Design, Preparation, and Evaluation of Osthol Poly-Butyl-Cyanoacrylate Nanoparticles with Improved In Vitro Anticancer Activity in Neuroblastoma Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Osthol Analysis
2.2.1. Determination of the Optimum Detection Wavelength for Osthol Analysis
2.2.2. Chromatographic Conditions and Calibration Curve
2.3. Preparation of Osthol-PBCA NPs
2.3.1. Single-Factor Experiment
2.3.2. Optimization of the Orthogonal Design
2.4. Evaluation of Nanoparticles
2.4.1. Particle Size and Zeta Potential
2.4.2. Encapsulation Efficiency (EE) and Drug Loading (DL)
2.4.3. TEM Studies of the Osthol-PBCA NPs
2.4.4. Differential Scanning Calorimetry (DSC) Analysis
2.4.5. Fourier Transform Infrared (FTIR) Analysis
2.4.6. Influencing Factors Test
2.5. In Vitro Release Study
2.6. Cell Absorption Studies In Vitro
2.7. Exploring the Cell Endocytosis Mechanism of NPs
2.8. Determining Cytotoxicity Effects In Vitro
2.9. Assessment of Membrane Integrity
2.10. Pharmacokinetic (PK) and Aera under the Curve (AUC) study in Rats
2.11. Statistical Analysis
3. Results and Discussion
3.1. Osthol Determination Method
3.2. Preparation of Osthol-PBCA NPs
3.2.1. The Relationship between Stirring Rate and Particle Size
3.2.2. Optimization of the Preparation Process by Orthogonal Design
3.3. Characterizations of Osthol-PBCA NPs
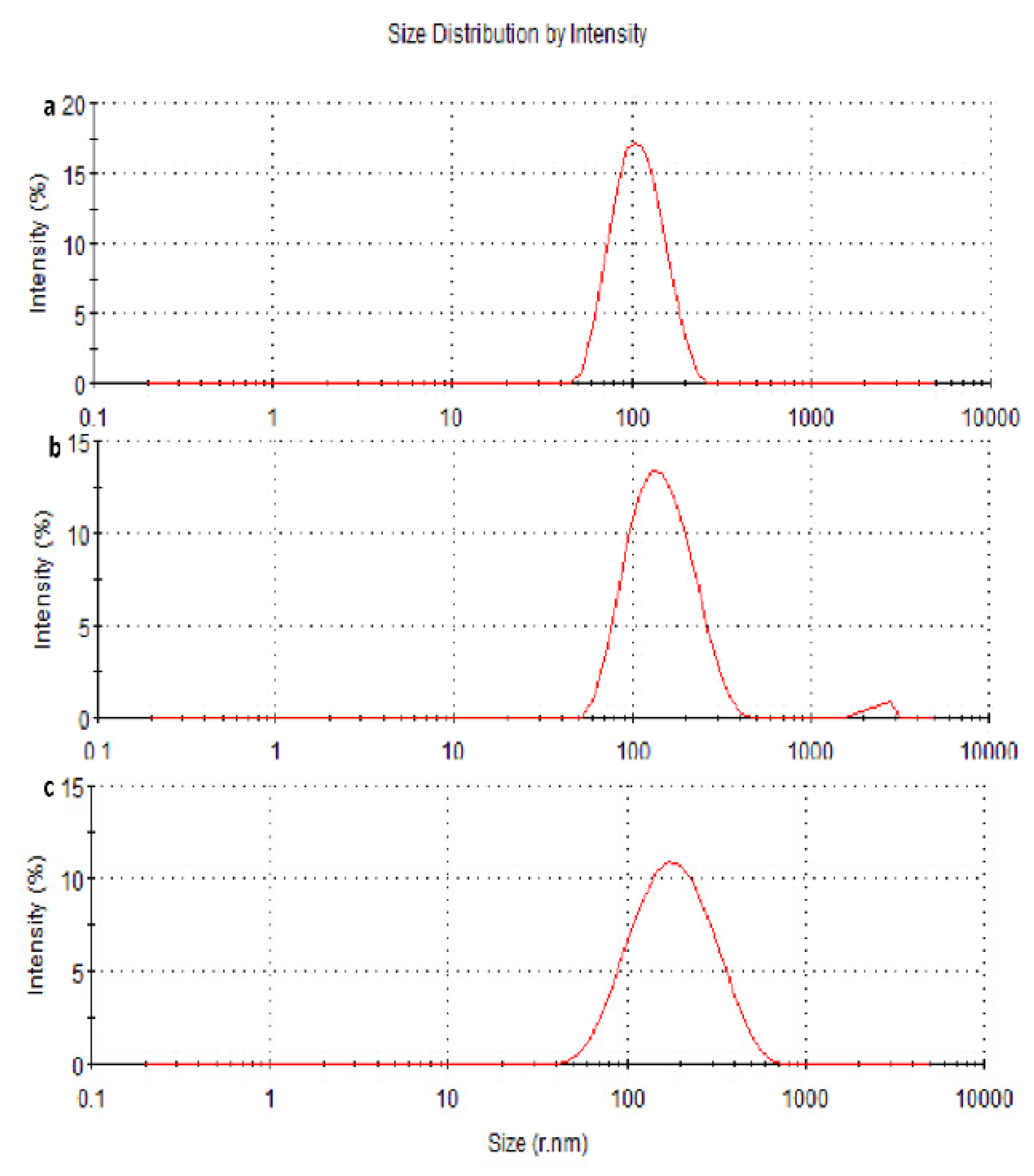
3.3.1. Particle Size Measurements and Morphology of the Nanoparticles
3.3.2. Zeta Potential and PDI Measurements
3.3.3. Encapsulation Efficiency (EE) and Drug Loading Efficiency (DL)
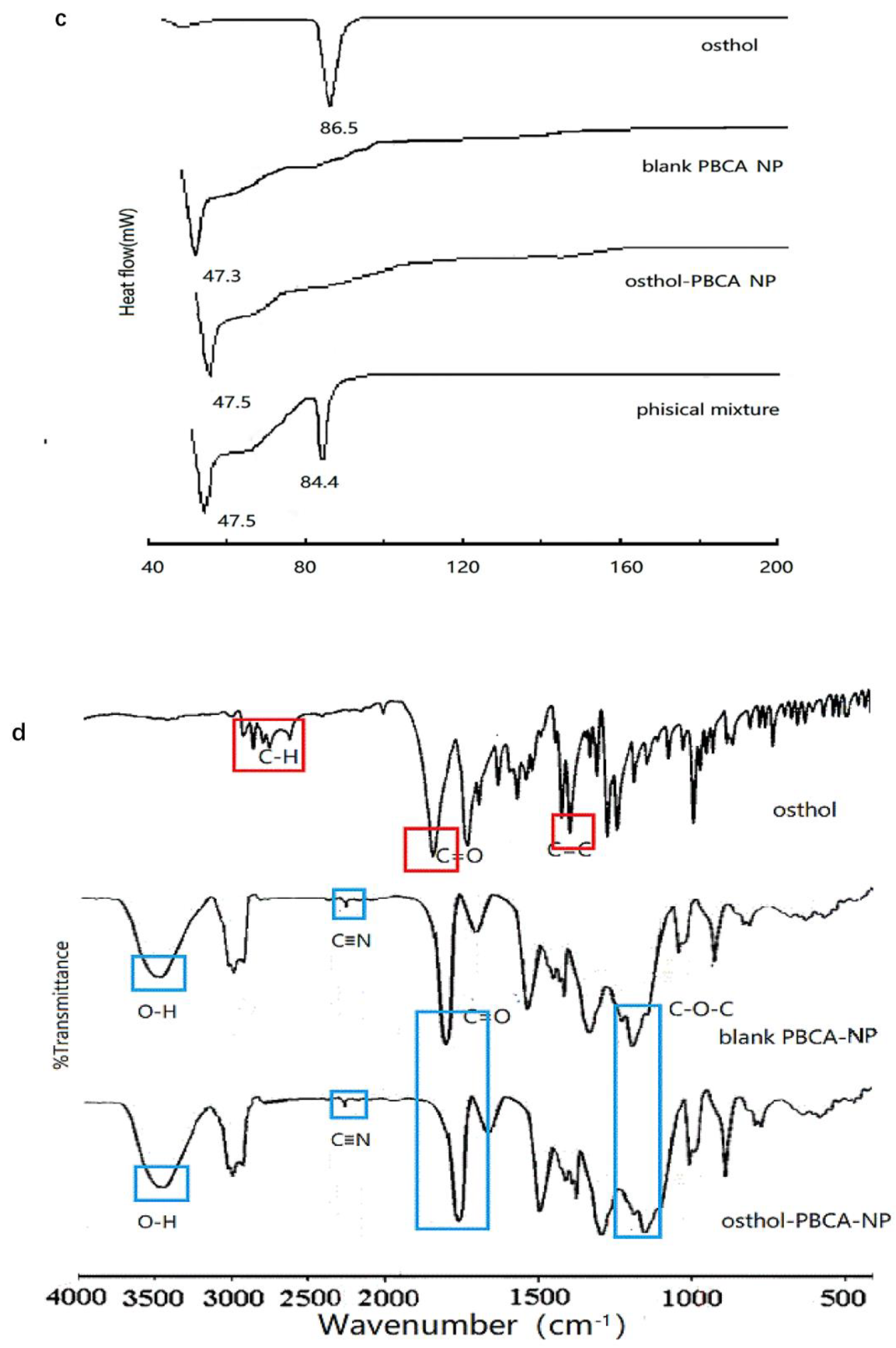
3.3.4. DSC and FTIR Analysis
Influencing Factors Test
3.4. In Vitro Drug Release Study
3.5. Kinetics of Intracellular Endocytosis of Osthol-PBCA NPs
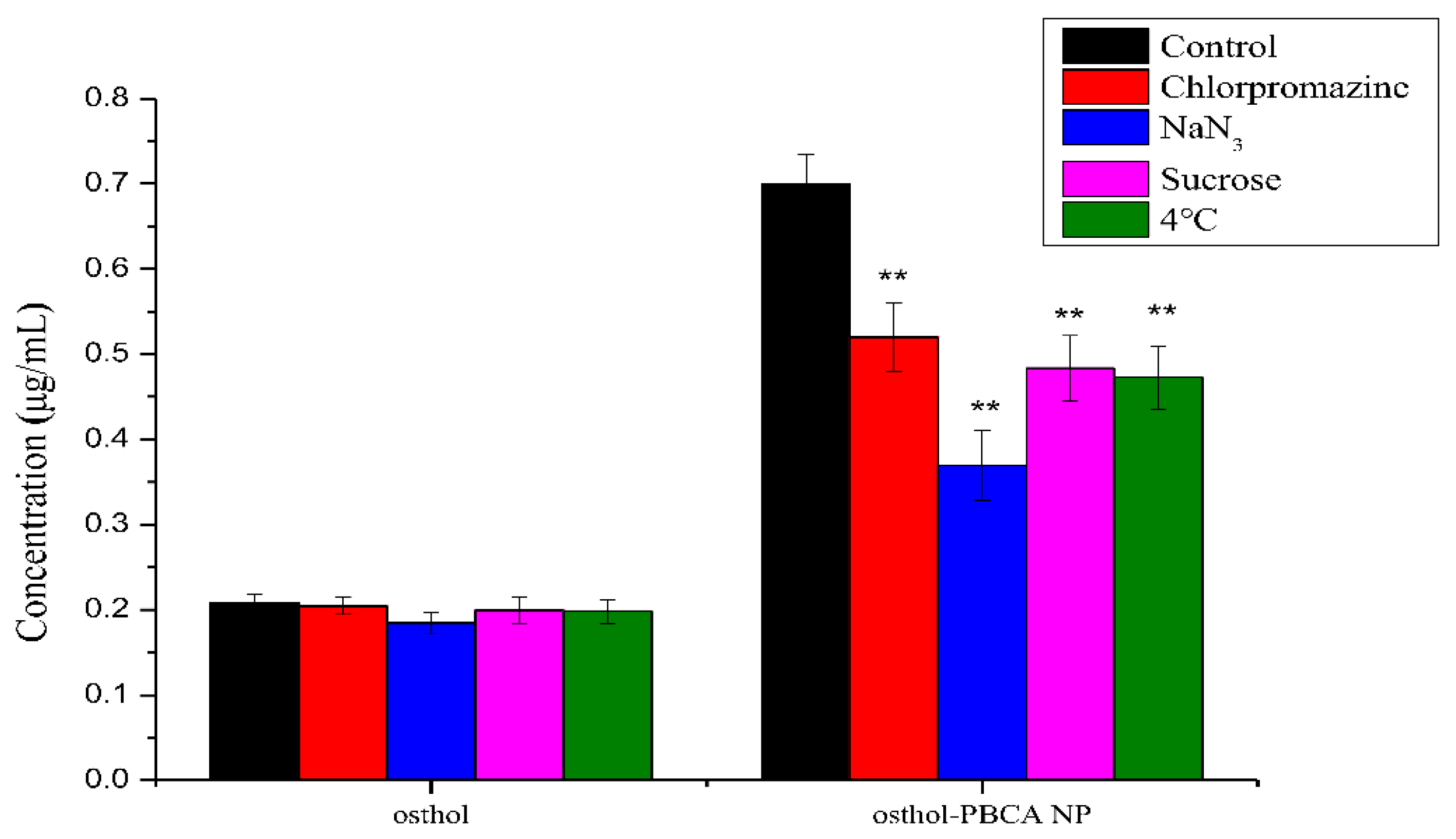
3.6. The Endocytosis Transport Mechanism of Osthol-PBCA NPs in Cells
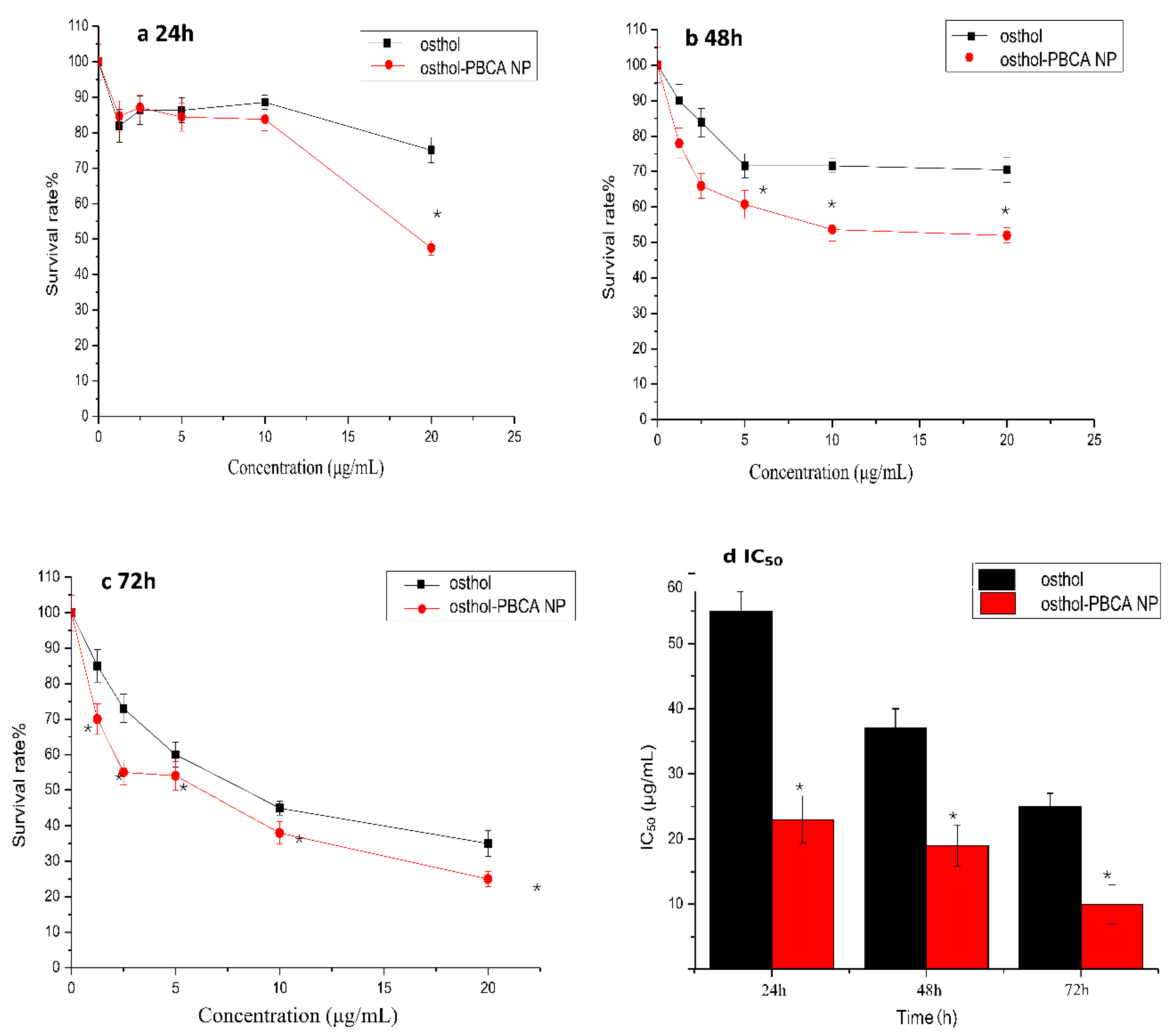
