Design, Synthesis and Structure—Activity Relationships of Phenylalanine-Containing Peptidomimetics as Novel HIV-1 Capsid Binders Based on Ugi Four-Component Reaction
Abstract
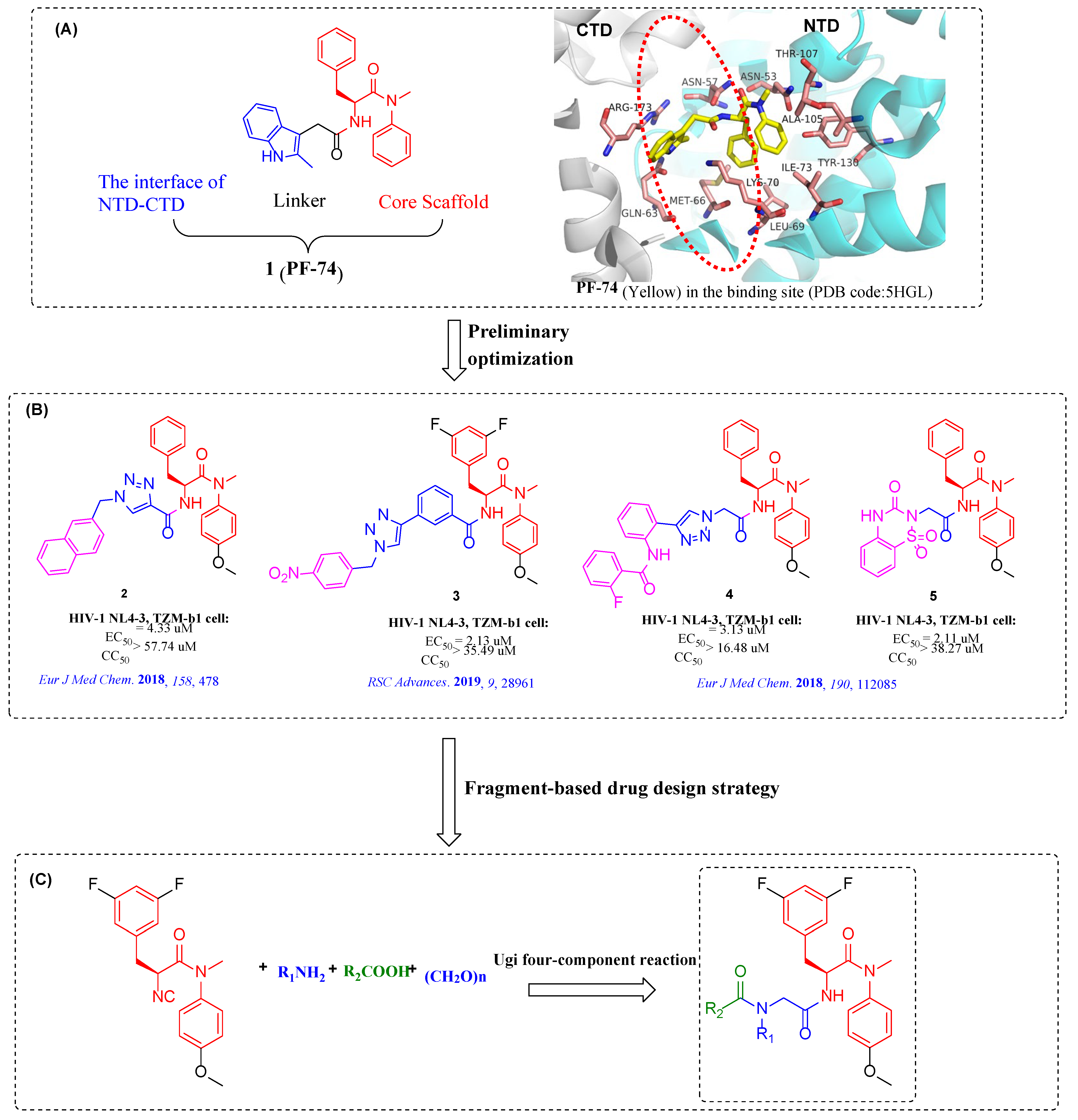
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Activity
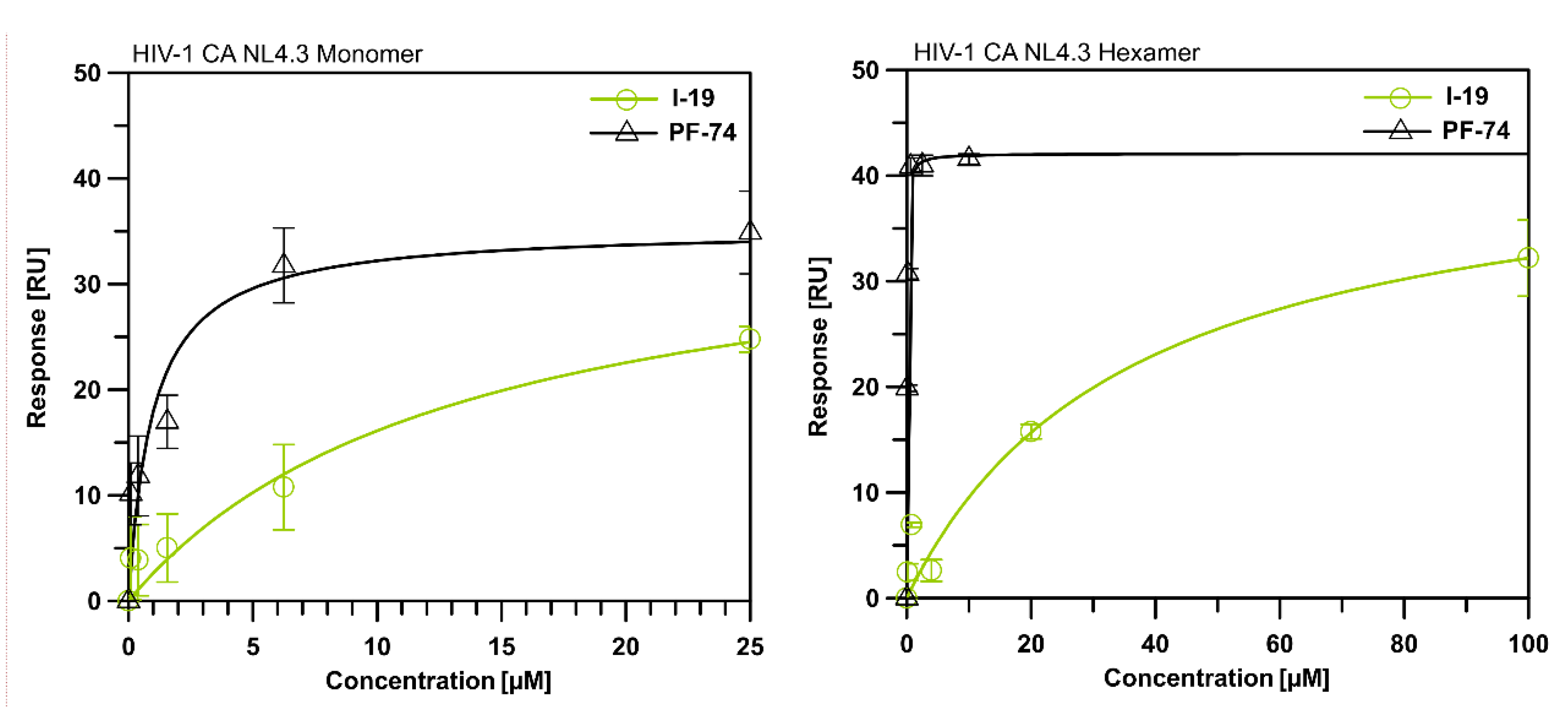
2.3. Surface Plasmon Resonance (SPR) Assay on HIV-1 CA
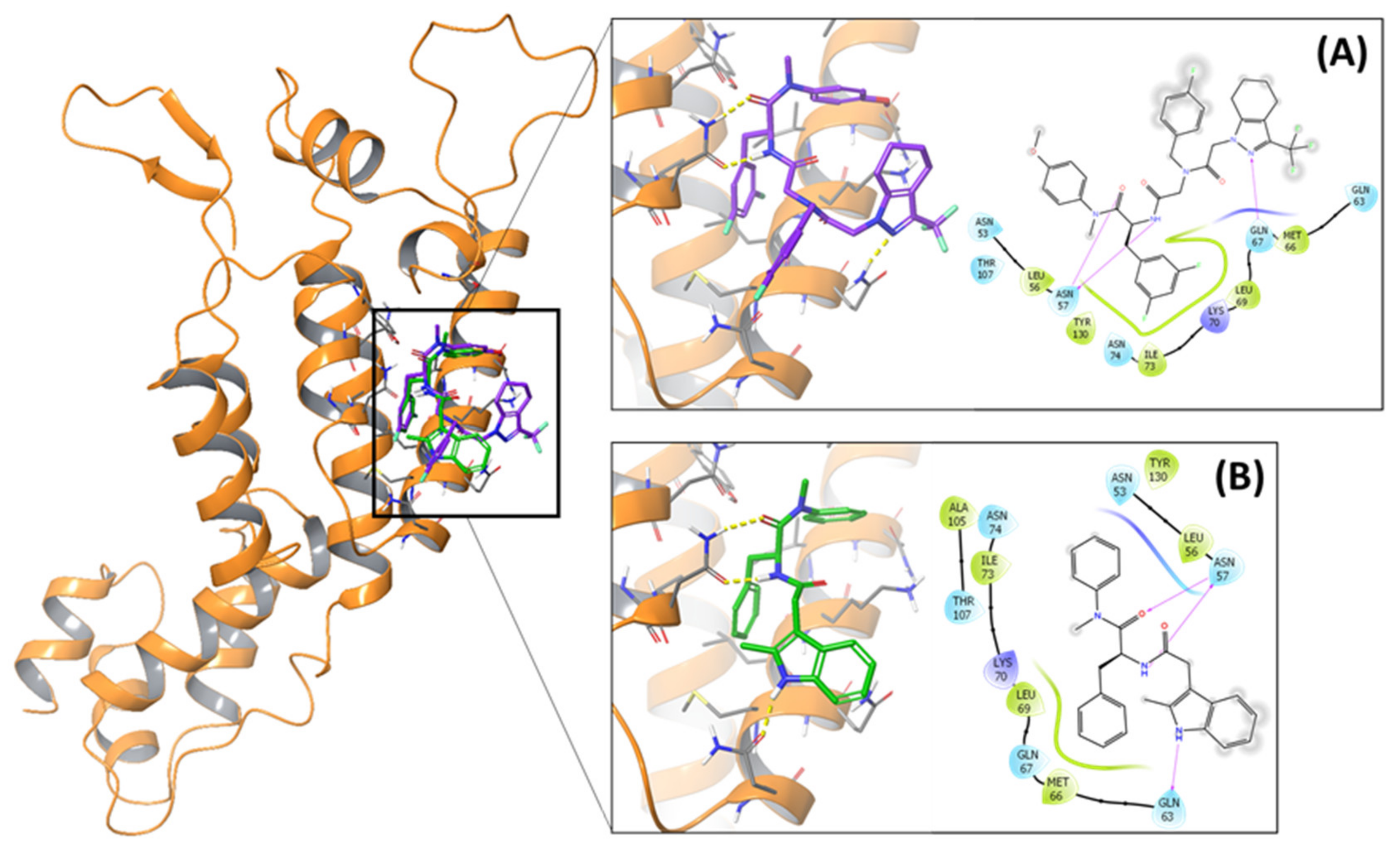
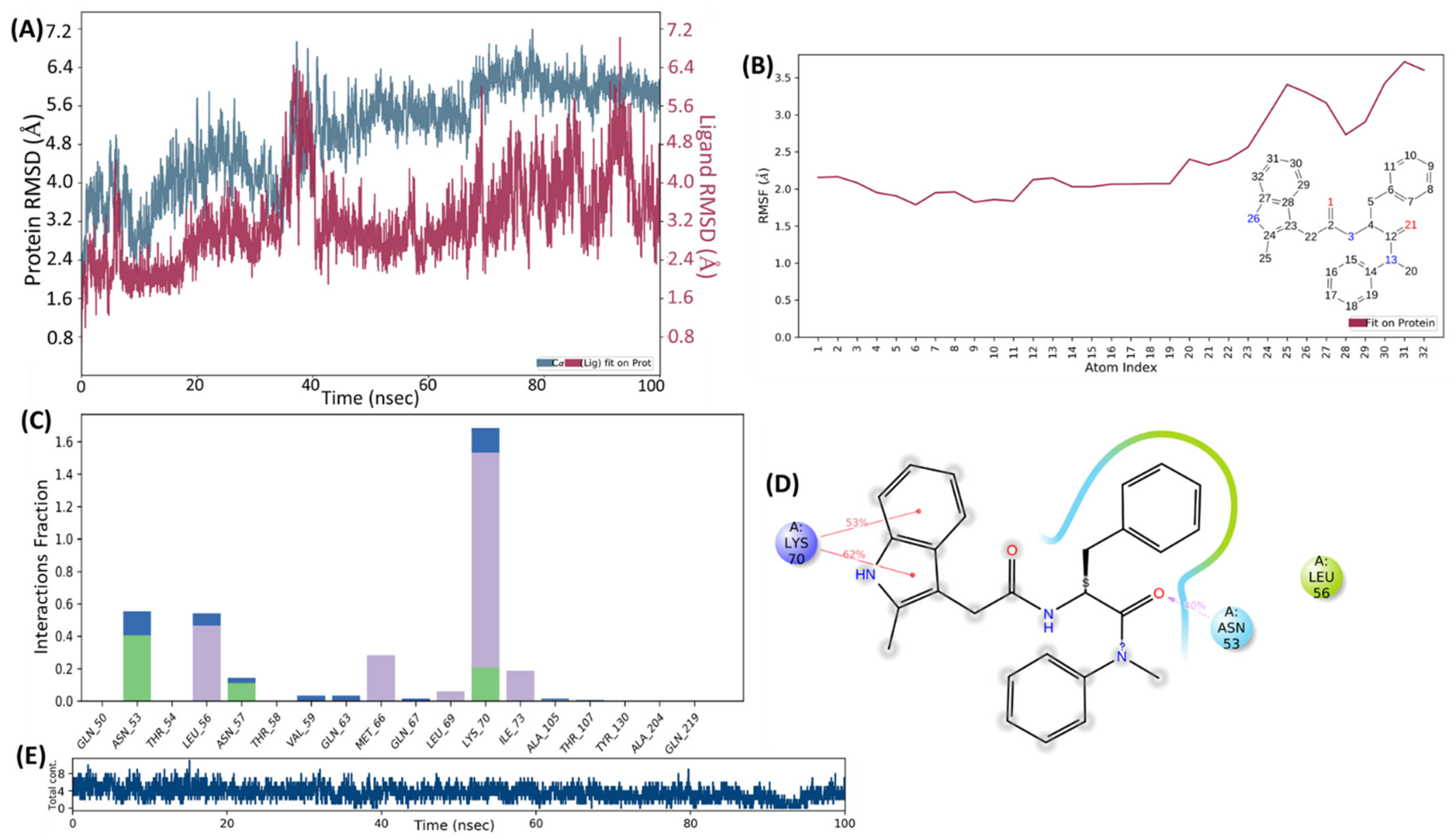
2.4. Molecular Docking and MD Simulation
3. Conclusions
4. Materials and Methods
4.1. Chemistry
4.1.1. Tert-butyl (S)-(3-(3,5-difluorophenyl)-1-((4-methoxyphenyl) (methyl)amino)-1-oxopropan-2-yl) carbamate (I-2)
4.1.2. (S)-2-amino-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (I-3)
4.1.3. (S)-3-(3,5-difluorophenyl)-2-formamido-N-(4-methoxyphenyl)-N-methylpropanamide (I-4)
4.1.4. (S)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methyl-2-(methylidyne-l4-azaneyl) propenamide (I-5)
4.1.5. General Procedure for the Synthesis of Target Compounds I-(6-23)
(S)-3-(3,5-difluorophenyl)-2-(2-(N-(4-fluorobenzyl)-2-(naphthalen-2-yl)acetamido)acetamido)-N-(4-methoxyphenyl)-N-methylpropanamide (I-6):
(S)-3-(3,5-difluorophenyl)-2-(2-(N-(4-fluorobenzyl)-2-(2-methyl-1H-indol-3-yl)acetamido)acetamido)-N-(4-methoxyphenyl)-N-methylpropanamide (I-7):
(S)-3-(3,5-difluorophenyl)-2-(2-(N-(4-fluorobenzyl)-2-(naphthalen-1-yl)acetamido)acetamido)-N-(4-methoxyphenyl)-N-methylpropanamide (I-8):
(S)-2-(2-(2-(5-bromo-1H-indol-3-yl)-N-(4-fluorobenzyl)acetamido)acetamido)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (I-9):
(S)-2-(2-(2-(5-bromo-1H-indol-3-yl)-N-(cyclohexylmethyl)acetamido)acetamido)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (I-10):
(S)-2-(2-(N-cyclopropyl-2-(2-methyl-1H-indol-3-yl)acetamido)acetamido)-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (I-11):
(S)-3-(3,5-difluorophenyl)-2-(2-(N-(4-fluorobenzyl)-2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)acetamido)acetamido)-N-(4-methoxyphenyl)-N-methylpropanamide (I-12):
(S)-2-(2-(N-(4-cyanobenzyl)-2-(naphthalen-2-yl)acetamido)acetamido)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (I-13):
(S)-2-(2-(N-(4-cyanobenzyl)-2-(naphthalen-1-yl)acetamido)acetamido)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (I-14):
(S)-2-(2-(N-(4-cyanobenzyl)-2-(5-fluoro-2-methyl-1H-indol-3-yl)acetamido)acetamido)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (I-15):
(S)-2-(2-(2-(5-bromo-2-methyl-1H-indol-3-yl)-N-(4-cyanobenzyl)acetamido)acetamido)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (I-16):
