Improved Mucoadhesion, Permeation and In Vitro Anticancer Potential of Synthesized Thiolated Acacia and Karaya Gum Combination: A Systematic Study
Abstract
1. Introduction
2. Result and Discussion
2.1. Synthesis of Thiolated Polymer Using Combination of Gum Acacia and Gum Karaya: Thiolated Reaction Mixture (TRM)
2.2. Characterization of Synthesized Thiomer (TRM)
2.2.1. Determination of Thiol Group Content (Ellman’s Method)
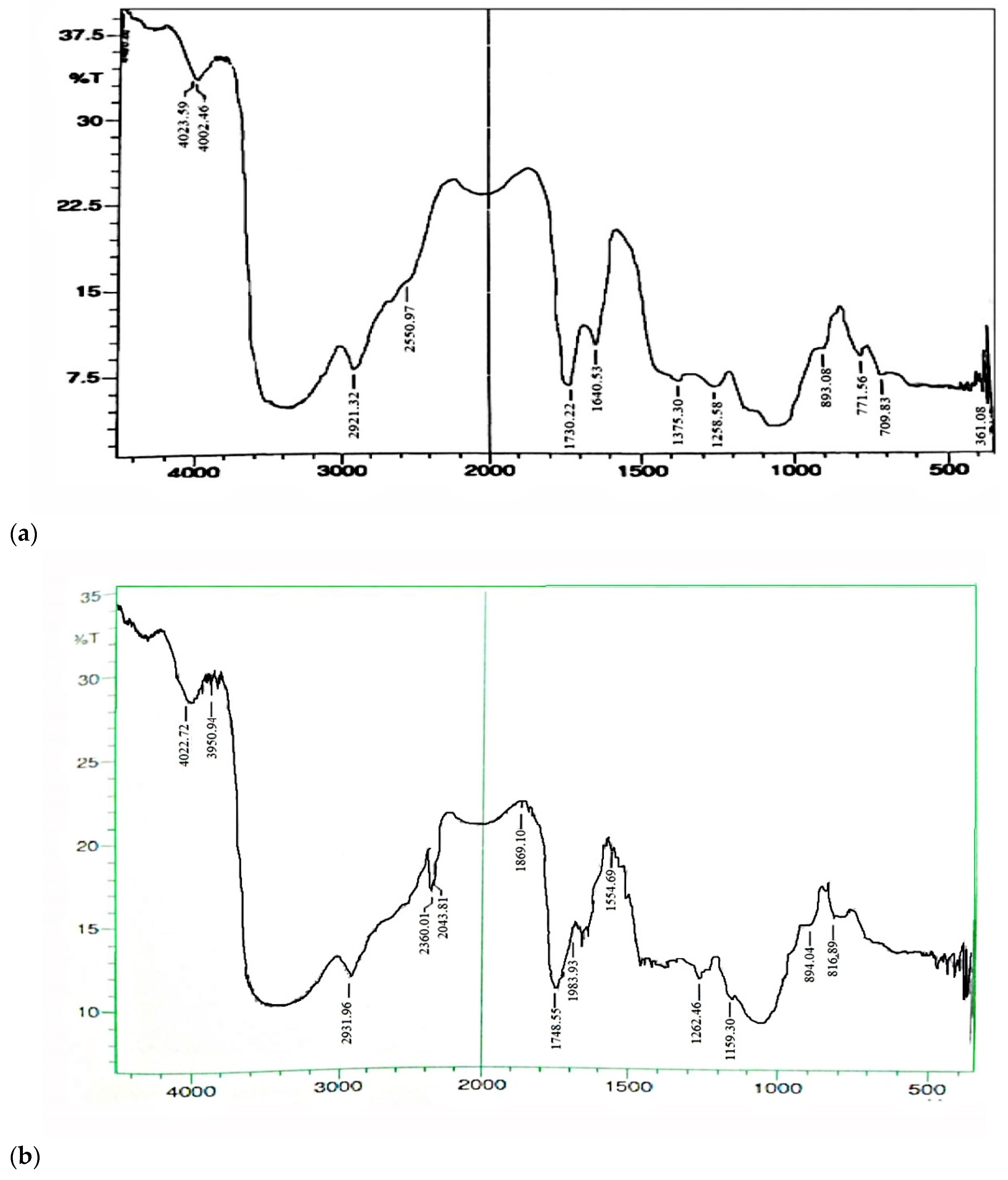
2.2.2. Fourier Transform Infra-Red Spectroscopy (FT-IR)
2.2.3. Differential Scanning Calorimetry (DSC)
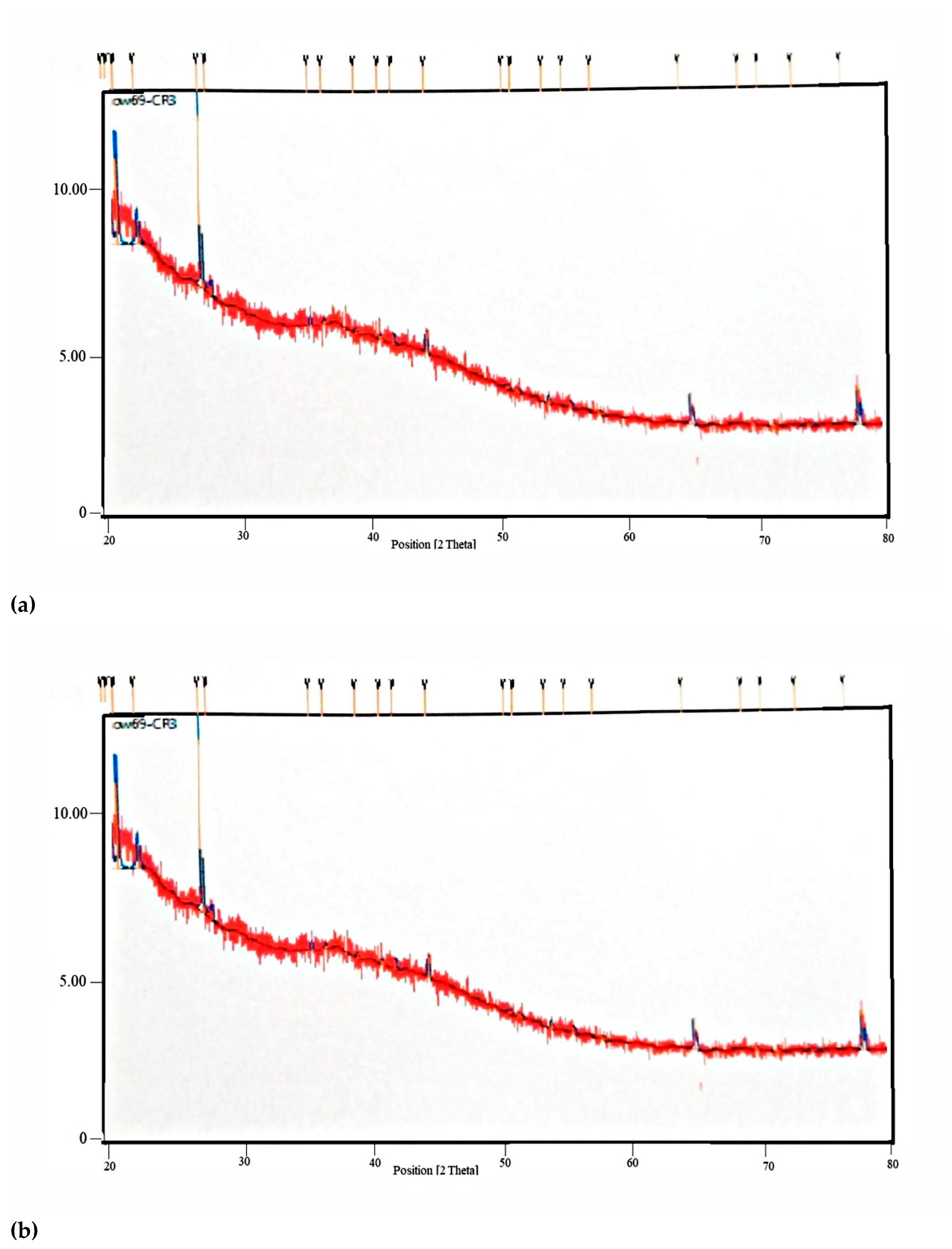
2.2.4. X-ray Diffraction-XRD Studies
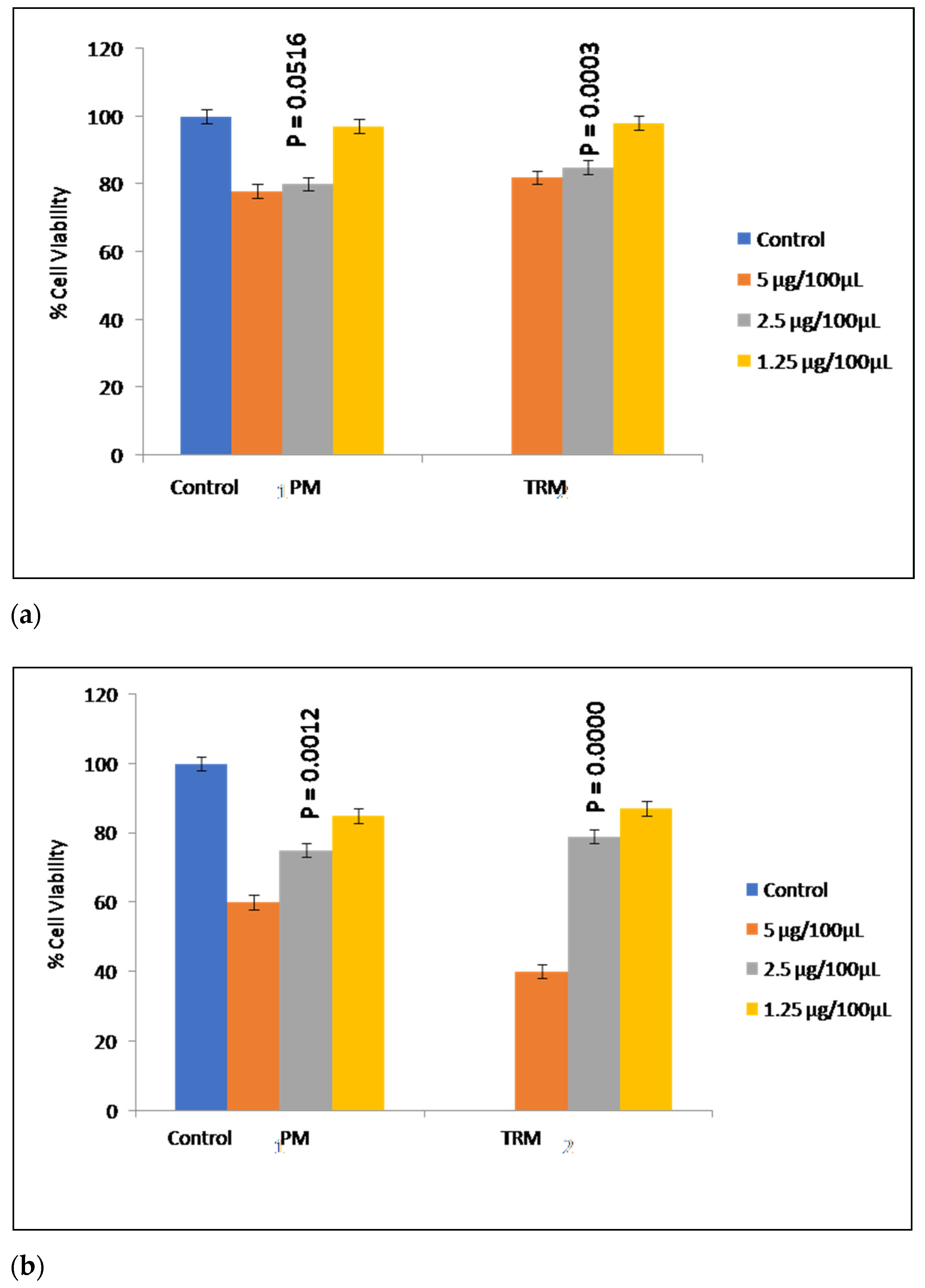
2.2.5. Cytotoxicity Study
2.2.6. Anticancer Activity
2.3. Formulation of Bilayered Buccal Tablets of Ivabradine HCl
2.3.1. Swelling Study
2.3.2. Mucoadhesive Strength
2.3.3. Ex Vivo Residence Time
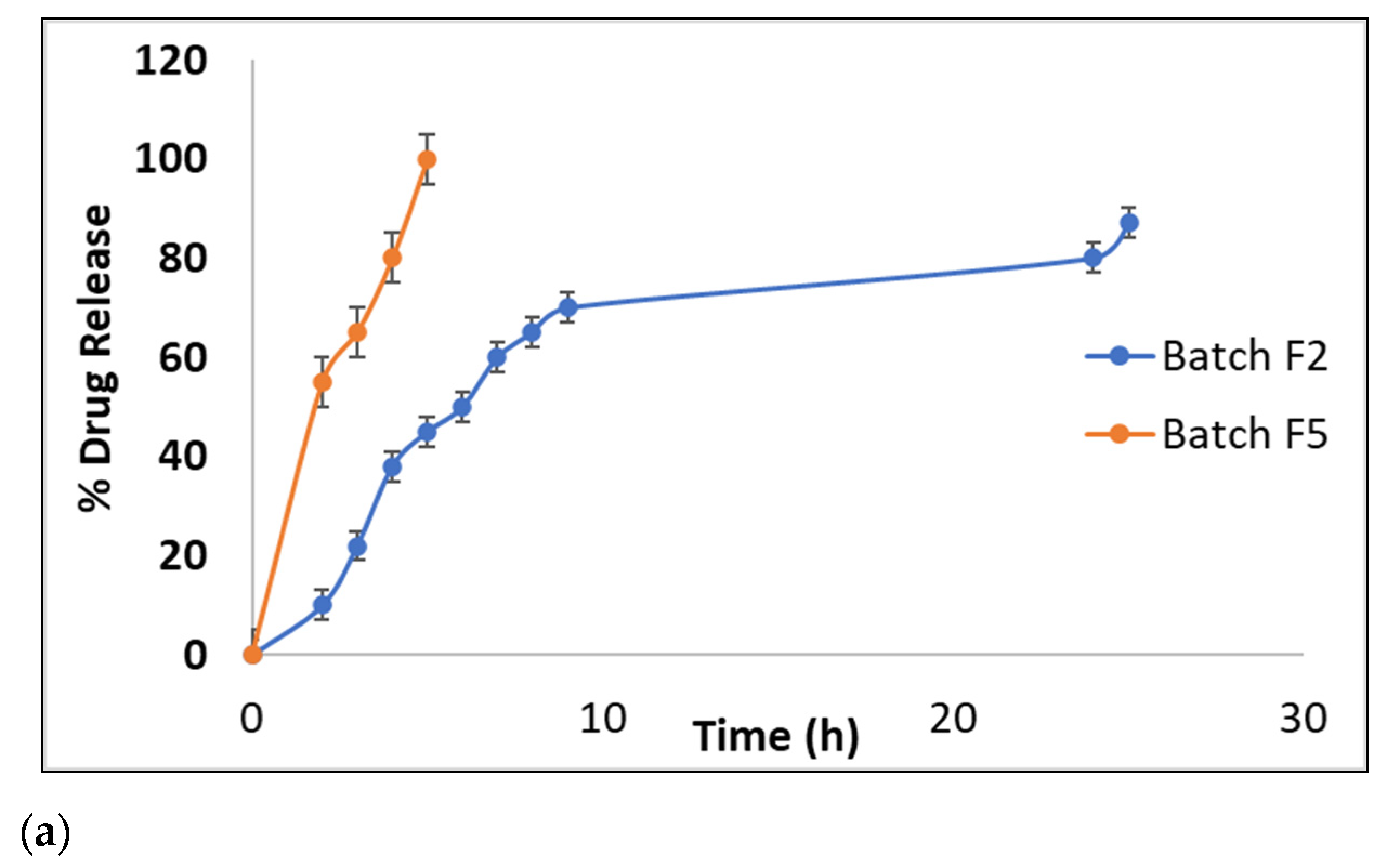
2.3.4. In Vitro Drug Release Study
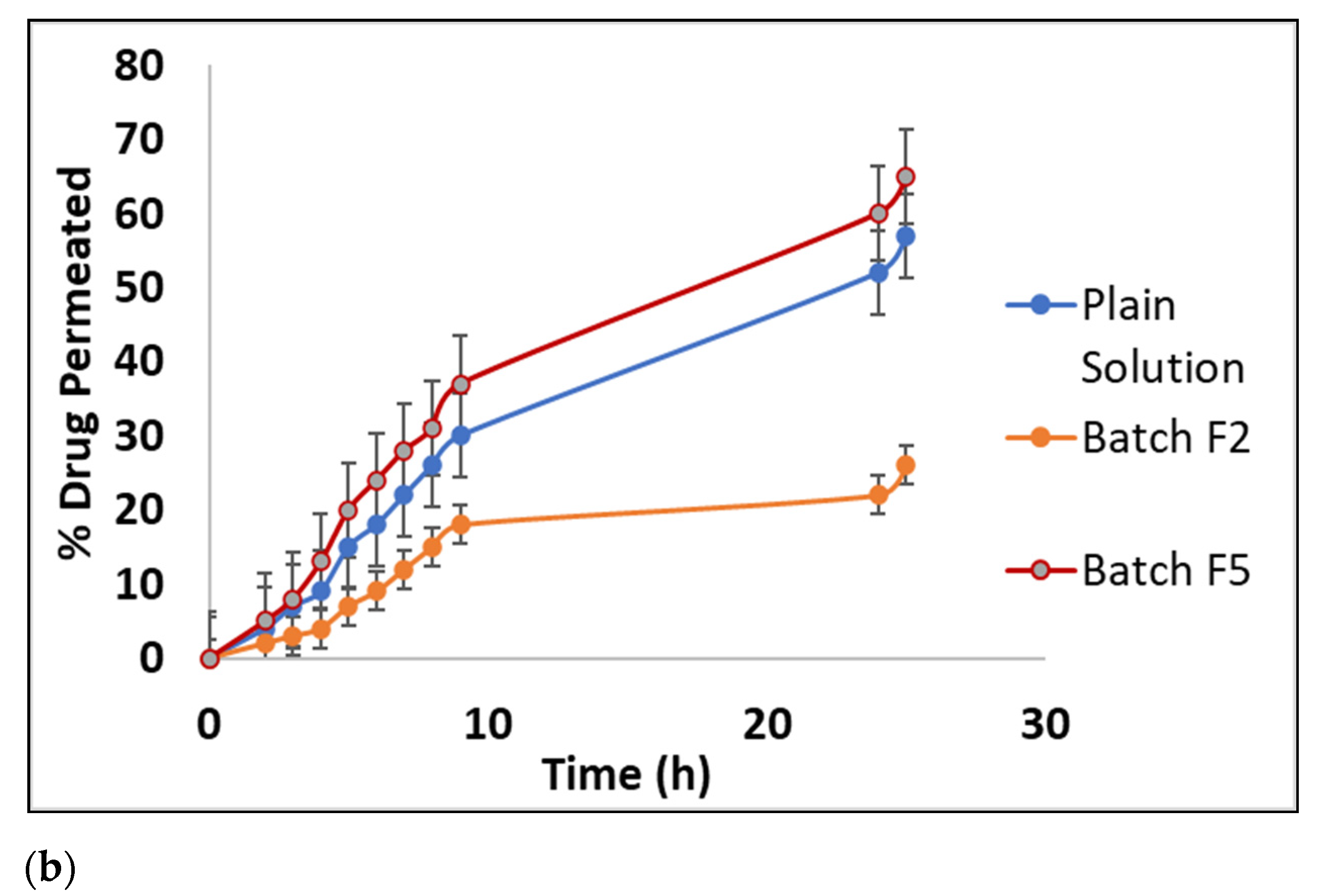
2.3.5. Ex Vivo Permeation of Ivabradine Hydrochloride Form Buccal Tablets
3. Materials and Methods
3.1. Synthesis of Thiolated Polymer Using Combination of Gum Acacia and Gum Karaya: Thiolated Reaction Mixture (TRM)
3.2. Characterization of Synthesized Thiomer (TRM)
3.2.1. Determination of Thiol Group Content (Ellman’s Method)
3.2.2. Fourier Transform Infra-Red Spectroscopy (FT-IR)
3.2.3. Differential Scanning Calorimetry (DSC)
3.2.4. X-ray Diffraction (XRD)
3.2.5. Cytotoxicity Studies