3.7. In Vitro Cytotoxicity Studies
3.8. Osthol-Induced LDH Determination
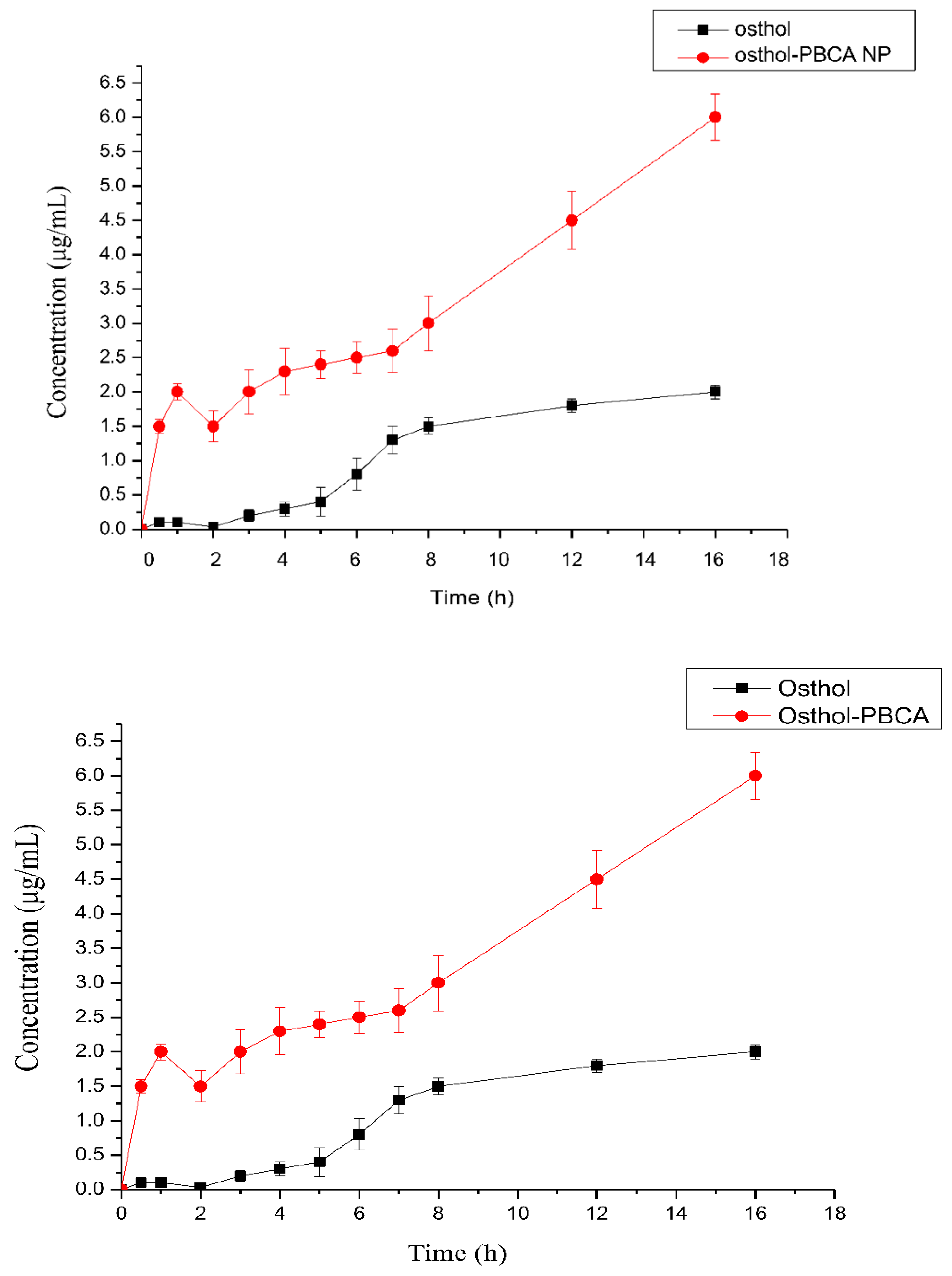
3.9. The PK and AUC Study in Rats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zafar, A.; Wang, W.; Liu, G.; Wang, X.; Xian, W.; McKeon, F.; Foster, J.; Zhou, J.; Zhang, R. Molecular targeting therapies for neuroblastoma: Progress and challenges. Med. Res. Rev. 2021, 41, 961–1021. [Google Scholar] [CrossRef] [PubMed]
- Jelodarian, Z.; Shokoohinia, Y.; Rashidi, M.; Ghiasvand, N.; Hosseinzadeh, L.; Iranshahi, M. New polyacetylenes from Echinophora cinerea (Boiss.) Hedge et Lamond. Nat. Prod. Res. 2017, 31, 2256–2263. [Google Scholar] [CrossRef]
- Sadraei, H.; Shokoohinia, Y.; Sajjadi, S.E.; Ghadirian, B. Antispasmodic effect of osthole and Prangos ferulacea extract on rat uterus smooth muscle motility. Res. Pharm. Sci. 2012, 7, 141–149. [Google Scholar] [PubMed]
- Shokoohinia, Y.; Jafari, F.; Mohammadi, Z.; Bazvandi, L.; Hosseinzadeh, L.; Chow, N.; Bhattacharyya, P.; Farzaei, M.H.; Farooqi, A.A.; Nabavi, S.M.; et al. Potential Anticancer Properties of Osthol: A Comprehensive Mechanistic Review. Nutrients 2018, 10, 36. [Google Scholar] [CrossRef]
- Mei, J.; Wang, T.; Zhao, S.; Zhang, Y. Osthole Inhibits Breast Cancer Progression through Upregulating Tumor Suppressor GNG7. J. Oncol. 2021, 27, 6610511. [Google Scholar] [CrossRef]
- Liang, L.; Yang, B.; Wu, Y.; Sun, L. Osthole suppresses the proliferation and induces apoptosis via inhibiting the PI3K/AKT signaling pathway of endometrial cancer JEC cells. Exp. Ther. Med. 2021, 22, 1171. [Google Scholar] [CrossRef]
- Bae, H.; Lee, J.Y.; Song, J.; Song, G.; Lim, W. Osthole interacts with an ER-mitochondria axis and facilitates tumor suppression in ovarian cancer. J. Cell. Physiol. 2021, 236, 1025–1042. [Google Scholar] [CrossRef] [PubMed]