(S)-2-(2-(N-(4-cyanobenzyl)-2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)acetamido)acetamido)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (I-17):
(S)-2-(2-(N-cyclopropyl-2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)acetamido)acetamido)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (I-18):
(S)-3-(3,5-difluorophenyl)-2-(2-(N-(4-fluorobenzyl)-2-(3-(trifluoromethyl)-4,5,6,7-tetrahydro-1H-indazol-1-yl)acetamido)acetamido)-N-(4-methoxyphenyl)-N-methylpropanamide (I-19):
(S)-2-(2-(N-(cyclohexylmethyl)-2-(5-methyl-3-(trifluoromethyl)-1H-pyrazol-1-yl)acetamido)acetamido)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (I-20):
(S)-2-(2-(N-benzyl-2-(2-methyl-1H-indol-3-yl)acetamido)acetamido)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (I-21):
(S)-2-(2-(N-benzyl-2-(naphthalen-2-yl)acetamido)acetamido)-3-(3,5-difluorophenyl)-N-(4-methoxyphenyl)-N-methylpropanamide (I-22):
(S)-(4-(2-((2-((3-(3,5-difluorophenyl)-1-((4-methoxyphenyl)(methyl)amino)-1-oxopropan-2-yl)amino)-2-oxoethyl)(4-fluorobenzyl)amino)-2-oxoethyl)phenyl) boronic acid (I-23):
4.2. In Vitro Anti-HIV Assay
4.3. Binding to CA Proteins Analysis via Surface Plasmon Resonance (SPR)
4.4. Molecular Docking and MD Simulation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- De Clercq, E. Antivirals: Past, present and future. Biochem. Pharmacol. 2013, 85, 727–744. [Google Scholar] [CrossRef]
- Smith, R.A.; Wu, V.H.; Zavala, C.G.; Raugi, D.N.; Ba, S.; Seydi, M.; Gottlieb, G.S. In vitro antiviral activity of cabotegravir against HIV-2. Antimicrob. Agents Chemother. 2018, 62, e01299-18. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Arias, L. Molecular basis of human immunodeficiency virus type 1 drug resistance: Overview and recent developments. Antiviral Res. 2013, 98, 93–120. [Google Scholar] [CrossRef] [PubMed]
- Lamoel, A.; Yaniv, O.; Berger, O.; Bacharach, E.; Gazit, E.; Frolow, F. A triclinic crystal structure of the carboxy-terminal domain of HIV-1 capsid protein with four molecules in the asymmetric unit reveals a novel packing interface. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69 Pt 6, 602–606. [Google Scholar]
- Chen, B. HIV capsid assembly, mechanism, and structure. Biochemistry 2016, 55, 2539–2552. [Google Scholar] [CrossRef]
- Chen, N.Y.; Zhou, L.; Gane, P.J.; Opp, S.; Ball, N.J.; Nicastro, G.; Zufferey, M.; Buffone, C.; Luban, J.; Selwood, D.; et al. HIV-1 capsid is involved in post-nuclear entry steps. Retrovirology 2016, 13, 28. [Google Scholar] [CrossRef]
