Bioactivity-Guided Fractionation and Identification of Antidiabetic Compound of Syzygium polyanthum (Wight.)’s Leaf Extract in Streptozotocin-Induced Diabetic Rat Model
Abstract
1. Introduction
2. Results and Discussion
2.1. Gas Chromatography–Mass Spectrometry Analysis
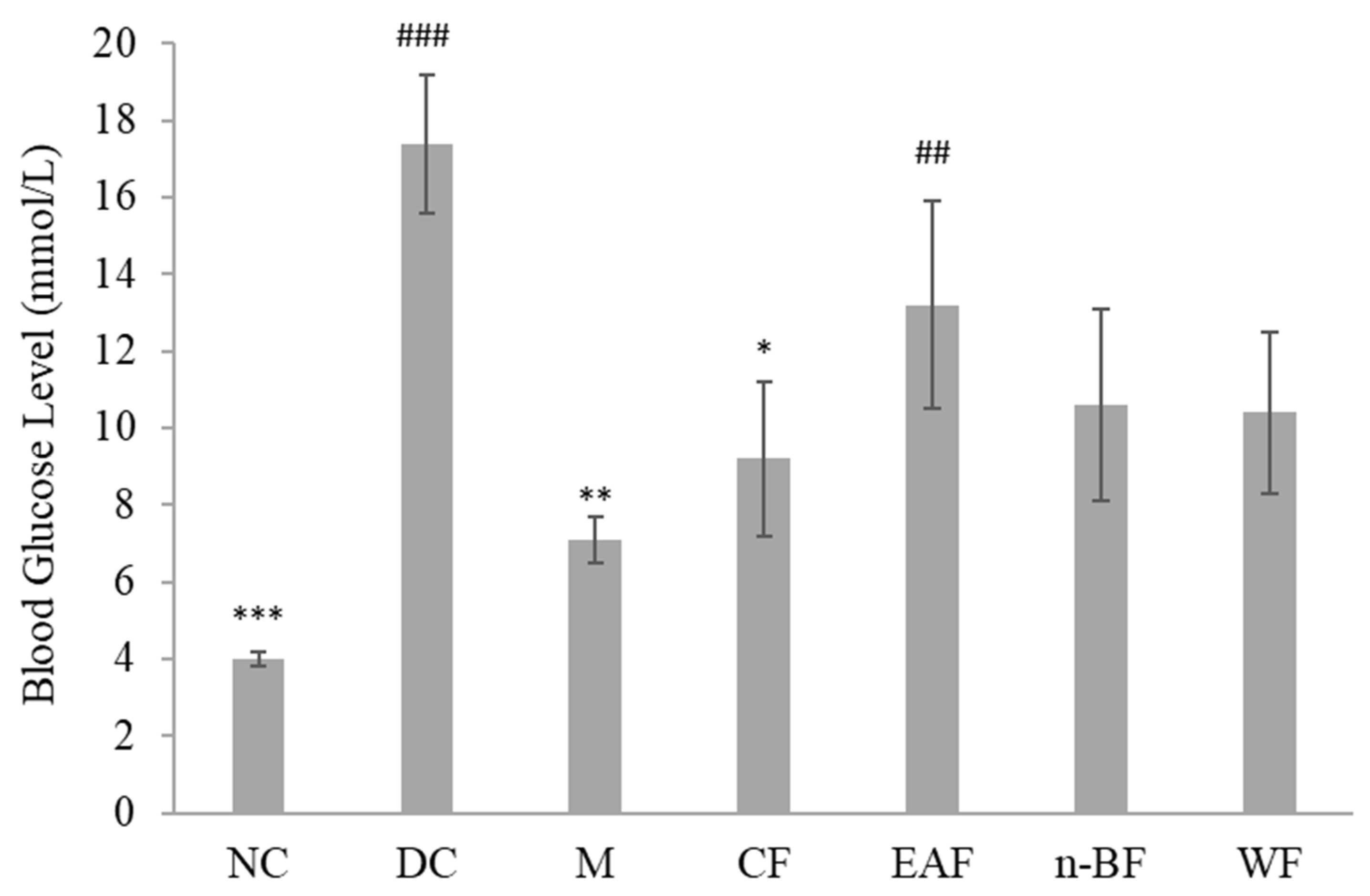
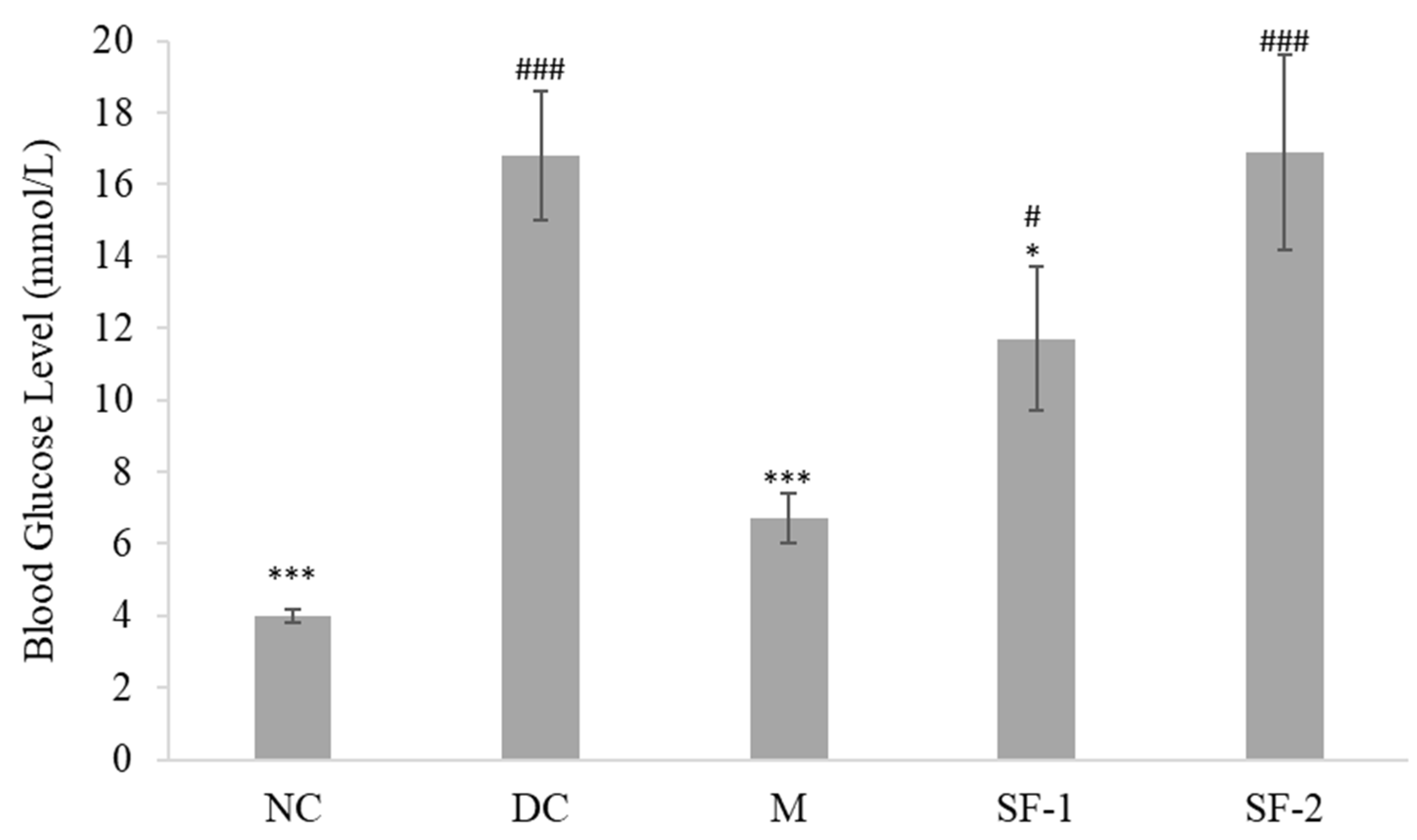
2.2. Effect on Blood Glucose Level
2.3. Effect on Body Weight
2.4. Effect on Lipid Profile
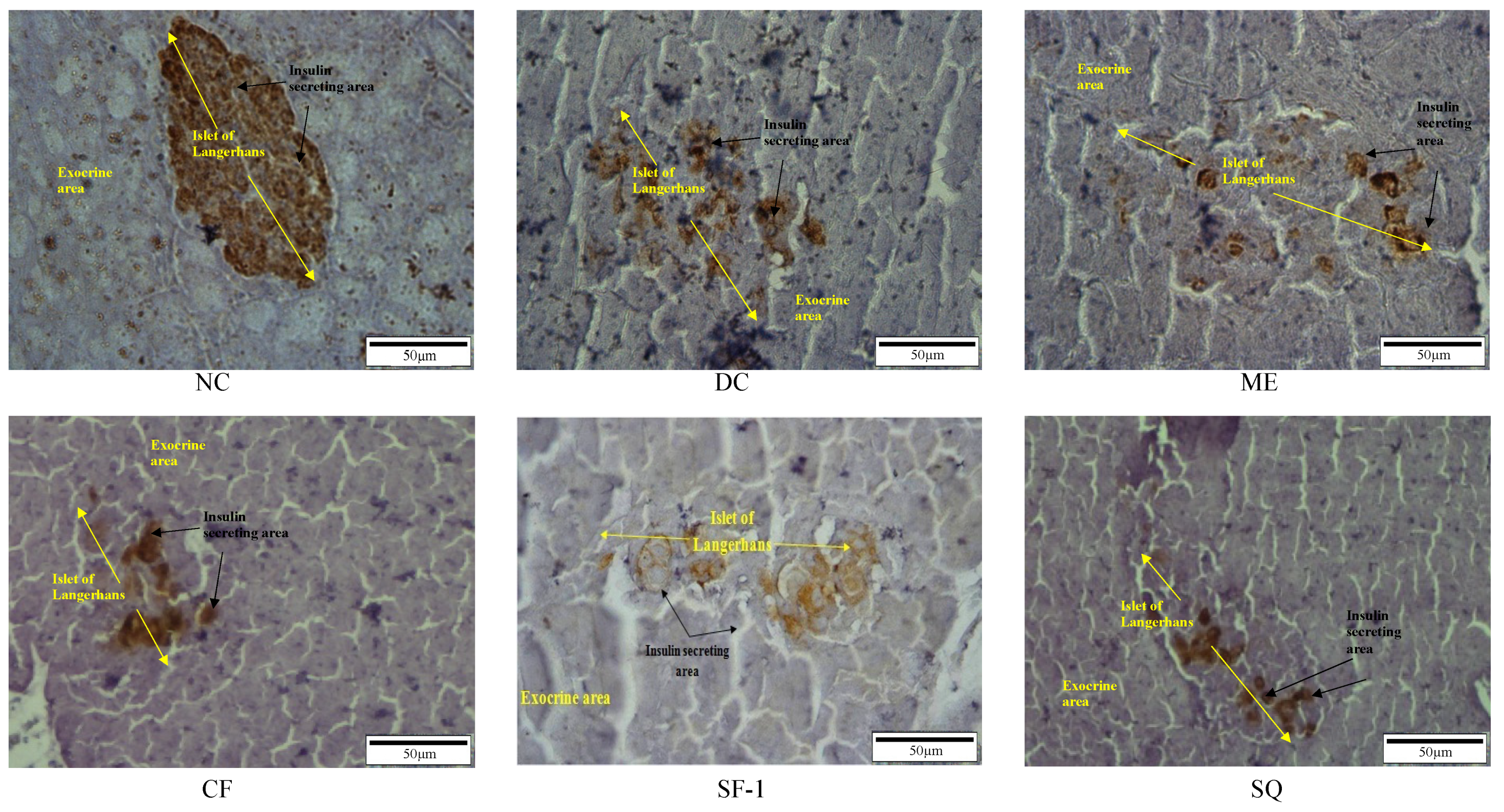
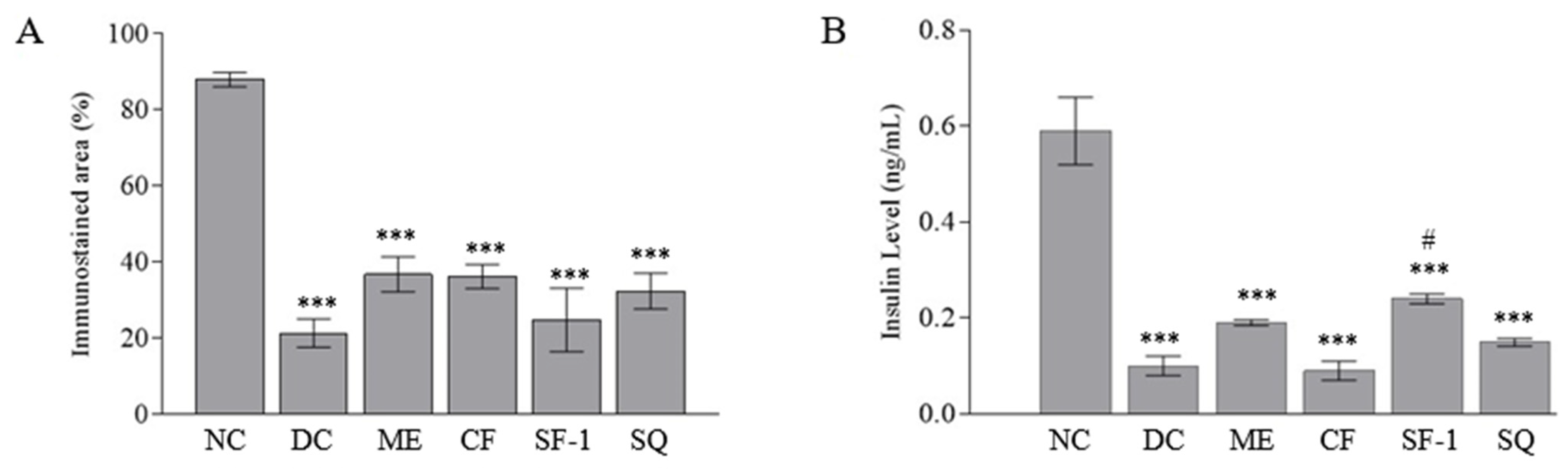
2.5. Insulin Level and Immunohistochemistry Assessment
2.6. Molecular Docking Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Plant Collection and Identification
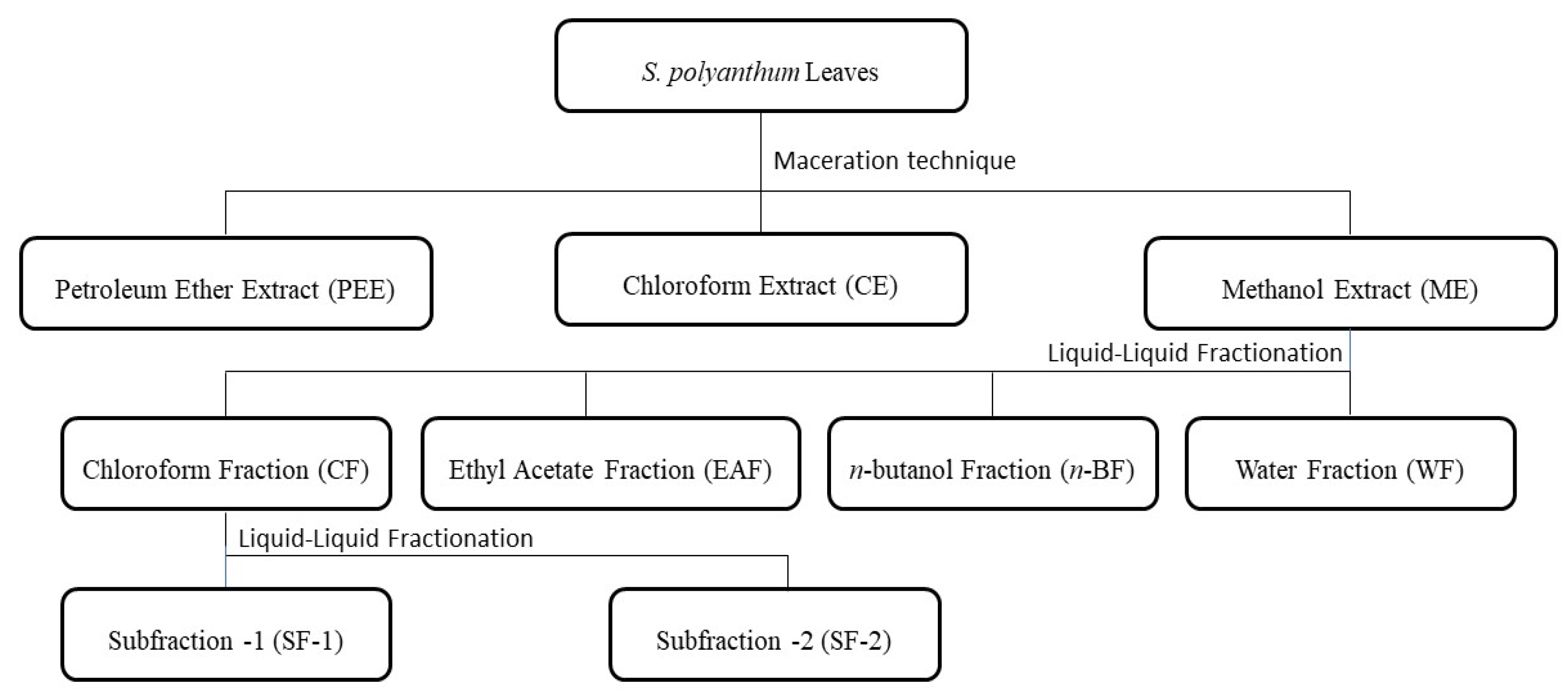
3.3. Preparation of Samples
3.4. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
3.5. In Vivo Antihyperglycemic Studies
3.5.1. Induction of Type 1 Diabetes Mellitus in Rats
3.5.2. Bioassay-Guided Antihyperglycemic Activity of S. polyanthum
3.5.3. Measurement of Fasting Blood Glucose
3.5.4. Measurement of Serum Insulin Level, Lipid Profile, and Body Weight
3.5.5. Immunohistochemistry Study of Pancreas
3.6. Molecular Docking Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| n-BF | n-butanol fraction |
| CF | Chloroform extract |
| Chol | Cholesterol |
| DC | Diabetic control |
| EAF | Ethyl acetate fraction |
| GCMS | Gas chromatography–mass spectrometry |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| M | Metformin |
| ME | Methanol extract |
| NC | Normal control |
| RT | Retention time |
| SEM | Standard error mean |
