4. Experimental Section
1H and
13C-NMR spectra were recorded on JEOL AL-400 NMR (400 MHz) and ECP-600 NMR (600 MHz) spectrometers (Tokyo, Japan).
1H-NMR spectra were referenced with (CH
3)
4Si (δ 0.00 ppm) or CHCl
3 (δ 7.26 ppm) as an internal standard.
13C-NMR spectra were referenced with deuterated solvent (δ 77.0 ppm for CDCl
3). IR spectra were recorded on a JASCO FT-IR-800 Fourier transform infrared spectrophotometer (Tokyo, Japan). High-resolution mass spectra were obtained on a SHIMADZU LCMS-IT-TOF mass spectrometer (Kyoto, Japan) with an electrospray ionization (ESI) method or atmospheric pressure chemical ionization (APCI). Optical rotations were measured on a JASCO DIP-370 digital polarimeter (Tokyo, Japan). Column chromatography was performed on silica gel 60N (Kanto Chemical Co., Inc., 40–50 μm, Tokyo, Japan) or silica gel 60 (Merck, 0.040–0.063 mm, Tokyo, Japan). All experiments were performed under anhydrous conditions under an atmosphere of argon unless otherwise stated. The supporting information of
1H and
13C NMR spectra of all new compounds:
5,
7–
11,
13,
20,
23,
24,
26,
28–
30, and
32–
36, as well as
19F NMR spectra of compounds:
5,
7, and
8 is available at the link in
Supplementary Materials.
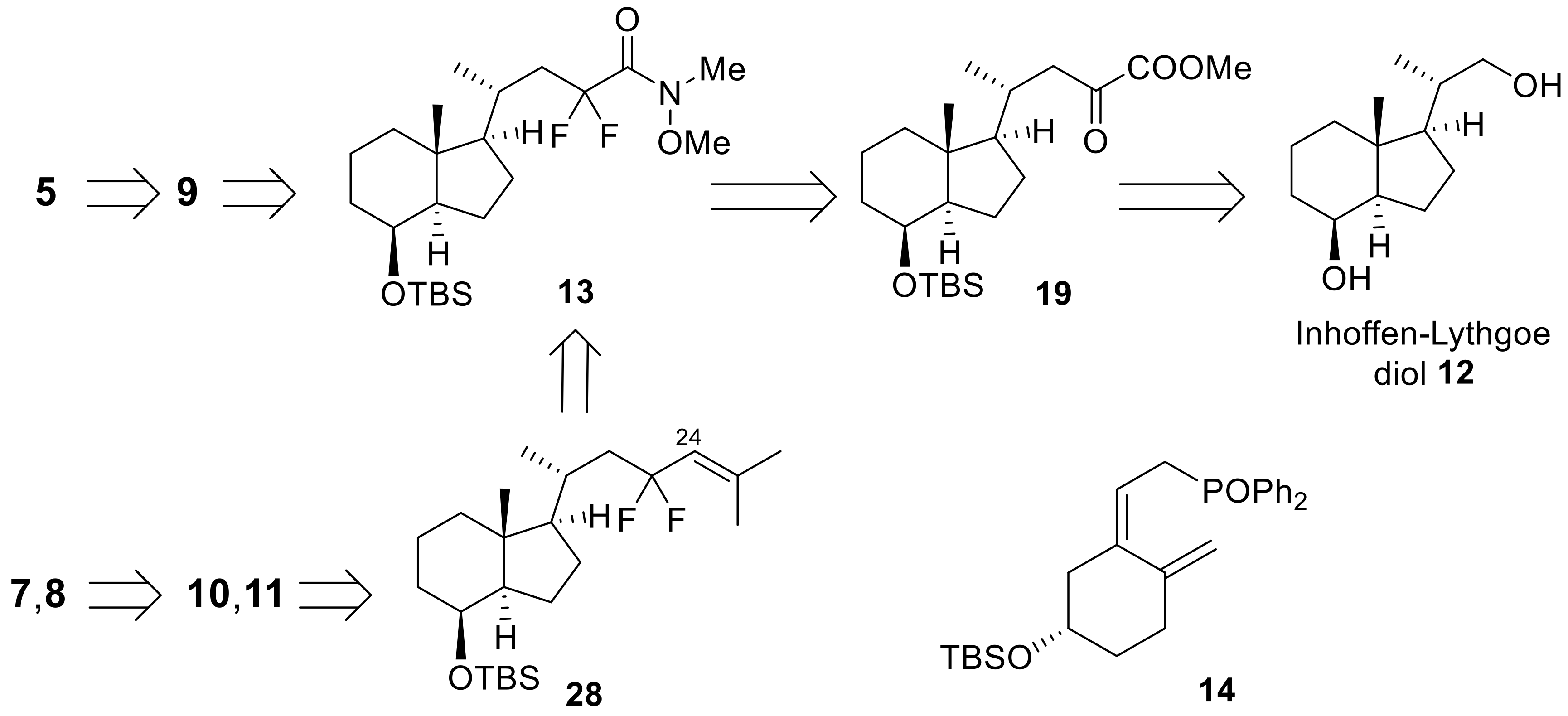
Methyl (R)-4-{(1R,3aR,4S,7aR)-4-[(tert-butyldimethylsilyl)oxy]-7a-methyloctahydro-1H-inden-1-yl}-2,2-difluoropentanoate (20)
To the solution of Horner–Emmons reagent (
17) (2.35 g, 7.24 mmol) in THF (3.5 mL), LDA (lithium diisopropylamide) (3.5 mL, 2 M THF/heptane/ethylbenzene solution, 1.75 mmol) was added at −40 °C; the mixture was stirred at the same temperature for 20 min, and a solution of
16 [
27] (977.0 mg, 3.01 mmol) in THF (3.5 mL) was added. The reaction mixture was stirred at 0 °C for 20 min. After the reaction was quenched with H
2O at 0 °C, the mixture was extracted with EtOAc three times. The organic layer was dried over Na
2SO
4, filtered, and concentrated. The crude residue was roughly purified by flash column chromatography on silica gel (hexanen:EtOAc = 3:1, 1% Et
3N) to obtain the crude coupling product
18, and it was used for the next reaction without further purification.
To the above coupling product 18 in CH2Cl2 (7 mL), AcOH (1.03 mL) and tetrabutylammonium fluoride (4.8 mL, 1 M THF solution, 4.8 mmol) were added at 0 °C under air, and the mixture was stirred at room temperature for 15 min. After the reaction was quenched with H2O at room temperature, the mixture was extracted with CH2Cl2 three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was partially purified by flash column chromatography on silica gel (hexane:EtOAc = 10:1) to obtain a crude residue of 19 (1.10 g).
To the solution of the above crude residue of 19 (1.10 g), CH2Cl2 (6.5 mL) was slowly added to N,N-diethylaminosulfur trifluoride (DAST) (2.8 mL, 3.4 g, 21.1 mmol) at 0 °C, and the mixture was stirred at room temperature for 16 h. The mixture was cooled to 0 °C, and MeOH and H2O were slowly added. The mixture was extracted with CH2Cl2 three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was partially purified by flash column chromatography on silica gel (hexane:EtOAc = 10:1) to obtain 20 (747.0 mg, 59%, three steps) as a colorless oil.
20: +36.5 (c 1.90, CHCl3); IR (neat) 1774, 1471, 1253, 1097, 1077, 1029, 838 cm−1; 1H-NMR (600 MHz, CDCl3) δ −0.01 (s, 3H), 0.01 (s, 3H), 0.88 (s, 9H), 0.90 (d, J = 5.4 Hz, 3H), 0.91 (s, 3H), 1.03 (q, J = 9.6 Hz, 1H), 1.10 (td, J = 3.6, 13.2 Hz, 1H), 1.17–1.27 (m, 2H), 1.31–1.38 (m, 2H), 1.43–1.49 (m, 1H), 1.51–1.59 (m, 2H), 1.65–1.67 (m, 1H), 1.74–1.84 (m, 2H), 1.88–1.98 (m, 2H), 2.04–2.16 (m, 1H), 3.86 (s, 3H), 3.98–4.00 (m, 1H); 13C-NMR (150 MHz, CDCl3) δ −5.2, −4.8, 13.7, 17.6, 18.0, 18.3, 23.0, 25.8, 27.0, 27.1, 31.1 (t, J = 22.3 Hz), 34.4, 34.5, 40.6, 42.1, 53.0, 53.2, 56.0, 69.4, 116.8 (t, J = 248.5 Hz), 165.0 (t, J = 33.8 Hz); HRMS (ESI−) calcd for C22H39O3F2Si [M − H]− 417.2642, found 417.2662.
(R)-4-{(1R,3aR,4S,7aR)-4-[(tert-Butyldimethylsilyl)oxy]-7a-methyloctahydro-1H-inden-1-yl}-2,2-difluoro-N-methoxy-N-methylpentanamide (13)
To the solution of compound 20 (1.01 g, 2.41 mmol) and Me(MeO)NH·HCl (934.3 mg, 9.58 mmol) in THF (20 mL), isopropyl magnesium chloride (19.0 mL, 1 M in THF, 19.0 mmol) was added at 0 °C, and the mixture was stirred at the same temperature for 23 h. After the reaction was quenched with water and aqueous saturated NH4Cl, the mixture was extracted with EtOAc three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 6:1) to obtain 13 (933.9 mg, 87%) as a colorless oil.
