Integrating Anti-Influenza Virus Activity and Chemical Pattern Recognition to Explore the Quality Evaluation Method of Lonicerae Japonicae Flos
Abstract
:1. Introduction
2. Results
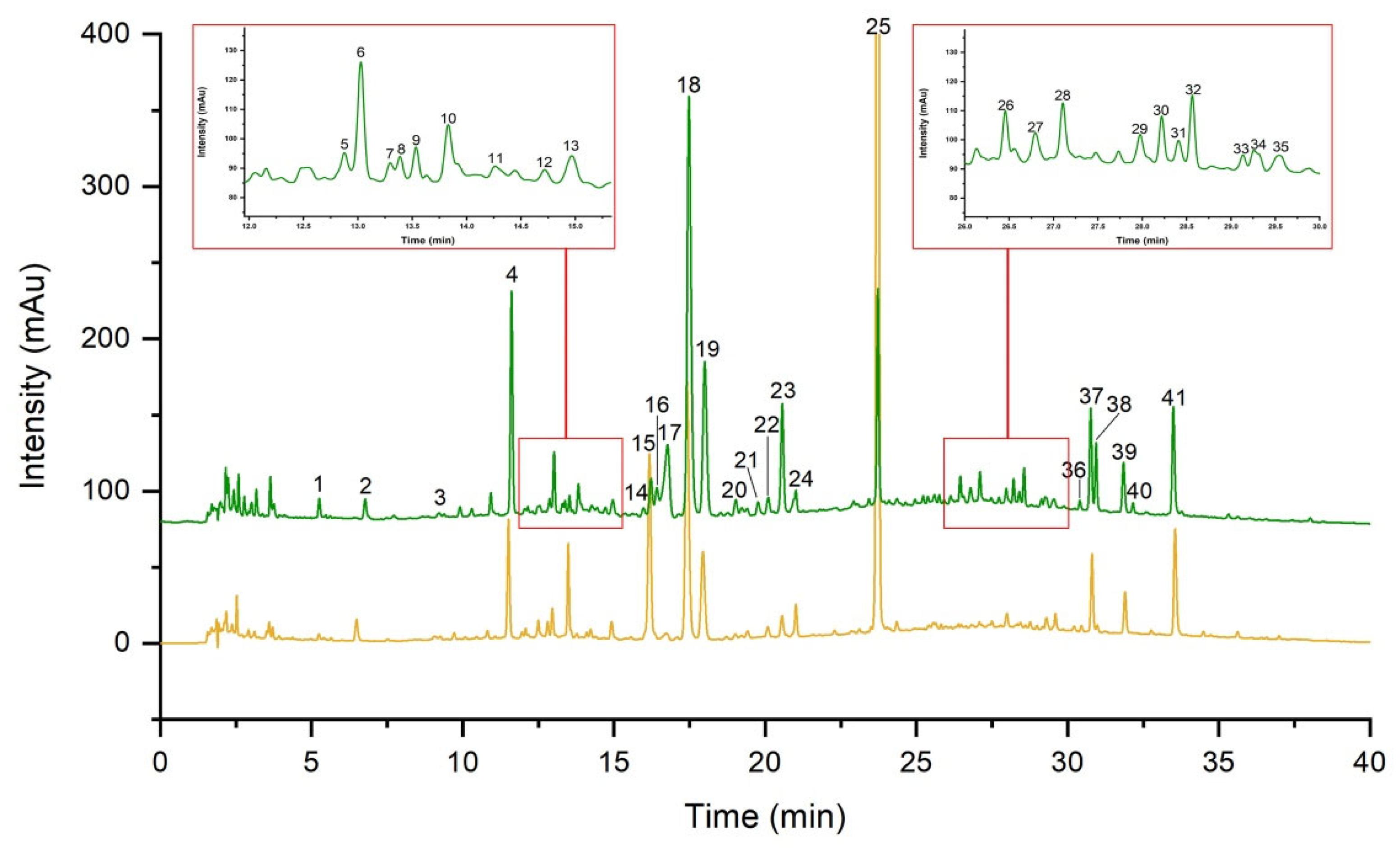
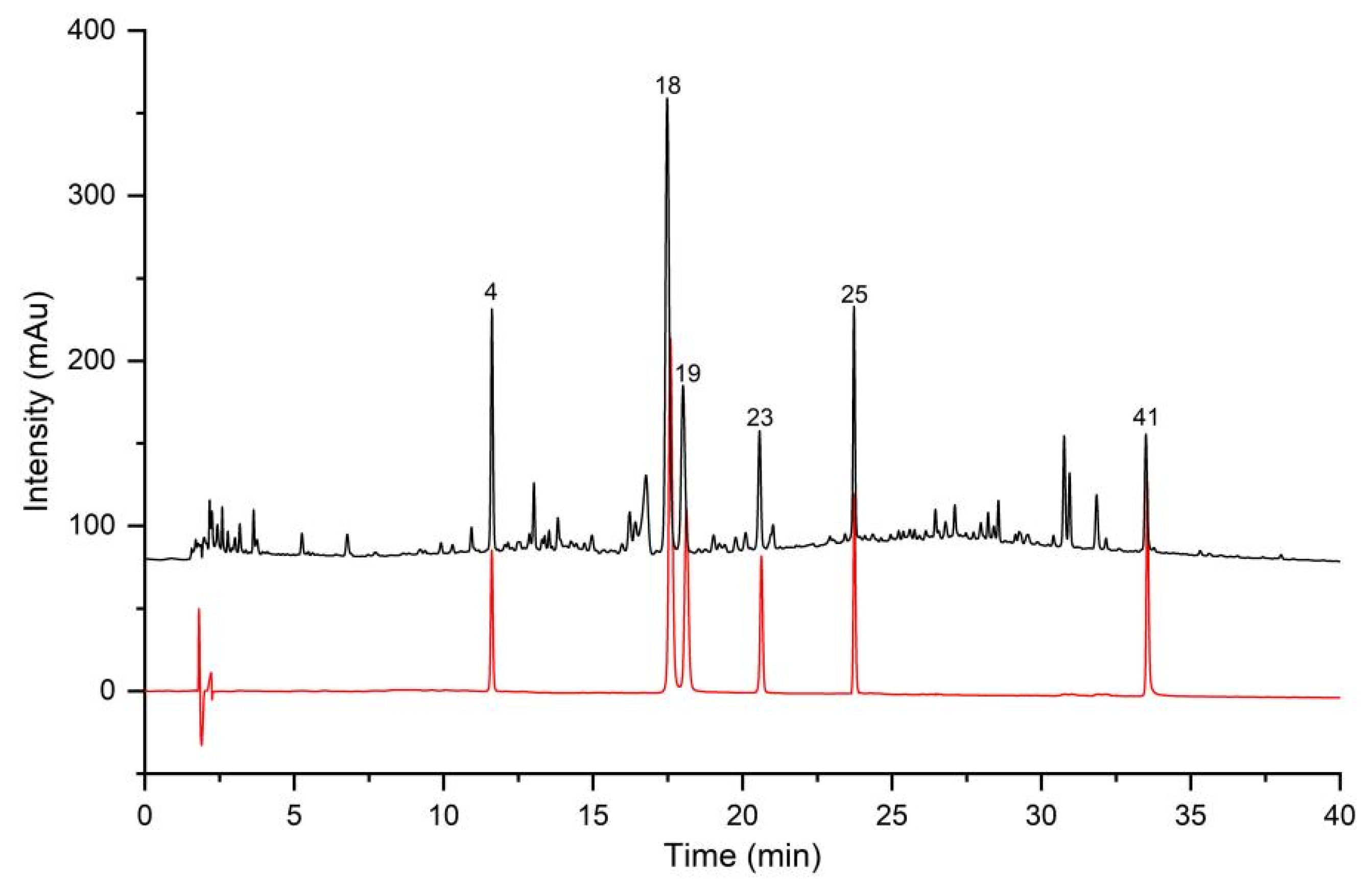
2.1. Method Validation for UHPLC Fingerprint
2.2. Similarity Analysis of UHPLC Fingerprint
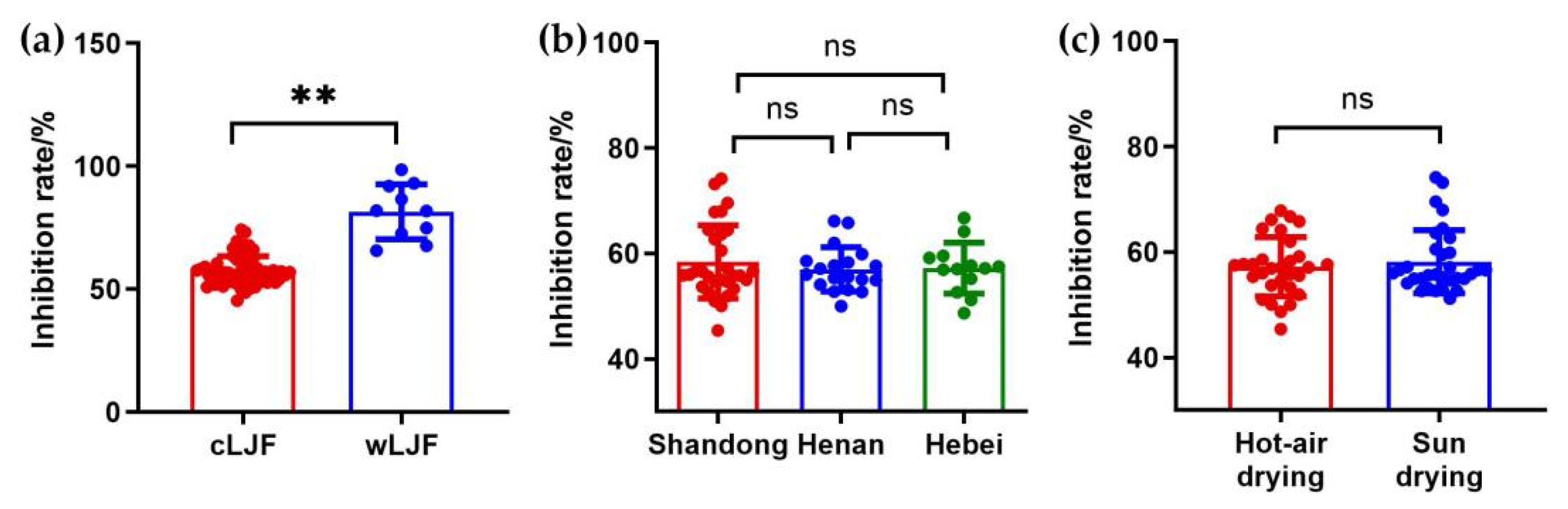
2.3. Anti-Influenza Virus Activity Determination
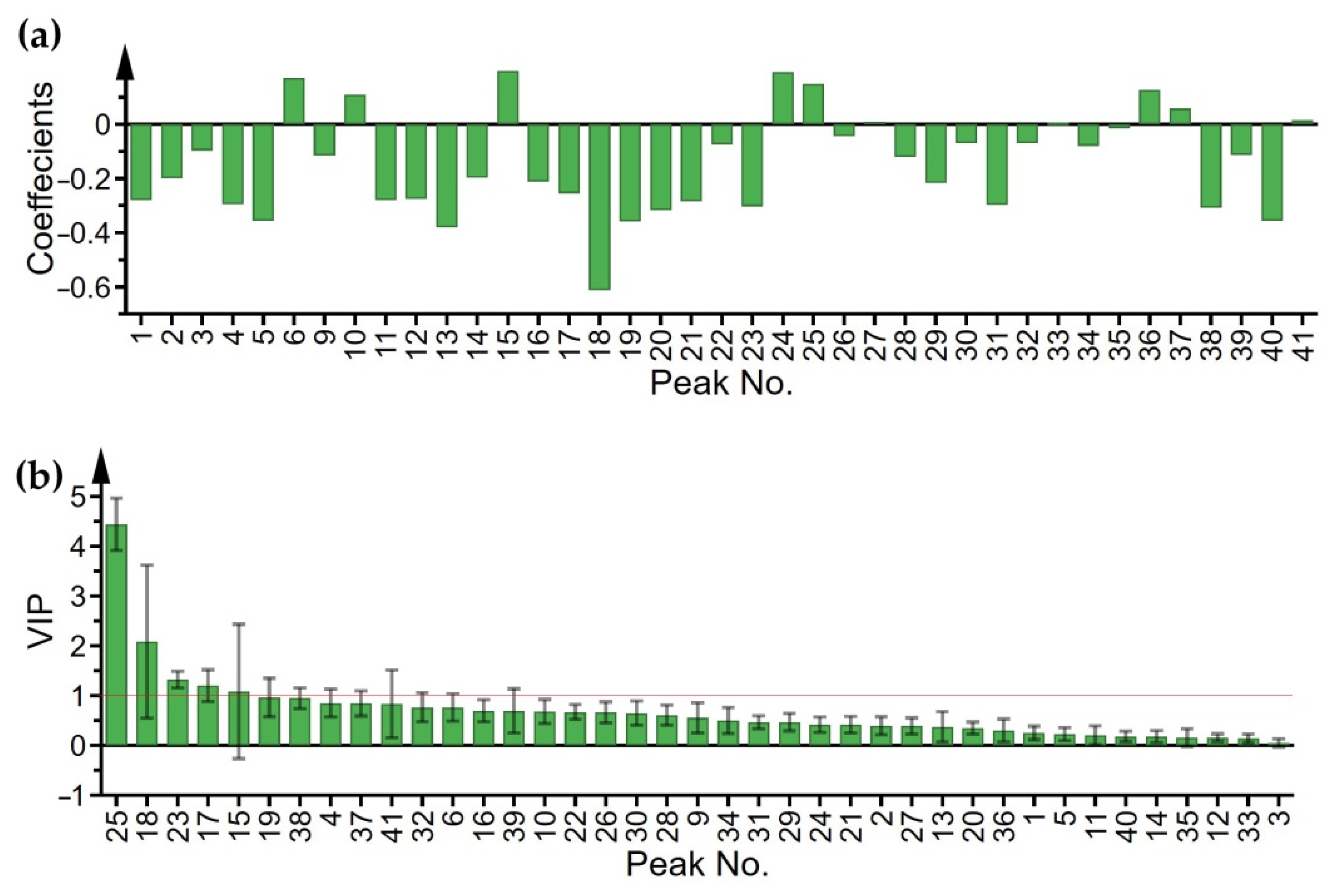
2.4. Spectrum-Effect Correlation Analysis
2.5. Identification of Bioactive Peaks in Lonicerae Japonicae Flos
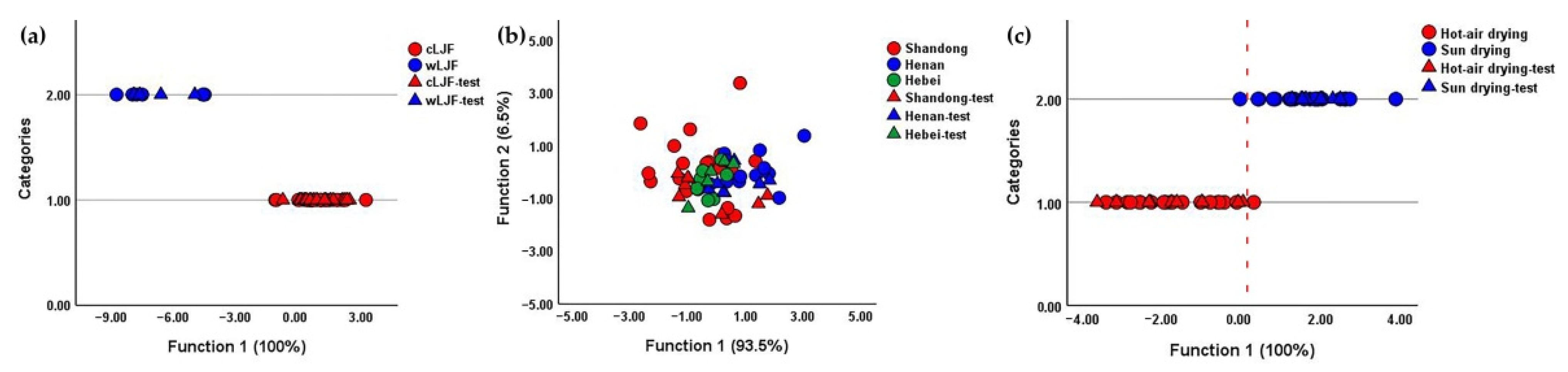
2.6. Quality Evaluation of Lonicerae Japonicae Flos by Chemical Pattern Recognition
2.7. Quantitative Analysis of Bioactive Compounds
2.8. Confirmation of Bioactive Compounds with NA inhibition
2.9. Methodological Validation of Quantification Procedures
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents and Materials
4.2. Preparation of Sample Solutions and Standard Solutions
4.3. Instrumentation and Chromatographic Conditions
4.4. Similarity Analysis
4.5. NA Inhibitor Screening Assay
4.6. Spectrum-Effect Correlation Analysis
4.7. Quality Evaluation of Lonicerae Japonicae Flos by Chemical Pattern Recognition
4.8. UPLC/Q-TOF/MS Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef]
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Liu, J.; Dong, L.; Chen, Q.; Liu, J.; Kong, H.; Zhang, Q.; Qi, X.; Hou, D.; et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza a viruses. Cell Res. 2015, 25, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Jin, J.; Dou, J.; Guo, Q.; Ke, X.; Zhou, C.; Guo, M. Inhibitory activity of honeysuckle extracts against influenza a virus in vitro and in vivo. Virol. Sin. 2021, 36, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Liu, B.; Qin, H.; Lee, S.; Wang, Y.; Du, G. Anti-Influenza Virus Activities of Flavonoids from the Medicinal Plant Elsholtzia rugulosa. Planta Med. 2008, 74, 847–851. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Omichi, Y.; Kurimoto, S.; Shibata, H.; Miyake, Y.; Kirimoto, T.; Takaishi, Y. Conjugates of a secoiridoid glucoside with a phenolic glucoside from the flower buds of Lonicera japonica Thunb. Phytochemistry 2013, 96, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lai, C.; Zhang, M.; Zhou, S.; Liu, Q.; Wang, D.; Geng, Y.; Wang, X. An improved 2D-HPLC-UF-ESI-TOF/MS approach for enrichment and comprehensive characterization of minor neuraminidase inhibitors from Flos Lonicerae Japonicae. J. Pharmaceut. Biomed. 2019, 175, 112758. [Google Scholar] [CrossRef]
- Zhou, W.; Yin, A.; Shan, J.; Wang, S.; Cai, B.; Di, L. Study on the rationality for antiviral activity of flos lonicerae Japonicae-Fructus forsythiae herb couple preparations improved by Chito-Oligosaccharide via integral pharmacokinetics. Molecules 2017, 22, 654. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.; Golonko, A.; Świsłocka, R.; Kalinowska, M.; Parcheta, M.; Swiergiel, A.; Lewandowski, W. Drug design strategies for the treatment of viral disease. Plant phenolic compounds and their derivatives. Front. Pharmacol. 2021, 12, 709104. [Google Scholar] [CrossRef]
- Chen, S.; Sun, X.; Liu, X.; Dai, X. Comparison and analysis of wild and cultivated lonicerae japonicae flos from xinmi. J. Chin. Med. Mater. 2014, 37, 587–589. [Google Scholar]
- Zhao, Z.; Guo, P.; Brand, E. The formation of daodi medicinal materials. J. Ethnopharmacol. 2012, 140, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Heinrich, M.; Zou, Y.; Reich, E.; Zhang, X.; Chen, Y.; Weckerle, C.S. Quality Variation of Goji (Fruits of Lycium spp.) in China: A Comparative Morphological and Metabolomic Analysis. Front. Pharmacol. 2018, 9, 151. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. 2021, 61, 1763–1786. [Google Scholar] [CrossRef]
- Jintao, X.; Quanwei, Y.; Chunyan, L.; Xiaolong, L.; Bingxuan, N. Rapid and simultaneous quality analysis of the three active components in Lonicerae Japonicae Flos by near-infrared spectroscopy. Food Chem. 2021, 342, 128386. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, X.; Hao, X.; Feng, S.; Hu, Y.; Yang, Y.; Wang, H.; Yu, Y. Untargeted metabolomics study of Lonicerae japonicae flos processed with different drying methods via GC-MS and UHPLC-HRMS in combination with chemometrics. Ind. Crop. Prod. 2022, 186, 115179. [Google Scholar] [CrossRef]