- Lahaye, X.; Satoh, T.; Gentili, M.; Cerboni, S.; Conrad, C.; Hurbain, I.; EI Marjou, A.; Lacabaratz, C.; Lelièvre, J.D.; Manel, N. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity 2013, 39, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Engelman, A.N. Capsid-dependent host factors in HIV-1 infection. Trends Microbiol. 2017, 25, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Pornillos, O.; Ganser-Pornillos, B.K.; Kelly, B.N.; Hua, Y.; Whitby, F.G.; Stout, C.D.; Sundquist, W.I.; Hill, C.P.; Yeager, M. X-ray structures of the hexameric building block of the HIV capsid. Cell 2009, 137, 1282–1292. [Google Scholar] [CrossRef]
- Zhao, G.; Perilla, J.R.; Yufenyuy, E.L.; Meng, X.; Chen, B.; Ning, J.; Ahn, J.; Gronenborn, A.M.; Schulten, K.; Aiken, C.; et al. Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature 2013, 497, 643–646. [Google Scholar] [CrossRef]
- Pornillos, O.; Ganser-Pornillos, B.K.; Yeager, M. Atomic-level modelling of the HIV capsid. Nature 2011, 469, 424–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Loeliger, E.; Kinde, I.; Kyere, S.; Mayo, K.; Barklis, E.; Sun, Y.; Huang, M.; Summers, M.F. Antiviral inhibition of the HIV-1 capsid protein. J. Mol. Biol. 2003, 327, 1013–1020. [Google Scholar] [CrossRef]
- Kelly, B.N.; Kyere, S.; Kinde, I.; Tang, C.; Howard, B.R.; Robinson, H.; Sundqist, W.I.; Summers, M.F.; Hill, C.P. Structure of the antiviral assembly inhibitor CAP-1 complex with the HIV-1 CA protein. J. Mol. Biol. 2007, 373, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Fader, L.D.; Bethell, R.; Bonneau, P.; Bös, M.; Bousquet, Y.; Cordingley, M.G.; Coulombe, R.; Deroy, P.; Faucher, A.M.; Gagnon, A.; et al. Discovery of a 1,5-dihydrobenzo[b][1,4]diazepine-2,4-dione series of inhibitors of HIV-1 capsid assembly. Bioorg. Med. Chem. Lett. 2011, 21, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Fader, L.D.; Landry, S.; Goulet, S.; Morin, S.; Kawai, S.H.; Bousquet, Y.; Dion, I.; Hucke, O.; Goudreau, N.; Lemke, C.T.; et al. Optimization of a 1,5-dihydrobenzo[b][1,4]diazepine-2,4-dione series of HIV capsid assembly inhibitors 2: Structure-activity relationships (SAR) of the C3-phenyl moiety. Bioorg. Med. Chem. Lett. 2013, 23, 3401–3405. [Google Scholar] [CrossRef] [PubMed]
- Lemke, C.T.; Titolo, S.; von Schwedler, U.; Goudreau, N.; Mercier, J.F.; Wardrop, E.; Faucher, A.M.; Coulombe, R.; Banik, S.S.; Fader, L.; et al. Distinct effects of two HIV-1 capsid assembly inhibitor families that bind the same site within the N-terminal domain of the viral CA protein. J. Virol. 2012, 86, 6643–6655. [Google Scholar] [CrossRef]