13: +39.9 (c 3.47, CHCl3); IR (neat) 1685, 1464, 1379, 1252, 1085, 1039, 984, 837 cm−1; 1H-NMR (600 MHz, CDCl3) δ −0.02 (s, 3H), 0.00 (s, 3H), 0.88 (s, 9H), 0.92 (s, 3H), 1.02 (d, J = 6.6 Hz, 3H), 1.06–1.13 (m, 2H), 1.21–1.26 (m, 2H), 1.30–1.37 (m, 3H), 1.51–1.59 (m, 1H), 1.64–1.66 (m, 1H), 1.74–1.87 (m, 4H), 1.94–1.96 (m, 1H), 2.22–2.32 (m, 1H) 3.24 (brs, 3H), 3.72 (s, 3H), 3.98–3.99 (m, 1H); 13C-NMR (150 MHz, CDCl3) δ −5.2, −4.8, 13.5, 17.6, 18.0, 20.2, 23.0, 25.8, 27.4, 30.6, 33.1, 34.3, 40.1 (t, J = 20.8 Hz), 40.6, 42.2, 53.1, 56.9, 61.9, 69.4, 118.6 (t, J = 249.2 Hz), 164.7 (t, J = 27.9 Hz); HRMS (ESI+) calcd for C23H43NO3F2SiNa [M + Na]+ 470.2872, found 470.2856.
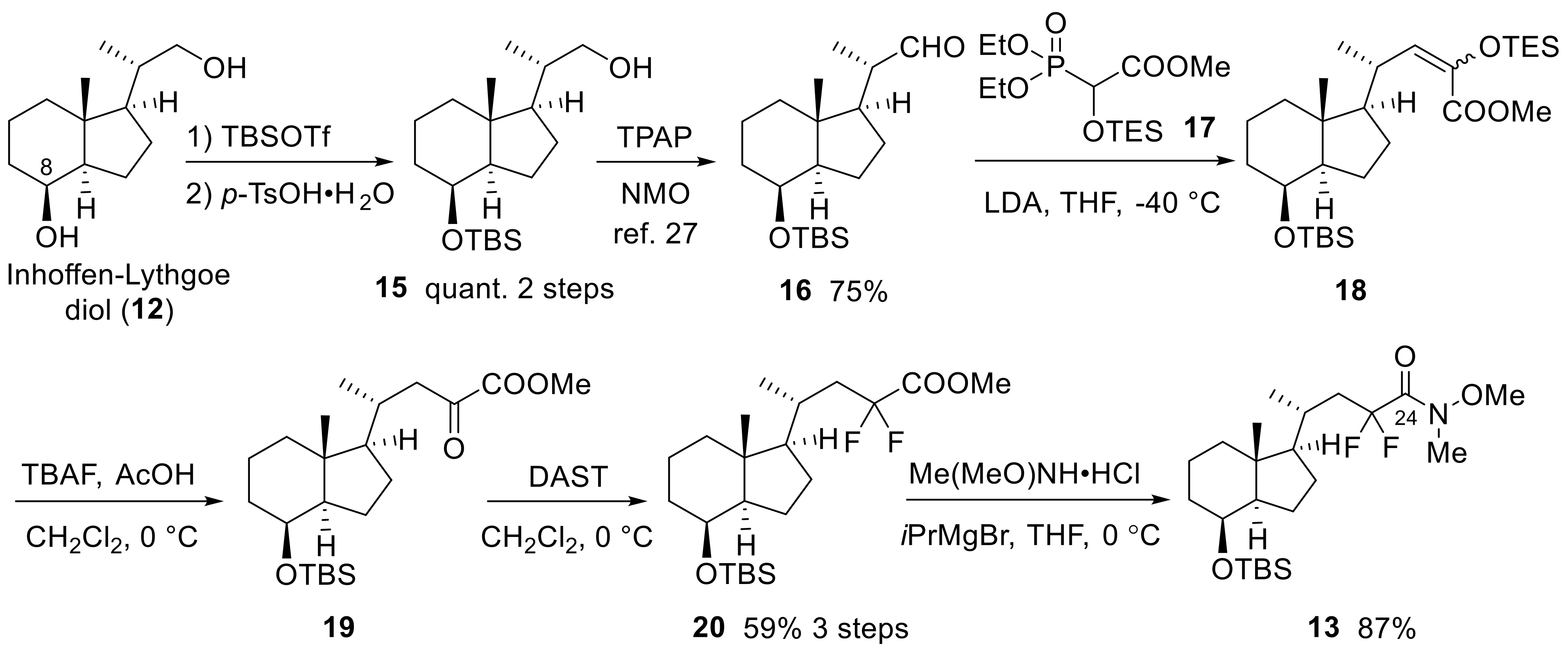
(6R)-6-{(1R,3aR,4S,7aR)-4-[(tert-Butyldimethylsilyl)oxy]-7a-methyloctahydro-1H-inden-1-yl}-4,4-difluoro-2-methylhept-1-en-3-yl acetate (23)
To the solution of compound 13 (251.5 mg, 0.56 mmol) in THF (20 mL), isopropenyl magnesium bromide (2.25 mL, 0.5 M in THF, 1.12 mmol) was added at 0 °C, and the mixture was stirred at room temperature for 1 h. After the reaction was quenched with water, the mixture was extracted with EtOAc three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 20:1) to obtain the crude product 21.
NaBH4 (45.1 mg, 1.19 mmol) was added to the solution of the above crude 21 and CeCl3·6H2O in EtOH (3 mL) and MeOH (3 mL) at 0 °C, and the mixture was stirred at the same temperature for 5 min. After the reaction was quenched with water, the mixture was extracted with EtOAc three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The crude 22 was used for the next reaction without further purification.
N,N-Dimethyl-4-aminopyridine (464.5 mg, 3.68 mmol) and Ac2O (1.85 g, 2 mL, 18.1 mmol) were added to the solution of the crude 22 in pyridine (4 mL) at 0 °C, and the mixture was stirred at room temperature for 10 min. After the reaction was quenched with water at room temperature, the mixture was extracted with EtOAc three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 20:1) to obtain 23 (mixture of diastereomers) (201.8 mg, 76%, three steps) as a colorless oil.
23: IR (neat) 1755, 1468, 1375, 1235, 1085, 1039, 841, 775 cm−1; 1H-NMR (600 MHz, CDCl3) δ −0.01 (s, 3H), 0.01 (s, 3H), 0.88 (s, 9H), 0.94 (s, 3H), 1.04–1.15 (m, 5H), 1.17–1.27 (m, 2H), 1.30–1.38 (m, 3H), 1.46–1.60 (m, 2H), 1.65–1.67 (m, 1H), 1.71–2.03 (m, 7H), 2.13–2.14 (m, 3H), 3.98–4.00 (m, 1H), 5.10–5.11 (m, 2H), 5.25–5.32 (m, 1H); 13C-NMR (150 MHz, CDCl3) δ −5.2, −4.8, 13.5, 13.5, 17.6, 18.0, 19.4, 19.7, 20.4, 20.5, 20.8, 23.0, 25.8, 27.5, 27.5, 30.3, 30.4, 34.3, 38.5 (t, J = 22.3 Hz), 38.7 (t, J = 22.3 Hz), 40.7, 42.2, 53.1, 57.0, 57.0, 69.4, 76.3 (t, J = 28.7 Hz), 76.8 (t, J = 27.3 Hz), 117.0, 117.4, 122.9 (t, J = 247.1 Hz), 122.9 (t, J = 246.4 Hz), 138.4, 138.5, 169.3, 169.4; HRMS (ESI+) calcd for C26H50O3NF2SiNa [M + Na]+ 490.3523, found 490.3543.
tert-Butyl({(1R,3aR,4S,7aR)-1-[(R)-4,4-difluoro-6-methylhept-6-en-2-yl]-7a-methyloctahydro-1H-inden-4-yl}oxy)dimethylsilane (24)
To a solution of sodium formate (155.8 mg, 2.29 mmol) and Pd(PPh3)4 (423.2 mg, 0.37 mmol) in dioxane (1.5 mL), nBu3P (345.5 mg, 427.0 μL, 1.77 mmol) was added at room temperature, and the mixture was stirred at 90 °C for 10 min. Acetate 23 (201.8 mg, 0.43 mmol) was dissolved in dioxane (1.5 mL), and the solution was added to the mixture. After being stirred at the same temperature for 17 h, the reaction mixture was quenched with H2O at room temperature. The mixture was extracted with EtOAc three times. The organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated. The residue was partially purified by flash column chromatography on silica gel (hexane:EtOAc = 100:1), and repurification by flash column chromatography on silica gel (hexane only) provided 24 (111.9 mg, 63%) as a colorless oil.
24: +36.0 (c 0.69, CHCl3); IR (neat) 1471, 1379, 1255, 1166, 1085, 1023, 837, 775 cm−1; 1H-NMR (600 MHz, CDCl3) δ 0.00 (s, 3H), 0.01 (s, 3H), 0.89 (s, 9H), 0.95 (s, 3H), 1.04 (d, J = 7.2 Hz, 3H), 1.07 (q, J = 9.6 Hz, 1H), 1.12 (td, J = 3.6, 13.2 Hz, 1H), 1.21–1.27 (m, 2H), 1.31–1.38 (m, 3H), 1.46–1.61 (m, 2H), 1.66–1.68 (m, 1H), 1.75–1.86 (m, 6H), 1.89–2.00 (m, 2H), 2.48–2.59 (m, 2H), 3.99–4.00 (m, 1H), 4.84 (brs, 1H), 4.59 (brs, 1H); 13C-NMR (150 MHz, CDCl3) δ −5.2, −4.8, 13.5, 17.6, 18.0, 20.3, 23.0, 23.3, 25.8, 27.5, 30.9, 34.4, 40.7, 41.7 (t, J = 23.0 Hz), 42.2, 45.6 (t, J = 25.9 Hz), 53.1, 57.1, 69.4, 116.3, 125.2 (t, J = 242.0 Hz), 138.7; HRMS (ESI+) calcd for C24H44OF2SiNa [M + Na]+ 437.3022, found 437.3033.
