MOBT Alleviates Pulmonary Fibrosis through an lncITPF–hnRNP-l-Complex-Mediated Signaling Pathway
Abstract
:1. Introduction
2. Results
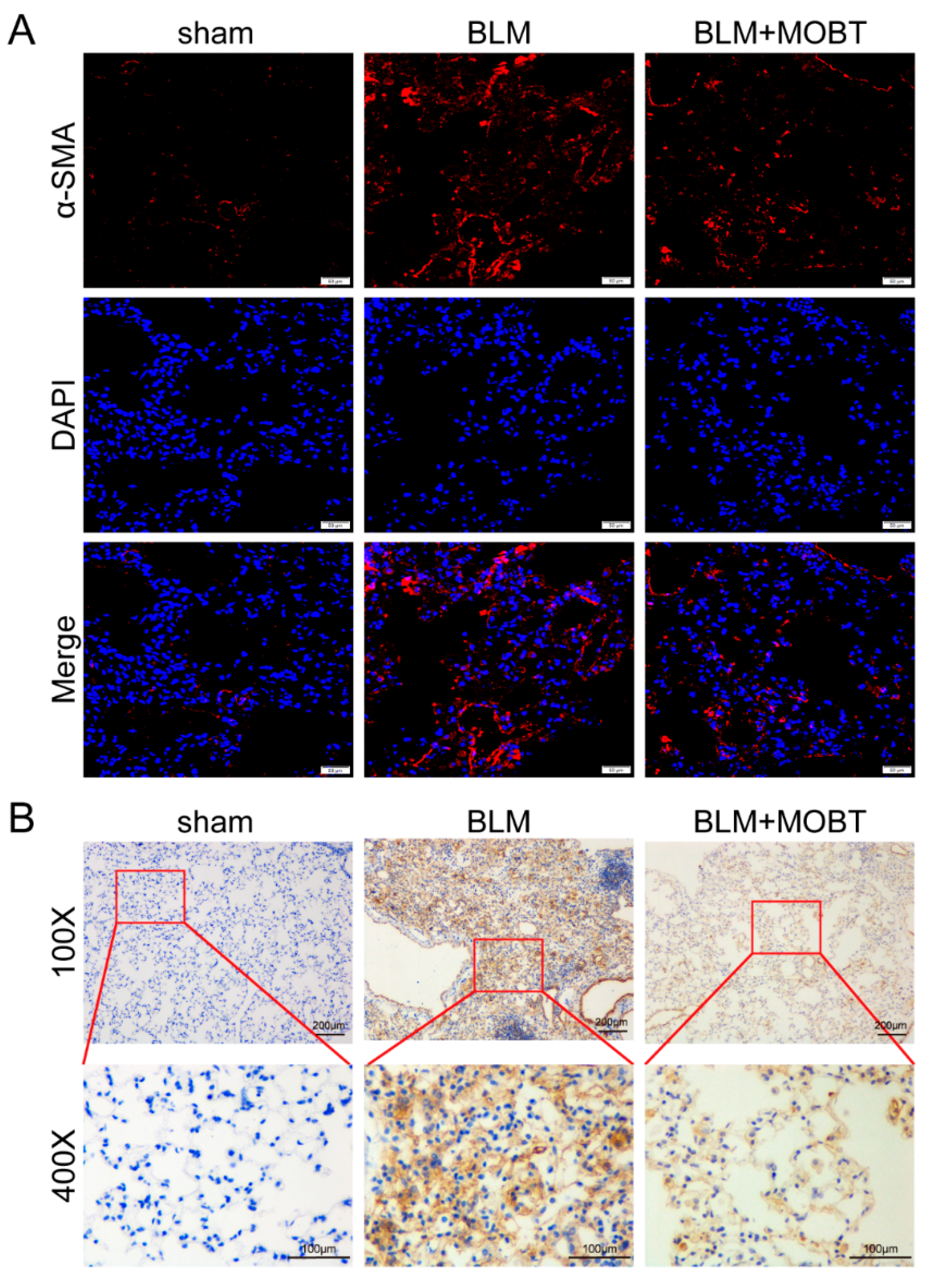
2.1. MOBT Prevented BLM-Induced Lung Fibrosis in Mice
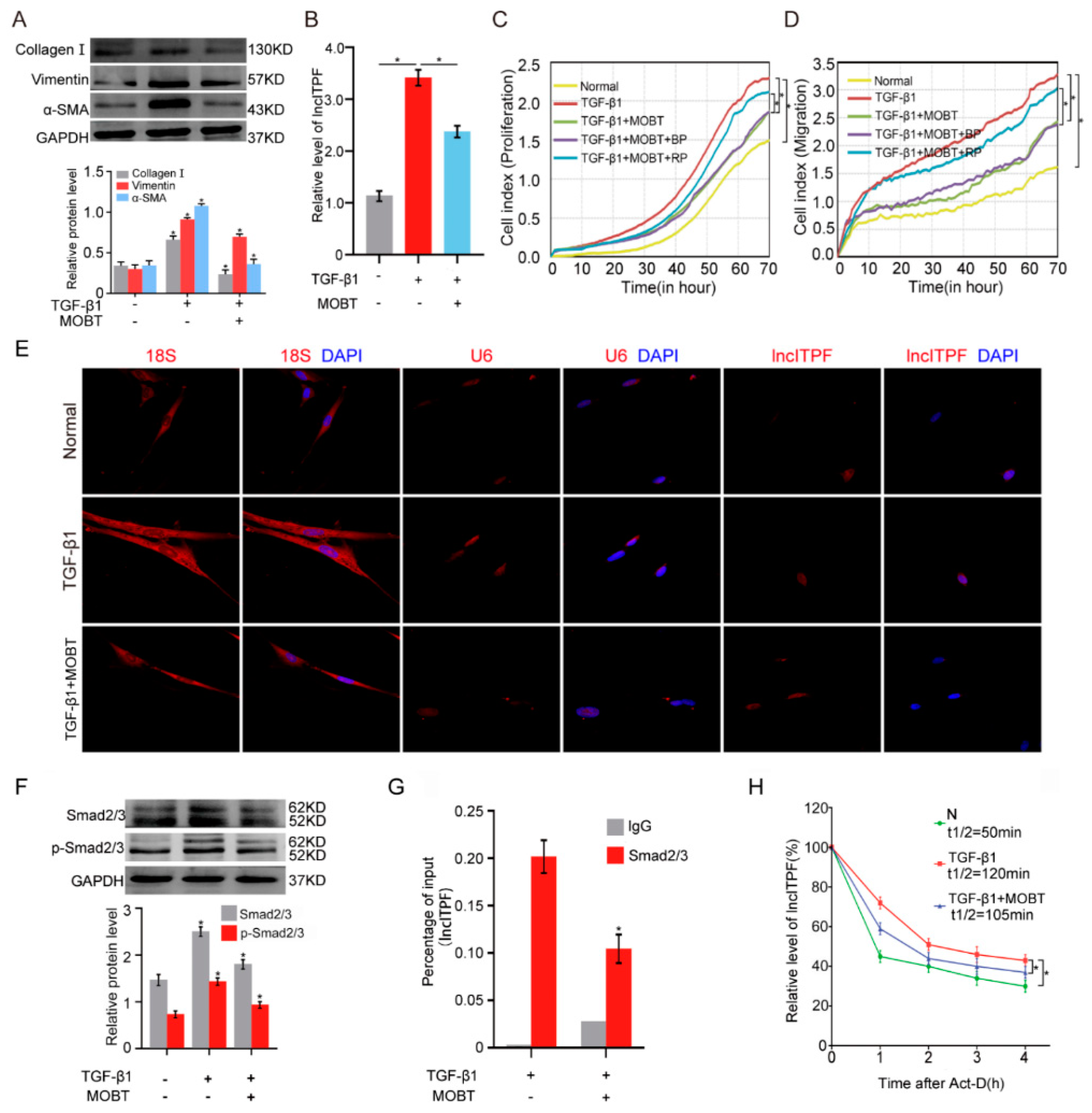