- Sun, L.; Dick, A.; Meuser, M.E.; Huang, T.; Zalloum, W.A.; Chen, C.H.; Cherukupalli, S.; Xu, S.; Ding, X.; Gao, P.; et al. Design, synthesis, and mechanism study of benzenesulfonamide-containing phenylalanine derivatives as novel HIV-1 capsid inhibitors with improved antiviral activities. J. Med. Chem. 2020, 63, 4790–4810. [Google Scholar] [CrossRef]
- Link, J.O.; Rhee, M.S.; Tse, W.C.; Zheng, J.; Somoza, J.R.; Rowe, W.; Begley, R.; Chiu, A.; Mulato, A.; Hansen, D.; et al. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature 2020, 584, 614–618. [Google Scholar] [CrossRef]
- Blair, W.S.; Pickford, C.; Irving, S.L.; Brown, D.G.; Anderson, M.; Bazin, R.; Cao, J.; Ciaramella, G.; Isaacson, J.; Jackson, L.; et al. HIV capsid is a tractable target for small molecule therapeutic intervention. PLoS Pathog. 2010, 6, e1001220. [Google Scholar] [CrossRef]
- Xu, J.P.; Francis, A.C.; Meuser, M.E.; Mankowski, M.; Ptak, R.G.; Rashad, A.A.; Melikyan, G.B.; Cocklin, S. Exploring modifications of an HIV-1 capsid inhibitor: Design, synthesis, and mechanism of action. J. Drug Des. Res. 2018, 5, 1070. [Google Scholar]
- Meuser, M.E.; Reddy, P.A.N.; Dick, A.; Maurancy, J.M.; Salvino, J.M.; Cocklin, S. Rapid optimization of the metabolic stability of a human immunodeficiency virus type-1 capsid inhibitor using a multistep computational workflow. J. Med. Chem. 2021, 64, 3747–3766. [Google Scholar] [CrossRef]
- Vernekar, S.K.V.; Sahani, R.L.; Casey, M.C.; Kankanala, J.; Wang, L.; Kirby, K.A.; Du, H.; Zhang, H.; Tedbury, P.R.; Xie, J.; et al. Toward structurally novel and metabolically stable HIV-1 capsid-targeting small molecules. Viruses 2020, 12, 452. [Google Scholar] [CrossRef]
- Marcelin, A.G.; Charpentier, C.; Jary, A.; Perrier, M.; Margot, N.; Callebaut, C.; Calvez, V.; Descamps, D. Frequency of capsid substitutions associated with GS-6207 in vitro resistance in HIV-1 from antiretroviral-naive and -experienced patients. J. Antimicrob. Chemother. 2020, 75, 1588–1590. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Casey, M.C.; Vernekar, S.K.V.; Do, H.T.; Sahani, R.L.; Kirby, K.A.; Du, H.; Hachiya, A.; Zhang, H.; Tedbury, P.R.; et al. Chemical profiling of HIV-1 capsid-targeting antiviral PF74. Eur. J. Med. Chem. 2020, 200, 112427. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zalloum, W.A.; Meuser, M.E.; Jing, L.; Kang, D.; Chen, C.H.; Tian, Y.; Zhang, F.; Cocklin, S.; Lee, K.H.; et al. Discovery of phenylalanine derivatives as potent HIV-1 capsid inhibitors from click chemistry-based compound library. Eur. J. Med. Chem. 2018, 158, 478–492. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Alam, S.L.; Fricke, T.; Zadrozny, K.; Sedzicki, J.; Taylor, A.B.; Demeler, B.; Pornillos, O.; Ganser-Pornillos, B.K.; Diaz-Griffero, F.; et al. Structural basis of HIV-1 capsid recognition by PF74 and CPSF6. Proc. Natl. Acad. Sci. USA 2014, 111, 18625–18630. [Google Scholar] [CrossRef]
