Effect-Directed Profiling of Strawberry Varieties and Breeding Materials via Planar Chromatography and Chemometrics
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPTLC Profiling
2.2. Effect-Directed Analysis
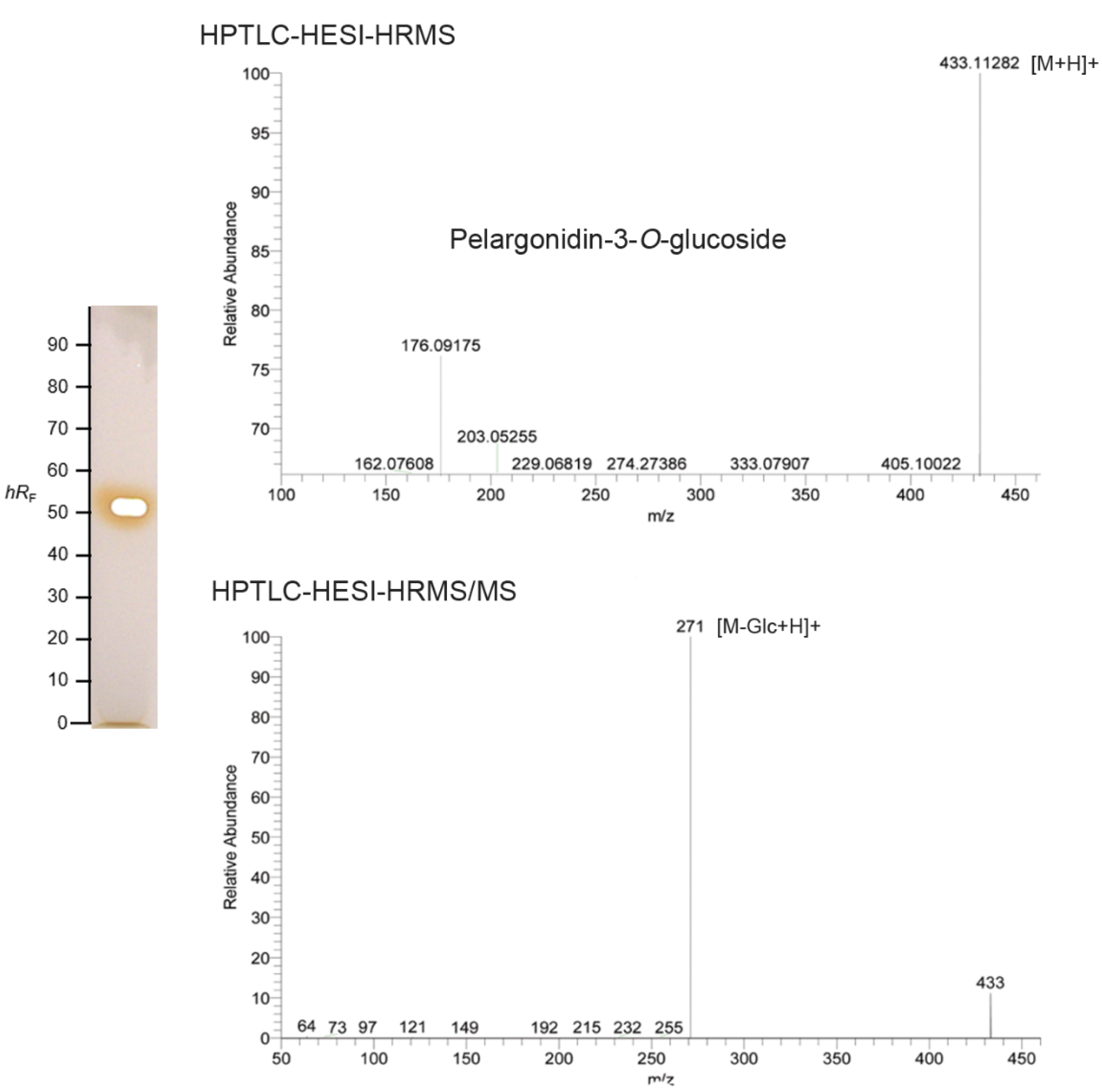
2.3. Characterization of the Main Bioactive Compound by HPTLC–UV/vis-HESI–HRMS/MS
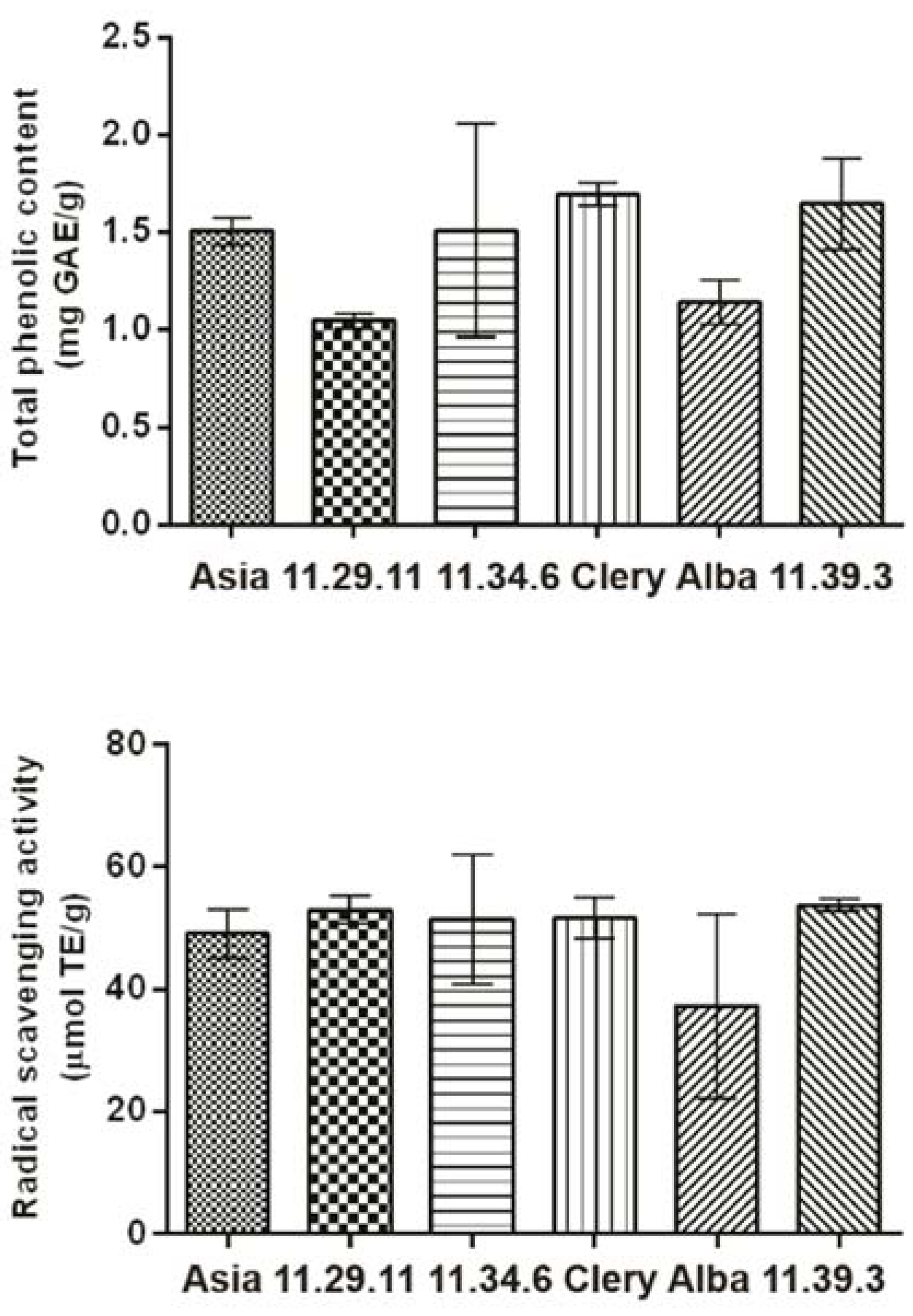
2.4. Total Phenolic Content and Radical Scavenging Activity
2.5. Mechanism of Antioxidant Activity of Pg-3-glc Studied by DFT
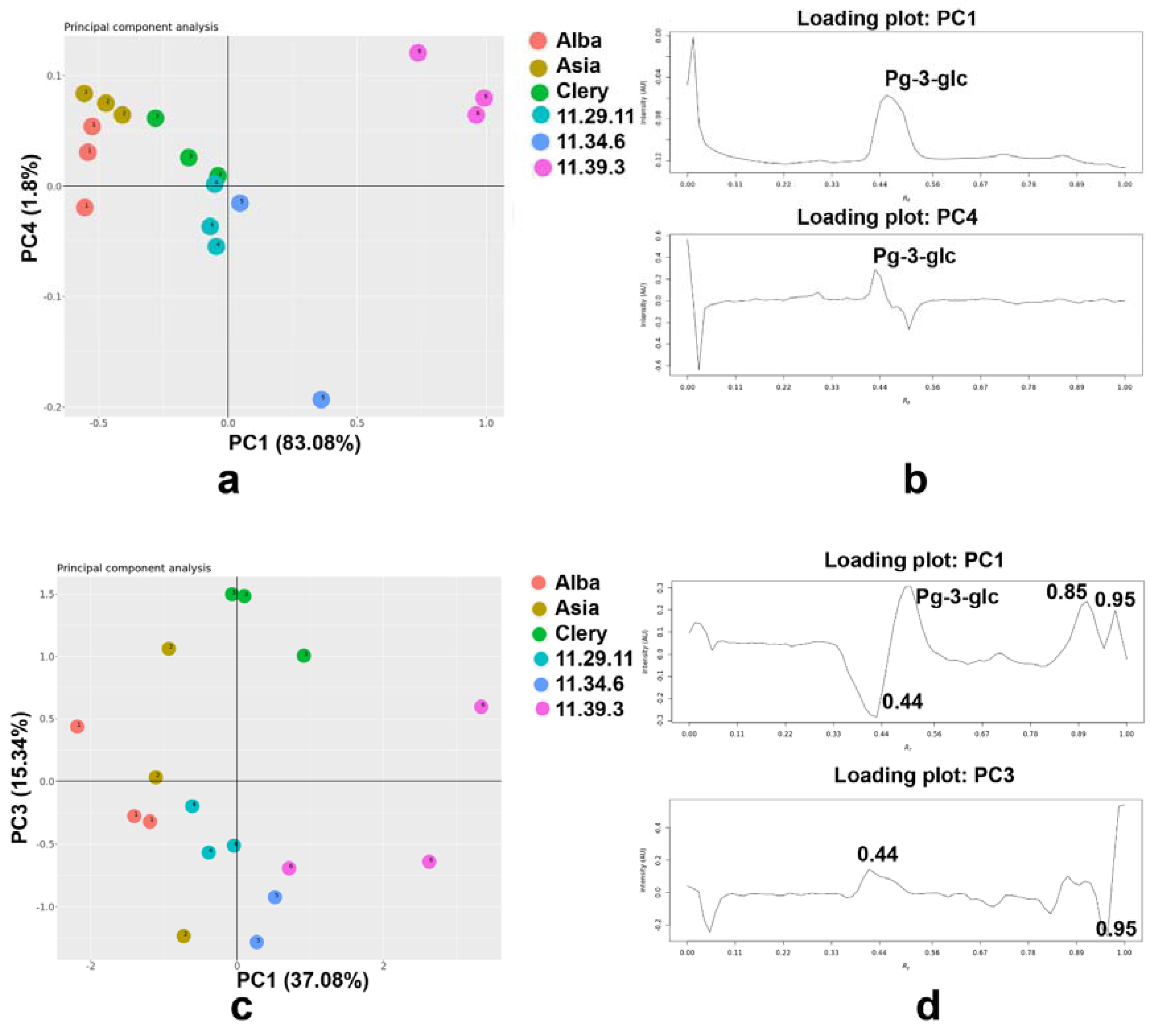
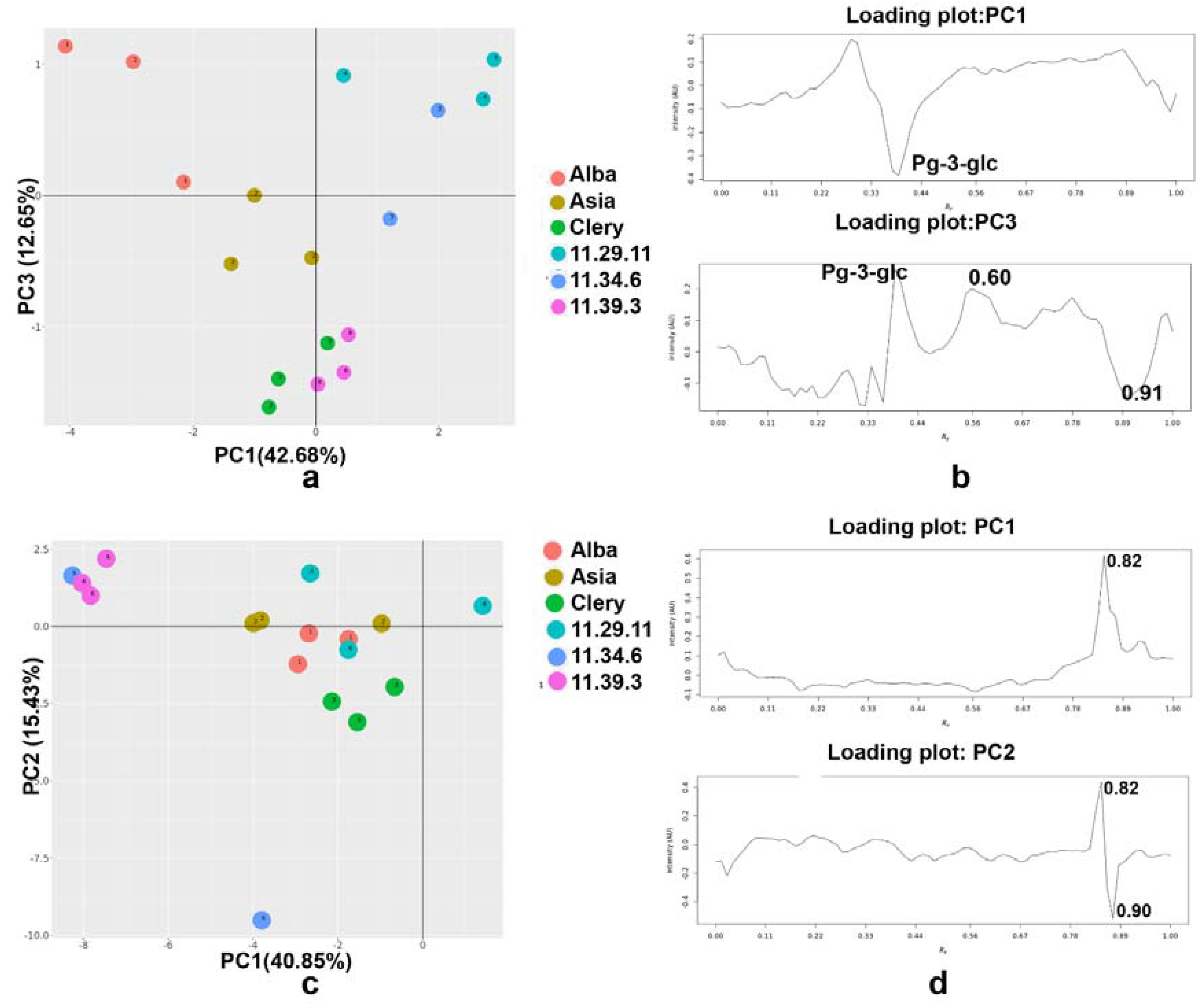
2.6. Principal Component Analysis
3. Material and Methods
3.1. Reagents and Chemicals
3.2. Samples Preparation
3.3. Extraction of Phenolic Compounds
3.4. Determination of Total Phenolic Content (TPC)
3.5. Determination of the Radical-Scavenging Activity (RSA)
3.6. High-Performance Thin-Layer Chromatography
3.7. Effect-Directed Analysis
3.8. Characterization of Active Zones via HPTLC-HESI-HRMS
3.9. Multivariate Analysis
3.10. Molecular Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- da Silva, F.L.; Escribano-Bailón, M.T.; Alonso, J.J.P.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT-Food Sci. Technol. 2007, 40, 374–382. [Google Scholar] [CrossRef]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.; Milivojević, J.; Ivanović, M. Strawberry production in Serbia—The state and perspectives. In Proceedings of the VI International Strawberry Symposium, Huelva, Spain, 3–7 March 2008; pp. 615–618. [Google Scholar]
- Andersen, M.; Fossen, T.; Torskangerpoll, K.; Fossen, A.; Hauge, U. Anthocyanin from strawberry (Fragaria ananassa) with the novel aglycone, 5-carboxypyranopelargonidin. Phytochemistry 2004, 65, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Tonutare, T.; Moor, U.; Szajdak, L. Strawberry anthocyanin determination by pH differential spectroscopic method—How to get true results? Acta Sci. Pol.-Hortorum Cultus 2014, 13, 35–47. [Google Scholar]