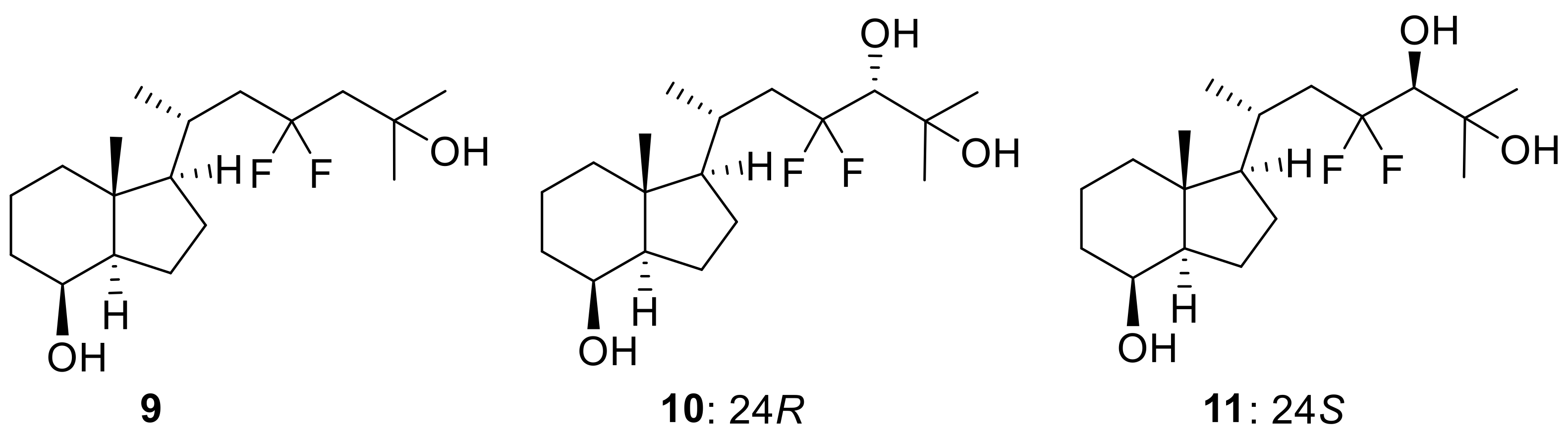
(1R,3aR,4S,7aR)-1-[(2R)-4,4-Difluoro-6-hydroxy-6-methylheptan-2-yl]-7a-methyloctahydro-1H-inden-4-ol (9)
mCPBA (286.1 mg, 1.66 mmol) was added to the mixture of 24 (111.9 mg, 0.27 mmol) and NaHCO3 (131.9 mg, 1.57 mmol) in CH2Cl2 (2 mL) at 0 °C, and the mixture was stirred at the same temperature for 100 min under air. After the reaction was quenched with H2O and saturated aqueous NaHCO3 at room temperature, the mixture was extracted with CH2Cl2 three times. The organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 20:1) to obtain the crude epoxide.
LiAlH4 (14.0 mg, 0.37 mmol) was added to the solution of the above crude epoxide in Et2O (3 mL) at 0 °C, and the mixture was stirred at the same temperature for 15 min, and then at room temperature for 20 min. After the reaction was quenched with MeOH, water, and saturated aqueous potassium sodium tartrate, the mixture was extracted with EtOAc three times. The organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated. The crude alcohol was used for the next reaction without further purification.
p-Toluenesulfonic acid monohydrate (981.0 mg, 5.16 mmol) was added to the solution of the above crude alcohol in MeOH (20 mL), and the mixture was stirred at room temperature for 25 h under air. After the reaction was quenched with H2O and saturated aqueous NaHCO3 at room temperature, the mixture was extracted with EtOAc three times. The organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 2:1) to obtain 9 (63.8 mg, 74%, three steps) as a colorless oil.
9: +26.9 (c 2.20, CHCl3); IR (neat) 3412, 1471, 1375, 1170, 987, 864 cm−1; 1H-NMR (600 MHz, CDCl3) δ 0.97 (s, 3H), 1.04 (d, J = 6.6 Hz, 3H), 1.08–1.18 (m, 2H), 1.23–1.38 (m, 8H), 1.39–1.49 (m, 3H), 1.54–1.88 (m, 8H), 1.92–2.12 (m, 4H), 4.06–4.08 (m, 1H); 13C-NMR (150 MHz, CDCl3) δ 13.3, 17.4, 20.3, 22.4, 27.5, 30.2, 30.4, 31.0, 33.5, 40.3, 41.9, 44.1 (t, J = 23.0 Hz), 48.6 (t, J = 23.0 Hz), 52.7, 56.8, 69.3, 69.9, 126.6 (t, J = 241.3 Hz); HRMS (ESI+) calcd for C18H32O2F2Na [M + Na]+ 341.2263 found 341.2249.
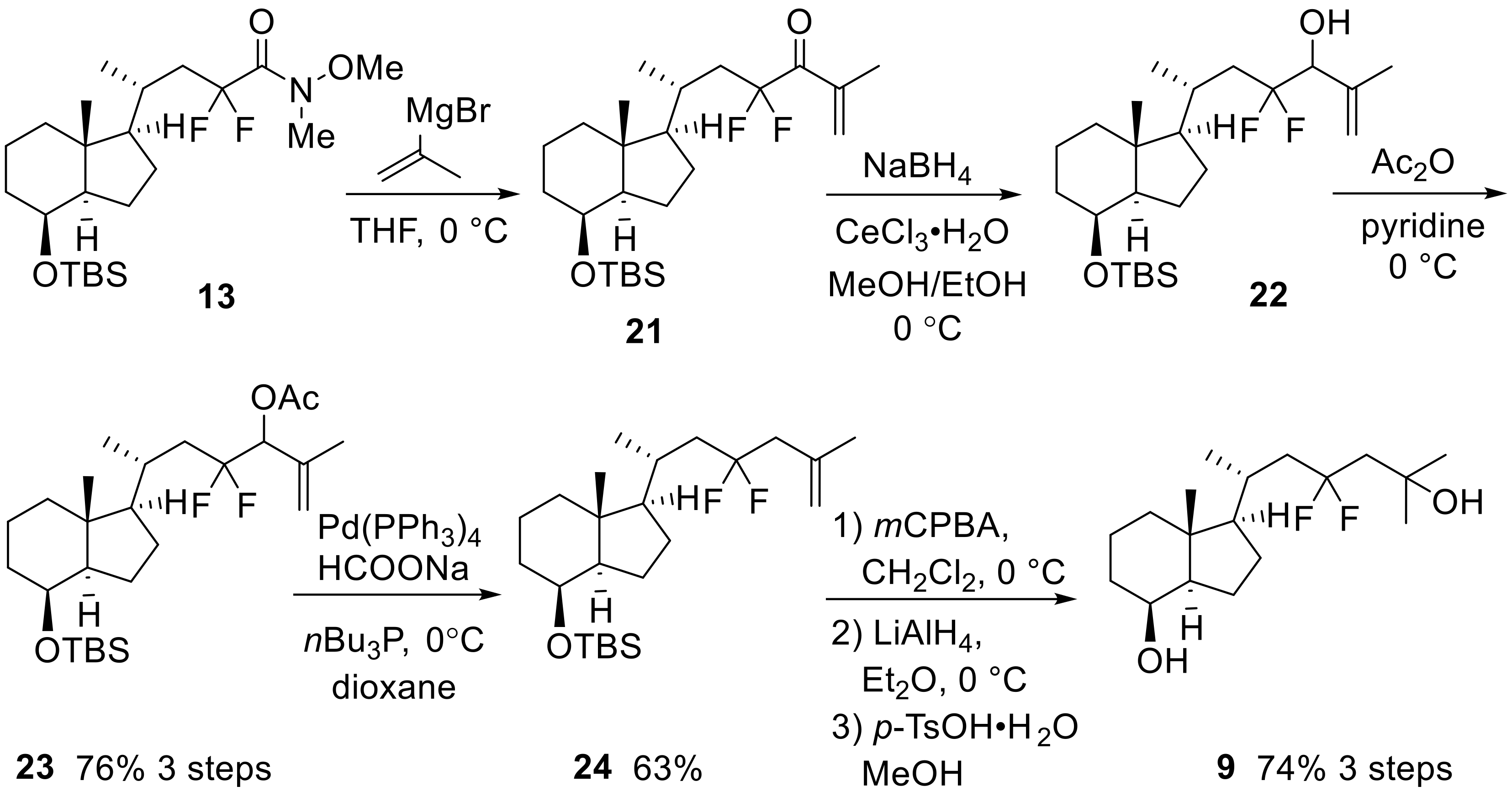
(6R)-6-{(1R,3aR,4S,7aR)-4-[(tert-Butyldimethylsilyl)oxy]-7a-methyloctahydro-1H-inden-1-yl}-4,4-difluoro-2-methylheptan-3-ol (26)
To the solution of compound 13 (933.9 mg, 2.09 mmol) in THF (10 mL), isopropyl magnesium chloride (6.3 mL, 1 M in THF, 6.26 mmol) was added at 0 °C, and the mixture was stirred at room temperature for 1 h. After the reaction was quenched with water and aqueous saturated NH4Cl at 0 °C, the mixture was extracted with EtOAc three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 10:1) to obtain the crude product 25.