3.2.6. Anticancer Activity
3.3. Formulation of Bilayered Buccal Tablets of Ivabradine HCl
3.4. Evaluation of Bilayer Buccal Tablets of Ivabradine Hydrochloride
3.4.1. Swelling Study
3.4.2. Mucoadhesive Strength
3.4.3. Ex Vivo Residence Time
3.4.4. In Vitro Drug Release Study
3.4.5. Ex Vivo Permeation of Ivabradine Hydrochloride form Buccal Tablets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mansour, H.M.; Sohn, M.; Al-Ghananeem, A.; DeLuca, P.P. Materials for pharmaceutical dosage forms: Molecular pharmaceutics and controlled release drug delivery aspects. Int. J. Mol. Sci. 2010, 11, 3298–3322. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Manjunath, K. Pharmaceutical applications of natural gums, mucilages and pectins—A review. Int. J. Pharm. Chem. Sci. 2013, 2, 1233–1239. [Google Scholar]
- Amiri, M.S.; Mohammadzadeh, V.; Yazdi, M.E.T.; Barani, M.; Rahdar, A.; Kyzas, G.Z. Plant-based gums and mucilages applications in pharmacology and nanomedicine: A review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef] [PubMed]
- Barak, S.; Mudgil, D.; Taneja, S. Exudate gums: Chemistry, properties and food applications—A review. J. Sci. Food Agric. 2020, 100, 2828–2835. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological effects of gum arabic: A review of some recent research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.; Thombare, N.; Sharma, S.C.; Kumar, S. Production, processing, properties and applications of karaya (Sterculia species) gum. Ind. Crops Prod. 2022, 177, 114467. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Applications of natural polymer Gum Arabic: A review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Setia, A.; Goyal, S.; Goyal, N. Applications of gum karaya in drug delivery systems: A review on recent research. Pharm. Lett. 2010, 2, 39–48. [Google Scholar]
- Hassanzadeh-Afruzi, F.; Maleki, A.; Zare, E.N. Novel eco-friendly acacia gum-grafted-polyamidoxime@copper ferrite nanocatalyst for synthesis of pyrazolopyridine derivatives. J. Nanostructure Chem. 2022, 1–12. [Google Scholar] [CrossRef]
- Hassanzadeh-Afruzi, F.; Maleki, A.; Zare, E.N. Efficient remediation of chlorpyrifos pesticide from contaminated water by superparamagnetic adsorbent based on Arabic gum-grafted-polyamidoxime. Int. J. Biol. Macromol. 2022, 203, 445–456. [Google Scholar] [CrossRef]
- Janani, N.; Zare, E.N.; Salimi, F.; Makvandi, P. Antibacterial tragacanth gum-based nanocomposite films carrying ascorbic acid antioxidant for bioactive food packaging. Carbohydr. Polym. 2020, 247, 116678. [Google Scholar] [CrossRef]
- Kumar, A.; Balakrishna, T.; Jash, R.; Murthy, T.E.G.K.; Kumar, A.; Sudheer, B. Formulation and evaluation of mucoadhesive microcapsules of metformin HCl with gum karaya. Int. J. Pharm. Pharm. Sci. 2011, 3, 150–155. [Google Scholar]