- Abosharaf, H.A.; Diab, T.; Atlam, F.M.; Mohamed, T.M. Osthole extracted from a citrus fruit that affects apoptosis on A549 cell line by histone deacetylasese inhibition (HDACs). Biotechnol. Rep. 2020, 28, e00531. [Google Scholar] [CrossRef]
- De la Cruz-Concepción, B.; Gutiérrez-Escobar, A.; Lorenzo-Moran, H.Y.; Navarro-Tito, N.; Martínez-Carrillo, D.N.; Ortuño-Pineda, C.; Zacapala-Gómez, A.E.; Torres-Rojas, F.I.; Dircio-Maldonado, R.; Jiménez-Wences, H.; et al. Use of coumarins as complementary medicine with an integrative approach against cervical cancer: Background and mechanisms of action. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7654–7667. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.M.; Kuo, Y.H.; Chao, C.C.; Tsou, Y.H.; Chou, C.H.; Lin, C.H.; Wong, K.L. Osthol is a Use-Dependent Blocker of Voltage-Gated Na+ Channels in Mouse Neuroblastoma N2A Cells. Planta Med. 2010, 76, 34–40. [Google Scholar] [CrossRef]
- Wu, S.; Lo, Y.K.; Chen, C.; Li, H.; Chiang, H. Inhibitory effect of the plant-extract osthole on L-type calcium current in NG108-15 neuronal cells. Biochem. Pharmacol. 2002, 15, 199–206. [Google Scholar] [CrossRef]
- Shokoohinia, Y.; Bazargan, S.; Miraghaee, S.; Javadirad, E.; Hosseinzadeh, L. Safety assessment of osthole isolated from Prangos ferulacea: Acute and subchronic toxicities and modulation of cytochrome P450. Jundishapur. J. Nat. Pharm. Prod. 2017, 12, e63764. [Google Scholar] [CrossRef]
- Shi, J.; Fu, Q.; Chen, W.; Yang, H.P.; Liu, J.; Wang, X.M.; He, X. Comparative study of pharmacokinetics and tissue distribution of osthole in rats after oral administration of pure osthole and Libanotis buchtormensis supercritical extract. J Ethnopharmacol. 2013, 145, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, X.; Wang, X.; Wang, J.; Hao, Q.; Hao, J.; Hou, X. Osthole-loaded nanoemulsion enhances brain target in the treatment of Alzheimer’s Disease via Intranasal administration. Oxid Med. Cell. Longev. 2021, 2021, 8844455. [Google Scholar] [CrossRef] [PubMed]