NaBH4 (45.1 mg, 1.19 mmol) was added to the solution of the above crude 25 in EtOH (3 mL) at 0 °C, and the mixture was stirred at the same temperature for 5 min. After the reaction was quenched with water, the mixture was extracted with EtOAc three times. The organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 10:1) to obtain 26 (mixture of diastereomers) (563.4 mg, 62%, two steps) as a colorless oil.
26: IR (neat) 3442, 1471, 1367, 1255, 1166, 1085, 1023, 837, 775 cm−1; 1H-NMR (600 MHz, CDCl3) δ −0.01 (s, 3H), 0.01 (s, 3H), 0.89 (s, 9H), 0.95–1.02 (m, 8H), 1.04–1.15 (m, 4H), 1.23–1.38 (m, 6H), 1.49–1.67 (m, 3H), 1.77–2.12 (m, 7H), 3.38–3.45 (m, 1H), 3.99–4.00 (m, 1H); 13C-NMR (150 MHz, CDCl3) δ −5.2, −4.8, 13.5, 13.6, 17.0, 17.5, 17.6, 18.0, 20.2, 20.3, 20.5, 20.7, 23.0, 25.8, 27.6, 27.6, 28.5, 28.6, 30.3, 30.5, 34.4, 38.4 (t, J = 21.5 Hz), 40.7, 40.7, 42.2, 53.1, 57.1, 69.4, 77.4 (t, J = 27.3 Hz), 77.6 (t, J = 28.9 Hz), 125.3 (t, J = 244.9 Hz), 125.5 (t, J = 244.9 Hz); HRMS (ESI+) calcd for C24H47O2F2Si [M + H]+ 433.3308, found 433.3290.
tert-Butyl({(1R,3aR,4S,7aR)-1-[(R)-4,4-difluoro-6-methylhept-5-en-2-yl]-7a-methyloctahydro-1H-inden-4-yl}oxy)dimethylsilane (28)
Trifluoromethanesulfonic anhydride (366.8 mg, 213 μL, 1.30 mmol) was added to the solution of 26 (467.3 mg, 1.08 mmol) and 2,6-di-tert-butylpyridine (464.5 mg, 3.68 mmol) in CH2Cl2 (4 mL) at 0 °C, and the mixture was stirred at the same temperature for 75 min. After the reaction was quenched with water and aqueous saturated NH4Cl at room temperature, the mixture was extracted with EtOAc three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The crude 27 was used for the next reaction without further purification.
1,8-Diazabicyclo [5.4.0]undec-7-ene (DBU) (600 μL) was added to the solution of the above crude 27 in CH2Cl2 (4 mL) at room temperature and the mixture was stirred at the same temperature for 15 h. After the reaction was quenched with water and aqueous saturated NH4Cl at room temperature, the mixture was extracted with EtOAc three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane only) to obtain 28 (269.8 mg, 60%, two steps) as a colorless oil.
28: +59.4 (c 0.79, CHCl3); IR (neat) 1468, 1375, 1255, 1166, 1085, 1027, 984, 837, 771 cm−1; 1H-NMR (600 MHz, CDCl3) δ −0.01 (s, 3H), 0.01 (s, 3H), 0.89 (s, 9H), 0.94 (s, 3H), 1.03 (d, J = 6.6 Hz, 3H), 1.07 (q, J = 9.6 Hz, 1H), 1.10 (td, J = 3.6, 13.2 Hz, 1H), 1.21–1.27 (m, 2H), 1.31–1.38 (m, 3H), 1.53–1.84 (m, 12H), 1.95–2.06 (m, 2H), 3.99–4.00 (m, 1H), 5.32 (t, J = 8.1 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ −5.2, −4.8, 13.5, 17.6, 18.0, 19.1, 20.4, 23.0, 25.8, 26.5, 27.6, 31.2, 34.4, 40.7, 42.2, 44.2 (t, J = 25.1 Hz), 53.1, 57.0, 69.4, 121.6 (t, J = 26.5 Hz), 123.2 (t, J = 238.5 Hz), 141.3 (t, J = 5.8 Hz); HRMS (ESI+) calcd for C24H44OF2SiNa [M + Na]+ 437.3022, found 437.3004.
(3R,6R)-6-{(1R,3aR,4S,7aR)-4-[(tert-Butyldimethylsilyl)oxy]-7a-methyloctahydro-1H-inden-1-yl}-4,4-difluoro-2-methylheptane-2,3-diol (29)
The mixture of AD-mix α (605.3 mg) in tBuOH (4 mL) and H2O (4 mL) was stirred at 0 °C for 20 min, and 28 (55.9 mg, 0.13 mmol) was added to the mixture at 0 °C with stirring at the same temperature for 4 h, and then at 4 °C for 20 h under air. After the reaction was quenched with water, the mixture was extracted with EtOAc three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 1:1) to obtain 29 (53.4 mg, 88%) as a colorless oil.
29: +35.0 (c 2.71, CHCl3); IR (neat) 3408, 1471, 1375, 1255, 1166, 1085, 1023, 833, 775 cm−1; 1H-NMR (600 MHz, CDCl3) δ −0.01 (s, 3H), 0.01 (s, 3H), 0.88 (s, 9H), 0.95 (s, 3H), 1.06 (d, J = 7.2 Hz, 3H), 1.09–1.16 (m, 2H), 1.22–1.38 (m, 11H), 1.53–1.67 (m, 3H), 1.67–1.88 (m, 3H), 1.95–2.00 (m, 2H), 2.26 (ddd, J = 7.8, 13.8, 34.2 Hz, 1H), 2.79 (d, J = 6.6 Hz, 1H), 3.41 (ddd, J = 5.4, 7.8, 19.2 Hz, 1H), 3.99–3.99 (m, 1H); 13C-NMR (150 MHz, CDCl3) δ −5.2, −4.8, 13.5, 17.6, 18.0, 21.1, 23.0, 25.6, 25.8, 27.6, 28.2, 30.1, 34.4, 39.5 (t, J = 22.3 Hz), 40.7, 42.2, 53.2, 57.1, 69.4, 72.1, 77.5 (t, J = 27.3 Hz), 126.3 (t, J = 245.6 Hz); HRMS (ESI−) calcd for C25H47O5F2Si [M + HCOO]− 439.3166, found 439.3170.
(3S,6R)-6-{(1R,3aR,4S,7aR)-4-[(tert-Butyldimethylsilyl)oxy]-7a-methyloctahydro-1H-inden-1-yl}-4,4-difluoro-2-methylheptane-2,3-diol (30)
The mixture of AD-mix β (704.8 mg) in tBuOH (4 mL) and H2O (4 mL) was stirred at 0 °C for 30 min, and 28 (51.8 mg, 0.13 mmol) was added to the mixture at 0 °C with stirring at the same temperature for 5 h, and then at 4 °C for 19 h under air. After the reaction was quenched with water, the mixture was extracted with EtOAc three times. The organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 2:1) to obtain 30 (53.8 mg, 96%) as a white powder.
30: +44.7 (c 0.30, CHCl3); IR (neat) 3431, 1471, 1375, 1255, 1162, 1081, 1019, 837, 771 cm−1; 1H-NMR (400 MHz, CDCl3)
δ −0.01 (s, 3H), 0.00 (s, 3H), 0.88 (s, 9H), 0.96 (s, 3H), 1.03 (d, J = 6.4 Hz, 3H), 1.07–1.16 (m, 2H), 1.21–1.39 (m, 11H), 1.52–1.86 (m, 6H), 1.95–2.19 (m, 3H), 2.84 (d, J = 7.6 Hz, 1H), 3.42 (ddd, J = 3.6, 8.0, 21.5 Hz, 1H), 3.99–3.99 (m, 1H); 13C-NMR (100 MHz, CDCl3) δ −5.2, −4.8, 13.6, 17.6, 18.0, 20.2, 23.0, 25.7, 25.8, 27.5, 28.2, 30.6, 34.4, 39.9 (t, J = 21.9 Hz), 40.7, 42.2, 53.1, 57.1, 69.4, 72.3, 76.6 (t, J = 28.2 Hz), 126.8 (t, J = 246.0 Hz); HRMS (ESI−) calcd for C25H47O5F2Si [M + HCOO]− 439.3166, found 439.3164.
(3R,6R)-4,4-Difluoro-6-[(1R,3aR,4S,7aR)-4-hydroxy-7a-methyloctahydro-1H-inden-1-yl]-2-methylheptane-2,3-diol (10)
To the solution of 29 (53.4 mg, 0.12 mmol) in MeOH (10 mL), p-toluenesulfonic acid monohydrate (222.8 mg, 1.17 mmol) was added, and the mixture was stirred at room temperature for 39 h under air. After the reaction was quenched with H2O and saturated aqueous NaHCO3 at room temperature, the mixture was extracted with CH2Cl2 three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 1:2) to obtain 10 (36.1 mg, 91%, in two steps) as a white powder.
10: +28.7 (c 2.34, CHCl3); IR (neat) 3415, 1471, 1371, 1267, 1162, 1073, 991, 741 cm−1; 1H-NMR (600 MHz, CDCl3) δ 0.96 (s, 3H), 1.05 (d, J = 7.2 Hz, 3H), 1.11–1.17 (m, 2H), 1.27–1.66 (m, 14H), 1.77–1.91 (m, 4H), 1.99–2.02 (m, 1H), 2.16 (brs, 1H), 2.21–2.30 (m, 1H), 3.03 (d, J = 7.2 Hz, 1H), 3.41 (dt, J = 6.0, 18.6 Hz, 1H), 4.06–4.07 (m, 1H); 13C-NMR (100 MHz, CDCl3) δ 13.6, 17.4, 20.1, 22.4, 25.6 (t, J = 3.8 Hz), 27.4, 28.2, 30.6, 33.5, 39.8 (t, J = 21.9 Hz), 40.3, 41.9, 52.6, 56.9, 69.3, 72.3, 76.7 (t, J = 30.5 Hz), 126.7 (t, J = 246.0 Hz); HRMS (ESI−) calcd for C18H32O3F2Cl [M + Cl]− 369.2014, found 369.1989.
