Integrated Hydrogeological, Hydrochemical, and Isotopic Assessment of Seawater Intrusion into Coastal Aquifers in Al-Qatif Area, Eastern Saudi Arabia
Abstract
1. Introduction
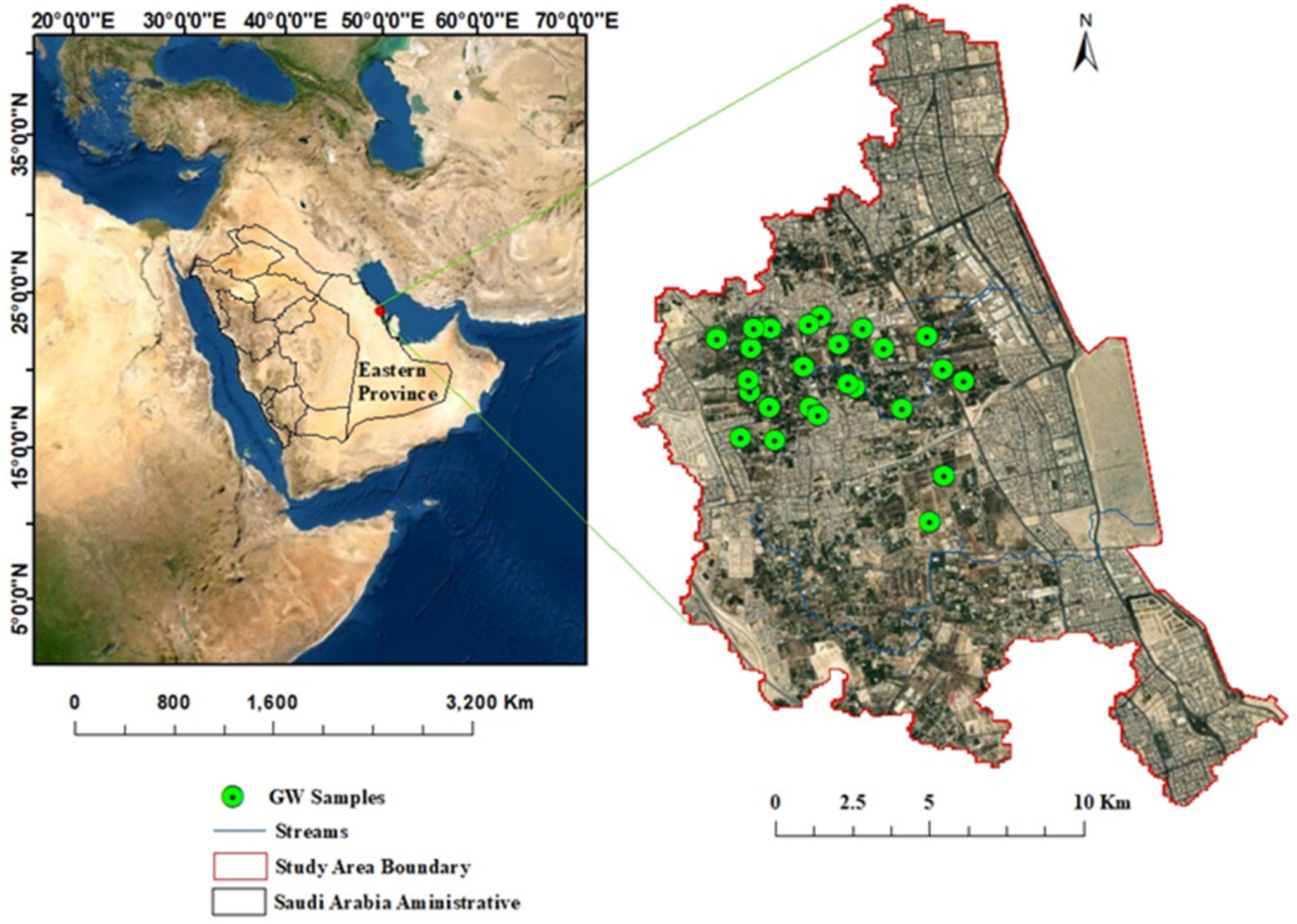
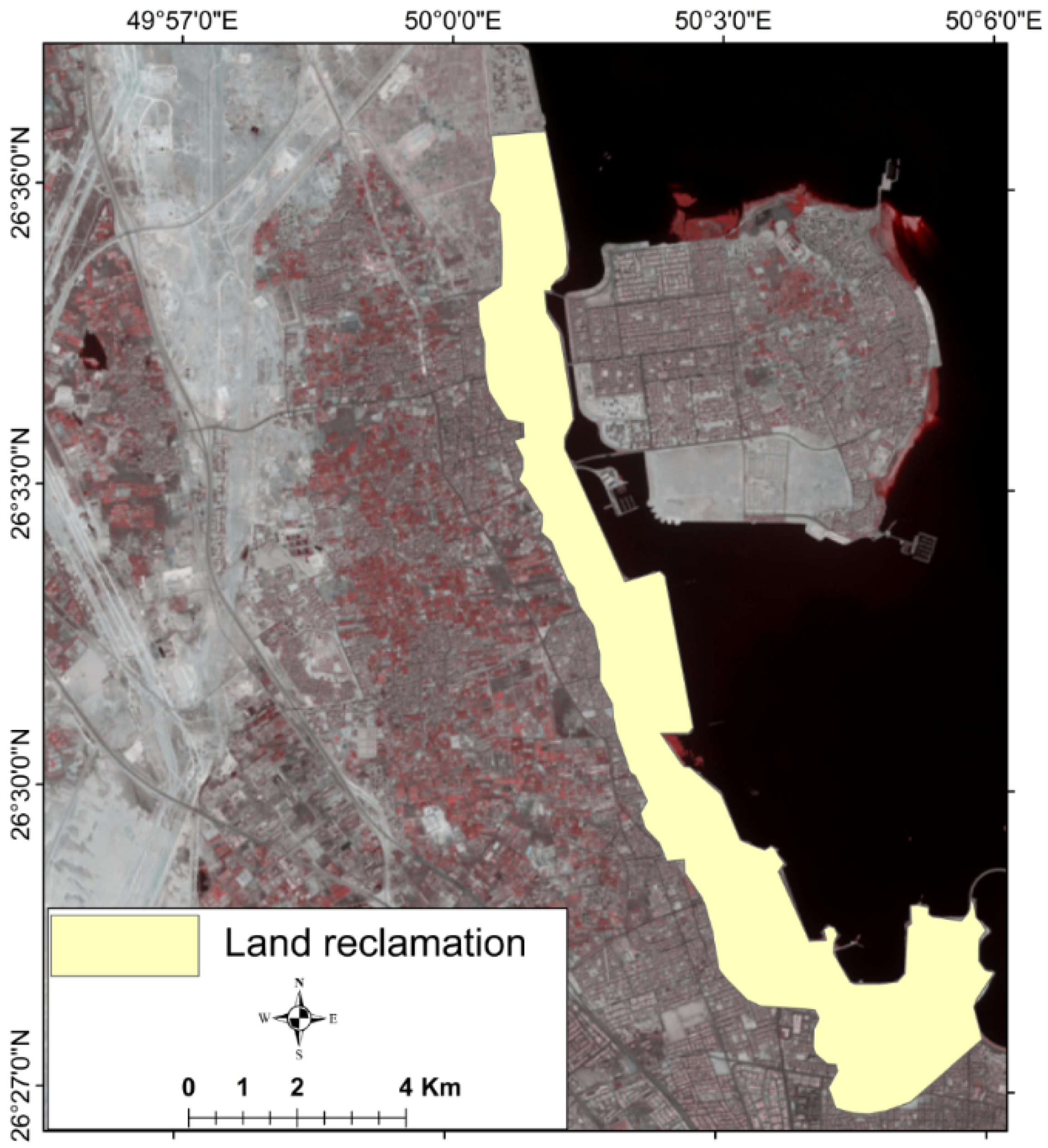
2. Study Area
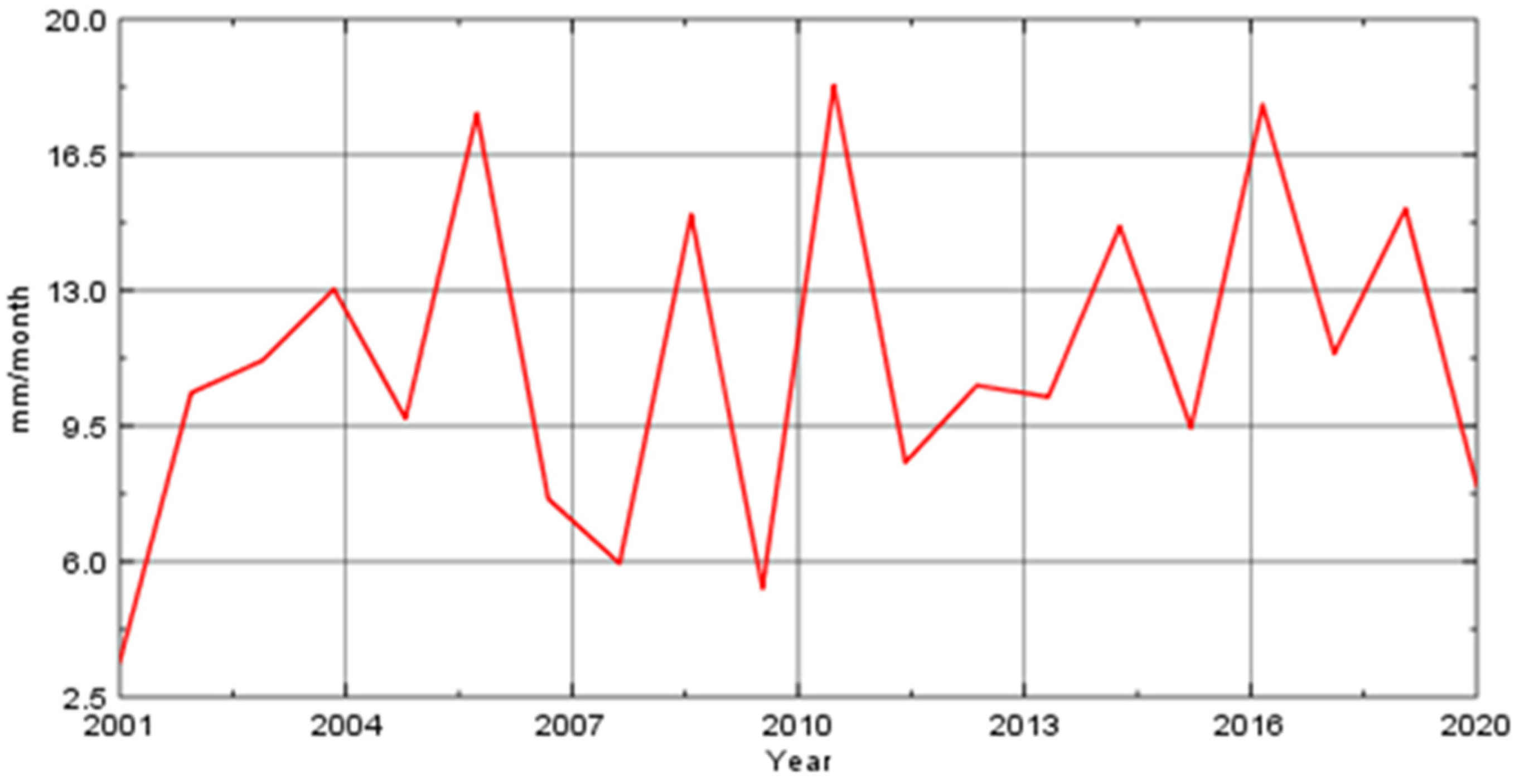
3. Climate and Land Use
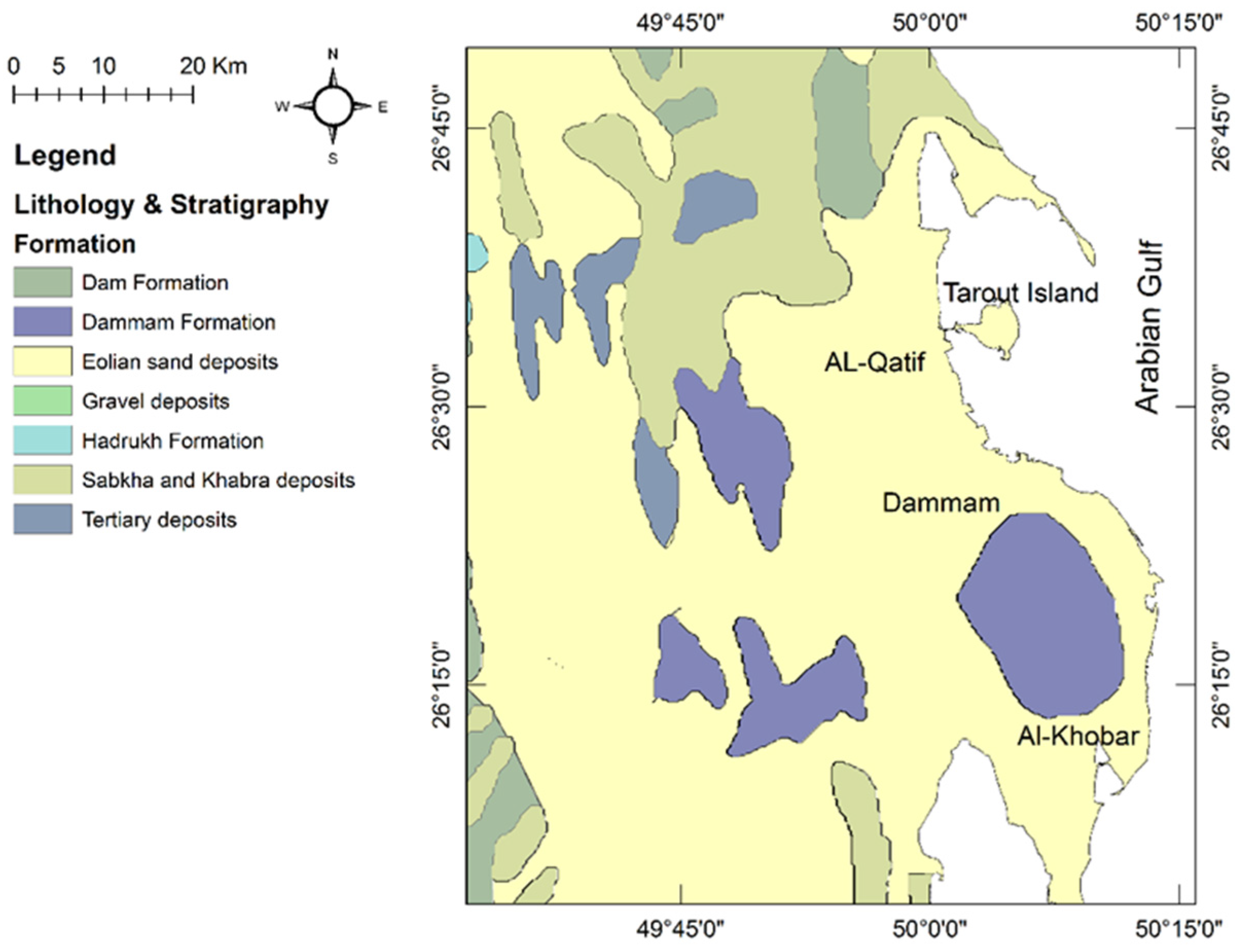
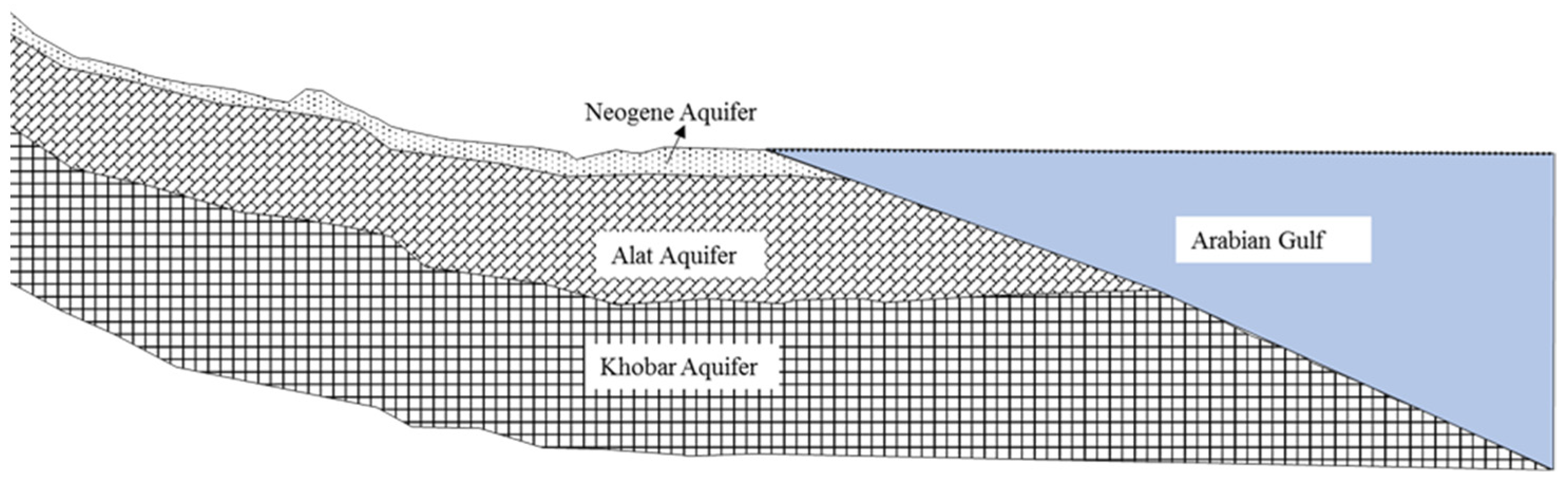
4. Geological and Hydrogeological Setting
5. Methodology
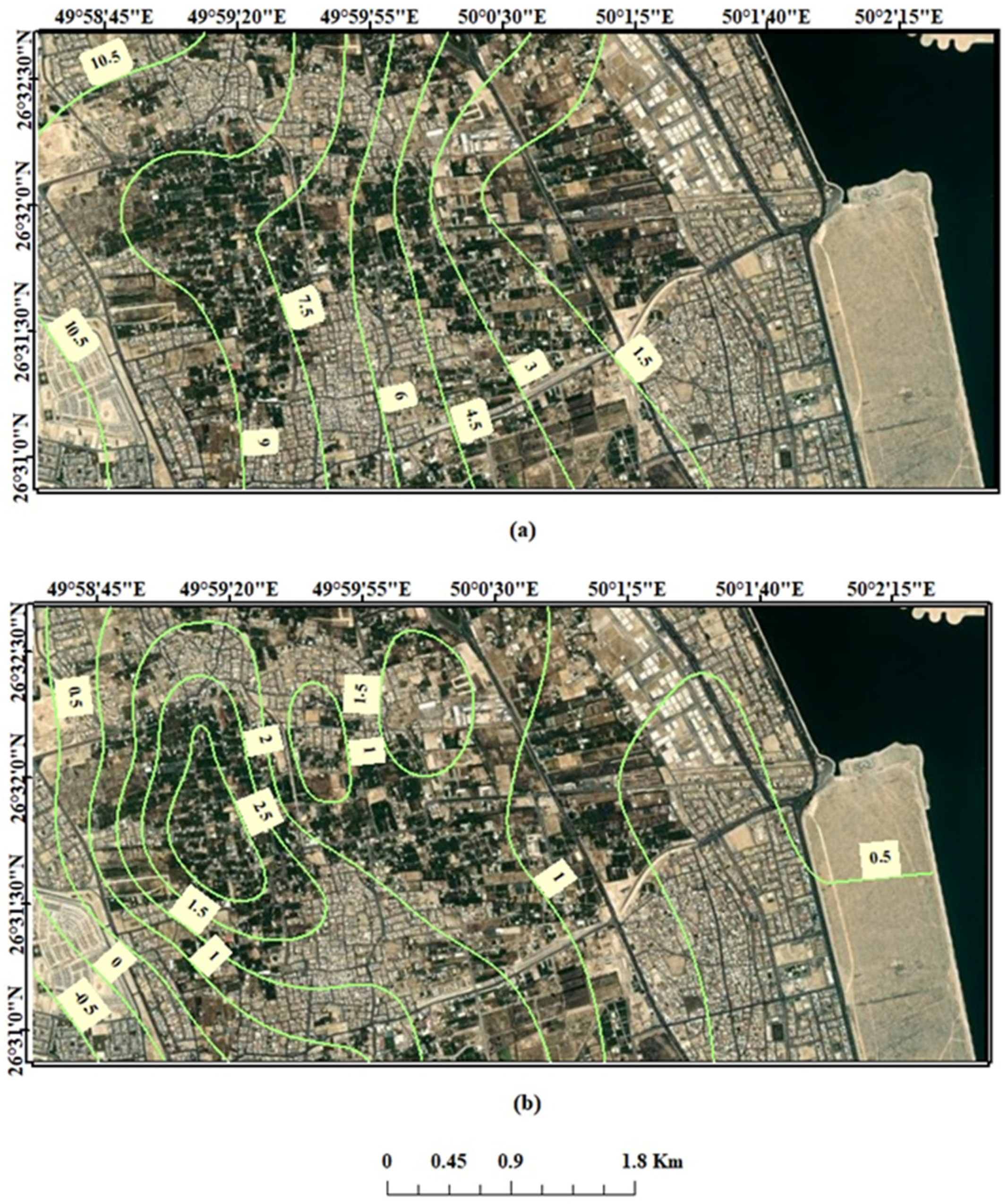
6. Results and Discussion
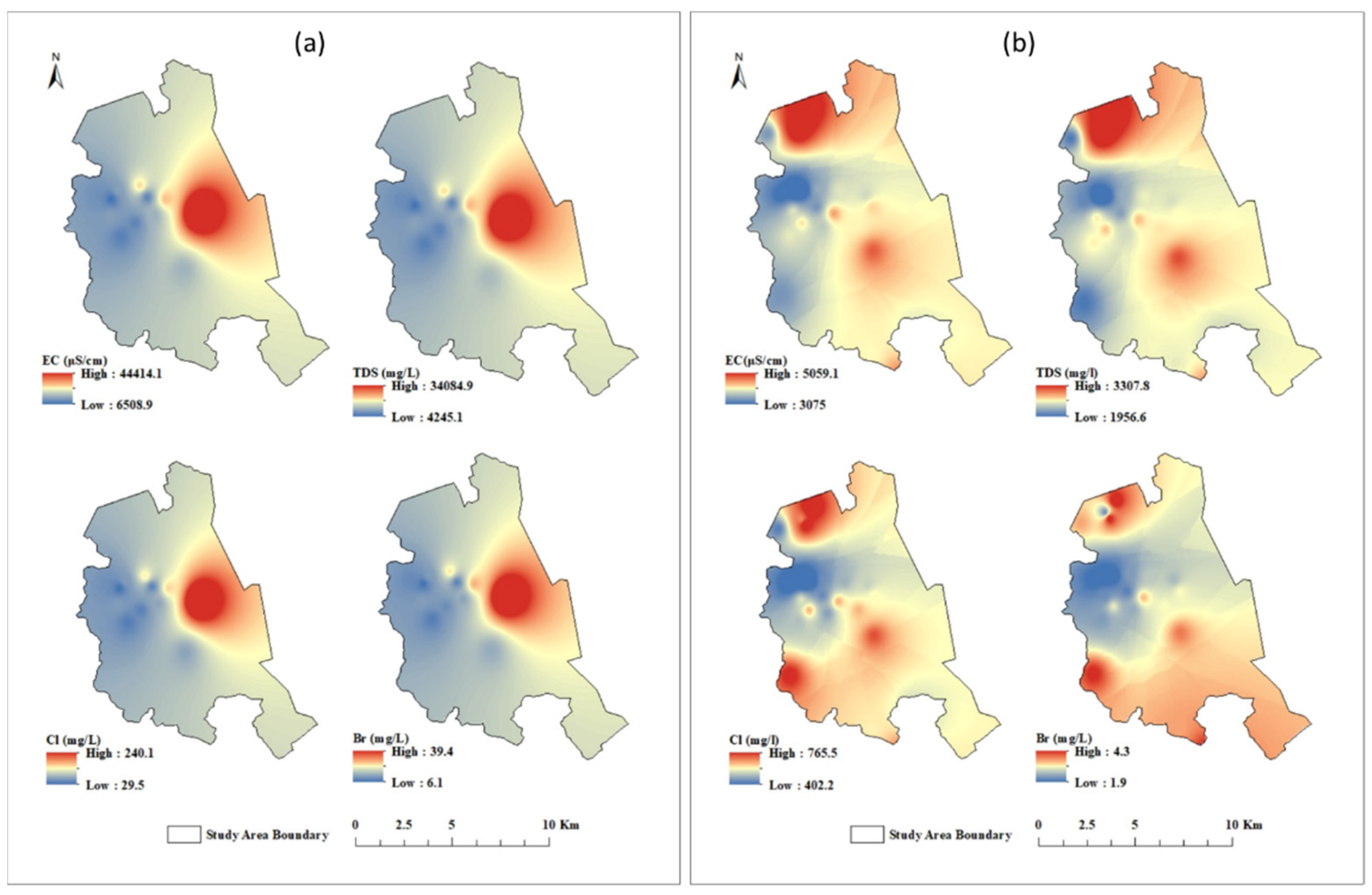
6.1. Hydrogeochemical Characteristics of Groundwater
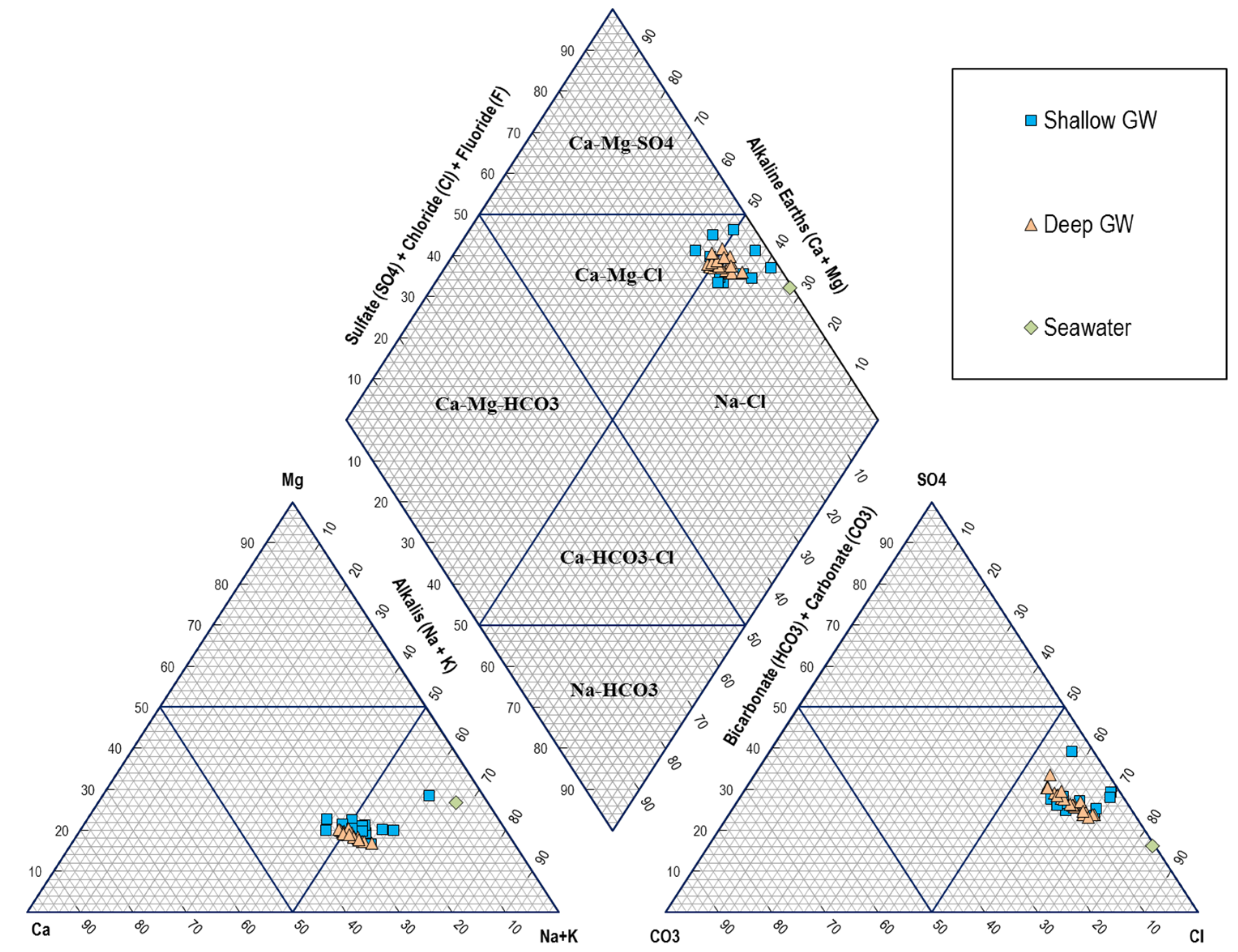
6.2. Hydrogeochemical Facies and Water Type
6.3. Seawater Intrusion Indicators
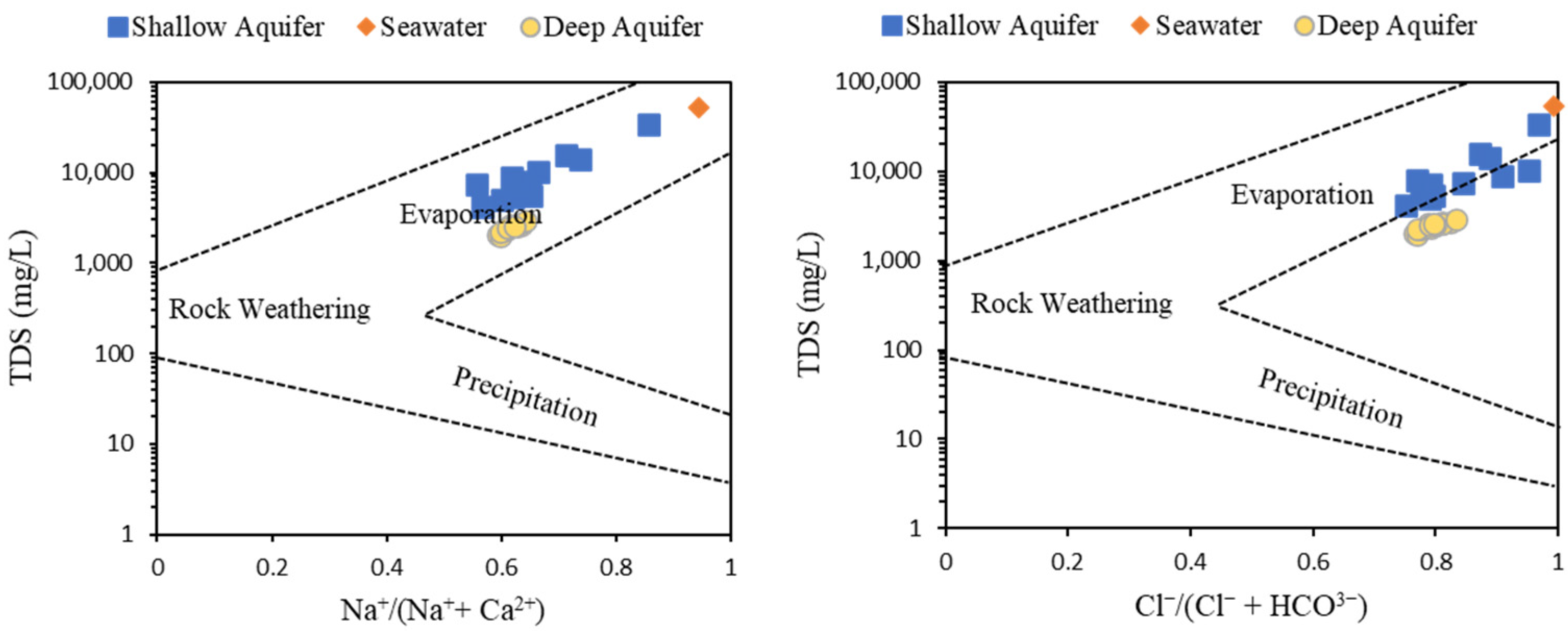
6.3.1. Gibbs Diagram
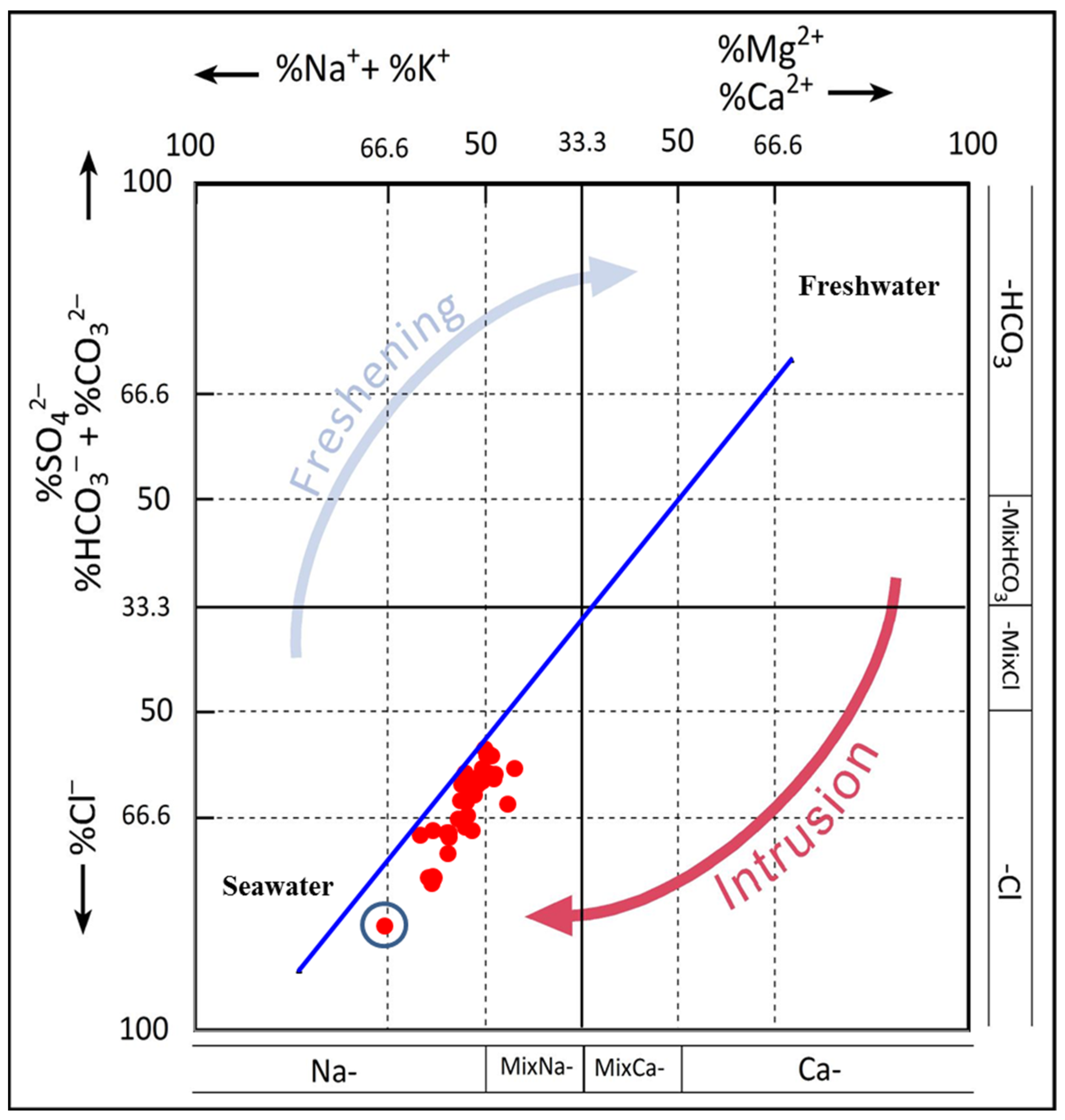
6.3.2. Hydrochemical Facies Evolution (HFE) Diagram
6.3.3. Chadha’s Diagram
6.3.4. Inverse of Simpson’s Ratio
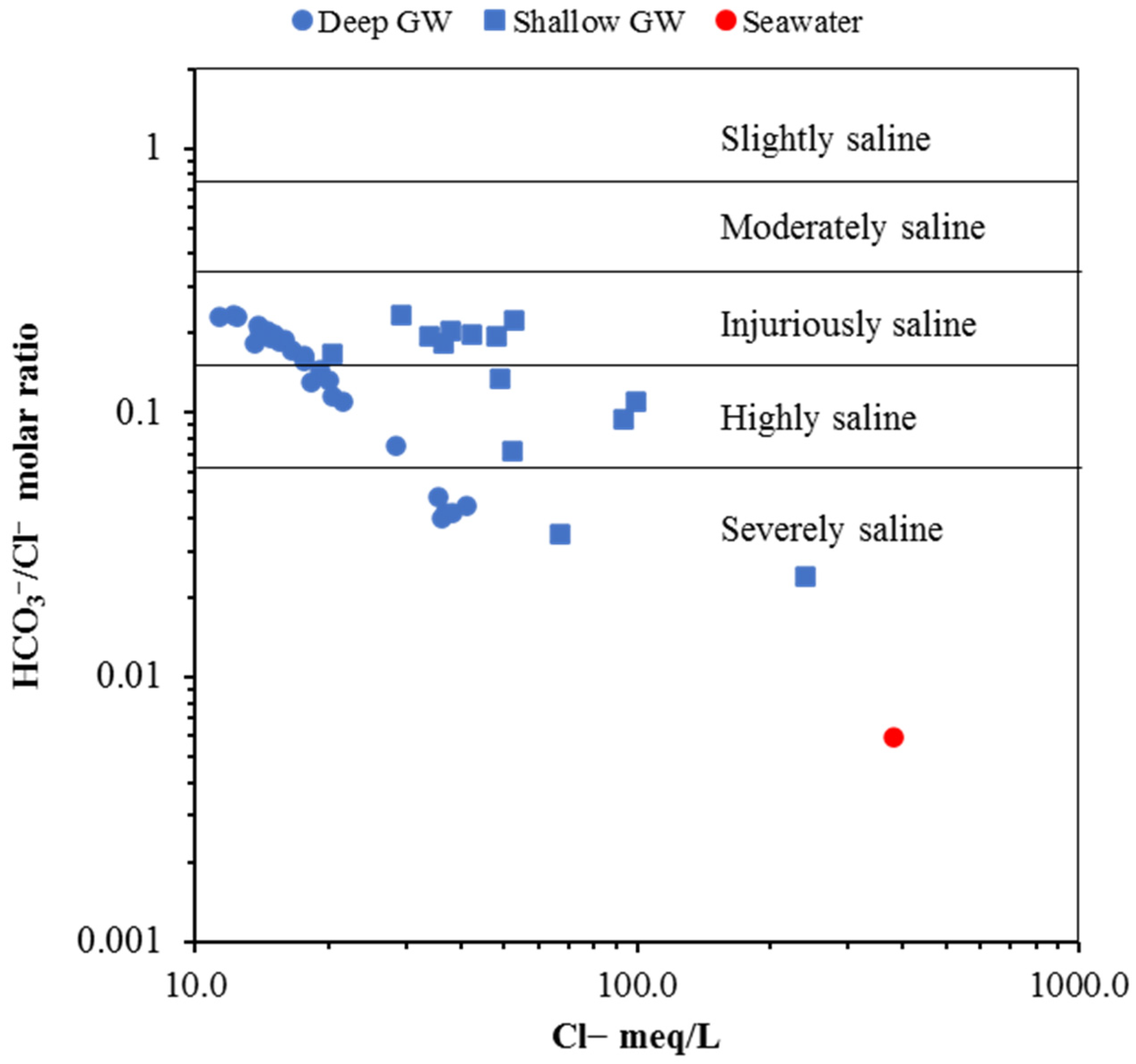
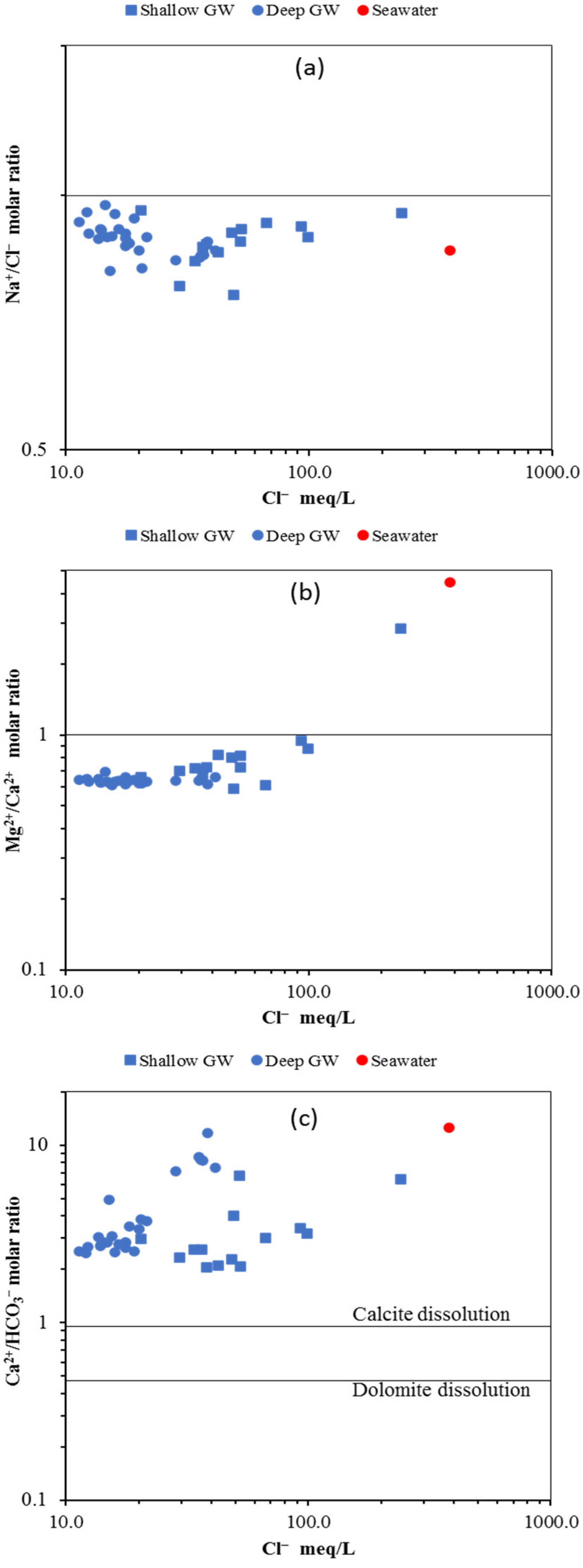
6.3.5. Ionic Ratio Diagram
Na+/Cl− vs. Cl−
Mg2+/Ca2+ vs. Cl−
Ca2+/HCO3− vs. Cl−
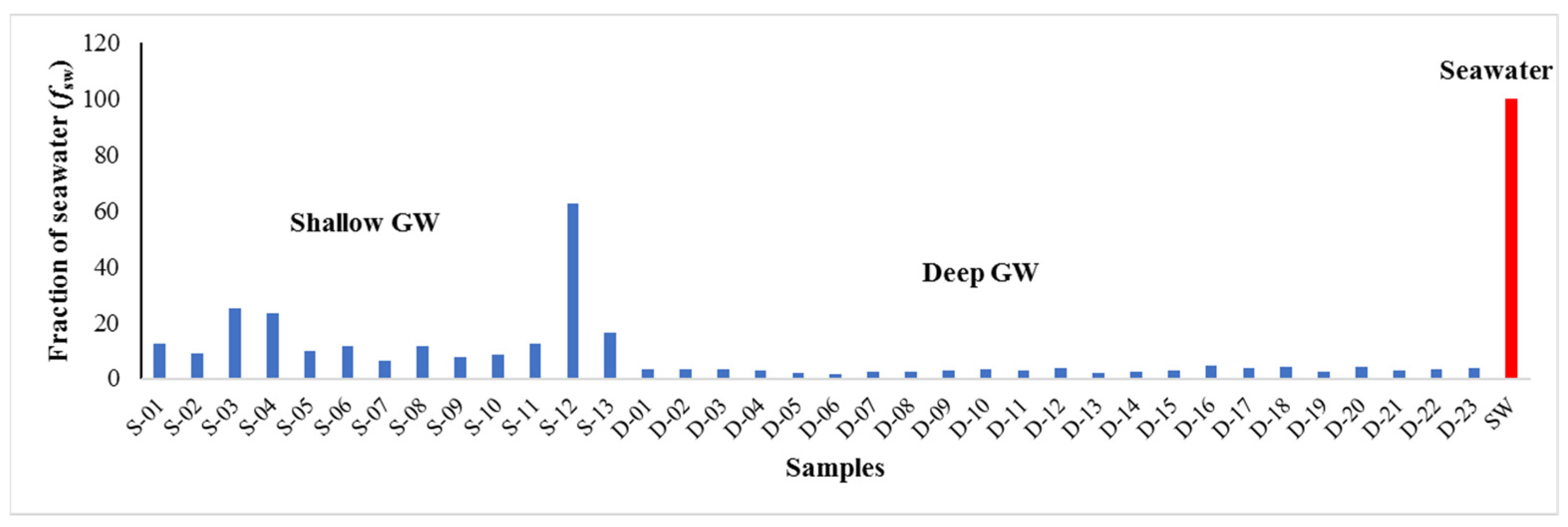
6.4. Quantification of Seawater Intrusion
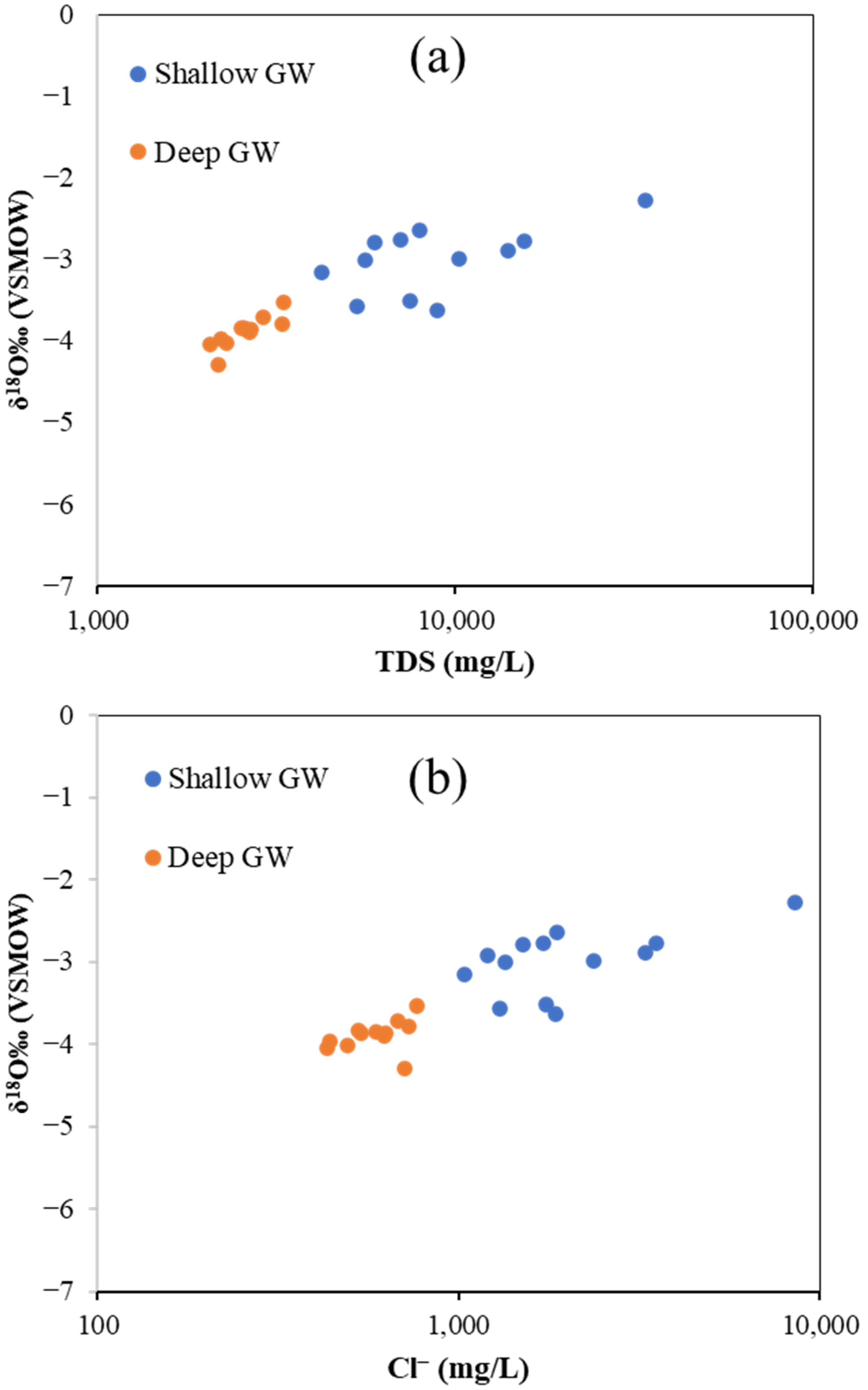
6.5. Isotopic Analyses
6.6. Management of Seawater Intrusion
7. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Famiglietti, J.S. The global groundwater crisis. Nat. Clim. Chang. 2014, 4, 945–948. [Google Scholar] [CrossRef]