- Zheng, C.; Li, W.; Yao, Y.; Zhou, Y. Quality evaluation of atractylodis macrocephalae rhizoma based on combinative method of HPLC fingerprint, quantitative analysis of Multi-Components and chemical pattern recognition analysis. Molecules 2021, 26, 7124. [Google Scholar] [CrossRef]
- Ma, D.; Wang, L.; Jin, Y.; Gu, L.; Yu, X.; Xie, X.; Yin, G.; Wang, J.; Bi, K.; Lu, Y.; et al. Application of UHPLC fingerprints combined with chemical pattern recognition analysis in the differentiation of six rhodiola species. Molecules 2021, 26, 6855. [Google Scholar] [CrossRef]
- Gu, L.; Xie, X.; Wang, B.; Jin, Y.; Wang, L.; Yin, G.; Wang, J.; Bi, K.; Wang, T. Chemical pattern recognition for quality analysis of lonicerae japonicae flos and lonicerae flos based on Ultra-High performance liquid chromatography and Anti-SARS-CoV2 main protease activity. Front. Pharmacol. 2022, 12, 810748. [Google Scholar] [CrossRef]
- Gubareva, L.V.; Webster, R.G.; Hayden, F.G. Detection of influenza virus resistance to neuraminidase inhibitors by an enzyme inhibition assay. Antivir. Res. 2002, 53, 47–61. [Google Scholar] [CrossRef]
- Lee, J.; Abundo, M.E.C.; Lee, C. Herbal medicines with antiviral activity against the influenza virus, a systematic review. Am. J. Chinese Med. 2019, 46, 1663–1700. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Chen, C.; Li, P. Structural characterization and identification of iridoid glycosides, saponins, phenolic acids and flavonoids in Flos Lonicerae Japonicae by a fast liquid chromatography method with diode-array detection and time-of-flight mass spectrometry. Rapid Commun. Mass Sp. 2009, 23, 3227–3242. [Google Scholar] [CrossRef]
- Pan, H.; Zhou, H.; Miao, S.; Cao, J.; Liu, J.; Lan, L.; Hu, Q.; Mao, X.; Ji, S. An integrated approach for global profiling of multi-type constituents: Comprehensive chemical characterization of Lonicerae Japonicae Flos as a case study. J. Chromatogr. A 2020, 1613, 460674. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, W.; Hao, M.; Liu, X.; Xiao, J.; Wang, T.; Dong, Y.; Zhao, J. How chinese herbal medicine prevents epidemics: From ancient pestilences to COVID-19 pandemic. Am. J. Chinese Med. 2021, 49, 1017–1044. [Google Scholar] [CrossRef]