(3S,6R)-4,4-Difluoro-6-[(1R,3aR,4S,7aR)-4-hydroxy-7a-methyloctahydro-1H-inden-1-yl]-2-methylheptane-2,3-diol (11)
To the solution of 30 (48.9 mg, 0.11 mmol) in MeOH (5 mL) and CH2Cl2 (5 mL), p-toluenesulfonic acid monohydrate (204.1 mg, 1.07 mmol) was added, and the mixture was stirred at room temperature for 66 h under air. After the reaction was quenched with H2O and saturated aqueous NaHCO3 at room temperature, the mixture was extracted with CH2Cl2 three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 1:2) to obtain 11 (30.4 mg, 83%, in two steps) as a white powder.
11: +22.6 (c 2.78, CHCl3); IR (neat) 3396, 1468, 1379, 1275, 1162, 1073, 987, 741 cm−1; 1H-NMR (600 MHz, CDCl3) δ 0.97 (s, 3H), 1.04 (d, J = 6.0 Hz, 3H), 1.08–1.20 (m, 2H), 1.28–1.91 (m, 18H), 2.00–2.19 (m, 3H), 2.93 (d, J = 7.8 Hz, 1H), 3.42 (ddd, J = 4.1, 7.8, 21.1 Hz, 1H), 4.06–4.08 (m, 1H); 13C-NMR (150 MHz, CDCl3) δ 13.3, 17.3, 21.0 (d, J = 3.0 Hz), 22.4, 25.5, 27.4, 28.2, 30.1 (d, J = 2.9 Hz), 33.5, 39.4 (t, J = 22.3 Hz), 40.3, 41.9, 52.7, 56.9, 69.3, 72.0, 77.5 (t, J = 25.0 Hz), 126.1 (t, J = 245.6 Hz); HRMS (ESI−) calcd for C18H32O3F2Cl [M + Cl]− 369.2014, found 369.1983.
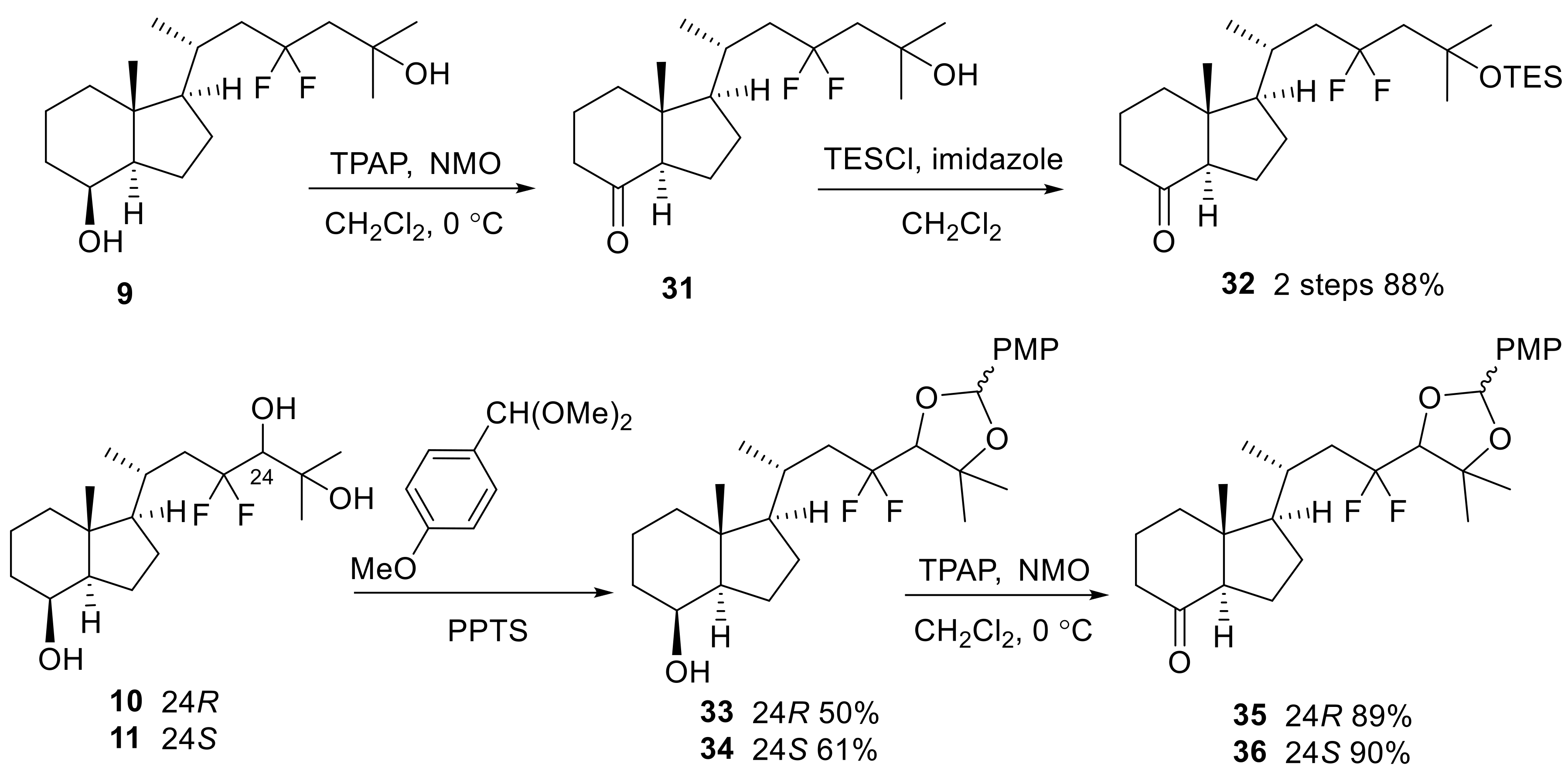
(1R,3aR,7aR)-1-{(2R)-4,4-Difluoro-6-methyl-6[(triethylsilyl)oxy] heptan-2-yl}-7a-methyloctahydro-4H-inden-4-one (32)
4-Methylmorpholine N-oxide (36.6 mg, 0.31 mmol) was added to the solution of 9 (63.8 mg, 0.20 mmol) in CH2Cl2 (2 mL), and the mixture was cooled to 0 °C. TPAP (37.8 mg, 0.11 mmol) was added to the mixture, and the mixture was stirred at 0 °C for 1 h. The reaction was diluted with an excess amount of Et2O. The mixture was directly purified by flash column chromatography on silica gel (Et2O only) to obtain the crude ketone (31), and this was used for the next reaction without further purification.
TESCl (211.0 mg, 234 μL, 1.4 mmol) was added to the 0 °C cooled solution of the above crude ketone (31) and imidazole (130.6 mg, 1.92 mmol) in CH2Cl2 (5 mL), and the mixture was stirred at room temperature for 28 h. After the reaction was quenched with H2O at 0 °C, the mixture was extracted with CH2Cl2 three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 20:1–10:1) to obtain 32 (76.1 mg, 88%, in two steps) as a colorless oil.
32: +6.4 (c 2.36, CHCl3); IR (neat) 1715, 1464, 1387, 1371, 1239, 1177, 1042, 748 cm−1; 1H-NMR (600 MHz, CDCl3) δ 0.58 (q, J = 7.8 Hz, 6H), 0.68 (s, 3H), 0.95 (t, J = 7.8 Hz, 9H), 1.10 (d, J = 6.0 Hz, 3H), 1.31–1.37 (m, 7H), 1.46 (q, J = 9.6 Hz, 1H), 1.50–1.78 (m, 4H), 1.82–2.06 (m, 7H), 2.12–2.16 (m, 1H), 2.19–2.30 (m, 2H), 2.46 (dd, J = 7.8, 12.0 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 6.7, 7.0, 12.3, 19.1, 21.0, 23.9, 27.7, 30.7, 30.9, 38.9, 40.9, 43.1 (t, J = 23.0 Hz), 49.8, 51.2 (t, J = 23.7 Hz), 57.0, 62.0, 72.1, 125.3 (t, J = 240.6 Hz), 211.8; HRMS (ESI+) calcd for C24H44O2F2SiNa [M + Na]+ 453.2971 found 453.2953.
(1R,3aR,4S,7aR)-1-{(2R)-4,4-Difluoro-4-[(4R)-2-(4-methoxyphenyl)-5,5-dimethyl-1,3-dioxolan-4-yl]butan-2-yl}-7a-methyloctahydro-1H-inden-4-ol (33)
Pyridinium p-toluenesulfonate (PPTS) (19.3 mg, 0.08 mmol) was added to a solution of 10 (45.7 mg, 0.14 mmol) in anisaldehyde dimethyl acetal (1.5 mL) at room temperature, and the mixture was stirred at the same temperature for 18 h. After the reaction was quenched with water and saturated aqueous NaHCO3, the mixture was extracted with EtOAc three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was partially purified by flash column chromatography on silica gel (hexane:EtOAc = 4:1–2:1), and repurification by flash column chromatography on silica gel (hexane:EtOAc = 3:1) provided 33 (mixture of diastereomers) (31.3 mg, 50%) as a colorless oil.