- Daskalaki, P.; Voudouris, K. Groundwater quality of porous aquifers in Greece: A synoptic review. Environ. Geol. 2008, 54, 505–513. [Google Scholar] [CrossRef]
- Van Camp, M.; Mtoni, Y.; Mjemah, I.C.; Bakundukize, C.; Walraevens, K. Investigating seawater intrusion due to groundwater pumping with schematic model simulations: The example of the Dar es Salaam coastal aquifer in Tanzania. J. Afr. Earth Sci. 2014, 96, 71–78. [Google Scholar] [CrossRef]
- Singh, A. Managing the environmental problem of seawater intrusion in coastal aquifers through simulation-optimization modeling. Ecol. Indic. 2015, 48, 498–504. [Google Scholar] [CrossRef]
- Balasubramanian, M.; Sridhar, S.G.D.; Ayyamperumal, R.; Karuppannan, S.; Gopalakrishnan, G.; Chakraborty, M.; Huang, X. Isotopic signatures, hydrochemical and multivariate statistical analysis of seawater intrusion in the coastal aquifers of Chennai and Tiruvallur District, Tamil Nadu, India. Mar. Pollut. Bull. 2022, 174, 113232. [Google Scholar] [CrossRef]
- Benaafi, M.; Yassin, M.A.; Usman, A.G.; Abba, S.I. Neurocomputing Modelling of Hydrochemical and Physical Properties of Groundwater Coupled with Spatial Clustering, GIS, and Statistical Techniques. Sustainability 2022, 14, 2250. [Google Scholar] [CrossRef]
- Cao, T.; Han, D.; Song, X. Past, present, and future of global seawater intrusion research: A bibliometric analysis. J. Hydrol. 2021, 603, 126844. [Google Scholar] [CrossRef]