| SF-1 | Subfraction-1 |
| SF-2 | Subfraction-2 |
| SQ | Squalene |
| TG | Triglycerides |
| WF | Water fraction |
References
- Dandekar, P.; Ramkumar, S.; RaviKumar, A. Chapter 12—Structure–Activity Relationships of Pancreatic α-Amylase and α-Glucosidase as Antidiabetic Targets. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 381–410. ISBN 9780323910989. [Google Scholar]
- Mouri, M.; Badireddy, M. Hyperglycemia. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430900/ (accessed on 1 June 2022).
- Kaul, K.; Tarr, J.M.; Ahmad, S.I.; Kohner, E.M.; Chibber, R. Introduction to Diabetes Mellitus. Adv. Exp. Med. Biol. 2012, 771, 1–11. [Google Scholar] [CrossRef] [PubMed]
- IDF. IDF Diabetes Atlas, 10th Edition. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 14 September 2022).
- Veeresham, C. Natural Products Derived from Plants as a Source of Drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200. [Google Scholar] [CrossRef]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Das, S.K.; Samantaray, D.; Sahoo, S.K.; Pradhan, S.K.; Samanta, L.; Thatoi, H. Bioactivity Guided Isolation of Antidiabetic and Antioxidant Compound from Xylocarpus Granatum, J. Koenig Bark. 3 Biotech 2019, 9, 198–206. [Google Scholar] [CrossRef] [PubMed]
- El Deeb, K.S.; Eid, H.H.; Ali, Z.Y.; Shams, M.M.; Elfiky, A.M. Bioassay-Guided Fractionation and Identification of Antidiabetic Compounds from the Rind of Punica granatum Var. nana. Nat. Prod. Res. 2019, 35, 2103–2106. [Google Scholar] [CrossRef] [PubMed]
