]
- Ford, C.H.J.; Al-Bader, M.; Al-Ayadhi, B.; Francis, I. Reassessment of Estrogen Receptor Expression in Human Breast Cancer Cell Lines. Anticancer Res. 2011, 31, 521–527. [Google Scholar]
- Al-Bader, M.; Ford, C.; Al-Ayadhy, B.; Francis, I. Analysis of Estrogen Receptor Isoforms and Variants in Breast Cancer Cell Lines. Exp. Ther. Med. 2011, 2, 537–544. [Google Scholar] [CrossRef]
- Comşa, Ş.; Cîmpean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 Years of Experience in Research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar]
- Tomé-Carneiro, J.; Larrosa, M.; González-Sarrías, A.; Tomás-Barberán, F.; García-Conesa, M.; Espín, J. Resveratrol and Clinical Trials: The Crossroad from In Vitro Studies to Human Evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of Three Wine-Related Polyphenols in Three Different Matrices by Healthy Subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and Safety Study of Resveratrol 500 Mg Tablets in Healthy Male and Female Volunteers. Exp. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.S.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I Dose Escalation Pharmacokinetic Study in Healthy Volunteers of Resveratrol, a Potential Cancer Chemopreventive Agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
- Borgert, C.J.; LaKind, J.S.; Witorsch, R.J. A Critical Review of Methods for Comparing Estrogenic Activity of Endogenous and Exogenous Chemicals in Human Milk and Infant Formula. Env. Health Perspect. 2003, 111, 1020–1036. [Google Scholar] [CrossRef]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol Acts as a Mixed Agonist/Antagonist for Estrogen Receptors α and Β*. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]
- Gehm, B.D.; Levenson, A.S.; Liu, H.; Lee, E.-J.; Amundsen, B.M.; Cushman, M.; Jordan, V.C.; Jameson, J.L. Estrogenic Effects of Resveratrol in Breast Cancer Cells Expressing Mutant and Wild-Type Estrogen Receptors: Role of AF-1 and AF-2. J. Steroid Biochem. Mol. Biol. 2004, 88, 223–234. [Google Scholar] [CrossRef]
- Ruotolo, R.; Calani, L.; Fietta, E.; Brighenti, F.; Crozier, A.; Meda, C.; Maggi, A.; Ottonello, S.; Del Rio, D. Anti-Estrogenic Activity of a Human Resveratrol Metabolite. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1086–1092. [Google Scholar] [CrossRef]
- Biagi, M.; Bertelli, A.A.E. Wine, Alcohol and Pills: What Future for the French Paradox? Life Sci. 2015, 131, 19–22. [Google Scholar] [CrossRef]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French Paradox Revisited. Front. Pharm. 2012, 3, 141. [Google Scholar] [CrossRef]
- Lu, R.; Serrero, G. Resveratrol, a Natural Product Derived from Grape, Exhibits Antiestrogenic Activity and Inhibits the Growth of Human Breast Cancer Cells. J. Cell Physiol. 1999, 179, 297–304. [Google Scholar] [CrossRef]