- Maurya, P.; Kumari, R.; Mukherjee, S. Hydrochemistry in integration with stable isotopes (δ18O and δD) to assess seawater intrusion in coastal aquifers of Kachchh district, Gujarat, India. J. Geochem. Explor. 2019, 196, 42–56. [Google Scholar] [CrossRef]
- Batayneh, A.; Zaman, H.; Zumlot, T.; Ghrefat, H.; Mogren, S.; Nazzal, Y.; Elawadi, E.; Qaisy, S.; Bahkaly, I.; Al-Taani, A. Hydrochemical facies and ionic ratios of the coastal groundwater aquifer of saudi gulf of aqaba: Implication for seawater intrusion. J. Coast. Res. 2014, 30, 75–87. [Google Scholar]
- De Filippis, G.; Foglia, L.; Giudici, M.; Mehl, S.; Margiotta, S.; Negri, S.L. Seawater intrusion in karstic, coastal aquifers: Current challenges and future scenarios in the Taranto area (southern Italy). Sci. Total Environ. 2016, 573, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Al Naeem, M.F.A.; Yusoff, I.; Ng, T.F.; Maity, J.P.; Alias, Y.; May, R.; Alborsh, H. A study on the impact of anthropogenic and geogenic factors on groundwater salinization and seawater intrusion in Gaza coastal aquifer, Palestine: An integrated multi-techniques approach. J. Afr. Earth Sci. 2019, 156, 75–93. [Google Scholar] [CrossRef]
- Xu, X.; Xiong, G.; Chen, G.; Fu, T.; Yu, H.; Wu, J.; Liu, W.; Su, Q.; Wang, Y.; Liu, S. Characteristics of coastal aquifer contamination by seawater intrusion and anthropogenic activities in the coastal areas of the Bohai Sea, eastern China. J. Asian Earth Sci. 2021, 217, 104830. [Google Scholar] [CrossRef]