- Bagherpour Doun, S.K.; Alavi, S.E.; Koohi Moftakhari Esfahani, M.; Ebrahimi Shahmabadi, H.; Alavi, F.; Hamzei, S. Efficacy of Cisplatin-loaded poly butyl cyanoacrylate nanoparticles on the ovarian cancer: An in vitro study. Tumour. Biol. 2014, 35, 7491–7497. [Google Scholar] [CrossRef]
- Cabeza, L.; Ortiz, R.; Arias, J.L.; Prados, J.; Ruiz Martínez, M.A.; Entrena, J.M.; Luque, R.; Melguizo, C. Enhanced anti-tumor activity of doxorubicin in breast cancer through the use of poly(butylcyanoacrylate) nanoparticles. Int. J. Nanomed. 2015, 10, 1291–1306. [Google Scholar] [CrossRef]
- Fatemeh, D.R.; Ebrahimi Shahmabadi, H.; Abedi, A.; Alavi, S.E.; Movahedi, F.; Koohi Mof-takhari Esfahani, M.; Zadeh Mehrizi, T.; Akbarzadeh, A. Polybutylcyanoacrylate nanoparticles and drugs of the platinum family: Last status. Indian J. Clin. Biochem. 2014, 29, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Xu, Y.; Asghar, S.; Chen, M.; Zou, L.; Eltayeb, S. Polybutylcyanoacrylate nanocarriers as promising targeted drug delivery systems. J. Drug Target 2015, 23, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, C.; Lee, Y. Synthesis of high loading and encapsulation efficient paclitaxel-loaded poly (n-butyl cyanoacrylate) nanoparticles via miniemulsion. Int. J. Pharm. 2007, 338, 267–275. [Google Scholar] [CrossRef]
- Ren, F.; Chen, R.; Wang, Y.; Sun, Y.; Jiang, Y.; Li, G. Paclitaxel-loaded poly(n-butylcyanoacrylate) nanoparticle delivery system to overcome multidrug resistance in ovarian cancer. Pharm. Res. 2011, 28, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, G. System of Experimental Design: Engineering Methods to Optimize Quality and Minimize Costs; UNIPUB/Kraus International Publications: Millwood, NY, USA, 1987. [Google Scholar]