2.2. MOBT Prevented Pulmonary Fibrosis via Inhibiting lncITPF Transcription
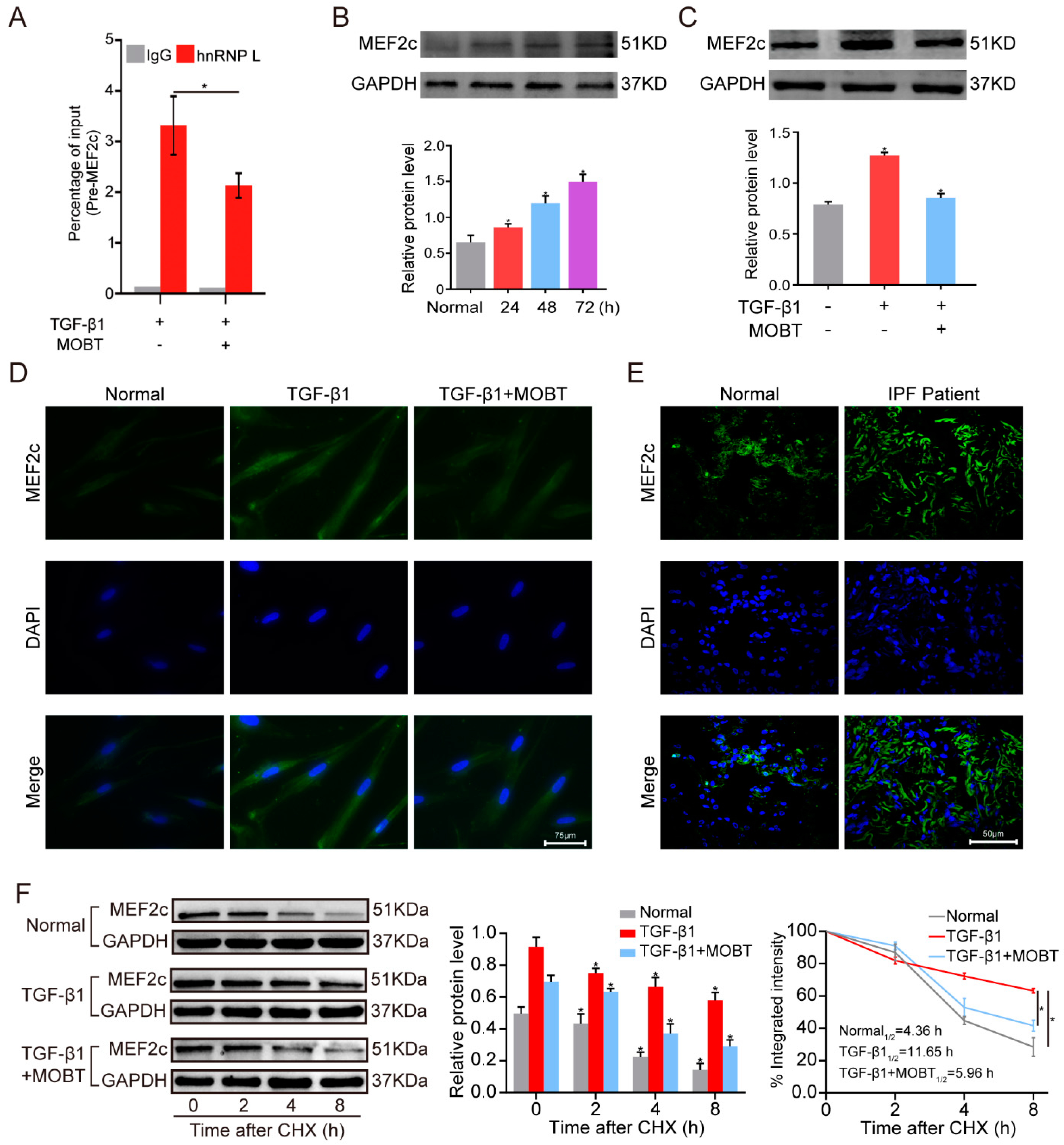