- Stahl, S.; Chun, T.-Y.; Gray, W.G. Phytoestrogens Act as Estrogen Agonists in an Estrogen-Responsive Pituitary Cell Line. Toxicol. Appl. Pharmacol. 1998, 152, 41–48. [Google Scholar] [CrossRef]
- Klinge, C.M.; Risinger, K.E.; Watts, M.B.; Beck, V.; Eder, R.; Jungbauer, A. Estrogenic Activity in White and Red Wine Extracts. J. Agric. Food Chem. 2003, 51, 1850–1857. [Google Scholar] [CrossRef]
- Maier-Salamon, A.; Böhmdorfer, M.; Riha, J.; Thalhammer, T.; Szekeres, T.; Jaeger, W. Interplay between Metabolism and Transport of Resveratrol. Ann. N. Y. Acad. Sci. 2013, 1290, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Murias, M.; Miksits, M.; Aust, S.; Spatzenegger, M.; Thalhammer, T.; Szekeres, T.; Jaeger, W. Metabolism of Resveratrol in Breast Cancer Cell Lines: Impact of Sulfotransferase 1A1 Expression on Cell Growth Inhibition. Cancer Lett. 2008, 261, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Murias, M.; Luczak, M.W.; Niepsuj, A.; Krajka-Kuzniak, V.; Zielinska-Przyjemska, M.; Jagodzinski, P.P.; Jäger, W.; Szekeres, T.; Jodynis-Liebert, J. Cytotoxic Activity of 3,3′,4,4′,5,5′-Hexahydroxystilbene against Breast Cancer Cells Is Mediated by Induction of P53 and Downregulation of Mitochondrial Superoxide Dismutase. Toxicol. Vitr. 2008, 22, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Ebrahimzadeh, M.A. Resveratrol—A Comprehensive Review of Recent Advances in Anticancer Drug Design and Development. Eur. J. Med. Chem. 2020, 200, 112356. [Google Scholar] [CrossRef]
- Kucinska, M.; Piotrowska, H.; Luczak, M.W.; Mikula-Pietrasik, J.; Ksiazek, K.; Wozniak, M.; Wierzchowski, M.; Dudka, J.; Jäger, W.; Murias, M. Effects of Hydroxylated Resveratrol Analogs on Oxidative Stress and Cancer Cells Death in Human Acute T Cell Leukemia Cell Line: Prooxidative Potential of Hydroxylated Resveratrol Analogs. Chem. Biol. Interact. 2014, 209, 96–110. [Google Scholar] [CrossRef]
- Murias, M.; Handler, N.; Erker, T.; Pleban, K.; Ecker, G.; Saiko, P.; Szekeres, T.; Jäger, W. Resveratrol Analogues as Selective Cyclooxygenase-2 Inhibitors: Synthesis and Structure-Activity Relationship. Bioorg. Med. Chem. 2004, 12, 5571–5578. [Google Scholar] [CrossRef]
- Gaussian 16 Rev. C.01/C.02 Release Notes|Gaussian.Com. Available online: https://gaussian.com/relnotes/ (accessed on 17 September 2022).
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A Program to Generate Schematic Diagrams of Protein-Ligand Interactions. Protein Eng. Des. Sel. 1995, 8, 127–134. [Google Scholar] [CrossRef]
- Psi4 1: An Open-Source Electronic Structure Program Emphasizing Automation, Advanced Libraries, and Interoperability|Journal of Chemical Theory and Computation. Available online: https://pubs.acs.org/doi/abs/10.1021/acs.jctc.7b00174 (accessed on 17 September 2022).