- Phukhatmuen, P.; Raksat, A.; Laphookhieo, S.; Charoensup, R.; Duangyod, T.; Maneerat, W. Bioassay-Guided Isolation and Identification of Antidiabetic Compounds from Garcinia Cowa Leaf Extract. Heliyon 2020, 6, e03625. [Google Scholar] [CrossRef]
- Milow, P.; Ramli, M.R.; Chooi, O.H. Preliminary Survey on Plants in Home Gardens in Pahang, Malaysia. J. Biodivers. 2017, 1, 19–25. [Google Scholar] [CrossRef]
- Widyawati, T.; Purnawan, W.W.; Atangwho, I.J.; Yusoff, N.A.; Ahmad, M.; Asmawi, M.Z. Antidiabetic Activity of Syzygium polyanthum (Wight) Leaf Extract, the Most Commonly Used Herb among Diabetic Patients in Medan, North Sumatera, Indonesia. Int. J. Pharm. Sci. Res. 2015, 6, 1698–1704. [Google Scholar]
- Widyawati, T.; Yusoff, N.A.; Asmawi, M.Z.; Ahmad, M. Antihyperglycemic Effect of Methanol Extract of Syzygium polyanthum (Wight.) Leaf in Streptozotocin-Induced Diabetic Rats. Nutrients 2015, 7, 7764–7780. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hosokawa, M.; Miyashita, K.; Liu, S.; Hosokawa, M.; Miyashita, K. Dietary Effect of Squalene on Lipid Metabolism of Obese/Diabetes KK-Ay Mice and Wild-Type C57BL/6J Mice. Food Nutr. Sci. 2018, 9, 1498–1513. [Google Scholar] [CrossRef]
- Mirmiranpour, H.; Ashoori, M.R.; Mikaeili, A.S.; Pezeshki, S.; Serani, A.; Vassar, R.; Martirosyan, D. The Effect of Squalene on Lipid Profile and Some Oxidative Biomarkers in Patients with Type 2 Diabetes Mellitus. Funct. Food Sci. 2022, 2, 144–156. [Google Scholar] [CrossRef]
- Song, X.; Xiao, H.; Luo, S.; Wang, X.; Wang, W.; Lin, S. Biosynthesis of Squalene-Type Triterpenoids in Saccharomyces Cerevisiae by Expression of CYP505D13 from Ganoderma Lucidum. Bioresour. Bioprocess. 2019, 6, 19. [Google Scholar] [CrossRef]