- Benaafi, M.; Al-Shaibani, A. Hydrochemical and Isotopic Investigation of the Groundwater from Wajid Aquifer in Wadi Al-Dawasir, Southern Saudi Arabia. Water 2021, 13, 1855. [Google Scholar] [CrossRef]
- Huang, G.; Sun, J.; Zhang, Y.; Chen, Z.; Liu, F. Impact of anthropogenic and natural processes on the evolution of groundwater chemistry in a rapidly urbanized coastal area, South China. Sci. Total Environ. 2013, 463, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Kloppmann, W.; Bourhane, A.; Schomburgk, S. Groundwater salinization in France. Procedia Earth Planet. Sci. 2013, 7, 440–443. [Google Scholar] [CrossRef]
- Comte, J.-C.; Cassidy, R.; Obando, J.; Robins, N.; Ibrahim, K.; Melchioly, S.; Mjemah, I.; Shauri, H.; Bourhane, A.; Mohamed, I. Challenges in groundwater resource management in coastal aquifers of East Africa: Investigations and lessons learnt in the Comoros Islands, Kenya and Tanzania. J. Hydrol. Reg. Stud. 2016, 5, 179–199. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Rayaleh, W.E.; Zghibi, A.; Ouddane, B. Assessment of chemical quality of groundwater in coastal volcano-sedimentary aquifer of Djibouti, Horn of Africa. J. Afr. Earth Sci. 2017, 131, 284–300. [Google Scholar] [CrossRef]
- Ziadi, A.; Hariga, N.T.; Tarhouni, J. Mineralization and pollution sources in the coastal aquifer of Lebna, Cap Bon, Tunisia. J. Afr. Earth Sci. 2019, 151, 391–402. [Google Scholar] [CrossRef]
