Neuroprotective Effects of a New Derivative of Chlojaponilactone B against Oxidative Damaged Induced by Hydrogen Peroxide in PC12 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Modification and Structure Elucidation
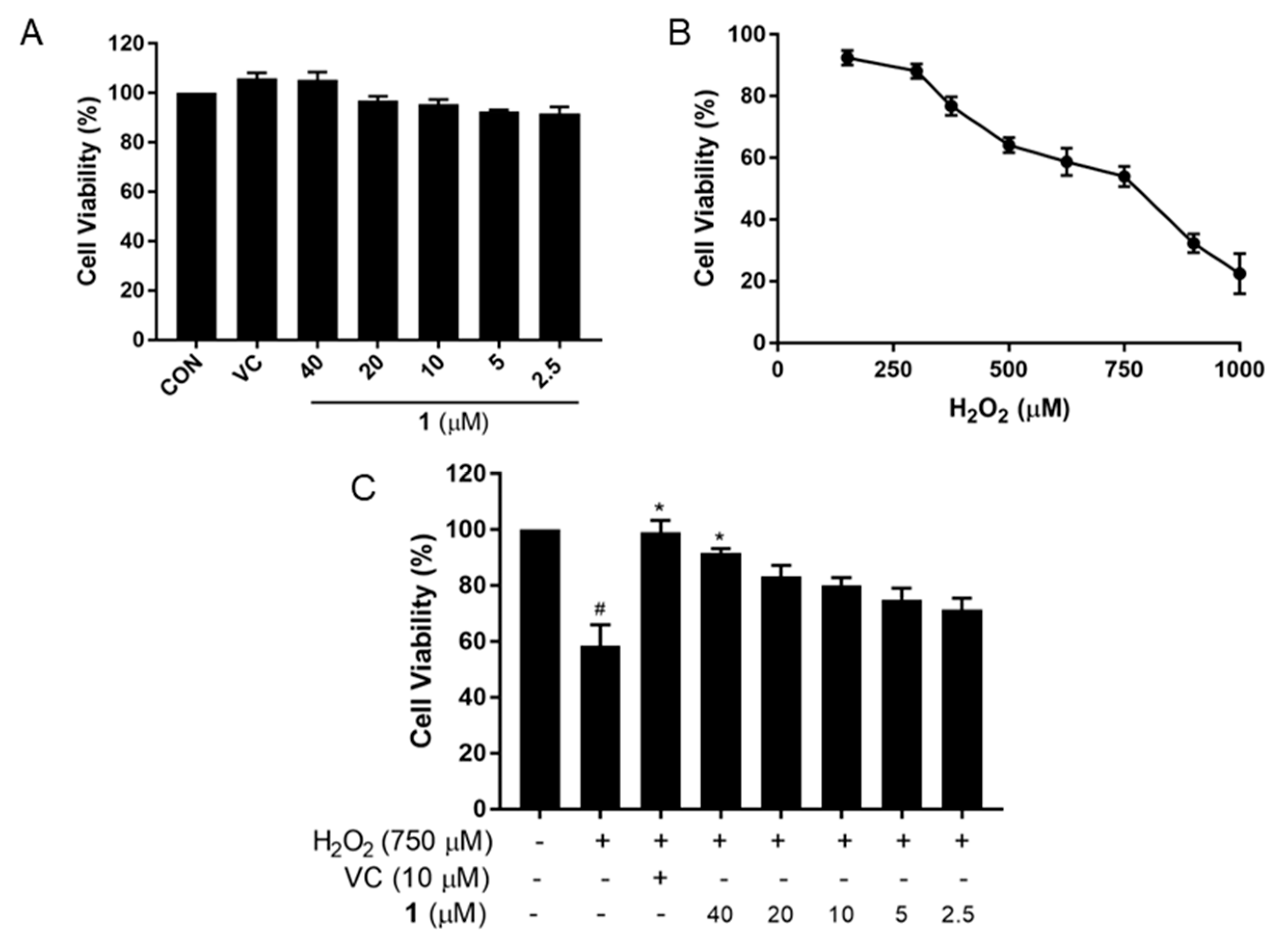
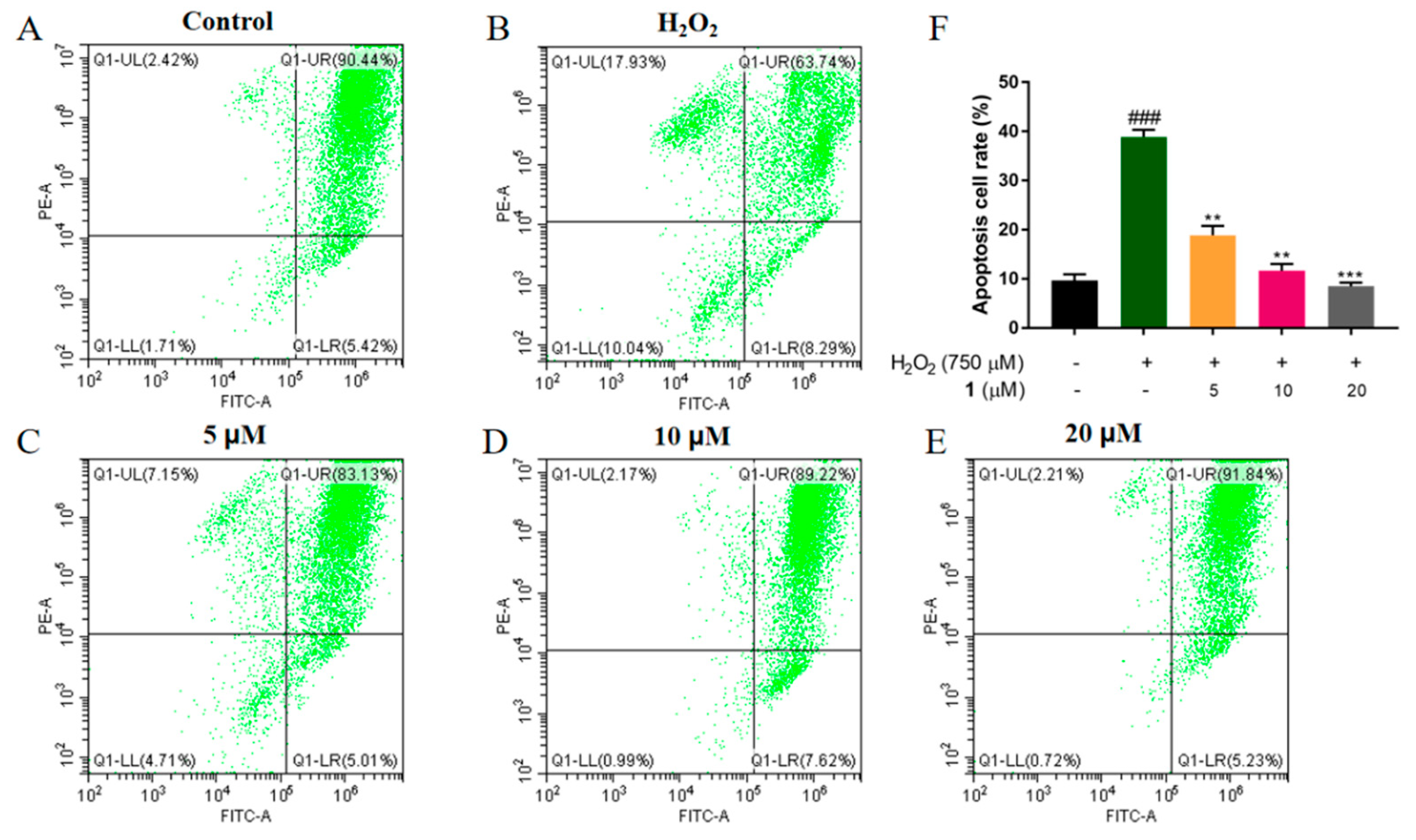
2.2. Neuroprotective Effect of Compound 1 against PC12 Cell Injury Induced by H2O2
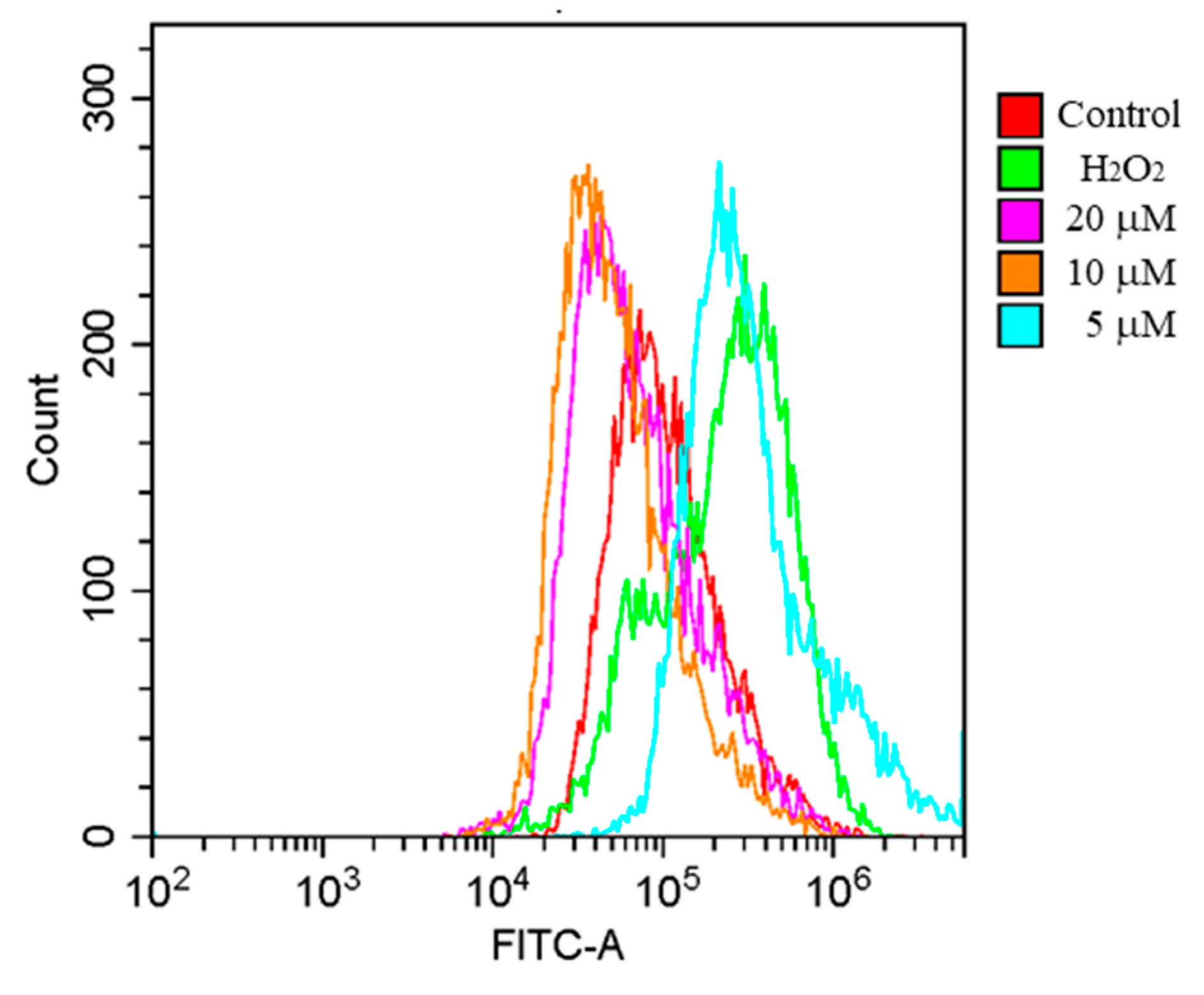
2.3. Effects of 1 on ROS Generation in H2O2-Induced PC12 Cells
2.4. Effects of 1 on the Recovery of the Loss of MMP in H2O2-Induced PC12 Cells
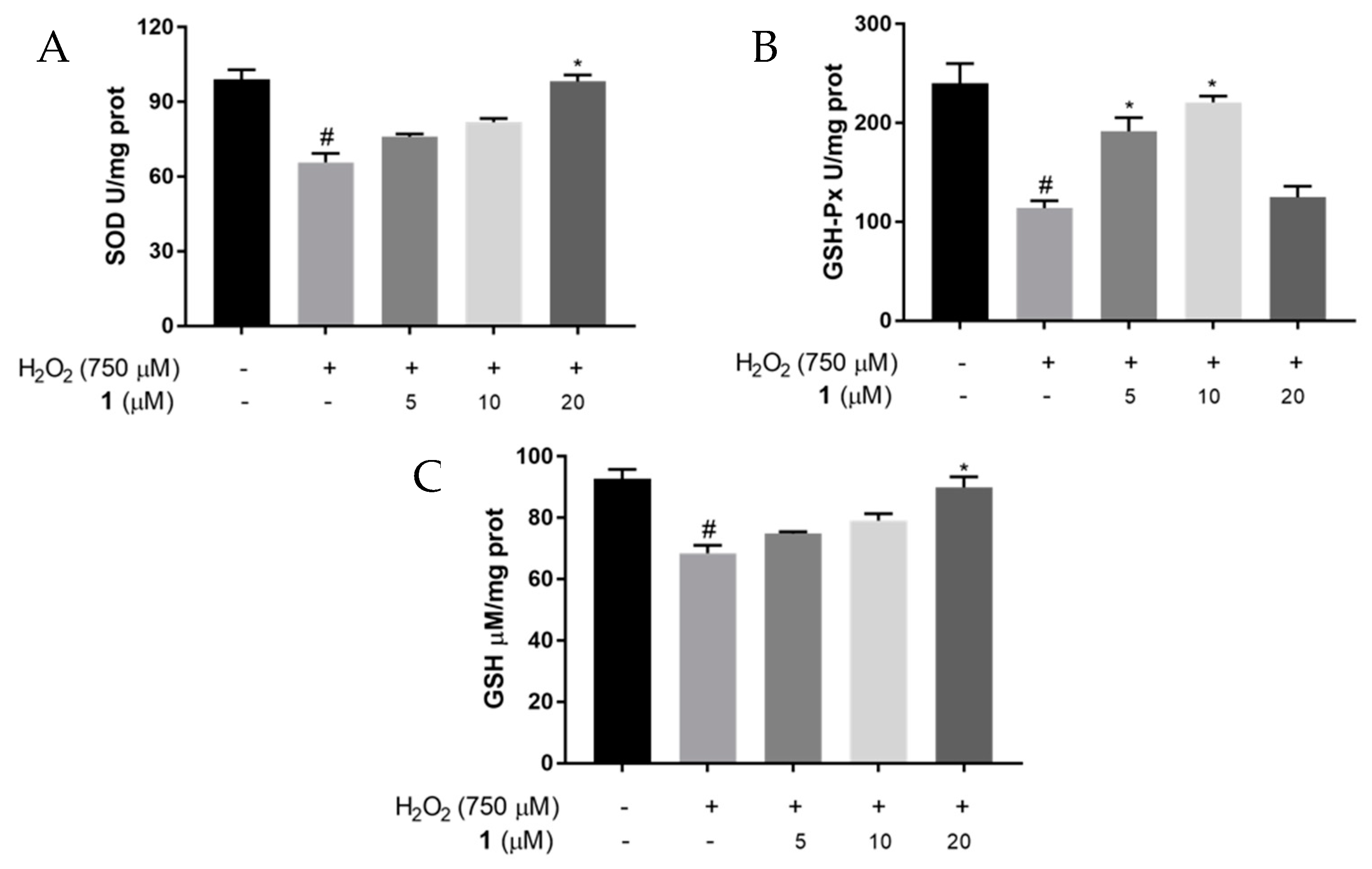
2.5. Effects of 1 on SOD and GSH-Px Activities, and GSH Levels in H2O2-Induced PC12 Cells
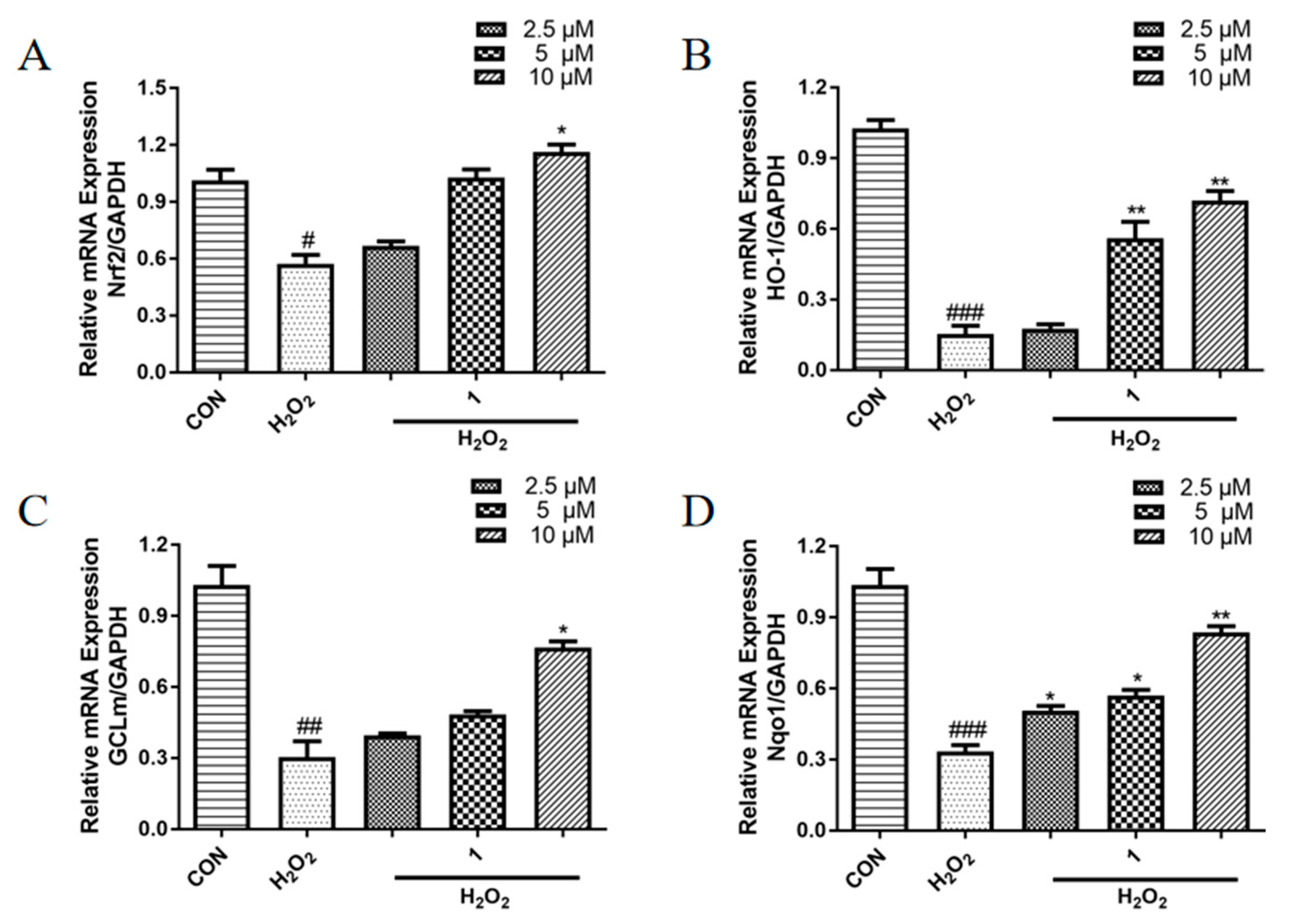
2.6. Effects of 1 on the mRNA Expression Levels of Antioxidant Proteins in PC12 Cells Induced with H2O2
3. Materials and Methods
3.1. Reagents
3.2. Cell Culture
3.3. Assay of Cell Viability
3.4. ROS Measurement
3.5. Mitochondrial Membrane Potential
3.6. Measurement of Intracellular Antioxidant Activity