- Maraei, R.W.; Elsawy, K.M. Chemical quality and nutrient composition of strawberry fruits treated by γ-irradiation. J. Radiat. Res. Appl. Sci. 2017, 10, 80–87. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and Human Health: Effects beyond Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef]
- Morlock, G.; Schwack, W. Hyphenations in planar chromatography. J. Chromatogr. A 2010, 1217, 6600–6609. [Google Scholar] [CrossRef]
- Filip, M.; Vlassa, M.; Copaciu, F.; Coman, V. Identification of anthocyanins and anthocyanidins from berry fruits by chromatographic and spectroscopic techniques to establish the juice authenticity from market. JPC-J. Planar Chromatogr.-Mod. TLC 2012, 25, 534–541. [Google Scholar] [CrossRef]
- Comandini, P.; Blanda, G.; Cardinali, A.; Cerretani, L.; Bendini, A.; Caboni, M.F. CZE separation of strawberry anthocyanins with acidic buffer and comparison with HPLC. J. Sep. Sci. 2008, 31, 3257–3264. [Google Scholar] [CrossRef]
- Garcia-Viguera, C.; Zafrilla, P.; Tomás-Barberán, F. Influence of processing and storage conditions in strawberry jam color/Influencia de las condiciones de tratamiento y de almacenamiento en el color de confituras de fresa. Food Sci. Technol. Int. 1999, 5, 487–492. [Google Scholar] [CrossRef]
- Bakker, J.; Bridle, P.; Koopman, A. Strawberry juice colour: The effect of some processing variables on the stability of anthocyanins. J. Sci. Food Agric. 1992, 60, 471–476. [Google Scholar] [CrossRef]
- Lopes-Da-Silva, F.; de Pascual-Teresa, S.; Rivas-Gonzalo, J.-C.; Santos-Buelga, C. Identification of anthocyanin pigments in strawberry (cv Camarosa) by LC using DAD and ESI-MS detection. Eur. Food Res. Technol. 2002, 214, 248–253. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Characterization of phenolic compounds in strawberry fruits by RP-HPLC-DAD and investigation of their antioxidant capacity. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2495–2504. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Angeloni, S.; Abouelenein, D.; Acquaticci, L.; Xiao, J.; Sagratini, G.; Maggi, F.; Vittori, S.; Caprioli, G. A new HPLC-MS/MS method for the simultaneous determination of 36 polyphenols in blueberry, strawberry and their commercial products and determination of antioxidant activity. Food Chem. 2022, 367, 130743. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Theoduloz, C.; Caligari, P.D.; Schmeda-Hirschmann, G. Comparison of phenolic composition and antioxidant properties of two native Chilean and one domestic strawberry genotypes. Food Chem. 2009, 113, 377–385. [Google Scholar] [CrossRef]
- Kosar, M.; Kafkas, E.; Paydas, S.; Baser, K.H.C. Phenolic Composition of Strawberry Genotypes at Different Maturation Stages. J. Agric. Food Chem. 2004, 52, 1586–1589. [Google Scholar] [CrossRef] [PubMed]
- Musilová, J.; Trebichalský, P.; Timoracká, M.; Bystrická, J. Cultivar as one of the factors affecting the anthocyanin content and antioxidant activity in strawberry fruits. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 1765–1775. [Google Scholar]
- Ristivojevic, P.; Andric, F.; Trifković, J.; Vovk, I.; Stanisavljević, L.; Tesic, Z.; Milojković-Opsenica, D.M. Pattern recognition methods and multivariate image analysis in HPTLC fingerprinting of propolis extracts. J. Chemom. 2014, 28, 301–310. [Google Scholar] [CrossRef]
- Taghavi, T.; Patel, H.; Akande, O.E.; Galam, D.C.A. Total Anthocyanin Content of Strawberry and the Profile Changes by Extraction Methods and Sample Processing. Foods 2022, 11, 1072. [Google Scholar] [CrossRef]
- Lindoo, S.J.; Caldwell, M.M. Ultraviolet-B Radiation-induced Inhibition of Leaf Expansion and Promotion of Anthocyanin Production: Lack of Involvement of the Low Irradiance Phytochrome System 1. Plant Physiol. 1978, 61, 278–282. [Google Scholar] [CrossRef]