- Rajmohan, N.; Al-Futaisi, A.; Al-Touqi, S. Geochemical process regulating groundwater quality in a coastal region with complex contamination sources: Barka, Sultanate of Oman. Environ. Earth Sci. 2009, 59, 385–398. [Google Scholar] [CrossRef]
- Abdalla, F. Ionic ratios as tracers to assess seawater intrusion and to identify salinity sources in Jazan coastal aquifer, Saudi Arabia. Arab. J. Geosci. 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Nogueira, G.; Stigter, T.Y.; Zhou, Y.; Mussa, F.; Juizo, D. Understanding groundwater salinization mechanisms to secure freshwater resources in the water-scarce city of Maputo, Mozambique. Sci. Total Environ. 2019, 661, 723–736. [Google Scholar] [CrossRef]
- Frollini, E.; Parrone, D.; Ghergo, S.; Masciale, R.; Passarella, G.; Pennisi, M.; Salvadori, M.; Preziosi, E. An integrated approach for investigating the salinity evolution in a Mediterranean coastal karst aquifer. Water 2022, 14, 1725. [Google Scholar] [CrossRef]
- Missimer, T.M.; Hoppe-Jones, C.; Jadoon, K.Z.; Li, D.; Al-Mashharawi, S.K. Hydrogeology, water quality, and microbial assessment of a coastal alluvial aquifer in western Saudi Arabia: Potential use of coastal wadi aquifers for desalination water supplies. Hydrogeol. J. 2014, 22, 1921–1934. [Google Scholar] [CrossRef]
- Alfaifi, H.; Kahal, A.; Albassam, A.; Ibrahim, E.; Abdelrahman, K.; Zaidi, F.; Alhumidan, S. Integrated geophysical and hydrochemical investigations for seawater intrusion: A case study in southwestern Saudi Arabia. Arab. J. Geosci. 2019, 12, 372. [Google Scholar] [CrossRef]