- Wang, F.; Li, H.; Zhao, H.; Zhang, Y.; Qiu, P.; Li, J.; Wang, S. Antidiabetic Activity and Chemical Composition of Sanbai Melon Seed Oil. Evid.-Based Complement. Altern. Med. 2018, 2018, 5434156. [Google Scholar] [CrossRef]
- Ravi Kumar, S.; Yamauchi, I.; Narayan, B.; Katsuki, A.; Hosokawa, M.; Miyashita, K. Squalene Modulates Fatty Acid Metabolism: Enhanced EPA/DHA in Obese/Diabetic Mice (KK-Ay) Model. Eur. J. Lipid Sci. Technol. 2016, 118, 1935–1941. [Google Scholar] [CrossRef]
- Sutrisna, E.; Trisharyanti, I.; Munawaroh, R. Antioxidant and Antidiabetic Activity of 70% Ethanolic Extract of Syzygium polyanthum (Wight) Leaf from Indonesia. Int. J. Res. Ayurveda Pharm. 2016, 7, 214–216. [Google Scholar] [CrossRef]
- Hikmah, N.; Yuliet, Y.; Khaerati, K. Effect of Administration of Bay Leaf Extract (Syzygium polyanthum Wight.) on Glibenclamide in Reducing Blood Glucose Levels of Mice (Mus Musculus) Induced by Alloxan. J. Farm. Galen. (Galen. J. Pharm.) 2016, 2, 24–30. [Google Scholar] [CrossRef]
- Wijaya, S.H.; Fadillah, C.; Kusuma, W.A. Prediction of Synergistic Effect between Multiple Compounds Related to Diabetes Mellitus. IOP Conf. Ser. Earth Environ. Sci. 2019, 299, 012038. [Google Scholar] [CrossRef]
- Lynch, K.L. Toxicology: Liquid Chromatography Mass Spectrometry. In Mass Spectrometry for the Clinical Laboratory; Nair, H., Clarke, W., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 109–130. [Google Scholar]