- Bester, S.M.; Wei, G.; Zhao, H.; Adu-Ampratwum, D.; Iqbal, N.; Courouble, V.V.; Francis, A.C.; Annamalai, A.S.; Singh, P.K.; Shkriabai, N.; et al. Structural and mechanistic bases for a potent HIV-1 capsid inhibitor. Science 2020, 370, 360–364. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, G.; Zalloum, W.A.; Meuser, M.E.; Dick, A.; Sun, L.; Chen, C.H.; Kang, D.; Jing, L.; Jia, R.; et al. Discovery of novel 1,4-disubstituted 1,2,3-triazole phenylalanine derivatives as HIV-1 capsid inhibitors. RSC Adv. 2019, 9, 28961–28986. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Casey, M.C.; Vernekar, S.K.V.; Sahani, R.L.; Kirby, K.A.; Du, H.; Zhang, H.; Tedbury, P.R.; Xie, J.; Sarafianos, S.G.; et al. Novel PF74-like small molecules targeting the HIV-1 capsid protein: Balance of potency and metabolic stability. Acta Pharm. Sin. B 2021, 11, 810–822. [Google Scholar] [CrossRef]
- Sun, L.; Huang, T.; Dick, A.; Meuser, M.E.; Zalloum, W.A.; Chen, C.H.; Ding, X.; Gao, P.; Cocklin, S.; Lee, K.H.; et al. Design, synthesis and structure-activity relationships of 4-phenyl-1H-1,2,3-triazole phenylalanine derivatives as novel HIV-1 capsid inhibitors with promising antiviral activities. Eur. J. Med. Chem. 2020, 190, 112085. [Google Scholar] [CrossRef]
- Clas, S.D.; Sanchez, R.I.; Nofsinger, R. Chemistry-enabled drug delivery (prodrugs): Recent progress and challenges. Drug Discov. Today 2014, 19, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana Birudukota, N.V.; Franke, R.; Hofer, B. An approach to “escape from flatland”: Chemo-enzymatic synthesis and biological profiling of a library of bridged bicyclic compounds. Org. Biomol. Chem. 2016, 14, 3821–3837. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Quinn, R.J. Fragment-based screening with natural products for novel anti-parasitic disease drug discovery. Expert. Opin. Drug Discov. 2019, 14, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Huang, B.; Olotu, F.A.; Li, J.; Kang, D.; Wang, Z.; De Clercq, E.; Soliman, M.E.S.; Pannecouque, C.; Liu, X.; et al. Exploiting the tolerant region I of the non-nucleoside reverse transcriptase inhibitor (NNRTI) binding pocket. Part 2: Discovery of diarylpyrimidine derivatives as potent HIV-1 NNRTIs with high Fsp(3) values and favorable drug-like properties. Eur. J. Med. Chem. 2021, 213, 113051. [Google Scholar] [CrossRef] [PubMed]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Yan, A.; Gasteiger, J. Prediction of aqueous solubility of organic compounds based on a 3D structure representation. J. Chem. Inf. Comput. Sci. 2003, 43, 429–434. [Google Scholar] [CrossRef]