33: IR (neat) 3481, 1523, 1460, 1379, 1252, 1170, 1096, 991, 829 cm−1; 1H-NMR (600 MHz, CDCl3) δ 0.96–0.96 (m, 3H), 1.06–1.18 (m, 5H), 1.25–1.35 (m, 3H), 1.41–1.62 (m, 12H), 1.79–1.93 (m, 4H), 1.99–2.20 (m, 1H), 2.17–2.28 (m, 1H), 3.79–3.83 (m, 4H), 4.06 (brs, 1H), 5.87 (s, 0.57H), 6.03 (s, 0.45H), 6.89–6.91 (m, 2H), 7.38–7.44 (m, 2H); 13C-NMR (150 MHz, CDCl3) δ 13.3, 17.4, 20.7, 20.8, 21.4, 21.4, 22.4, 23.4, 27.1, 27.3, 27.4, 28.0, 30.1, 30.2, 30.3, 30.3, 33.5, 40.3, 40.3, 40.5 (t, J = 23.0 Hz), 41.3 (t, J = 22.3 Hz), 41.9, 41.9, 52.7, 55.3, 56.8, 56.9, 69.3, 80.9, 81.6, 83.3, 83.5, 83.6, 83.6, 83.7, 83.8, 83.9, 84.0, 101.8, 102.1, 113.7, 113.7, 123.5 (dd, J = 241.2, 251.3 Hz), 123.7 (dd, J = 241.4, 251.4 Hz), 127.9, 128.4, 129.3, 130.5, 160.4, 160.5; HRMS (ESI+) calcd for C26H38O4F2Na [M + Na]+ 475.2630, found 475.2636.
(1R,3aR,4S,7aR)-1-{(2R)-4,4-Difluoro-4-[(4S)-2-(4-methoxyphenyl)-5,5-dimethyl-1,3-dioxolan-4-yl]butan-2-yl}-7a-methyloctahydro-1H-inden-4-ol (34)
Pyridinium p-toluenesulfonate (PPTS) (158.6 mg, 0.63 mmol) was added to a solution of 11 (36.1 mg, 0.11 mmol) in anisaldehyde dimethyl acetal (3 mL) at room temperature, and the mixture was stirred at the same temperature for 18 h. After the reaction was quenched with water and saturated aqueous NaHCO3, the mixture was extracted with EtOAc three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was partially purified by flash column chromatography on silica gel (hexane only–hexane:EtOAc = 4:1–2:1), and repurification by flash column chromatography on silica gel (hexane:EtOAc = 4:1) provided 34 (mixture of diastereomers) (22.9 mg, 61%) as a colorless oil.
34: IR (neat) 3516, 1519, 1460, 1375, 1252, 1166, 1093, 987, 829 cm−1; 1H-NMR (600 MHz, CDCl3) δ 0.96 (s, 3H), 1.05–1.35 (m, 8H), 1.40–1.88 (m, 15H), 1.96–2.07 (m, 2H), 3.81–3.84 (m, 4H), 4.05–4.07 (m, 1H), 5.55 (s, 0.56H), 6.04 (s, 0.44H), 6.90 (d, J = 7.8 Hz, 2H), 7.39–7.45 (m, 2H); 13C-NMR (150 MHz, CDCl3) δ 13.3, 13.3, 17.3, 21.5, 21.5, 22.4, 23.3 (t, J = 4.4 Hz), 27.1, 27.3, 27.4, 28.1, 30.6, 33.5, 40.3, 40.4 (t, J = 21.5 Hz), 41.2 (t, J = 23.0 Hz), 41.9, 52.6, 55.3, 56.8, 56.8, 69.3, 80.9, 81.6, 82.8, 82.9, 83.0, 83.0, 83.2, 83.2, 83.3, 83.5, 101.9, 102.2, 113.7, 113.8, 123.7 (dd, J = 239.9, 251.4 Hz), 123.9 (dd, J = 241.2, 251.3 Hz), 127.8, 128.4, 129.4, 130.5, 160.4, 160.5; HRMS (ESI+) calcd for C26H38O4F2Na [M + Na]+ 475.2630, found 475.2604.
(1R,3aR,7aR)-1-{(2R)-4,4-Difluoro-4-[(4R)-2-(4-methoxyphenyl)-5,5-dimethyl-1,3-dioxolan-4-yl]butan-2-yl}-7a-methyloctahydro-4H-inden-4-one (35)
4-Methylmorpholine N-oxide (14.4 mg, 0.12 mmol) was added to the solution of 33 (29.9 mg, 0.07 mmol) in CH2Cl2 (3 mL), and the mixture was cooled to 0 °C. TPAP (14.2 mg, 0.04 mmol) was added to the mixture, and the mixture was stirred at 0 °C for 40 min. The reaction was diluted with an excess amount of Et2O. The mixture was directly purified by flash column chromatography on silica gel (Et2O only) followed by purification on flash column chromatography on silica gel (hexane:EtOAc = 4:1) to obtain 35 (mixture of diastereomers) (26.5 mg, 89%) as a colorless oil.
35: IR (neat) 1712, 1615, 1519, 1468, 1387, 1252, 1170, 1096, 1031, 833, 736 cm−1; 1H-NMR (600 MHz, CDCl3) δ 0.65–0.67 (m, 3H), 1.12 (t, J = 6.9 Hz, 3H), 1.22–1.38 (m, 1H), 1.46–1.77 (m, 11H), 1.82–1.94 (m, 3H), 1.98–2.03 (m, 1H), 2.10–2.30 (m, 4H), 2.42–2.46 (m, 1H), 3.78–3.82 (m, 4H), 5.87 (s, 0.55H), 6.03 (s, 0.44H), 6.88–6.91 (m, 2H), 7.38–7.44 (m, 2H); 13C-NMR (150 MHz, CDCl3) δ 12.3, 12.3, 19.0, 20.9, 21.0, 21.4, 21.4, 23.3, 23.9, 27.1, 27.5, 27.6, 28.0, 30.2, 30.2, 30.4, 30.4, 38.8, 38.9, 40.4 (t, J = 22.3 Hz), 40.9, 41.3 (t, J = 23.0 Hz), 49.8, 55.3, 56.8, 56.8, 62.0, 80.9, 81.6, 83.3, 83.4, 83.5, 83.7, 83.8, 84.0, 101.9, 102.2, 113.7, 113.8, 121.8, 121.9, 123.4, 123.5, 123.6, 125.0, 125.2, 127.9, 128.4, 129.2, 130.3, 160.4, 160.6, 211.7; HRMS (ESI+) calcd for C26H36O4F2Na [M + Na]+ 473.2474, found 473.2492.
(1R,3aR,7aR)-1-{(2R)-4,4-Difluoro-4-[(4S)-2-(4-methoxyphenyl)-5,5-dimethyl-1,3-dioxolan-4-yl]butan-2-yl}-7a-methyloctahydro-4H-inden-4-one (36)
4-Methylmorpholine N-oxide (13.0 mg, 0.11 mmol) was added to the solution of 34 (31.3 mg, 0.07 mmol) in CH2Cl2 (3 mL), and the mixture was cooled to 0 °C. TPAP (13.1 mg, 0.04 mmol) was added to the mixture, and the mixture was stirred at 0 °C for 35 min. The reaction was diluted with an excess amount of Et2O. The mixture was directly purified by flash column chromatography on silica gel (Et2O only) followed by purification on flash column chromatography on silica gel (hexane:EtOAc = 4:1) to obtain 36 (mixture of diastereomers) (28.2 mg, 90%) as a colorless oil.
36: IR (neat) 1712, 1611, 1519, 1464, 1383, 1248, 1174, 1096, 1035, 837 cm−1; 1H-NMR (600 MHz, CDCl3) δ 0.66 (s, 3H), 1.10–1.12 (m, 3H), 1.24–1.36 (m, 1H), 1.40–1.61 (m, 10H), 1.66–2.04 (m, 7H), 2.11–2.14 (m, 1H), 2.18–2.24 (m, 1H), 2.27–2.30 (m, 1H), 2.41–2.46 (m, 1H), 3.79–3.83 (m, 4H), 5.88 (s, 0.53H), 6.04 (s, 0.44H), 6.89–6.91 (m, 2H), 7.38–7.44 (m, 2H); 13C-NMR (150 MHz, CDCl3) δ 12.4, 19.0, 20.5, 20.6, 21.4, 21.5, 23.2, 23.9, 27.1, 27.5, 27.6, 28.1, 30.7, 38.9, 40.4 (t, J = 22.3 Hz), 41.0, 41.1 (t, J = 23.0 Hz), 49.8, 55.3, 55.3, 56.7, 56.8, 62.0, 80.4, 81.6, 82.8, 82.9, 83.0, 83.0, 83.2, 83.2, 83.3, 83.5, 101.9, 102.2, 113.7, 113.8, 122.0, 122.1, 123.6, 123.6, 123.7, 123.8, 125.2, 125.4, 127.8, 128.4, 129.3, 130.4, 160.4, 160.6, 211.7, 211.7; HRMS (ESI+) calcd for C26H36O4F2Na [M + Na]+ 473.2474, found 473.2499.