- Pantelić, M.; Zagorac, D.D.; Natić, M.; Gašić, U.; Jović, S.; Vujović, D.; Djordjević, J.P. Impact of Clonal Variability on Phenolics and Radical Scavenging Activity of Grapes and Wines: A Study on the Recently Developed Merlot and Cabernet Franc Clones (Vitis vinifera L.). PLoS ONE 2016, 11, e0163823. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Goertz, S.; Moradtalab, N.; Walker, F.; Höglinger, B.; Ludewig, U.; Neumann, G. Drought-protective effects of nutrient seed treatments during early growth of oilseed rape. J. Plant Nutr. 2022, 1–20. [Google Scholar] [CrossRef]
- Krüger, S.; Mirgos, M.; Morlock, G. Effect-directed analysis of fresh and dried elderberry (Sambucus nigra L.) via hyphenated planar chromatography. J. Chromatogr. A 2015, 1426, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Ivanova, S.A.; Shikov, A.N.; Makarov, V.G. Separation and free radical-scavenging activity of major curcuminoids ofCurcuma longa using HPTLC-DPPH method. Phytochem. Anal. 2008, 19, 236–243. [Google Scholar] [CrossRef] [PubMed]
- DIN EN ISO 11348-1; Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent Bacteria Test)—Part 1: Method Using Freshly Prepared Bacteria. Beuth Verlag: Berlin, Germany, 2009.
- Reguigui, A.; Heil, J.; Gorai, M.; Mabrouk, M.; Romdhane, M.; Morlock, G.E. Profile comparison and valorization of Tunisian Salvia aegyptiaca and S. verbenaca aerial part extracts via hyphenated high-performance thin-layer chromatography. J. Chromatogr. A 2022, 1673, 463057. [Google Scholar] [CrossRef]
- Jamshidi-Aidji, M.; Morlock, G.E. Bioprofiling of unknown antibiotics in herbal extracts: Development of a streamlined direct bioautography using Bacillus subtilis linked to mass spectrometry. J. Chromatogr. A 2015, 1420, 110–118. [Google Scholar] [CrossRef]