- Dömling, A.; Ugi, I.I. Multicomponent reactions with isocyanides. Angew. Chem. Int. Ed. Engl. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Rocha, R.O.; Rodrigues, M.O.; Neto, B.A.D. Review on the Ugi multicomponent reaction mechanism and the use of fluorescent derivatives as functional chromophores. ACS Omega 2020, 5, 972–979. [Google Scholar] [CrossRef]
- Carnes, S.K.; Sheehan, J.H.; Aiken, C. Inhibitors of the HIV-1 capsid, a target of opportunity. Curr. Opin. HIV AIDS 2018, 13, 359–365. [Google Scholar] [CrossRef]
- Xu, J.P.; Branson, J.D.; Lawrence, R.; Cocklin, S. Identification of a small molecule HIV-1 inhibitor that targets the capsid hexamer. Bioorg. Med. Chem. Lett. 2016, 26, 824–828. [Google Scholar] [CrossRef]
- Bravman, T.; Bronner, V.; Lavie, K.; Notcovich, A.; Papalia, G.A.; Myszka, D.G. Exploring “one-shot” kinetics and small molecule analysis using the ProteOn XPR36 array biosensor. Anal. Biochem. 2006, 358, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Sharma, P.P.; Rathi, B.; Ji, X.; Hu, L.; Gao, Z.; Kang, D.; Wang, Z.; Xie, M.; Xu, S.; et al. Discovery of novel 1,2,4-triazole phenylalanine derivatives targeting an unexplored region within the interprotomer pocket of the HIV capsid protein. J. Med. Virol. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Miyake, A.; Doi, N.; Koma, T.; Uchiyama, T.; Adachi, A.; Nomaguchi, M. Comparison of biochemical properties of HIV-1 and HIV-2 capsid proteins. Front. Microbiol. 2017, 8, 1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]



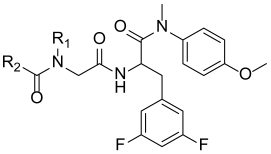 | |||||||
|---|---|---|---|---|---|---|---|
| Compound ID | R1 | R2 | EC50 (µM) a | CC50 (µM) b | SI c | ||
| HIV-1 | HIV-2 | HIV-1 | HIV-2 | ||||
| I-6 | 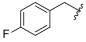 |  | >160.20 | >160.20 | >160.20 | X1 d | X1 |
| I-7 | 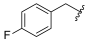 | 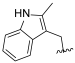 | 2.79 ± 0.19 | >22.59 | 22.59 ± 0.96 | 8 | X1 |
| I-8 | 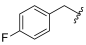 | 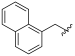 | >191.35 | 5.87 ± 1.79 | >191.35 | X1 | >33 |
| I-9 | 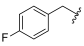 | 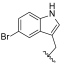 | 2.93 ± 0.32 | >17.84 | 17.84 ± 1.04 | 6 | <1 |
| I-10 | 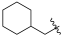 | 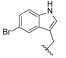 | >17.61 | >17.61 | 17.61 ± 2.41 | <1 | <1 |