- Wie, Y.M.; Lee, K.G.; Lee, K.H.; Ko, T.; Lee, K.H. The experimental process design of artificial lightweight aggregates using an orthogonal array table and analysis by machine learning. Materials 2020, 13, 5570. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Q.; Jiang, Y.; Shi, H.; Peng, T.; Wang, M. Optimization of early mobilization program for patients with acute Ischemic Stroke: An Orthogonal Design. Front Neurol. 2021, 12, 645811. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Guidelines for Stability Testing of Apis and Preparations. In Chinese Pharmacopoeia; China Medical Science Press: Beijing, China, 2020; (Part Four). [Google Scholar]
- Kim, S.; Jeong, D.; Lee, H.; Kim, D.; Jung, S. Succinoglycan dialdehyde-reinforced gelatin hydrogels with toughness and thermal stability. Int. J. Biol. Macromol. 2020, 149, 281–289. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J.; Yang, Y.; Zeng, F.; Liu, C. Highly efficient delivery of functional cargoes by a novel cell-penetrating peptide derived from SP140-Like Protein. Bioconjugate Chem. 2016, 27, 1373–1381. [Google Scholar] [CrossRef]
- Li, R.; Chen, C.; Zhu, S.; Wang, X.; Yang, Y.; Shi, W.; Chen, S.; Wang, C.; Yan, L.; Shi, J. CGA-N9, an antimicrobial peptide derived from chromogranin A: Direct cell penetration of and endocytosis by Candida tropicalis. Biochem. J. 2019, 476, 483–497. [Google Scholar] [CrossRef]
- Boyd, M.; Morris, J.M.; Santoro, D. Plasma membrane integrity and oxidative stress index outcomes of canine progenitor epidermal keratinocytes (CPEKs) exposed to virgin coconut oil (VCO). Vet. Dermatol. 2019, 30, 553-e166. [Google Scholar] [CrossRef]
- Alavi, S.E.; Cabot, P.J.; Moyle, P.M. Glucagon-like peptide-1 receptor agonists and strategies to improve their efficiency. Mol. Pharm. 2019, 16, 2278–2295. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Yang, B.; Deng, F.; Ji, J.; Yang, Y.; Huang, Z.; Zhang, X.; Wei, Y. Luminescence tunable fluorescent organic nanoparticles from polyethyleneimine and maltose: Facile preparation and bioimaging applications. RSC Adv. 2014, 43, 22294–22298. [Google Scholar] [CrossRef]