3.7. Analysis of Antioxidant Gene Expression by Quantitative Real-Time Reverse Transcription PCR (qRT-PCR)
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—sources, functions, oxidative damage. Pol. Merkur. Lekarski 2020, 48, 124–127. [Google Scholar]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. Febs Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.J.; Yuan, S.; Zi, H.; Gu, J.M.; Fang, C.; Zeng, X.T. Oxidative Stress Links Aging-Associated Cardiovascular Diseases and Prostatic Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 5896136. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, J.J.; Zhang, R.; Zhang, L.L.; Zhang, Q.; Yang, H.; An, J. Neuroprotective effects of natural compounds on neurotoxin-induced oxidative stress and cell apoptosis. Nutr. Neurosci. 2022, 25, 1078–1099. [Google Scholar] [CrossRef]
- Kryl’skii, E.D.; Chupandina, E.E.; Popova, T.N.; Shikhaliev, K.S.; Mittova, V.O.; Popov, S.S.; Verevkin, A.N.; Filin, A.A. Neuroprotective effect of 6-hydroxy-2,2,4-trimethyl-1,2-dihydroquinoline mediated via regulation of antioxidant system and inhibition of inflammation and apoptosis in a rat model of cerebral ischemia/reperfusion. Biochimie 2021, 186, 130–146. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, Y.Q.; Liu, D.Q.; Yao, X.T.; Jiang, H.; Zhang, Y.B. Demethylenetetrahydroberberine protects dopaminergic neurons in a mouse model of Parkinson’s disease. Chin. J. Nat. Med. 2022, 20, 111–119. [Google Scholar] [CrossRef]
- Chen, L.; Wei, M.L.; Zhao, J.J.; Hong, H.; Qu, W.; Feng, F.; Liu, W.Y. GTS40, an active fraction of Gou Teng-San (GTS), protects PC12 from H2O2-induced cell injury through antioxidative properties. Chin. J. Nat. Med. 2017, 15, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Li, X.Y.; Wang, J.; He, Y.; Chi, C.F.; Wang, B. Antioxidant peptides from protein hydrolysate of skipjack tuna milt: Purification, identification, and cytoprotection on H2O2 damaged human umbilical vein endothelial cells. Process Biochem. 2022, 113, 258–269. [Google Scholar] [CrossRef]
- Karthika, C.; Appu, A.P.; Akter, R.; Rahman, M.H.; Tagde, P.; Ashraf, G.M.; Abdel-Daim, M.M.; ul Hassan, S.S.; Abid, A.; Bungau, S. Potential innovation against Alzheimer’s disorder: A tricomponent combination of natural antioxidants (vitamin E, quercetin, and basil oil) and the development of its intranasal delivery. Environ. Sci. Pollut. Res. 2022, 29, 10950–10965. [Google Scholar] [CrossRef] [PubMed]
- Flora of China Editorial Committee. Flora of China; Science Press: Beijing, China, 1982; Volume 20, p. 85. [Google Scholar]
- Chen, F.Y.; Bian, Y.T.; Huang, W.M.; Chen, Z.C.; Shuang, P.C.; Feng, Z.G.; Luo, Y.M. Research progress on chemical constituents from Chloranthus plants and their biological activities. China J. Chin. Mater. Med. 2021, 46, 3789–3796. [Google Scholar] [CrossRef]
- Zhao, J.J.; Guo, Y.Q.; Yang, D.P.; Xue, X.; Liu, Q.; Zhu, L.P.; Yin, S.; Zhao, Z.M. Chlojaponilactone B from Chloranthus japonicus: Suppression of Inflammatory Responses via Inhibition of the NF-κB Signaling Pathway. J. Nat. Prod. 2016, 79, 2257–2263. [Google Scholar] [CrossRef]