- Bahulkar, S.S.; Munot, N.M.; Surwase, S.S. Synthesis, characterization of thiolated karaya gum and evaluation of effect of pH on its mucoadhesive and sustained release properties. Carbohydr. Polym. 2015, 130, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A. Thiomers: A new generation of mucoadhesive polymers. Adv. Drug Deliv. Rev. 2005, 57, 1569–1582. [Google Scholar] [CrossRef]
- Rahmat, D.; Sakloetsakun, D.; Shahnaz, G.; Perera, G.; Kaindl, R.; Bernkop-Schnürch, A. Design and synthesis of a novel cationic thiolated polymer. Int. J. Pharm. 2011, 411, 10–17. [Google Scholar] [CrossRef]
- Viral, H.S.; Pragna, S.; Gaurang, B.S. Design and evaluation of thiolated chitosan based mucoadhesive and permeation enhancing bilayered buccal drug delivery system. Afr. J. Pharm. Pharmacol. 2013, 6, 491–501. [Google Scholar]
- Patel, V.M.; Prajapati, B.G.; Patel, M.M. Formulation, evaluation, and comparison of bilayered and multilayered mucoadhesive buccal devices of propranolol hydrochloride. AAPS Pharm. Sci. Tech. 2007, 8, E147–E154. [Google Scholar] [CrossRef] [PubMed]
- Thekkae Padil, V.V.; Černík, M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013, 8, 889–898. [Google Scholar]
- Bashir, M.; Haripriya, S. Assessment of physical and structural characteristics of almond gum. Int. J. Biol. Macromol. 2016, 93, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Munir, H.; Mumtaz, A.; Rashid, R.; Najeeb, J.; Zubair, M.T.; Munir, S.; Bilal, M.; Cheng, H. Eucalyptus camaldulensis gum as a green matrix to fabrication of zinc and silver nanoparticles: Characterization and novel prospects as antimicrobial and dye-degrading agents. J. Mater. Res. Technol. 2020, 9, 15513–15524. [Google Scholar] [CrossRef]