- Aldekhail, N.M.N.P. Pharmacotherapy and Weight Management: Efficacy and Clinical Effectiveness in Patients with Obesity and Type 2 Diabetes; University of Glasgow: Glasgow, Scotland, UK, 2018. [Google Scholar]
- Raj, R.; Bhatti, J.S.; Badada, S.K.; Ramteke, P.W. Genetic Basis of Dyslipidemia in Disease Precipitation of Coronary Artery Disease (CAD) Associated Type 2 Diabetes Mellitus (T2DM). Diabetes. Metab. Res. Rev. 2015, 31, 663–671. [Google Scholar] [CrossRef]
- Holmes, A.; Coppey, L.J.; Davidson, E.P.; Yorek, M.A. Rat Models of Diet-Induced Obesity and High Fat/Low Dose Streptozotocin Type 2 Diabetes: Effect of Reversal of High Fat Diet Compared to Treatment with Enalapril or Menhaden Oil on Glucose Utilization and Neuropathic Endpoints. J. Diabetes Res. 2015, 2015, 307285. [Google Scholar] [CrossRef]
- Pournaghi, P.; Sadrkhanlou, R.-A.; Hasanzadeh, S.; Foroughi, A. An Investigation on Body Weights, Blood Glucose Levels and Pituitary-Gonadal Axis Hormones in Diabetic and Metformin-Treated Diabetic Female Rats. Vet. Res. Forum 2012, 3, 79–84. [Google Scholar]
- Hossein Dashti-Rahmatabadi, M.; Morshedi, A. Chronic Consumption of Kombucha and Black Tea Prevents Weight Loss in Diabetic Rats. Iran. J. Diabetes Obes. 2010, 2, 23–26. [Google Scholar]
- Taher, M.; Tg Zakaria, T.M.F.S.; Susanti, D.; Zakaria, Z.A. Hypoglycaemic Activity of Ethanolic Extract of Garcinia Mangostana Linn. in Normoglycaemic and Streptozotocin-Induced Diabetic Rats. BMC Complement. Altern. Med. 2016, 16, 135. [Google Scholar] [CrossRef]