- Shan, S.; Yao, J.; Xu, L.; Han, S.; Cao, J.; Zhang, G.; Sun, Y. Preparation of Icaritin-Loaded mPEG-PLA Micelles and Evaluation on Ischemic Brain Injury. J. Biomed. Nanotechnol. 2019, 15, 674–685. [Google Scholar] [CrossRef]
- Casamento, A.; Boucrot, E. Molecular mechanism of fast endophilin-mediated endocytosis. Biochem. J. 2020, 477, 2327–2345. [Google Scholar] [CrossRef]
- Jo, M.J.; Lee, Y.J.; Park, C.W.; Chung, Y.B.; Kim, J.S.; Lee, M.K.; Shin, D.H. Evaluation of the Physicochemical Properties, Pharmacokinetics, and In Vitro Anticancer Effects of Docetaxel and Osthol Encapsulated in Methoxy Poly (ethylene glycol)-b-Poly(caprolactone) Polymeric Micelles. Int. J. Mol. Sci. 2020, 22, 231. [Google Scholar] [CrossRef]
- Duan, C.; Townley, H.E. Nanoparticles as vectors to tackle cancer. Biomolecules 2021, 11, 1729. [Google Scholar] [CrossRef]
- Renard, H.F.; Boucrot, E. Unconventional endocytic mechanisms. Curr. Opin. Cell. Biol. 2021, 71, 120–129. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, Y.; Qin, H.; Liu, K.; Guo, M.; Ge, Y.; Xu, M.; Sun, Y.; Zheng, X. Cytotoxicity of CdTe quantum dots in human umbilical vein endothelial cells: The involvement of cellular uptake and induction of pro-apoptotic endoplasmic reticulum stress. Int. J. Nanomed. 2016, 11, 529–542. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Bao, G. Physical principles of nanoparticle cellular endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef]





| Variable | A/Speed (r/min) | B/pH | C/α-BCA (μL) | D/Poloxamer-127 (mg) | |
|---|---|---|---|---|---|
| Levels | |||||
| 1 | 800 | 2 | 10 | 10 | |
| 2 | 1000 | 4 | 15 | 20 | |
| 3 | 1200 | 6 | 20 | 40 | |
| Speed (r/min) | Average Size (nm) | PDI | Maximum Size (nm) | Size Distribution |