- Ye, S.X.; Zheng, Q.Y.; Zhou, Y.; Bai, B.; Yang, D.P.; Zhao, Z.M. Chlojaponilactone B Attenuates Lipopolysaccharide-Induced Inflammatory Responses by Suppressing TLR4-Mediated ROS Generation and NF-κB Signaling Pathway. Molecules 2019, 24, 3731. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Li, B. Neuroprotection of two C21 steroidal glycosides from Cynanchum auriculatum against H2O2-induced damage on PC12 cells. Nat. Prod. Res. 2021, 35, 1752–1755. [Google Scholar] [CrossRef]
- Li, K.; Wang, L.Y.; Hu, Y.; Zhu, Z.Y. Structural characterization and protective effect on PC12 cells against H2O2-induced oxidative damage of a polysaccharide extracted from mycelia of Lactarius deliciosus Gray. Int. J. Biol. Macromol. 2022, 209, 1815–1825. [Google Scholar] [CrossRef]
- Qi, S.D.; Zhang, X.J.; Fu, Z.Z.; Pi, A.R.; Shi, F.Y.; Fan, Y.A.; Zhang, J.H.; Xiao, T.T.; Shang, D.; Lin, M.; et al. (+/−)-5-bromo-2-(5-fluoro-1-hydroxyamyl) Benzoate Protects Against Oxidative Stress Injury in PC12 Cells Exposed to H2O2 Through Activation of Nrf2 Pathway. Front. Pharmacol. 2022, 13, 13. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.X.; Zheng, Y.F.; Zhao, J.W.; Yu, H.L.; Zhu, J.J. Relationship between Neuroprotective Effects and Structure of Procyanidins. Molecules 2022, 27, 18. [Google Scholar] [CrossRef]