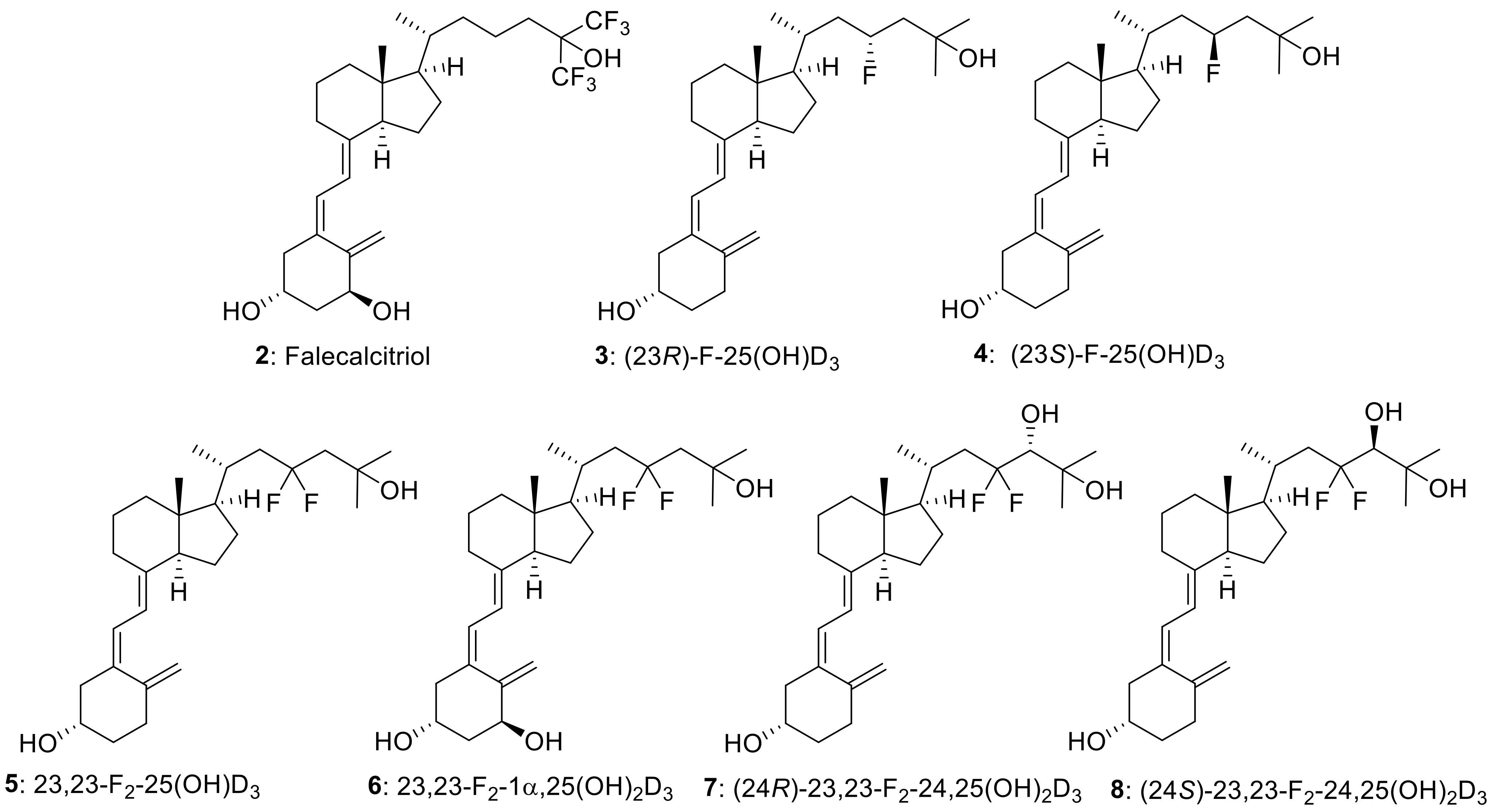
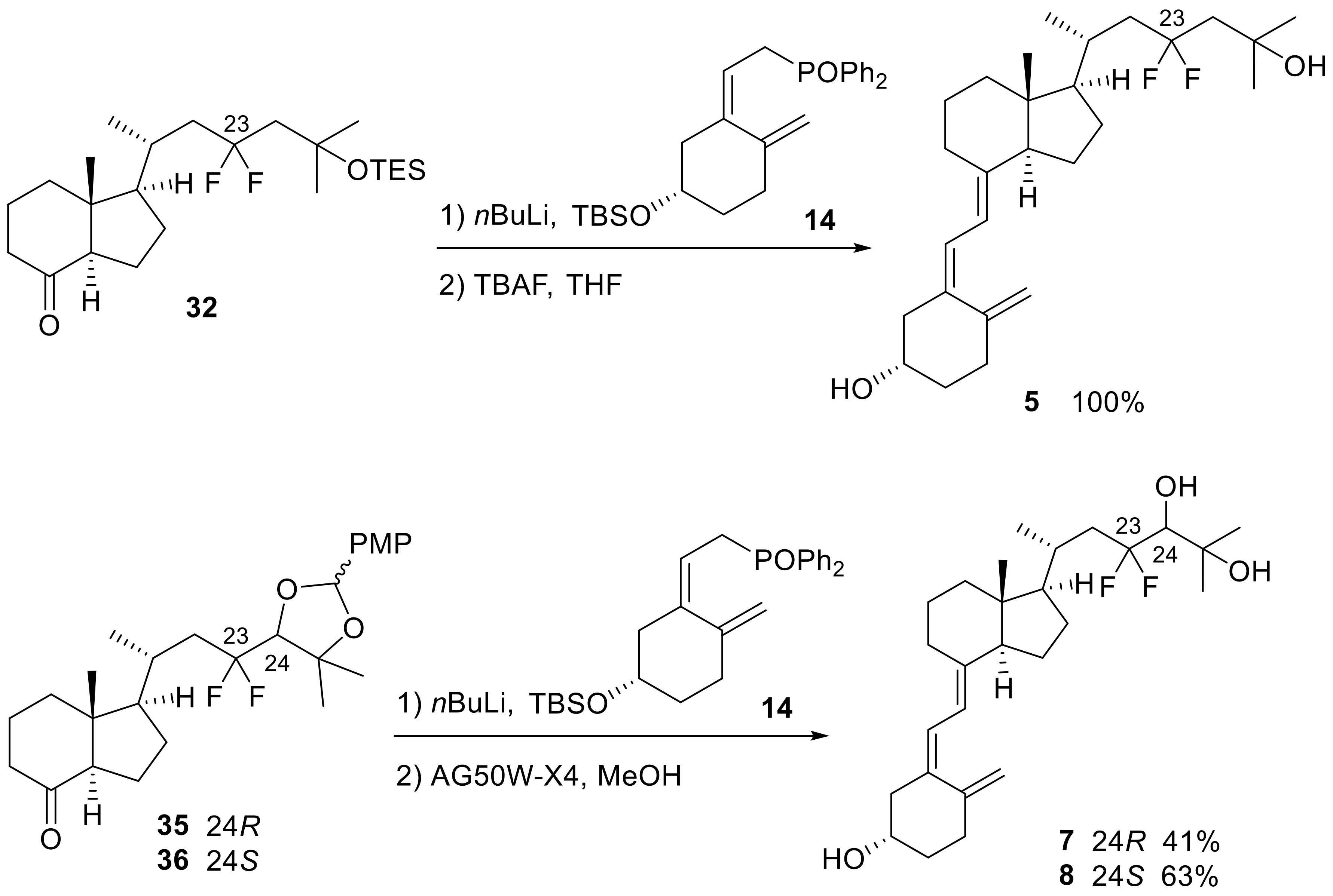
(3R,5Z,7E)-23,23-Difluoro-9,10-seco-5,7,10(19)-cholestatriene-3,25-diol: 23,23-Difluoro-25-hydroxyvitamin D3 (5)
nBuLi (183 μL, 1.55 M hexane solution, 0.29 mmol) was added to a solution of A-ring phosphine oxide 14 (129.1 mg, 0.29 mmol) in THF (1 mL) at −78 °C. After stirring for 20 min, a solution of 32 (30.7 mg, 0.07 mmol) in THF (1.5 mL) was added to the reaction mixture, and the mixture was stirred at −78 °C for 1 h. After the reaction was quenched with H2O at the same temperature, the mixture was extracted with EtOAc three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 20:1) to obtain the crude coupling product (52.0 mg), and it was used for the next reaction without further purification.
Tetrabutylammonium fluoride (710 μL, 1 M THF solution, 0.71 mmol) was added to the solution of the above crude coupling product (52.0 mg) in THF (4 mL), and the mixture was stirred at room temperature for 16 h. After the reaction was quenched with H2O and aqueous saturated NH4Cl at room temperature, the mixture was extracted with EtOAc three times. The organic layer was dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 1:1) to afford 5 (30.8 mg, 100%, in two steps) as a white powder.
5: +53.4 (c 2.37, EtOH); IR (neat) 3381, 1441, 1379, 1220, 1174, 1054, 895, 860, 741 cm−1; 1H-NMR (600 MHz, CD3OD) δ 0.63 (s, 3H), 1.12 (d, J = 7.2 Hz, 3H), 1.33–1.76 (m, 16H), 1.83–2.18 (m, 9H), 2.23 (dd, J = 9.6, 12.0 Hz, 1H), 2.44 (dt, J = 5.1, 13.2 Hz, 1H), 2.57 (dd, J = 3.9, 12.9 Hz, 1H), 2.88–2.91 (m, 1H), 3.78–3.82 (m, 1H), 4.78 (d, J = 1.2 Hz, 1H), 5.08 (brs, 1H), 6.08 (d, J = 10.8 Hz, 1H), 6.26 (d, J = 10.8 Hz, 1H); 13C-NMR (150 MHz, CD3OD) δ 12.6, 21.7, 23.5, 24.8, 29.2, 30.2, 30.6, 30.8, 33.1, 33.9, 36.9, 42.1, 45.2 (t, J = 23.0 Hz), 47.2, 47.3, 50.6 (t, J = 23.7 Hz), 57.9, 58.5, 70.4, 70.9, 113.0, 119.4, 122.9, 127.0 (t, J = 240.5 Hz), 137.8, 142.6, 147.3; 19F NMR (565 MHz, CD3OD) δ −94.3 (d, J = 224.7 Hz), −92.7 (d, J = 243.4 Hz); HRMS (ESI+) calcd for C27H42O4F2Na [M + Na]+ 459.3045, found 459.3033.