- Ligasová, A.; Koberna, K. DNA Dyes—Highly Sensitive Reporters of Cell Quantification: Comparison with Other Cell Quantification Methods. Molecules 2021, 26, 5515. [Google Scholar] [CrossRef]

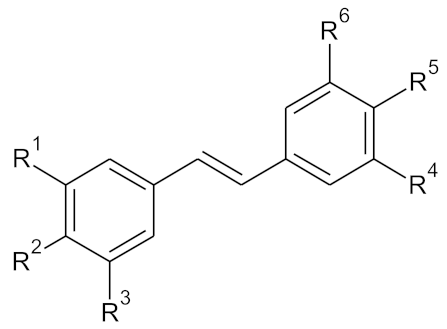 | ||||||
|---|---|---|---|---|---|---|
| Compound | Pos. 3 (=R1) | Pos. 4 (=R2) | Pos. 5 (=R3) | Pos. 3′ (=R4) | Pos. 4′ (=R5) | Pos. 5′ (=R6) |
| M1 | -OCH₃ | -H | -OCH₃ | -H | -OCH₃ | -H |
| M2 | -OCH₃ | -OCH₃ | -OCH₃ | -H | -OCH₃ | -H |
| M3 | -OCH₃ | -H | -OCH₃ | -OCH₃ | -H | -OCH₃ |
| M4 | -OCH₃ | -H | -OCH₃ | -OCH₃ | -OCH₃ | -H |
| M5 | -OCH₃ | -OCH₃ | -OCH₃ | -OCH₃ | -H | -OCH₃ |
| M6 | -OCH₃ | -OCH₃ | -OCH₃ | -OCH₃ | -OCH₃ | -OCH₃ |
| M7 | -OH | -H | -OH | -H | -OH | -H |
| M8 | -OH | -OH | -OH | -H | -OH | -H |
| M9 | -OH | -H | -OH | -OH | -H | -OH |
| M10 | -OH | -H | -OH | -OH | -OH | -H |
| M11 | -OH | -OH | -OH | -OH | -H | -OH |
| M12 | -OH | -OH | -OH | -OH | -OH | -OH |
| Compound | ERα Agonist EC50 (nM) | ERβ Agonist EC50 (nM) |
|---|---|---|
| resveratrol | 21.2 ± 2.2 | 32.3 ± 3.6 |
| M8 | 108.5 ± 10.2 | 8.1 ± 1.6 |
| estradiol | 8.3 ± 2.1 | 3.1 ± 0.9 |
| Compound | ERα antagonist EC50 (nM) | ERβ antagonist EC50 (nM) |
| M11 | 1012.3 ± 30.5 | >5000 |
| M12 | 110.2 ± 15.5 | >5000 |
| tamoxifen | 25.1 ± 3.6 | 240.2 ± 14.5 |
| Contacts | Electrostatics | Exchange | Induction | Dispersion | Total SAPT0 |
|---|---|---|---|---|---|
| Glu353_estradiol | −16.48923 | 20.23436 | −8.87314 | −4.35977 | −15.11974 |
| Glu353_M11 | −5.58802 | 8.24529 | −1.55134 | −6.79997 | −9.07403 |
| Glu353_M12 | −7.23545 | 7.55212 | −1.67306 | −7.19391 | −13.62578 |
| Glu353_M8 | −3.10338 | 28.25146 | −9.06124 | −6.54009 | 15.21372 |
| Glu353_resveratrol | −0.04736 | 7.90314 | −1.92755 | −3.18726 | 4.36802 |
| Glu353_tamoxifen | 0.06031 | 0.00046 | −0.02167 | −0.24192 | −0.32322 |
| Leu346_estradiol | −2.98294 | 4.04051 | −0.88196 | −4.95162 | −7.61105 |
| Leu346_M11 | −0.12331 | 0 | −0.00235 | −0.01384 | −0.22231 |
| Leu346_M12 | 0.17741 | 0 | −0.00204 | −0.01417 | 0.25688 |
| Leu346_M8 | 1.61081 | 0.8349 | −0.41181 | −2.44566 | −0.65619 |
| Leu346_resveratrol | −0.69714 | 0.04584 | −0.11532 | −1.24237 | −3.20152 |
| Leu346_tamoxifen | −0.09257 | 0.00032 | −0.01715 | −0.16193 | −0.43239 |
| Leu387_estradiol | −7.32042 | 27.15115 | −4.08131 | −11.49336 | 6.78245 |
| Leu387_M11 | −1.97959 | 8.37737 | −2.2794 | −5.13834 | −1.62543 |
| Leu387_M12 | −1.93474 | 7.15897 | −1.66628 | −4.70677 | −1.83076 |
| Leu387_M8 | 0.70104 | 2.78983 | −0.66255 | −4.62453 | −2.86244 |
| Leu387_resveratrol | −4.72523 | 14.06595 | −2.517 | −9.62002 | −4.45619 |
| Leu387_tamoxifen | −0.0311 | 0.03712 | −0.00821 | −0.37893 | −0.60736 |
| Phe404_estradiol | −5.86981 | 26.08135 | −3.4992 | −12.04759 | 7.43375 |
| Phe404_M11 | −0.06108 | −0.00002 | −0.01645 | −0.09533 | −0.2755 |
| Phe404_M12 | 0.0765 | −0.00002 | −0.01385 | −0.09806 | −0.05646 |