- Azad, A.K.; Azizi, W.M.; Sulaiman, W. Antidiabetic Effects of P. Macrocarpa Ethanolic Fruit Extract in Streptozotocin-Induced Diabetic Rats. Futur. J. Pharm. Sci. 2020, 6, 57. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Bi, D.; Wang, X.; ZHang, X.; Dai, H.; Chen, S.; Zhang, W. Influence of Squalene Feeding on Plasma Leptin, Testosterone & Blood Pressure in Rats—PubMed. Indian J. Med. Res. 2009, 129, 150–153. [Google Scholar]
- Gabás-Rivera, C.; Barranquero, C.; Martínez-Beamonte, R.; Navarro, M.A.; Surra, J.C.; Osada, J. Dietary Squalene Increases High Density Lipoprotein-Cholesterol and Paraoxonase 1 and Decreases Oxidative Stress in Mice. PLoS ONE 2014, 9, e104224. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mishra, M.; Kowluru, A.; Kumar, B. Hyperlipidemia and the Development of Diabetic Retinopathy: Comparison between Type 1 and Type 2 Animal Models. Metabolism 2016, 65, 1570. [Google Scholar] [CrossRef]
- Vergès, B. Dyslipidemia in Type 1 Diabetes: A Masked Danger. Trends Endocrinol. Metab. 2020, 31, 422–434. [Google Scholar] [CrossRef]
- Semova, I.; Levenson, A.E.; Krawczyk, J.; Bullock, K.; Gearing, M.E.; Ling, A.V.; Williams, K.A.; Miao, J.; Adamson, S.S.; Shin, D.J.; et al. Insulin Prevents Hypercholesterolemia by Suppressing 12-Hydroxylated Bile Acids. Circulation 2022, 145, 969–982. [Google Scholar] [CrossRef]
- Khandelwal, P.; Khanna, S. Diabetic Peripheral Neuropathy: An Insight into the Pathophysiology, Diagnosis, and Therapeutics. In Wound Healing, Tissue Repair, and Regeneration in Diabetes; Academic Press: Cambridge, MA, USA, 2020; pp. 49–77. [Google Scholar] [CrossRef]