- Gubareva, L.V.; Kaiser, L.; Hayden, F.G. Influenza virus neuraminidase inhibitors. Lancet 2000, 355, 827–835. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, A.; Kong, L.; Han, Y.; Yan, G.; Sun, H.; Wang, X. Analytical strategies for the discovery and validation of quality-markers of traditional Chinese medicine. Phytomedicine 2020, 67, 153165. [Google Scholar] [CrossRef]
- An, L.; Yu, X.; Liu, W.; Li, J.; Chang, Y. Identification and Screening of Natural Neuraminidase Inhibitors from Reduning Injection via One-Step High-Performance Liquid Chromatography-Fraction Collector and UHPLC/Q-TOF-MS. Int. J. Anal. Chem. 2020, 2020, 8838025. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, Z.; Cao, L.; Ding, G.; Wang, Z.; Xiao, W. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Sci. Rep. 2017, 7, 45723. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Masteikova, R.; Majiene, D.; Savickas, A.; Kevelaitis, E.; Bernatoniene, R.; Dvorácková, K.; Civinskiene, G.; Lekas, R.; Vitkevicius, K.; et al. Free radical-scavenging activities of Crataegus monogyna extracts. Medicina 2008, 44, 706–712. [Google Scholar] [CrossRef]
- Nagata, H.; Inagaki, Y.; Yamamoto, Y.; Maeda, K.; Kataoka, K.; Osawa, K.; Shizukuishi, S. Inhibitory effects of macrocarpals on the biological activity of Porphyromonas gingivalis and other periodontopathic bacteria. Oral Microbiol. Immunol. 2006, 21, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Mallik, A.U.; Hamilton, J. Harvest date and storage effect on fruit size, phenolic content and antioxidant capacity of wild blueberries of NW Ontario, Canada. J. Food Sci. Technol. 2017, 54, 1545–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.H.; Lee, H.K.; Kim, J.A.; Hong, S.I.; Kim, H.C.; Jo, T.H.; Park, Y.I.; Lee, C.K.; Kim, Y.B.; Lee, S.Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef]
- Hao, J.; Wu, Z.; Li, W.; Zhang, Z.; Gu, J. A new PAT application: Optimization of processing methods for honeysuckle flower (Lonicerae Japonicae Flos) and wild honeysuckle flower (Lonicerae Flos). J. Tradit. Chinese Med. Sci. 2018, 5, 199–205. [Google Scholar] [CrossRef]
- Souihi, N.; Lindegren, A.; Eriksson, L.; Trygg, J. OPLS in batch monitoring–Opens up new opportunities. Anal. Chim. Acta 2015, 857, 28–38. [Google Scholar] [CrossRef]