- Alhumimidi, M.S. An integrated approach for identification of seawater intrusion in coastal region: A case study of northwestern Saudi Arabia. J. King Saud Univ. 2020, 32, 3187–3194. [Google Scholar] [CrossRef]
- Alshehri, F.; Almadani, S.; El-Sorogy, A.S.; Alwaqdani, E.; Alfaifi, H.J.; Alharbi, T. Influence of seawater intrusion and heavy metals contamination on groundwater quality, Red Sea coast, Saudi Arabia. Mar. Pollut. Bull. 2021, 165, 112094. [Google Scholar] [CrossRef]
- Ebert, C.H. V Water resources and land use in the Qatif Oasis of Saudi Arabia. Geogr. Rev. 1965, 55, 496–509. [Google Scholar] [CrossRef]
- Al-Shaibani, A. Economic potential of brines of Sabkha Jayb Uwayyid, Eastern Saudi Arabia. Arab. J. Geosci. 2013, 6, 2607–2618. [Google Scholar] [CrossRef]
- Weijermars, R. Surface geology, lithostratigraphy and Tertiary growth of the Dammam Dome, Saudi Arabia: A new field guide. GeoArabia 1999, 4, 199–226. [Google Scholar] [CrossRef]
- Edgell, H.S. Aquifers of Saudi Arabia and their geological framework. Arab. J. Sci. Eng. 1997, 22, 3–31. [Google Scholar]
- Abderrahman, W.A.; Rasheeduddin, M.; Al-Harazin, I.M.; Esuflebbe, M.; Eqnaibi, B.S. Impacts of management practices on groundwater conditions in the eastern province, Saudi Arabia. Hydrogeol. J. 1995, 3, 32–41. [Google Scholar] [CrossRef]
- USEPA Groundwater Sampling Guidelines. Environ. Prot. Auth. 2000, 36.