- Bharti, S.K.; Kumar, A.; Sharma, N.K.; Prakash, O.; Jaiswal, S.K.; Krishnan, S.; Gupta, A.K.; Kumar, A. Tocopherol from Seeds of Cucurbita Pepo against Diabetes: Validation by In Vivo Experiments Supported by Computational Docking. J. Formos. Med. Assoc. 2013, 112, 676–690. [Google Scholar] [CrossRef]
- Guasch, L.; Sala, E.; Mulero, M.; Valls, C.; Salvadó, M.J.; Pujadas, G.; Garcia-Vallvé, S. Identification of PPARgamma Partial Agonists of Natural Origin (II): In Silico Prediction in Natural Extracts with Known Antidiabetic Activity. PLoS ONE 2013, 8, e55889. [Google Scholar] [CrossRef]
- Kumar, R.; Ramachandran, U.; Khanna, S.; Bharatam, P.V.; Raichur, S.; Chakrabarti, R. Synthesis, In Vitro and In Silico Evaluation of l-Tyrosine Containing PPARalpha/Gamma Dual Agonists. Bioorg. Med. Chem. 2007, 15, 1547–1555. [Google Scholar] [CrossRef]
- Yusoff, N.A.; Lim, V.; Al-Hindi, B.; Razak, K.N.A.; Widyawati, T.; Anggraini, D.R.; Ahmad, M.; Asmawi, M.Z. Nypa Fruticans Wurmb. Vinegar’s Aqueous Extract Stimulates Insulin Secretion and Exerts Hepatoprotective Effect on STZ-Induced Diabetic Rats. Nutrients 2017, 9, 925. [Google Scholar] [CrossRef]
- Rao, M.M.V.; Hariprasad, T.P.N. In Silico Analysis of a Potential Antidiabetic Phytochemical Erythrin against Therapeutic Targets of Diabetes. Silico Pharmacol. 2021, 9, 5. [Google Scholar] [CrossRef]





| Samples | RT * | Peak (%) | Compound Name | Molecular Formula |
|---|---|---|---|---|
| ME | 14.96 | 7.60 | Squalene | C30H50 |
| CF | 10.74 | 6.90 | Hexadecanoic acid, methyl ester | C17H34O2 |
| 11.49 | 2.03 | 9,12-Octadecadienoic acid, methyl ester | C19H34O2 | |
| 14.97 | 8.92 | Squalene | C30H50 | |