- Freitas, A.A.R.; Ribeiro, A.J.; Santos, A.C.; Veiga, F.; Nunes, L.C.C.; Silva, D.A.; Soares-Sobrinho, J.L.; Silva-Filho, E.C. Sterculia striata gum as a potential oral delivery system for protein drugs. Int. J. Biol. Macromol. 2020, 164, 1683–1692. [Google Scholar] [CrossRef]
- Naveen, N.R.; Gopinath, C.; Kurakula, M. Okra-Thioglycolic acid conjugate—Synthesis, characterization, and evaluation as a mucoadhesive polymer. Processes 2020, 8, 316. [Google Scholar] [CrossRef]
- Jindal, A.B.; Wasnik, M.N.; Nair, H.A. Synthesis of thiolated alginate and evaluation of mucoadhesiveness, cytotoxicity and release retardant properties. Indian J. Pharm. Sci. 2010, 72, 766–774. [Google Scholar] [PubMed]
- Lin, R.K.; Zhou, N.; Lyu, Y.L.; Tsai, Y.-C.; Lu, C.-H.; Kerrigan, J.; Chen, Y.-T.; Guan, Z.; Hsieh, T.-S.; Liu, L.F. Dietary isothiocyanate-induced apoptosis via thiol modification of DNA topoisomerase IIα. J. Biol. Chem. 2011, 286, 33591–33600. [Google Scholar] [CrossRef] [PubMed]
- Kafedjiiski, K.; Hoffer, M.; Werle, M.; Bernkop-Schnürch, A. Improved synthesis and in vitro characterization of chitosan–thioethylamidine conjugate. Biomaterials 2006, 27, 127–135. [Google Scholar] [CrossRef]
- Khan, H.; Khan, K.A.; Khalid Khan, M.; Ahmad, A.; Niazi, Z.; Mangi, A.A.; Rehman, F.; Shah, K.U.; Gul, R. Thiol-disulfide exchange reactions occurring at modified bovine serum albumin detected using ellman’s reagent (5,5′-dithiobis (2-itrobenzoic acid). Pak. J. Pharm. Sci. 2020, 33, 2767–2772. [Google Scholar] [PubMed]