- Ortiz-Herrero, L.; Cardaba, I.; Setien, S.; Bartolomé, L.; Alonso, M.L.; Maguregui, M.I. OPLS multivariate regression of FTIR-ATR spectra of acrylic paints for age estimation in contemporary artworks. Talanta 2019, 205, 120114. [Google Scholar] [CrossRef]
- Qiao, R.; Zhou, L.; Zhong, M.; Zhang, M.; Yang, L.; Yang, Y.; Chen, H.; Yang, W.; Yuan, J. Spectrum-effect relationship between UHPLC-Q-TOF/MS fingerprint and promoting gastrointestinal motility activity of Fructus aurantii based on multivariate statistical analysis. J. Ethnopharmacol. 2021, 279, 114366. [Google Scholar] [CrossRef]
- Tunnisa, F.; Nur Faridah, D.; Afriyanti, A.; Rosalina, D.; Ana Syabana, M.; Darmawan, N.; Dewi Yuliana, N. Antioxidant and antidiabetic compounds identification in several Indonesian underutilized Zingiberaceae spices using SPME-GC/MS-based volatilomics and in silico methods. Food Chem. X 2022, 14, 100285. [Google Scholar] [CrossRef] [PubMed]
- Guenaou, I.; Nait Irahal, I.; Errami, A.; Lahlou, F.A.; Hmimid, F.; Bourhim, N. Bioactive Compounds from Ephedra fragilis: Extraction Optimization, Chemical Characterization, Antioxidant and AntiGlycation Activities. Molecules 2021, 26, 5998. [Google Scholar] [CrossRef]
- Shi, J.; Li, Z.; Wu, J.; Jin, W.; Chang, X.; Sun, H.; Dong, L.; Jiang, Z.; Shi, Y. Identification of the bioactive components of Banxia Xiexin Decoction that protect against CPT-11-induced intestinal toxicity via UPLC-based spectrum-effect relationship analyses. J. Ethnopharmacol. 2021, 266, 113421. [Google Scholar] [CrossRef]
- Wu, X.; Chen, H.; Zhou, X.; Huang, Y.; Hu, E.; Jiang, Z.; Zhao, C.; Gong, X.; Deng, Q. Studies on Chromatographic Fingerprint and Fingerprinting Profile-Efficacy Relationship of Saxifraga stolonifera Meerb. Molecules 2015, 20, 22781–22798. [Google Scholar] [CrossRef]
- Elena Arce, M.; Saavedra, Á.; Míguez, J.L.; Granada, E. The use of grey-based methods in multi-criteria decision analysis for the evaluation of sustainable energy systems: A review. Renew. Sust. Energ. Rev. 2015, 47, 924–932. [Google Scholar] [CrossRef]
- Lü, S.; Dong, S.; Xu, D.; Duan, J.; Li, G.; Guo, Y.; Kuang, H.; Wang, Q. Spectrum-Effect Relationships between Fingerprints of Caulophyllum robustum Maxim and Inhabited Pro-Inflammation Cytokine Effects. Molecules 2017, 22, 1826. [Google Scholar] [CrossRef]