|---|---|---|---|---|
| 800 | 163.4 | 0.148 | 201.3 | 102.2 |
| 1000 | 137.3 | 0.135 | 153.8 | 62.13 |
| 1200 | 101.8 | 0.125 | 113.0 | 35.32 |
| Variable | A (r/min) | B (pH) | C (α-BCA μL) | D (mg) | Encapsulation Rate% | |
|---|---|---|---|---|---|---|
| Levels | ||||||
| 1 | 800 | 2 | 10 | 10 | 70.53 | |
| 2 | 800 | 4 | 15 | 20 | 73.99 | |
| 3 | 800 | 6 | 20 | 40 | 72.45 | |
| 4 | 1000 | 2 | 15 | 40 | 79.08 | |
| 5 | 1000 | 4 | 20 | 10 | 77.52 | |
| 6 | 1000 | 6 | 10 | 20 | 77.19 | |
| 7 | 1200 | 2 | 20 | 20 | 77.43 | |
| 8 | 1200 | 4 | 10 | 40 | 77.85 | |
| 9 | 1200 | 6 | 15 | 10 | 78.81 | |
| K1 | 72.312 | 75.679 | 75.189 | 75.621 | ||
| K2 | 77.932 | 76.452 | 77.296 | 76.203 | ||
| K3 | 78.028 | 76.142 | 75.787 | 76.449 | ||
| R | 5.716 | 0.773 | 2.107 | 0.828 | ||
| Factor | SS | DOF | F Ratio | Critical Value | Significance |
|---|---|---|---|---|---|
| A (r/min) | 64.242 | 2 | 16.098 | 6.94 | <0.05 |
| B (pH) | 0.907 | 2 | 0.227 | 6.94 | >0.05 |
| C (α-BCA μL) | 7.077 | 2 | 1.773 | 6.94 | >0.05 |
| D (mg) | 1.085 | 2 | 0.272 | 6.94 | >0.05 |
| Error | 7.98 | 4 |
| Formulations | Size (nm) | Size Distribution | PDI | Zeta Potential (mV) | Encapsulation Efficiency (%) | Loading Efficiency (%) |
|---|---|---|---|---|---|---|
| Osthol-loaded PBCA nanoparticles | 110 ± 6.7 nm | 20.3 nm | 0.126 ± 0.014 | −13 ± 0.32 mV | 80.59 | 40% |
| PBCA nanoparticles | 96 ± 5.3 nm | 18.3 nm | 0.123 ± 0.013 | −7 ± 0.40 mV | − | − |
| (a) Optical Stability Test (3000 lux) | |||||||
| Time (day) | 0 | 2 | 7 | 14 | 30 | Degradation Rate (%) | |
| Content (%) | |||||||
| Osthol | 100 ± 5.70 | 86 ± 6.70 | 60 ± 4.50 | 43 ± 5.50 | 32 ± 6.80 | 68 | |
| Osthol-PBCA-NP suspensions | 100 ± 6.10 | 94 ± 6.80 | 90 ± 5.41 | 88 ± 6.40 | 86 ± 5.65 | 14 | |
| Osthol-PBCA-NP powder | 100 ± 5.30 | 99 ± 4.60 | 90 ± 5.40 | 98 ± 3.12 | 94 ± 5.60 | 6 | |
| (b) Thermal stability test (60 °C) | |||||||