- Puri, V.; Sharma, A.; Kumar, P.; Singh, I.; Huanbutta, K. Synthesis and characterization of thiolated gum ghatti as a novel excipient: Development of compression-coated mucoadhesive tablets of domperidone. ACS Omega 2021, 6, 15844–15854. [Google Scholar] [CrossRef]
- Kandekar, U.Y.; Tapkir, M.H.; Bhalerao, P.H.; Rukhe, N.B.; Kad, S.K. Colocasia esculenta starch: Novel alternative disintegrant for pharmaceutical application. Indian Drugs 2021, 58, 41–53. [Google Scholar] [CrossRef]
- Jahan, F.; Zaman, S.U.; Arshad, R.; Tabish, T.A.; Naseem, A.A.; Shahnaz, G. Mapping the potential of thiolated pluronic based nanomicelles for the safe and targeted delivery of vancomycin against staphylococcal blepharitis. J. Drug Deliv. Sci. Technol. 2021, 61, 102220. [Google Scholar] [CrossRef]
- Devi, R.; Bhatia, M. Thiol functionalization of flaxseed mucilage: Preparation, characterization and evaluation as mucoadhesive polymer. Int. J. Biol. Macromol. 2019, 126, 101–106. [Google Scholar] [CrossRef]
- Salleh, N.F.A.M.; Azlina, A.; Harun, M.H.; Al-Tayar, B.A.; Hashim, S.N.M.; Yusoff, M.E.; Mohamad, N.K.; Abdullah, S.F.; Zainal, S.A. Cytotoxicity and morphological effects of aqueous areca nut crude extract on L929 fibroblast cell line. Asian J. Med. Biomed. 2020, 4, 34–41. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Kumar, P. Preparation and evaluation of a novel buccal adhesive system. AAPS Pharm. Sci. Tech. 2004, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Wroblewska, M.; Trofimiuk, M.; Basa, A.; Winnicka, K. Alginate oligosaccharides affect mechanical properties and antifungal activity of alginate buccal films with Posaconazole. Mar. Drugs 2019, 17, 692. [Google Scholar] [CrossRef] [PubMed]
- Samanthula, K.S.; Kumar CB, M.; Bairi, A.G.; Satla, S.R. Development, in-vitro and ex-vivo evaluation of muco-adhesive buccal tablets of hydralazine hydrochloride. Braz. J. Pharm. Sci. 2022, 58, e18635. [Google Scholar] [CrossRef]
- Patil, A.P.; Disouza, J.D.; Pawar, S.H. Evaluation of Lactobacillus plantarum growth in milk of Indian buffalo breeds based on its physico-chemical content. Buffalo Bull. 2019, 38, 345–352. [Google Scholar]
- Patil, A.P.; Disouza, J.D.; Pawar, S.H. Health benefits of Probiotics by Antioxidant Activity: A review. Pharma Times 2018, 50, 1–3. [Google Scholar]


| Batches | Hardness (kg/cm2) | Swelling Index | Mucoadhesive Strength (gm) | Force of Adhesion (N) |
|---|---|---|---|---|
| F1 | 4.5 | 53.67% ± 1.2 * | 54.41 ± 0.5 | 0.5337 |
| F2 | 4.5 | 58.54% ± 1.4 * | 57.45 ± 0.6 | 0.5635 |
| F3 | 4.8 | 58.52% ± 1.3 * | 60.14 ± 0.4 | 0.5899 |
| F4 | 4.5 | 77.54% ± 1.4 # | 79.28 ± 0.3 | 0.7801 |
| F5 | 4.8 | 86.02% ± 1.3 # | 99.78 ± 0.2 | 0.9788 |
| F6 | 4.8 | 80.35% ± 1.2 # | 90.54 ± 0.4 | 0.8881 |
| Batch | Polymer | Polymer (mg) | Drug (mg) | Ethyl Cellulose (mg) | Total Weight of Tablet (mg) |
|---|---|---|---|---|---|
| F1 | PM | 59 | 11 | 180 | 250 |