| I-11 | 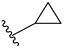 | 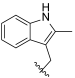 | 3.08 ± 0.15 | 17.33 ± 3.79 | 111.60 ± 7.99 | 36 | 6 |
| I-12 | 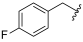 | 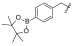 | 11.73 ± 2.07 | 13.21 ± 2.60 | 65.40 ± 16.77 | 6 | 5 |
| I-13 | 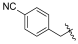 | 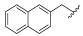 | >189.32 | 161.33 ± 26.04 | >189.32 | X1 | >1 |
| I-14 |  | 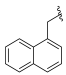 | >189.33 | 2.30 ± 0.11 | >189.32 | X1 | >82 |
| I-15 |  | 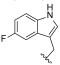 | 17.25 ± 9.98 | 5.81 ± 1.11 | 148.30 ± 16.63 | 9 | 26 |
| I-16 | 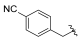 | 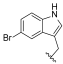 | 5.61 ± 1.18 | 15.71 ± 13.30 | 87.59 ± 62.25 | 16 | 6 |
| I-17 | 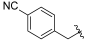 | 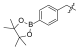 | 17.65 ± 4.60 | 16.16 ± 4.59 | 52.41 ± 14.22 | 3 | 3 |
| I-18 | 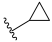 |  | 5.18 ± 1.11 | 9.71 ± 0.80 | 89.05 ± 17.06 | 17 | 9 |
| I-19 | 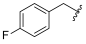 | 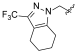 | 2.53 ± 0.84 | 12.93 ± 3.72 | 107.61 ± 27.43 | 42 | 8 |
| I-20 | 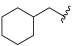 | 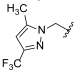 | 30.29 ± 3.24 | 24.90 ± 12.04 | 45.66 ± 5.36 | 2 | 2 |
| I-21 |  | 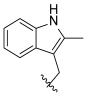 | 2.77 ± 0.77 | 6.52 ± 0.60 | 28.63 ± 0.86 | 10 | 4 |
| I-22 | 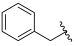 | 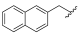 | >176.28 | >18.87 | 176.28 ± 7.35 | <1 | <1 |
| I-23 | 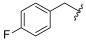 |  | 4.33 ± 1.32 | 6.80 ± 0.36 | 18.91 ± 3.59 | 4 | 3 |
| PF-74 | 0.26 ± 0.08 | 2.22 ± 0.31 | 73.83 ± 7.54 | 297 | 33 | ||
| NVP | 0.12 ± 0.027 | >15.02 | >15.02 | >123 | X1 | ||
| Compound ID | KD (nM) a | Kon (M−1S−1) | Koff (S−1) | Ratio b | |||
|---|---|---|---|---|---|---|---|
| Monomer | Hexamer | Monomer | Hexamer | Monomer | Hexamer | ||
| I-19 | 13,338.0 ± 7793.4 | 36,866.7 ± 12012.6 | 4160 ± 1670 | 628 ± 152 | 0.0301 ± 0.0172 | 0.00758 ± 0.000504 | 0.36 |
| PF-74 | 968.5 ± 446.7 | 47.0 ± 0.6 | 1,770,000 ± 1,460,000 | 756,000 ± 45,500 | 1.58 ± 0.628 | 0.0363 ± 0.00692 | 21 |
| I-19 | PF-74 | I-19 | PF-74 |
|---|---|---|---|
| −56.63870297 | −48.4265 | −47.64271626 | −42.9916 |
| −58.18585033 | −47.3288 | −59.70576824 | −42.4198 |
| −44.63984043 | −50.9128 | −53.24015651 | −38.9422 |
| −51.85506041 | −48.0358 | −50.34292794 | −39.1304 |
| −69.09500139 | −50.5226 | −48.40161062 | −40.9924 |
| −52.42678458 | -42.4339 | −48.80203871 | −40.818 |
| −37.40863362 | −48.288 | −51.18628072 | −45.1481 |