- Boukerche, A.; Zheng, L.; Alfandi, O. Outlier detection. ACM Comput. Surv. 2021, 53, 1–37. [Google Scholar] [CrossRef]
- Fawcett, T. An introduction to ROC analysis. Pattern Recogn. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Cerqueira, B.V.; Barbon, S.; Barbin, D.F. Classification of fermented cocoa beans (cut test) using computer vision. J. Food Compos. Anal. 2021, 97, 103771. [Google Scholar] [CrossRef]

| Sample No. | Inhibition Rate (%) | Sample No. | Inhibition Rate (%) | Sample No. | Inhibition Rate (%) |
|---|---|---|---|---|---|
| S1 | 69.59 ± 1.44 | S26 | 50.06 ± 1.20 | S51 | 56.90 ± 0.36 |
| S2 | 55.72 ± 1.19 | S27 | 50.98 ± 0.84 | S52 | 64.20 ± 0.59 |
| S3 | 64.52 ± 1.04 | S28 | 45.40 ± 0.66 | S53 | 66.73 ± 0.27 |
| S4 | 73.20 ± 2.43 | S29 | 55.80 ± 1.10 | S54 | 57.74 ± 1.29 |
| S5 | 74.16 ± 1.17 | S30 | 57.68 ± 1.41 | S55 | 57.52 ± 0.55 |
| S6 | 55.98 ± 0.82 | S31 | 55.98 ± 0.40 | S56 | 48.70 ± 0.70 |
| S7 | 68.02 ± 0.40 | S32 | 57.70 ± 0.39 | S57 | 51.17 ± 0.30 |
| S8 | 62.72 ± 1.44 | S33 | 62.03 ± 0.13 | S58 | 52.61 ± 0.92 |
| S9 | 52.67 ± 1.42 | S34 | 66.17 ± 1.35 | S59 | 59.57 ± 0.59 |
| S10 | 55.04 ± 1.31 | S35 | 57.13 ± 0.09 | S60 | 55.21 ± 1.87 |
| S11 | 54.68 ± 1.07 | S36 | 58.55 ± 0.27 | S61 | 57.21 ± 0.35 |
| S12 | 56.66 ± 0.57 | S37 | 65.83 ± 0.46 | S62 | 93.20 ± 1.34 |
| S13 | 55.90 ± 1.08 | S38 | 58.28 ± 1.75 | S63 | 86.71 ± 0.73 |
| S14 | 60.52 ± 0.45 | S39 | 50.02 ± 0.73 | S64 | 81.91 ± 0.14 |
| S15 | 55.64 ± 0.34 | S40 | 55.37 ± 0.28 | S65 | 81.96 ± 0.21 |
| S16 | 57.09 ± 0.66 | S41 | 55.56 ± 0.40 | S66 | 92.04 ± 0.39 |
| S17 | 56.68 ± 0.32 | S42 | 52.72 ± 1.40 | S67 | 67.71 ± 0.39 |
| S18 | 56.59 ± 0.22 | S43 | 54.98 ± 0.42 | S68 | 74.98 ± 0.09 |
| S19 | 63.68 ± 0.64 | S44 | 50.86 ± 0.08 | S69 | 72.72 ± 1.42 |
| S20 | 64.44 ± 0.55 | S45 | 52.77 ± 1.34 | S70 | 65.73 ± 0.35 |
| S21 | 67.89 ± 0.16 | S46 | 55.07 ± 0.68 | S71 | 98.66 ± 0.46 |
| S22 | 53.71 ± 1.83 | S47 | 54.10 ± 0.68 | ||
| S23 | 54.63 ± 0.92 | S48 | 53.12 ± 1.17 | ||
| S24 | 51.90 ± 0.30 | S49 | 59.18 ± 0.52 | ||
| S25 | 53.27 ± 0.74 | S50 | 57.04 ± 1.39 |
| Peak No. | Coefficient (OPLS) | VIP (OPLS) | r (Pearson) | GRA |
|---|---|---|---|---|
| 2 | 0.989 | |||
| 4 | −0.295 | 0.993 | ||
| 5 | −0.357 | |||
| 13 | −0.381 | 0.993 | ||
| 16 | −0.650 | |||