| Time (day) | 0 | 2 | 7 | 14 | 30 | Degradation Rate (%) | |
| Content(%) | |||||||
| Osthol | 100 ± 5.40 | 76 ± 6.50 | 52 ± 3.50 | 35 ± 5.50 | 28 ± 6.80 | 72 | |
| Osthol-PBCA-NP suspensions | 100 ± 5.30 | 93 ± 6.40 | 89 ± 5.40 | 87 ± 6.40 | 84 ± 5.65 | 16 | |
| Osthol-PBCA-NP powder | 100 ± 4.70 | 99 ± 3.60 | 97 ± 5.80 | 94 ± 3.52 | 90 ± 4.60 | 10 | |
| Parameters | Unit | Osthol | Osthol-PBCA NP |
|---|---|---|---|
| t1/2 α | min | 0.328 ± 0.235 | 0.269 ± 2:035 |
| t1/2 β | min | 28.135 ± 5.65 | 230.8 ± 7.54 |
| AUC | μg/mL∙h | 6.90 ± 1.831 | 22.52 ± 5.76 |
| CL | (μg∙h /mL) | 1.89 ± 0.4 | 0.278 ± 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Shen, L.; Li, Z.; Zhang, X.; Wu, M.; Zhang, Y.; Liu, J. Design, Preparation, and Evaluation of Osthol Poly-Butyl-Cyanoacrylate Nanoparticles with Improved In Vitro Anticancer Activity in Neuroblastoma Treatment. Molecules 2022, 27, 6908. https://doi.org/10.3390/molecules27206908
Zheng L, Shen L, Li Z, Zhang X, Wu M, Zhang Y, Liu J. Design, Preparation, and Evaluation of Osthol Poly-Butyl-Cyanoacrylate Nanoparticles with Improved In Vitro Anticancer Activity in Neuroblastoma Treatment. Molecules. 2022; 27(20):6908. https://doi.org/10.3390/molecules27206908
Chicago/Turabian StyleZheng, Liqing, Lixia Shen, Ze Li, Xiaoli Zhang, Miaomiao Wu, Yuanyuan Zhang, and Jianhua Liu. 2022. "Design, Preparation, and Evaluation of Osthol Poly-Butyl-Cyanoacrylate Nanoparticles with Improved In Vitro Anticancer Activity in Neuroblastoma Treatment" Molecules 27, no. 20: 6908. https://doi.org/10.3390/molecules27206908
APA StyleZheng, L., Shen, L., Li, Z., Zhang, X., Wu, M., Zhang, Y., & Liu, J. (2022). Design, Preparation, and Evaluation of Osthol Poly-Butyl-Cyanoacrylate Nanoparticles with Improved In Vitro Anticancer Activity in Neuroblastoma Treatment. Molecules, 27(20), 6908. https://doi.org/10.3390/molecules27206908