| −56.59643919 | −43.116 | −57.96423626 | |
| −58.20409472 | −48.9477 | −51.31042621 | |
| −61.13273155 | −44.6422 | −55.20319794 | |
| −56.26117453 | −50.9619 | −47.39770192 | |
| −42.66745202 | −47.8149 | −46.58669711 | |
| −39.143513 | −49.8492 | −67.89125429 | |
| −52.88091489 | −40.7626 | −58.99503816 | |
| −57.51528574 | −39.4291 | −45.4356079 | |
| −48.50930805 | −43.8134 | −59.82486366 | |
| −46.0713223 | −45.8949 | −57.89411038 | |
| −61.01377527 | −45.0506 | −58.09693178 | |
| −59.25622436 | −44.6718 | −60.99124817 | |
| −52.76749689 | −41.9282 | −66.82186876 | |
| −56.22260111 | −36.215 | −50.50954655 | |
| −44.7446381 | −43.4079 | −52.43726697 | |
| −58.74678608 | −40.6441 | −56.73687394 |
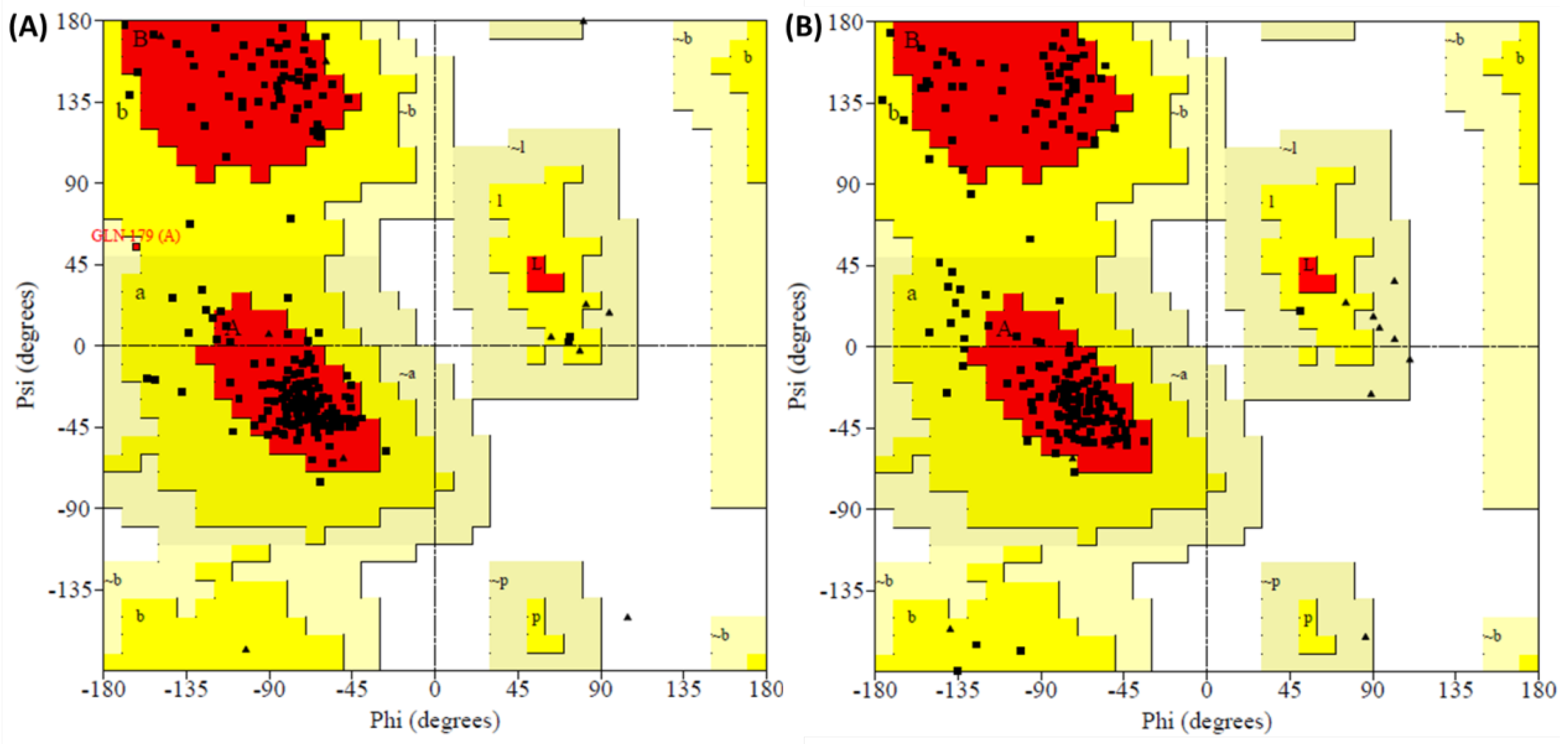
| Complex | Residues in Favored Region | Residues in Additional Allowed Region | Residues in Generously Allowed Region | Residues in Disallowed Region |
|---|---|---|---|---|
| I-19-5TSX | 89.3% (167) | 10.2% (19) | 0.5% (1) | 0.0% (0) |
| PF-74-5TSX | 85.6% (160) | 14.4% (27) | 0.0% (0) | 0.0% (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Li, J.; Sharma, P.P.; Jiang, X.; Rathi, B.; Gao, Z.; Hu, L.; Kang, D.; De Clercq, E.; Cocklin, S.; et al. Design, Synthesis and Structure—Activity Relationships of Phenylalanine-Containing Peptidomimetics as Novel HIV-1 Capsid Binders Based on Ugi Four-Component Reaction. Molecules 2022, 27, 5995. https://doi.org/10.3390/molecules27185995
Ji X, Li J, Sharma PP, Jiang X, Rathi B, Gao Z, Hu L, Kang D, De Clercq E, Cocklin S, et al. Design, Synthesis and Structure—Activity Relationships of Phenylalanine-Containing Peptidomimetics as Novel HIV-1 Capsid Binders Based on Ugi Four-Component Reaction. Molecules. 2022; 27(18):5995. https://doi.org/10.3390/molecules27185995
Chicago/Turabian StyleJi, Xiangkai, Jing Li, Prem Prakash Sharma, Xiangyi Jiang, Brijesh Rathi, Zhen Gao, Lide Hu, Dongwei Kang, Erik De Clercq, Simon Cocklin, and et al. 2022. "Design, Synthesis and Structure—Activity Relationships of Phenylalanine-Containing Peptidomimetics as Novel HIV-1 Capsid Binders Based on Ugi Four-Component Reaction" Molecules 27, no. 18: 5995. https://doi.org/10.3390/molecules27185995
APA StyleJi, X., Li, J., Sharma, P. P., Jiang, X., Rathi, B., Gao, Z., Hu, L., Kang, D., De Clercq, E., Cocklin, S., Liu, C., Pannecouque, C., Dick, A., Liu, X., & Zhan, P. (2022). Design, Synthesis and Structure—Activity Relationships of Phenylalanine-Containing Peptidomimetics as Novel HIV-1 Capsid Binders Based on Ugi Four-Component Reaction. Molecules, 27(18), 5995. https://doi.org/10.3390/molecules27185995