| F2 | PM | 89 | 11 | 150 | 250 |
| F3 | PM | 119 | 11 | 120 | 250 |
| F4 | TRM | 59 | 11 | 180 | 250 |
| F5 | TRM | 89 | 11 | 150 | 250 |
| F6 | TRM | 119 | 11 | 120 | 250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munot, N.; Kandekar, U.; Rikame, C.; Patil, A.; Sengupta, P.; Urooj, S.; Bilal, A. Improved Mucoadhesion, Permeation and In Vitro Anticancer Potential of Synthesized Thiolated Acacia and Karaya Gum Combination: A Systematic Study. Molecules 2022, 27, 6829. https://doi.org/10.3390/molecules27206829
Munot N, Kandekar U, Rikame C, Patil A, Sengupta P, Urooj S, Bilal A. Improved Mucoadhesion, Permeation and In Vitro Anticancer Potential of Synthesized Thiolated Acacia and Karaya Gum Combination: A Systematic Study. Molecules. 2022; 27(20):6829. https://doi.org/10.3390/molecules27206829
Chicago/Turabian StyleMunot, Neha, Ujjwala Kandekar, Chaitali Rikame, Abhinandan Patil, Poulomi Sengupta, Shabana Urooj, and Anusha Bilal. 2022. "Improved Mucoadhesion, Permeation and In Vitro Anticancer Potential of Synthesized Thiolated Acacia and Karaya Gum Combination: A Systematic Study" Molecules 27, no. 20: 6829. https://doi.org/10.3390/molecules27206829
APA StyleMunot, N., Kandekar, U., Rikame, C., Patil, A., Sengupta, P., Urooj, S., & Bilal, A. (2022). Improved Mucoadhesion, Permeation and In Vitro Anticancer Potential of Synthesized Thiolated Acacia and Karaya Gum Combination: A Systematic Study. Molecules, 27(20), 6829. https://doi.org/10.3390/molecules27206829