| 17 | 1.202 | −0.722 | 0.989 | |
| 18 | −0.612 | 2.086 | −0.654 | 0.994 |
| 19 | −0.358 | 0.994 | ||
| 20 | −0.317 | |||
| 22 | −0.706 | |||
| 23 | −0.303 | 1.322 | −0.772 | 0.990 |
| 25 | 4.441 | 0.623 | ||
| 31 | −0.296 | |||
| 34 | −0.614 | |||
| 37 | 0.993 | |||
| 38 | −0.309 | |||
| 39 | 0.993 | |||
| 40 | −0.356 | |||
| 41 | −0.745 | 0.994 |
| Classification Items | Accuracy (%) | ||
|---|---|---|---|
| Training Set | Cross-Validation | Testing Set | |
| Cultivation pattern | 100.00 | 100.00 | 100.00 |
| Geographical origin (hot-air-dried samples) | 90.00 | 70.00 | 70.00 |
| Geographical origin (sun-dried samples) | 85.70 | 50.00 | 57.10 |
| Geographical origin | 65.90 | 40.00 | 39.00 |
| Processing method | 95.10 | 92.70 | 95.00 |
| Classification Items | Categories | Precision | Recall | F-Score |
|---|---|---|---|---|
| Cultivation pattern | Training set | |||
| cLJF | 1.000 | 1.000 | 1.000 | |
| wLJF | 1.000 | 1.000 | 1.000 | |
| Testing set | ||||
| cLJF | 1.000 | 1.000 | 1.000 | |
| wLJF | 1.000 | 1.000 | 1.000 | |
| Geographical origin (hot-air-dried samples) | Training set | |||
| Shandong | 1.000 | 0.833 | 0.909 | |
| Henan | 1.000 | 0.889 | 0.941 | |
| Hebei | 0.714 | 1.000 | 0.833 | |
| Testing set | ||||
| Shandong | 1.000 | 0.250 | 0.400 | |
| Henan | 0.600 | 1.000 | 0.750 | |
| Hebei | 0.750 | 1.000 | 0.857 | |
| Geographical origin (sun-dried samples) | Training set | |||
| Shandong | 1.000 | 0.929 | 0.963 | |
| Henan | 0.667 | 0.500 | 0.572 | |
| Hebei | 0.600 | 1.000 | 0.750 | |
| Testing set | ||||
| Shandong | 1.000 | 0.800 | 0.889 | |
| Henan | 0.000 | 0.000 | 0.000 | |
| Hebei | 0.250 | 0.500 | 0.333 | |
| Geographical origin | Training set | |||
| Shandong | 0.800 | 0.600 | 0.686 | |
| Henan | 0.750 | 0.692 | 0.720 | |
| Hebei | 0.429 | 0.750 | 0.546 | |
| Testing set | ||||
| Shandong | 0.600 | 0.333 | 0.428 | |
| Henan | 0.500 | 0.500 | 0.500 | |
| Hebei | 0.222 | 0.400 | 0.286 | |
| Processing method | Training set | |||
| Hot-air drying | 0.950 | 0.950 | 0.950 | |
| Sun drying | 0.952 | 0.952 | 0.952 | |
| Testing set | ||||
| Hot-air drying | 1.000 | 0.900 | 0.947 | |
| Sun drying | 0.909 | 1.000 | 0.952 |
| Peak No. | Compound | Inhibition Rate (%) 1 | IC50(μM) |
|---|---|---|---|