- Gallo, G.; Sprovieri, P.; Martino, G. 4-hydroxynonenal and oxidative stress in several organelles and its damaging effects on cell functions. J. Physiol. Pharmacol. 2020, 71, 15–33. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zeevalk, G.D.; Razmpour, R.; Bernard, L. Glutathione and Parkinson’s disease: Is this the elephant in the room? Biomed. Pharmacother. 2008, 62, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.M. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic. Biol. Med. 2022, 179, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.E.; Lazzarino, G.; Sampaolese, B.; Brancato, A.; Tringali, G. DHA protects PC12 cells against oxidative stress and apoptotic signals through the activation of the NFE2L2/HO-1 axis. Int. J. Mol. Med. 2019, 43, 2523–2531. [Google Scholar] [CrossRef]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef]
- Peng, S.; Hou, Y.; Yao, J.; Fang, J. Activation of Nrf2 by costunolide provides neuroprotective effect in PC12 cells. Food Funct. 2019, 10, 4143–4152. [Google Scholar] [CrossRef]
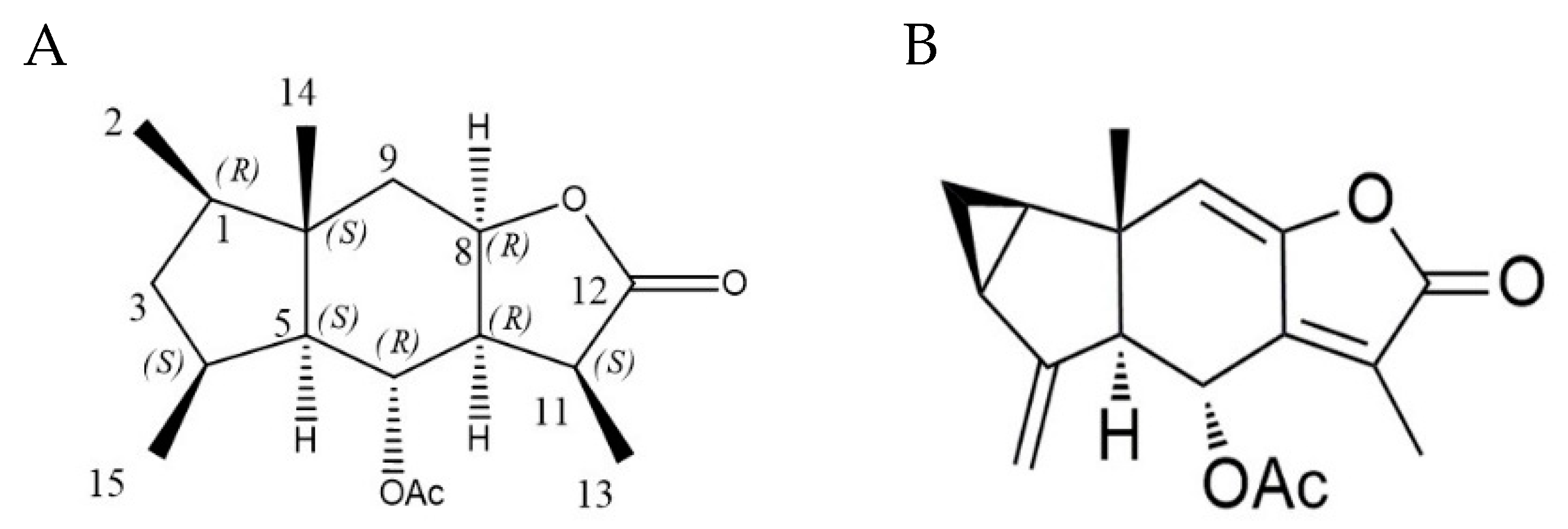
| Position | δ C | δH, multi. (J in Hz) |
|---|---|---|
| 1 | 47.0 | 1.48, m |
| 2 | 13.3 | 0.86, d (6.76) |
| 3 | 41.3 | 0.99, m 2.16, m |
| 4 | 30.4 | 2.14, m |
| 5 | 54.3 | 1.81, dd (12.08, 8.64) |
| 6 | 70.3 | 5.10, dd (12.08, 8.76) |
| 7 | 48.1 | 2.62, ddd (12.32, 5.00, 1.00) |
| 8 | 80.3 | 4.65, td (4.56, 1.52) |
| 9 | 40.7 | 1.30, m 2.21, m |
| 10 | 43.8 | |
| 11 | 40.9 | 2.83, m |
| 12 | 178.4 | |
| 13 | 10.4 | 1.25, d (7.36) |
| 14 | 16.2 | 0.80, s |
| 15 | 19.2 | 0.92, d (7.00) |
| COOCH3 | 169.9 | |
| COOCH3 | 21.8 | 2.02, s |
| Gene Symbol | Forward Primer (5’-3’) | Reverse Primer (5’-3’) |
|---|---|---|
| GADPH | GGTGAAGGTCGGTGTGAACG | CTCGCTCCTGGAAGATGGTG |
| GCLm | CTTCGCCTCCGATTGAAGATG | AAAGGCAGTCAAATCTGGTGG |
| HO-1 | AGATGGCGTCACTTCGTCAG | GCTGATCTGGGGTTTCCCTC |
| Nrf2 | TTGGCAGAGACATTCCCAT | GCTGCCACCGTCACTGGG |