(3R,24R,5Z,7E)-23,23-Difluoro-9,10-seco-5,7,10(19)-cholestatriene-3,24,25-triol: (24R)-23,23-Difluoro-24,25-dihydroxyvitamin D3 (7)
nBuLi (152 μL, 1.55 M hexane solution, 0.24 mmol) was added to a solution of A-ring phosphine oxide 14 (112.9 mg, 0.25 mmol) in THF (1 mL) at −78 °C. After stirring for 20 min, a solution of 35 (26.5 mg, 0.06 mmol) in THF (1 mL) was added to the reaction mixture, and the mixture was stirred at −78 °C for 2 h. After the reaction was quenched with H2O at the same temperature, the mixture was extracted with EtOAc three times. The organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 5:1) to obtain the crude coupling product (25.5 mg), and it was used for the next reaction without further purification.
The above crude residue was dissolved in MeOH (5 mL), and AG 50W-X4 resin H+ form (183.1 mg) was added. The mixture was stirred for 3 h, insoluble materials were filtered off, and the solution was diluted with H2O and saturated aqueous NaHCO3. The mixture was extracted with EtOAc three times. The organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography (hexane:EtOAc = 1:1) to afford 7 (10.8 mg, 41%, two steps) as a white solid. Crystal of 7 was obtained by dissolving 7 in EtOH and allowing the solvent to slowly evaporate at room temperature (colorless needles).
7: +90.0 (c 0.83, EtOH); IR (neat) 3388, 1433, 1375, 1158, 1058, 1000, 957, 895 cm−1; 1H-NMR (600 MHz, CD3OD) δ 0.63 (s, 3H), 1.13 (d, J = 7.2 Hz, 3H), 1.29–1.63 (m, 13H), 1.70–1.79 (m, 3H), 1.88–2.33 (m, 9H), 2.44 (dt, J = 5.4, 13.8 Hz, 1H), 2.57 (dd, J = 3.6, 12.6 Hz, 1H), 2.88–2.91 (m, 1H), 3.44 (dd, J = 9.0, 13.8 Hz, 1H), 3.78–3.82 (m, 1H), 4.78 (d, J = 1.2 Hz, 1H), 5.08 (brs, 1H), 6.07 (d, J = 11.4 Hz, 1H), 6.26 (d, J = 10.8 Hz, 1H); 13C-NMR (150 MHz, CD3OD) δ 12.5, 22.2, 23.5, 24.8, 27.0 (d, J = 4.4 Hz), 27.8, 29.2, 30.2, 32.4, 33.9, 36.9, 41.1 (t, J = 22.3 Hz), 42.1, 47.2, 47.3, 57.9, 58.7, 70.9, 73.0, 79.5 (t, J = 26.6 Hz), 113.0, 119.4, 122.9, 127.1 (t, J = 244.1 Hz), 137.7, 142.6, 147.3; 19F-NMR (565 MHz, CD3OD) δ −107.1 (d, J = 251.8 Hz), −101.8 (d, J = 251.2 Hz); HRMS (ESI−) calcd for C27H42O3F2Cl [M + Cl]− 487.2796, found 487.2778.
4.1. Crystal Data of 7 (CCDC 2202393)
C27H42F2O3: Mr = 452.63, CuKα (λ = 1.54187 Å), orthorhombic, Pbca, colorless block 0.200 × 0.150 × 0.040 mm, crystal dimensions a = 6.74568(15) Å, b = 10.5822(3) Å, c = 36.4192(7) Å, α = 90°, β = 90°, γ = 90°, T = 173 K, Z = 4, V = 32599.74(10) Å3, Dcalc = 1.156 g/cm3, μCuKα = 6.723 cm−1, F000 = 984.00, GOF = 1.162, Rint = 0.0686, R1 = 0.0614, wR2 = 0.1337.
All measurements were taken on a Rigaku Raxis Rapid imaging plate area detector with graphite monochromated Cu-Kα radiation. The data were collected at a temperature of –100 °C. The structure was solved by direct-method SIR97 and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. All calculations were performed using the Crystal Structure (Crystal Structure 4.2.2) crystallographic software package except for refinement, which was performed using SHELXL97.
4.2. Accession Codes of Compound 7
CCDC 2202393 contains the supplementary crystallographic data for this study. These data can be obtained free of charge via
www.ccdc.cam.ac.uk/data_request/cif (accessed on 21 August 2022), by emailing
data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033.
(3R,24S,5Z,7E)-23,23-Difluoro-9,10-seco-5,7,10(19)-cholestatriene-3,24,25-triol: (24S)-23,23-Difluoro-24,25-dihydroxyvitamin D3 (8)
nBuLi (161 μL, 1.55 M hexane solution, 0.25 mmol) was added to a solution of A-ring phosphine oxide 14 (115.4 mg, 0.25 mmol) in THF (1 mL) at −78 °C. After stirring for 20 min, a solution of 36 (28.2 mg, 0.06 mmol) in THF (1 mL) was added to the reaction mixture, and the mixture was stirred at −78 °C for 100 min. After the reaction was quenched with H2O at the same temperature, the mixture was extracted with EtOAc three times. The organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel (hexane:EtOAc = 4:1) to obtain the crude coupling product (41.6 mg), and it was used for the next reaction without further purification.
The above crude residue was dissolved in MeOH (5 mL), and AG 50W-X4 resin H+ form (188.3 mg) was added. The mixture was stirred for 3 h 10 min, insoluble materials were filtered off, and the solution was diluted with H2O and saturated aqueous NaHCO3. The mixture was extracted with EtOAc three times. The organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography (hexane:EtOAc = 1:1) to afford 8 (19.6 mg, 63%, two steps) as a white powder.
8: +12.0 (c 0.25, CHCl3); IR (neat) 3388, 1437, 1375, 1263, 1162, 1066, 996, 891, 741 cm−1; 1H-NMR (600 MHz, CD3OD) δ 0.64 (s, 3H), 1.12 (d, J = 7.2 Hz, 3H), 1.30–1.42 (m, 10H), 1.47–1.63 (m, 4H), 1.68–1.79 (m, 3H), 1.87–2.31 (m, 9H), 2.44 (dt, J = 5.1, 13.2 Hz, 1H), 2.57 (dd, J = 3.6, 12.6 Hz, 1H), 2.88–2.91 (m, 1H), 3.46 (dd, J = 7.2, 16.8 Hz, 1H), 3.78–3.82 (m, 1H), 4.79 (d, J = 2.4 Hz, 1H), 5.08 (brs, 1H), 6.08 (d, J = 10.8 Hz, 1H), 6.26 (d, J = 10.8 Hz, 1H); 13C-NMR (150 MHz, CD3OD) δ 12.6, 21.6, 23.6, 24.8, 26.7 (d, J = 4.2 Hz), 27.9, 29.1, 30.2, 32.7, 33.9, 36.9, 41.3 (t, J = 22.2 Hz), 42.2, 47.2, 47.3, 57.9, 58.7, 70.9, 73.1, 79.0 (t, J = 27.3 Hz), 113.0, 119.4, 122.9, 127.3 (t, J = 244.9 Hz), 137.7, 142.6, 147.3; 19F-NMR (565 MHz, CD3OD) δ −105.0 (d, J = 251.5 Hz), −101.6 (d, J = 250.9 Hz); HRMS (ESI−) calcd for C27H42O3F2Cl [M + Cl]− 487.2796, found 487.2794.
4.3. Metabolism of 25(OH)D3 (1) and 5 by Recombinant hCYP24A1
The metabolism of 25(OH)D
3 and its analogue
5 by CYP24A1 was analyzed using the membrane fraction prepared from recombinant
Escherichia coli cells expressing human CYP24A1, as described in our previous study [
29]. Briefly, the reaction mixture containing 0.02 µM of human CYP24A1, 2.0 µM of adrenodoxin (ADX), 0.2 µM of NADPH-adrenodoxin reductase (ADR), 1 mM of EDTA, 1 mM of NADPH, and 5.0 µM of each substrate in 100 mM Tris-HCl (pH 7.4) was incubated at 37 °C for 5 or 15 min. The metabolites were extracted with four volumes of CHCl
3-CH
3OH (3:1) and analyzed by HPLC under the following conditions: column, CAPCELL PAK C18 UG120 (5 μm) (4.6 × 250 mm) (SHISEIDO, Tokyo, Japan); UV detection, 265 nm; flow-rate, 1.0 mL min
−1; column temperature, 40 °C; mobile phase, CH
3CN: a linear gradient of 20–100% CH
3CN aqueous solution per 25 min and 100% CH
3CN for 10 min.
4.4. Measurement of hVDR Binding Affinity of 25(OH)D3 (1) and 5
The binding affinity of each analogue for hVDR was evaluated using the in vitro system based on the split-luciferase technique described in our previous study [
30]. Briefly, 50 μL of cell lysate prepared from recombinant
E. coli expressing split-luciferase vitamin D biosensor protein was added to each well of a 96-well plate and left for 10 min at room temperature. Then, 50 μL of the Luciferin solution containing 20 mM of MgSO
4, 2 mM of D-luciferin, and 4 mM of adenosine triphosphate in 25 mM Tris-HCl (pH 7.4) were injected into each well and incubated for 15 min at room temperature. The luminescence (photon counts) was measured using a luminometer (Infinite 200 Pro 96-microplate luminometer, Tecan). The relative hVDR binding affinity of each analogue was evaluated based on the concentration at which the luminescence showed 50% of the maximum value.