| 18.37 | 4.73 | Vitamin E | C29H50O2 | |
| 21.66 | 22.57 | Stigmasterol, 22,23-dihydro- | C29H50O | |
| SF-1 | 14.92 | 4.54 | Squalene | C30H50 |
| 18.28 | 4.26 | Vitamin E | C29H50O2 | |
| 21.50 | 33.37 | Stigmasterol, 22,23-dihydro- | C29H50O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widyawati, T.; Yusoff, N.A.; Bello, I.; Asmawi, M.Z.; Ahmad, M. Bioactivity-Guided Fractionation and Identification of Antidiabetic Compound of Syzygium polyanthum (Wight.)’s Leaf Extract in Streptozotocin-Induced Diabetic Rat Model. Molecules 2022, 27, 6814. https://doi.org/10.3390/molecules27206814
Widyawati T, Yusoff NA, Bello I, Asmawi MZ, Ahmad M. Bioactivity-Guided Fractionation and Identification of Antidiabetic Compound of Syzygium polyanthum (Wight.)’s Leaf Extract in Streptozotocin-Induced Diabetic Rat Model. Molecules. 2022; 27(20):6814. https://doi.org/10.3390/molecules27206814
Chicago/Turabian StyleWidyawati, Tri, Nor Adlin Yusoff, Idris Bello, Mohd Zaini Asmawi, and Mariam Ahmad. 2022. "Bioactivity-Guided Fractionation and Identification of Antidiabetic Compound of Syzygium polyanthum (Wight.)’s Leaf Extract in Streptozotocin-Induced Diabetic Rat Model" Molecules 27, no. 20: 6814. https://doi.org/10.3390/molecules27206814
APA StyleWidyawati, T., Yusoff, N. A., Bello, I., Asmawi, M. Z., & Ahmad, M. (2022). Bioactivity-Guided Fractionation and Identification of Antidiabetic Compound of Syzygium polyanthum (Wight.)’s Leaf Extract in Streptozotocin-Induced Diabetic Rat Model. Molecules, 27(20), 6814. https://doi.org/10.3390/molecules27206814