| P4 | Neochlorogenic acid | 76.00 | 157.3 |
| P18 | Chlorogenic acid | 74.81 | 139.0 |
| P19 | Cryptochlorogenic acid | 54.89 | 289.9 |
| P23 | Sweroside | 44.16 | - |
| P25 | Secoxyloganin | 37.74 | - |
| P41 | 4,5-Di-O-caffeoylquinic acid | 75.90 | 131.8 |
| Sample No. | Species | Cultivation Patterns | Processing Methods | Geographical Origins |
|---|---|---|---|---|
| S1–S19 | Lonicerae japonicae flos | Cultivated | Sun drying | Shandong province |
| S20–S29 | Lonicerae japonicae flos | Cultivated | Hot-air drying | Shandong province |
| S30–S41 | Lonicerae japonicae flos | Cultivated | Sun drying | Henan province |
| S42–S48 | Lonicerae japonicae flos | Cultivated | Hot-air drying | Henan province |
| S49–S56 | Lonicerae japonicae flos | Cultivated | Sun drying | Hebei province |
| S57–S61 | Lonicerae japonicae flos | Cultivated | Hot-air drying | Hebei province |
| S62–S71 | Lonicerae japonicae flos | Wild | Sun drying | Hubei province |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Gu, L.; Xu, W.; Yu, X.; Yin, G.; Wang, J.; Jin, Y.; Wang, L.; Wang, B.; Wang, T. Integrating Anti-Influenza Virus Activity and Chemical Pattern Recognition to Explore the Quality Evaluation Method of Lonicerae Japonicae Flos. Molecules 2022, 27, 5789. https://doi.org/10.3390/molecules27185789
Xie X, Gu L, Xu W, Yu X, Yin G, Wang J, Jin Y, Wang L, Wang B, Wang T. Integrating Anti-Influenza Virus Activity and Chemical Pattern Recognition to Explore the Quality Evaluation Method of Lonicerae Japonicae Flos. Molecules. 2022; 27(18):5789. https://doi.org/10.3390/molecules27185789
Chicago/Turabian StyleXie, Xueqing, Lifei Gu, Wanyi Xu, Xiean Yu, Guo Yin, Jue Wang, Yibao Jin, Lijun Wang, Bing Wang, and Tiejie Wang. 2022. "Integrating Anti-Influenza Virus Activity and Chemical Pattern Recognition to Explore the Quality Evaluation Method of Lonicerae Japonicae Flos" Molecules 27, no. 18: 5789. https://doi.org/10.3390/molecules27185789
APA StyleXie, X., Gu, L., Xu, W., Yu, X., Yin, G., Wang, J., Jin, Y., Wang, L., Wang, B., & Wang, T. (2022). Integrating Anti-Influenza Virus Activity and Chemical Pattern Recognition to Explore the Quality Evaluation Method of Lonicerae Japonicae Flos. Molecules, 27(18), 5789. https://doi.org/10.3390/molecules27185789