| Nqo1 | TGAAGAAGAGAGGATGGGAGG | GATGACTCGGAAGGATACTGAAAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, S.; Wen, Q.; Zhu, L.; Qian, C.; Yang, D.; Zhao, Z. Neuroprotective Effects of a New Derivative of Chlojaponilactone B against Oxidative Damaged Induced by Hydrogen Peroxide in PC12 Cells. Molecules 2022, 27, 6049. https://doi.org/10.3390/molecules27186049
Ye S, Wen Q, Zhu L, Qian C, Yang D, Zhao Z. Neuroprotective Effects of a New Derivative of Chlojaponilactone B against Oxidative Damaged Induced by Hydrogen Peroxide in PC12 Cells. Molecules. 2022; 27(18):6049. https://doi.org/10.3390/molecules27186049
Chicago/Turabian StyleYe, Shaoxia, Qiyin Wen, Longping Zhu, Chunguo Qian, Depo Yang, and Zhimin Zhao. 2022. "Neuroprotective Effects of a New Derivative of Chlojaponilactone B against Oxidative Damaged Induced by Hydrogen Peroxide in PC12 Cells" Molecules 27, no. 18: 6049. https://doi.org/10.3390/molecules27186049
APA StyleYe, S., Wen, Q., Zhu, L., Qian, C., Yang, D., & Zhao, Z. (2022). Neuroprotective Effects of a New Derivative of Chlojaponilactone B against Oxidative Damaged Induced by Hydrogen Peroxide in PC12 Cells. Molecules, 27(18), 6049. https://doi.org/10.3390/molecules27186049