- Glavnik, V.; Vovk, I.; Albreht, A. High performance thin-layer chromatography—Mass spectrometry of Japanese knotweed flavan-3-ols and proanthocyanidins on silica gel plates. J. Chromatogr. A 2017, 1482, 97–108. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Stewart, J.J.P. MOPAC: A semiempirical molecular orbital program. J. Comput. Mol. Des. 1990, 4, 1–103. [Google Scholar] [CrossRef]
- Pedretti, A.; Mazzolari, A.; Gervasoni, S.; Fumagalli, L.; Vistoli, G. The VEGA suite of programs: An versatile platform for cheminformatics and drug design projects. Bioinformatics 2021, 37, 1174–1175. [Google Scholar] [CrossRef]
- Spiegel, M.; Gamian, A.; Sroka, Z. A Statistically Supported Antioxidant Activity DFT Benchmark—The Effects of Hartree—Fock Exchange and Basis Set Selection on Accuracy and Resources Uptake. Molecules 2021, 26, 5058. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.; Tošović, J.; Milenkovic, D.; Markovic, S. Revisiting the solvation enthalpies and free energies of the proton and electron in various solvents. Comput. Theor. Chem. 2016, 1077, 11–17. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]


| Site | Gas Phase | Water | Pentyl Ethanoate | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDE | IP | PDE | PA | ETE | BDE | IP | PDE | PA | ETE | BDE | IP | PDE | PA | ETE | |
| 4′-OH | 409.3 | 988.1 | 734.0 | 1024.8 | 697.2 | 407.3 | 524.1 | 41.8 | 96.1 | 469.9 | 402.8 | 630.6 | 15.6 | 123.2 | 523.0 |
| 5-OH | 383.2 | 702.2 | 1016.2 | 674.1 | 384.9 | 19.4 | 88.0 | 455.5 | 376.2 | −10.9 | 114.7 | 505.0 | |||
| 7-OH | 414.9 | 728.3 | 1041.5 | 675.0 | 400.0 | 34.5 | 116.4 | 442.3 | 396.1 | 8.9 | 140.5 | 499.1 | |||
| ID | Genotype Patent No. Material Type | Main Characteristics | Use |
|---|---|---|---|
| 1 | Alba NF 311 Variety | Variety with very early season of ripening, characterized by high productivity and yield. Fruits are large, very uniform, with intense red color and average taste. Soluble Solids Content (°Brix) is lower than in new, promising breeding materials, and usually without ideally balanced sugar/acidity ratio. Due to its good shelf-life and consistency, variety is well suitable for long-distant markets. | Fresh market |