| Phe404_M8 | −1.37589 | 3.62802 | −0.65048 | −5.83775 | −6.75066 |
| Phe404_resveratrol | −2.67406 | 10.12713 | −1.38583 | −7.92448 | −2.9597 |
| Phe404_tamoxifen | −0.01185 | 0 | −0.00042 | −0.01658 | −0.04596 |
| Trp383_estradiol | −0.08804 | 0.02432 | −0.02605 | −0.6719 | −1.21379 |
| Trp383_M11 | −0.6325 | 0.00003 | −0.03516 | −0.12425 | −1.26194 |
| Trp383_M12 | 0.51117 | 0.00002 | −0.0319 | −0.12425 | 0.5658 |
| Trp383_M8 | −0.29856 | 0.00114 | −0.02108 | −0.44586 | −1.21807 |
| Trp383_resveratrol | −0.30921 | 0.00078 | −0.02223 | −0.31586 | −1.0303 |
| Trp383_tamoxifen | −3.32377 | 11.98675 | −1.40812 | −10.75084 | −5.57119 |
| Contacts | Electrostatics | Exchange | Induction | Dispersion | Total SAPT0 |
|---|---|---|---|---|---|
| Gly472_estradiol | 0.67436 | 2.07195 | −0.42113 | −2.26001 | 0.10386 |
| Gly472_M11 | −0.35218 | 0.93438 | −0.26853 | −1.18058 | −1.38151 |
| Gly472_M12 | 1.32746 | 0.85342 | −0.27736 | −1.22162 | 1.08668 |
| Gly472_M8 | 2.18831 | 4.18735 | −0.84722 | −2.52704 | 4.78306 |
| Gly472_resveratrol | 1.18342 | 3.43909 | −0.56283 | −2.29321 | 2.81503 |
| Gly472_tamoxifen | −0.00946 | 0 | −0.00005 | −0.00121 | −0.01708 |
| His475_estradiol | 2.6531 | 0.32233 | −0.2649 | −1.1753 | 2.44654 |
| His475_M11 | −8.82514 | 7.30577 | −2.10095 | −3.77149 | −11.7796 |
| His475_M12 | −9.74329 | 16.46084 | −4.11932 | −6.04703 | −5.49602 |
| His475_M8 | −5.37217 | 7.33722 | −1.4282 | −4.28748 | −5.97701 |
| His475_resveratrol | −7.87121 | 7.70495 | −1.86333 | −3.38573 | −8.62985 |
| His475_tamoxifen | 0.00019 | 0 | 0 | −0.00103 | −0.00132 |
| Ile376_estradiol | −1.30063 | 6.40377 | −1.14341 | −3.91051 | 0.07843 |
| Ile376_M11 | −0.79526 | 2.10206 | −0.40792 | −2.30483 | −2.24053 |
| Ile376_M12 | −0.01665 | 1.65675 | −0.38003 | −2.25141 | −1.57979 |
| Ile376_M8 | −0.19615 | 1.61684 | −0.22421 | −2.36771 | −1.86646 |
| Ile376_resveratrol | −0.62198 | 4.52934 | −0.69196 | −3.92697 | −1.13396 |
| Ile376_tamoxifen | 0.02073 | 0 | −0.00017 | −0.00308 | 0.02785 |
| Ile380_estradiol | 0.03227 | 0.01127 | −0.00866 | −0.50132 | −0.74332 |
| Ile380_M11 | 0.08539 | 0.00143 | −0.00339 | −0.22014 | −0.21785 |
| Ile380_M12 | 0.0868 | 0.00136 | −0.00482 | −0.2241 | −0.22432 |
| Ile380_M8 | 0.04291 | 0.0004 | −0.00576 | −0.21957 | −0.29007 |
| Ile380_resveratrol | 0.13932 | 0.00055 | −0.0065 | −0.24107 | −0.17163 |
| Ile380_tamoxifen | 0.01516 | 0 | −0.00034 | −0.01263 | 0.00349 |
| Leu298_estradiol | −0.04451 | 0.23119 | −0.12607 | −1.75834 | −2.70551 |
| Leu298_M11 | −3.442 | 0.51532 | −0.49191 | −2.31155 | −9.13157 |
| Leu298_M12 | 0.86562 | 0.34692 | −0.21914 | −2.18667 | −1.90159 |
| Leu298_M8 | 0.57419 | 1.13771 | −0.37985 | −2.36568 | −1.64719 |
| Leu298_resveratrol | −3.26084 | 2.76471 | −0.95337 | −2.90107 | −6.93308 |
| Leu298_tamoxifen | −0.03978 | 0 | −0.00095 | −0.01014 | −0.08108 |