- Clescerl, L.; Greenberg, A.; Eaton, A. Standard Methods for Examining Water and Wastewater; APHA: Washington, DC, USA, 1999.
- Fritz, S.J. A survey of charge-balance errors on published analyses of potable ground and surface waters. Groundwater 1994, 32, 539–546. [Google Scholar] [CrossRef]
- Clark, I. Groundwater Geochemistry and Isotopes; CRC Press: Boca Raton, FL, USA, 2015; ISBN 1466591749. [Google Scholar]
- Khan, A.F.; Srinivasamoorthy, K.; Rabina, C. Hydrochemical characteristics and quality assessment of groundwater along the coastal tracts of Tamil Nadu and Puducherry, India. Appl. Water Sci. 2020, 10, 1–21. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Rao, V.V.S.G. Hydrogeochemical, seawater intrusion and oxygen isotope studies on a coastal region in the Puri District of Odisha, India. Catena 2019, 172, 558–571. [Google Scholar] [CrossRef]
- El Waheidi, M.M.; Ghrefat, H.; Qaysi, S.; Batayneh, A.T.; Russo, G.; Jallouli, C.; Badhris, O.; Maeshi, T.; Elawadhi, E.A.; Bahkali, I. Hydrogeophysical Characterization of the Coastal Aquifer System in the Northeast Gulf of Aqaba (Saudi Arabia): An Investigation in the Context of Groundwater Resources Management. J. Coast. Res. 2021, 37, 41–51. [Google Scholar] [CrossRef]
- Mondal, N.C.; Singh, V.P.; Singh, V.S.; Saxena, V.K. Determining the interaction between groundwater and saline water through groundwater major ions chemistry. J. Hydrol. 2010, 388, 100–111. [Google Scholar] [CrossRef]
- Alfarrah, N.; Walraevens, K. Groundwater overexploitation and seawater intrusion in coastal areas of arid and semi-arid regions. Water 2018, 10, 143. [Google Scholar] [CrossRef]
- Appelo, C.; Postma, D. Geochemistry Groundwater and Pollution, 2nd, ed.; Balkema Publication: Leiden, The Netherlands, 2005. [Google Scholar]
- Abu Salem, H.S.; Gemail, K.S.; Junakova, N.; Ibrahim, A.; Nosair, A.M. An Integrated Approach for Deciphering Hydrogeochemical Processes during Seawater Intrusion in Coastal Aquifers. Water 2022, 14, 1165. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Trans. Am. Geophys. Union 1944, 25, 914. [Google Scholar] [CrossRef]
- Mahlknecht, J.; Merchán, D.; Rosner, M.; Meixner, A.; Ledesma-Ruiz, R. Assessing seawater intrusion in an arid coastal aquifer under high anthropogenic influence using major constituents, Sr and B isotopes in groundwater. Sci. Total Environ. 2017, 587, 282–295. [Google Scholar] [CrossRef]
- Firat Ersoy, A.; Ayyildiz Turan, N.; Arslan, H.; Kuleyin, A. Assessment of seawater intrusion in Kızılırmak delta coastal area (North Turkey) using hydrochemical and isotopic data. Environ. Earth Sci. 2021, 80, 1–15. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Gueddari, H.; Akodad, M.; Baghour, M.; Moumen, A.; Skalli, A.; El Yousfi, Y.; Ismail, A.; Chahban, M.; Azizi, G.; Hmeid, H.A. The salinity origin and hydrogeochemical evolution of groundwater in the Oued Kert basin, North-eastern of Morocco. Sci. Afr. 2022, 16, e01226. [Google Scholar] [CrossRef]
- Giménez-Forcada, E. Dynamic of sea water interface using hydrochemical facies evolution diagram. Groundwater 2010, 48, 212–216. [Google Scholar] [CrossRef]
- Hajji, S.; Allouche, N.; Bouri, S.; Aljuaid, A.M.; Hachicha, W. Assessment of Seawater Intrusion in Coastal Aquifers Using Multivariate Statistical Analyses and Hydrochemical Facies Evolution-Based Model. Int. J. Environ. Res. Public Health 2021, 19, 155. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Forcada, E.; Sánchez San Román, F.J. An Excel Macro to Plot the HFE-Diagram to Identify Sea Water Intrusion Phases. Groundwater 2015, 53, 819–824. [Google Scholar] [CrossRef]
- Chadha, D.K. A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol. J. 1999, 7, 431–439. [Google Scholar] [CrossRef]
- Thilagavathi, R.; Chidambaram, S.; Prasanna, M.V.; Thivya, C. A study on groundwater geochemistry and water quality in layered aquifers system of Pondicherry region, southeast India. Appl. water Sci. 2012, 2, 253–269. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Vinodh, K.; Johnson Babu, G.; Gowtham, B.; Arulprakasam, V. Integrated seawater intrusion study of coastal region of Thiruvallur district, Tamil Nadu, South India. Appl. Water Sci. 2019, 9, 1–20. [Google Scholar] [CrossRef]
- Aju, C.D.; Reghunath, R.; Achu, A.L.; Rajaneesh, A. Understanding the hydrogeochemical processes and physical parameters controlling the groundwater chemistry of a tropical river basin, South India. Environ. Sci. Pollut. Res. 2022, 29, 23561–23577. [Google Scholar] [CrossRef]
- Todd, D.K.; Mays, L.W. Groundwater Hydrology; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 0471059374. [Google Scholar]
- Moujabber, M.E.; Samra, B.B.; Darwish, T.; Atallah, T. Comparison of different indicators for groundwater contamination by seawater intrusion on the Lebanese coast. Water Resour. Manag. 2006, 20, 161–180. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Ale, S. Long term (1960–2010) trends in groundwater contamination and salinization in the Ogallala aquifer in Texas. J. Hydrol. 2014, 513, 376–390. [Google Scholar] [CrossRef]
- Abu-Alnaeem, M.F.; Yusoff, I.; Ng, T.F.; Alias, Y.; Raksmey, M. Assessment of groundwater salinity and quality in Gaza coastal aquifer, Gaza Strip, Palestine: An integrated statistical, geostatistical and hydrogeochemical approaches study. Sci. Total Environ. 2018, 615, 972–989. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Pisciotta, A.; De Maio, M. Evaluation of groundwater salinization and pollution level on Favignana Island, Italy. Environ. Pollut. 2019, 249, 969–981. [Google Scholar] [CrossRef]
- Mercado, A. The use of hydrogeochemical patterns in carbonate sand and sandstone aquifers to identify intrusion and flushing of saline water. Groundwater 1985, 23, 635–645. [Google Scholar] [CrossRef]
- Zghibi, A.; Tarhouni, J.; Zouhri, L. Assessment of seawater intrusion and nitrate contamination on the groundwater quality in the Korba coastal plain of Cap-Bon (North-east of Tunisia). J. Afr. Earth Sci. 2013, 87, 1–12. [Google Scholar] [CrossRef]
- Arslan, H.; Cemek, B.; Demir, Y. Determination of seawater intrusion via hydrochemicals and isotopes in Bafra Plain, Turkey. Water Resour. Manag. 2012, 26, 3907–3922. [Google Scholar] [CrossRef]
- Sankoh, A.A.; Derkyi, N.S.A.; Frazer-Williams, R.A.D.; Laar, C.; Kamara, I. A review on the application of isotopic techniques to trace groundwater pollution sources within developing countries. Water 2022, 14, 35. [Google Scholar] [CrossRef]
- Izbicki, J.A. Seawater Intrusion in a Coastal California Aquifer; US Department of the Interior, US Geological Survey: Reston, VA, USA, 1996.
- IAEA/WMO Global Network of Isotopes in Precipitation–the GNIP Database. Available online: https://www.iaea.org/services/networks/gnip (accessed on 15 May 2022).