| 2 | Asia NF 421 Variety | Variety with medium-late season of ripening, characterized by plants of erect and vigorous habitus. The flavor is good, with well balanced sugar/acidity ratio. Variety is sensitive to powdery mildew, but well resistant to frosts. | Fresh market |
| 3 | Clery - Variety | Variety with very early season of ripening and medium productivity. Fruits are firm, intense red in color and with long-conical fruits of standard good taste. Variety is sensitive to high temperatures and late spring frosts. However, it is characterized as resistant to most strawberry root diseases. | Fresh market |
| 4 | 11.29.11 BL 29 Breeding material | Perspective new genotype with very early season of ripening and high productivity and yield. Fruits are large, long-conical in shape, with very attractive bright color and great consistency, which suggests them for long-distant markets. It is characterized by premium quality fruits of good taste and well-balanced sugar/acidity ratio. Due to its resistance to rain, it is adapted for both open field and greenhouse cultivation. | Fresh market, organic cultivation, gardening |
| 5 | 11.34.6 BL 34 Breeding material | Perspective new genotype with extremely early season of ripening, especially in cases of cultivation under tunnels or in greenhouses. Genotype is characterized by strong and rustic plants, medium productivity and without susceptibility to common strawberry fungal diseases. Fruits are uniform, long-conical in shape, bright-red colored, very sweet and aromatic of premium quality. This genotype is suitable for organic cultivation and resistant to rain damage. | Fresh market, organic cultivation, gardening |