| Leu339_estradiol | −3.67128 | 8.14513 | −1.27868 | −9.21987 | −9.60098 |
| Leu339_M11 | −1.31019 | 8.0409 | −1.70312 | −7.86206 | −4.51701 |
| Leu339_M12 | −6.77811 | 7.65731 | −1.62827 | −7.81049 | −13.6405 |
| Leu339_M8 | −2.9227 | 5.78307 | −0.93685 | −6.63826 | −7.51341 |
| Leu339_resveratrol | −1.34789 | 7.12232 | −1.18906 | −7.29872 | −4.324 |
| Leu339_tamoxifen | 0.21284 | 0.14239 | −0.0332 | −0.84432 | −0.83233 |
| Leu343_estradiol | −2.18674 | 13.09303 | −2.21457 | −6.18217 | 3.99922 |
| Leu343_M11 | −2.36232 | 5.97714 | −0.90422 | −3.47968 | −1.22563 |
| Leu343_M12 | −1.89879 | 6.0387 | −0.83481 | −3.65332 | −0.55493 |
| Leu343_M8 | −3.74278 | 7.31482 | −1.18679 | −3.67933 | −2.06224 |
| Leu343_resveratrol | 0.54534 | 8.09066 | −2.46044 | −4.20701 | 3.13709 |
| Leu343_tamoxifen | 0.06469 | 0.21826 | −0.10876 | −1.02673 | −1.3586 |
| Met336_estradiol | −4.83937 | 13.30994 | −1.95439 | −7.8959 | −2.19874 |
| Met336_M11 | −0.87805 | 0.07175 | −0.13742 | −1.25052 | −3.49673 |
| Met336_M12 | −0.3888 | 0.08958 | −0.13754 | −1.31122 | −2.78558 |
| Met336_M8 | −0.61499 | 2.07571 | −0.46877 | −3.18665 | −3.49747 |
| Met336_resveratrol | −0.62168 | 1.34148 | −0.35293 | −2.64949 | −3.6376 |
| Met336_tamoxifen | 0.00693 | −0.00001 | −0.00365 | −0.03468 | −0.05005 |
| Met340_estradiol | −0.00016 | 0 | 0.00001 | 0 | −0.00023 |
| Met340_M11 | −0.93804 | 8.09994 | −1.91689 | −3.86824 | 2.19404 |
| Met340_M12 | −1.22265 | 6.13644 | −1.08073 | −3.37871 | 0.72404 |
| Met340_M8 | −1.21279 | 3.35209 | −0.42284 | −2.76341 | −1.66842 |
| Met340_resveratrol | −1.47083 | 2.07677 | −0.34852 | −2.7766 | −4.01457 |
| Met340_tamoxifen | 0.23072 | 0.00275 | −0.00888 | −0.23187 | −0.0116 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobylka, P.; Kucinska, M.; Kujawski, J.; Lazewski, D.; Wierzchowski, M.; Murias, M. Resveratrol Analogues as Selective Estrogen Signaling Pathway Modulators: Structure–Activity Relationship. Molecules 2022, 27, 6973. https://doi.org/10.3390/molecules27206973
Kobylka P, Kucinska M, Kujawski J, Lazewski D, Wierzchowski M, Murias M. Resveratrol Analogues as Selective Estrogen Signaling Pathway Modulators: Structure–Activity Relationship. Molecules. 2022; 27(20):6973. https://doi.org/10.3390/molecules27206973
Chicago/Turabian StyleKobylka, Paulina, Malgorzata Kucinska, Jacek Kujawski, Dawid Lazewski, Marcin Wierzchowski, and Marek Murias. 2022. "Resveratrol Analogues as Selective Estrogen Signaling Pathway Modulators: Structure–Activity Relationship" Molecules 27, no. 20: 6973. https://doi.org/10.3390/molecules27206973
APA StyleKobylka, P., Kucinska, M., Kujawski, J., Lazewski, D., Wierzchowski, M., & Murias, M. (2022). Resveratrol Analogues as Selective Estrogen Signaling Pathway Modulators: Structure–Activity Relationship. Molecules, 27(20), 6973. https://doi.org/10.3390/molecules27206973