- Al-Rashed, M.F.; Sherif, M.M. Water resources in the GCC countries: An overview. Water Resour. Manag. 2000, 14, 59–75. [Google Scholar] [CrossRef]
- Todd, D.K. Salt-water intrusion and its control. J. -Am. Water Work. Assoc. 1974, 66, 180–187. [Google Scholar] [CrossRef]
- Sun, Q.; Zheng, T.; Zheng, X.; Walther, M. Effectiveness and comparison of physical barriers on seawater intrusion and nitrate accumulation in upstream aquifers. J. Contam. Hydrol. 2021, 243, 103913. [Google Scholar] [CrossRef]
- Hussain, M.S.; Abd-Elhamid, H.F.; Javadi, A.A.; Sherif, M.M. Management of Seawater Intrusion in Coastal Aquifers: A Review. Water 2019, 11, 2467. [Google Scholar] [CrossRef]
- Abd-Elaty, I.; Javadi, A.A.; Abd-Elhamid, H. Management of saltwater intrusion in coastal aquifers using different wells systems: A case study of the Nile Delta aquifer in Egypt. Hydrogeol. J. 2021, 29, 1767–1783. [Google Scholar] [CrossRef]
- Abdalla, O.A.E.; Al-Rawahi, A.S. Groundwater recharge dams in arid areas as tools for aquifer replenishment and mitigating seawater intrusion: Example of AlKhod, Oman. Environ. Earth Sci. 2013, 69, 1951–1962. [Google Scholar] [CrossRef]
- Chen, K.P.; Jiao, J.J. Seawater intrusion and aquifer freshening near reclaimed coastal area of Shenzhen. Water Sci. Technol. Water Supply 2007, 7, 137–145. [Google Scholar] [CrossRef]

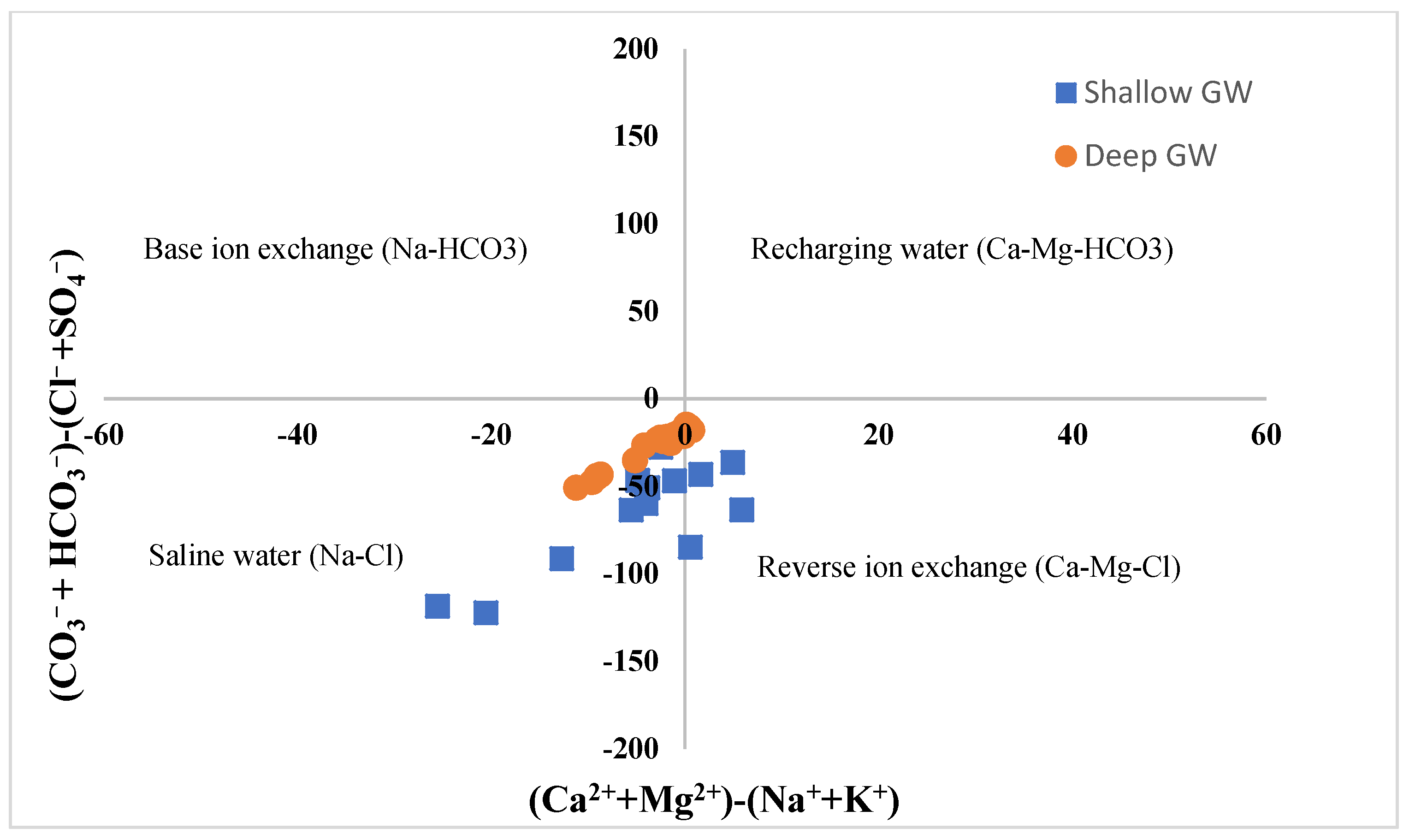


| Ca2+ (mg/L) | Mg2+ (mg/L) | Na+ (mg/L) | K+ (mg/L) | HCO3− (mg/L) | Cl− (mg/L) | SO42− (mg/L) | NO32− (mg/L) | Br− (mg/L) | EC (µS/cm) | TDS (mg/L) | pH | δ18O‰ | δ2H‰ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shallow aquifer | Min | 319.8 | 136.2 | 498.2 | 22.6 | 142.0 | 1041.9 | 666.0 | 7.5 | 6.1 | 6475.7 | 4220.0 | 6.5 | −3.6 | −26.8 |
| Max | 739.9 | 1267.6 | 5025.5 | 220.5 | 712.6 | 8523.7 | 4877.7 | 45.2 | 39.4 | 44,420.5 | 34,090.0 | 7.7 | −2.3 | −20.6 | |
| Avg | 479.2 | 292.8 | 1328.5 | 64.1 | 448.9 | 2405.7 | 1394.4 | 14.8 | 12.6 | 13,936.1 | 10,108.5 | 6.8 | −3.0 | −23.0 | |
| SD | 479.2 | 292.8 | 1328.5 | 64.1 | 448.9 | 2405.7 | 1394.4 | 14.8 | 12.6 | 13,936.1 | 10,108.5 | 6.8 | 0.4 | 2.2 | |
| Deep aquifer | Min | 129.7 | 51.3 | 237.3 | 9.9 | 136.0 | 401.8 | 294.2 | 10.3 | 1.9 | 3072.5 | 1955.0 | 6.8 | −4.3 | −31.1 |
| Max | 183.4 | 71.5 | 421.4 | 16.4 | 183.4 | 765.8 | 440.5 | 15.9 | 4.3 | 5060.3 | 3308.7 | 8.0 | −3.5 | −25.0 | |
| Avg | 161.6 | 61.7 | 315.3 | 13.1 | 165.7 | 574.3 | 337.9 | 12.8 | 3.0 | 3993.3 | 2544.7 | 7.2 | −3.9 | −28.2 | |
| SD | 161.6 | 61.7 | 315.3 | 13.1 | 165.7 | 574.3 | 337.9 | 12.8 | 3.0 | 3993.3 | 2544.7 | 7.2 | 0.2 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benaafi, M.; Tawabini, B.; Abba, S.I.; Humphrey, J.D.; AL-Areeq, A.M.; Alhulaibi, S.A.; Usman, A.G.; Aljundi, I.H. Integrated Hydrogeological, Hydrochemical, and Isotopic Assessment of Seawater Intrusion into Coastal Aquifers in Al-Qatif Area, Eastern Saudi Arabia. Molecules 2022, 27, 6841. https://doi.org/10.3390/molecules27206841
Benaafi M, Tawabini B, Abba SI, Humphrey JD, AL-Areeq AM, Alhulaibi SA, Usman AG, Aljundi IH. Integrated Hydrogeological, Hydrochemical, and Isotopic Assessment of Seawater Intrusion into Coastal Aquifers in Al-Qatif Area, Eastern Saudi Arabia. Molecules. 2022; 27(20):6841. https://doi.org/10.3390/molecules27206841
Chicago/Turabian StyleBenaafi, Mohammed, Bassam Tawabini, S. I. Abba, John D. Humphrey, Ahmed M. AL-Areeq, Saad A. Alhulaibi, A. G. Usman, and Isam H. Aljundi. 2022. "Integrated Hydrogeological, Hydrochemical, and Isotopic Assessment of Seawater Intrusion into Coastal Aquifers in Al-Qatif Area, Eastern Saudi Arabia" Molecules 27, no. 20: 6841. https://doi.org/10.3390/molecules27206841
APA StyleBenaafi, M., Tawabini, B., Abba, S. I., Humphrey, J. D., AL-Areeq, A. M., Alhulaibi, S. A., Usman, A. G., & Aljundi, I. H. (2022). Integrated Hydrogeological, Hydrochemical, and Isotopic Assessment of Seawater Intrusion into Coastal Aquifers in Al-Qatif Area, Eastern Saudi Arabia. Molecules, 27(20), 6841. https://doi.org/10.3390/molecules27206841