| 6 | 11.39.3 BL 39 Breeding material | Perspective new genotype with early season of ripening, strong plants, and medium/high productivity. Plants are described as rustic and strong, as well as not susceptible to common strawberry diseases. Fruits are short conical-round in shape, firm, very uniform, intensively bright-red colored, with premium quality and great shelf life. Due to its great organoleptic characteristics and interesting aroma, this genotype is suitable for industry/processing. Due to its resistance to rain, it is adapted for both open field and greenhouse production, but also suggested for organic cultivation. | Fresh market, processing, organic cultivation, gardening |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ristivojević, P.; Lekić, N.; Cvijetić, I.; Krstić, Đ.; Andrić, F.; Milojković-Opsenica, D.; Morlock, G.E. Effect-Directed Profiling of Strawberry Varieties and Breeding Materials via Planar Chromatography and Chemometrics. Molecules 2022, 27, 6062. https://doi.org/10.3390/molecules27186062
Ristivojević P, Lekić N, Cvijetić I, Krstić Đ, Andrić F, Milojković-Opsenica D, Morlock GE. Effect-Directed Profiling of Strawberry Varieties and Breeding Materials via Planar Chromatography and Chemometrics. Molecules. 2022; 27(18):6062. https://doi.org/10.3390/molecules27186062
Chicago/Turabian StyleRistivojević, Petar, Nevena Lekić, Ilija Cvijetić, Đurđa Krstić, Filip Andrić, Dušanka Milojković-Opsenica, and Gertrud E. Morlock. 2022. "Effect-Directed Profiling of Strawberry Varieties and Breeding Materials via Planar Chromatography and Chemometrics" Molecules 27, no. 18: 6062. https://doi.org/10.3390/molecules27186062
APA StyleRistivojević, P., Lekić, N., Cvijetić, I., Krstić, Đ., Andrić, F., Milojković-Opsenica, D., & Morlock, G. E. (2022). Effect-Directed Profiling of Strawberry Varieties and Breeding Materials via Planar Chromatography and Chemometrics. Molecules, 27(18), 6062. https://doi.org/10.3390/molecules27186062