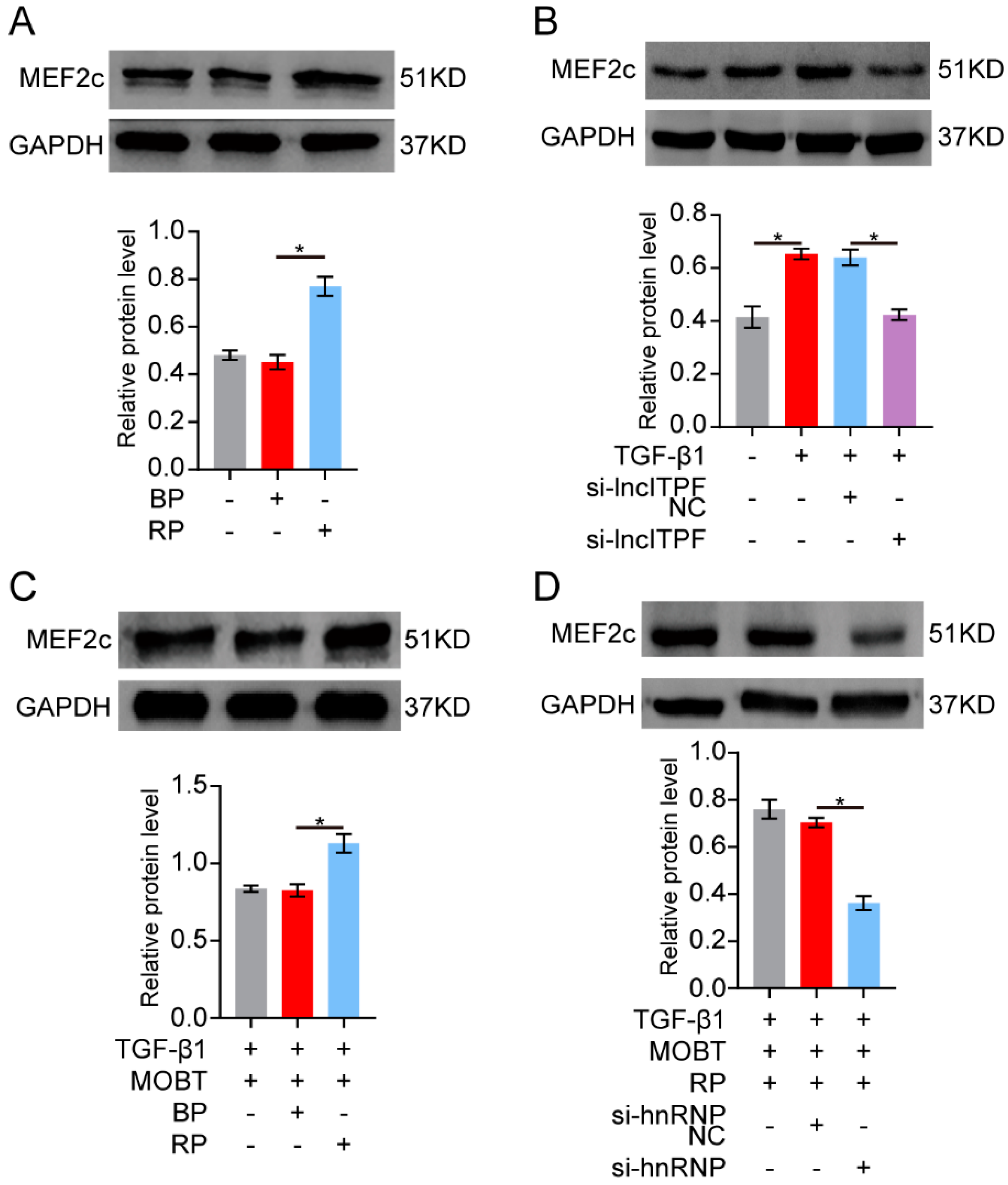
2.3. MOBT Downregulated lncITPF–hnRNP-l-Complex-Targeted MEF2c Splicing
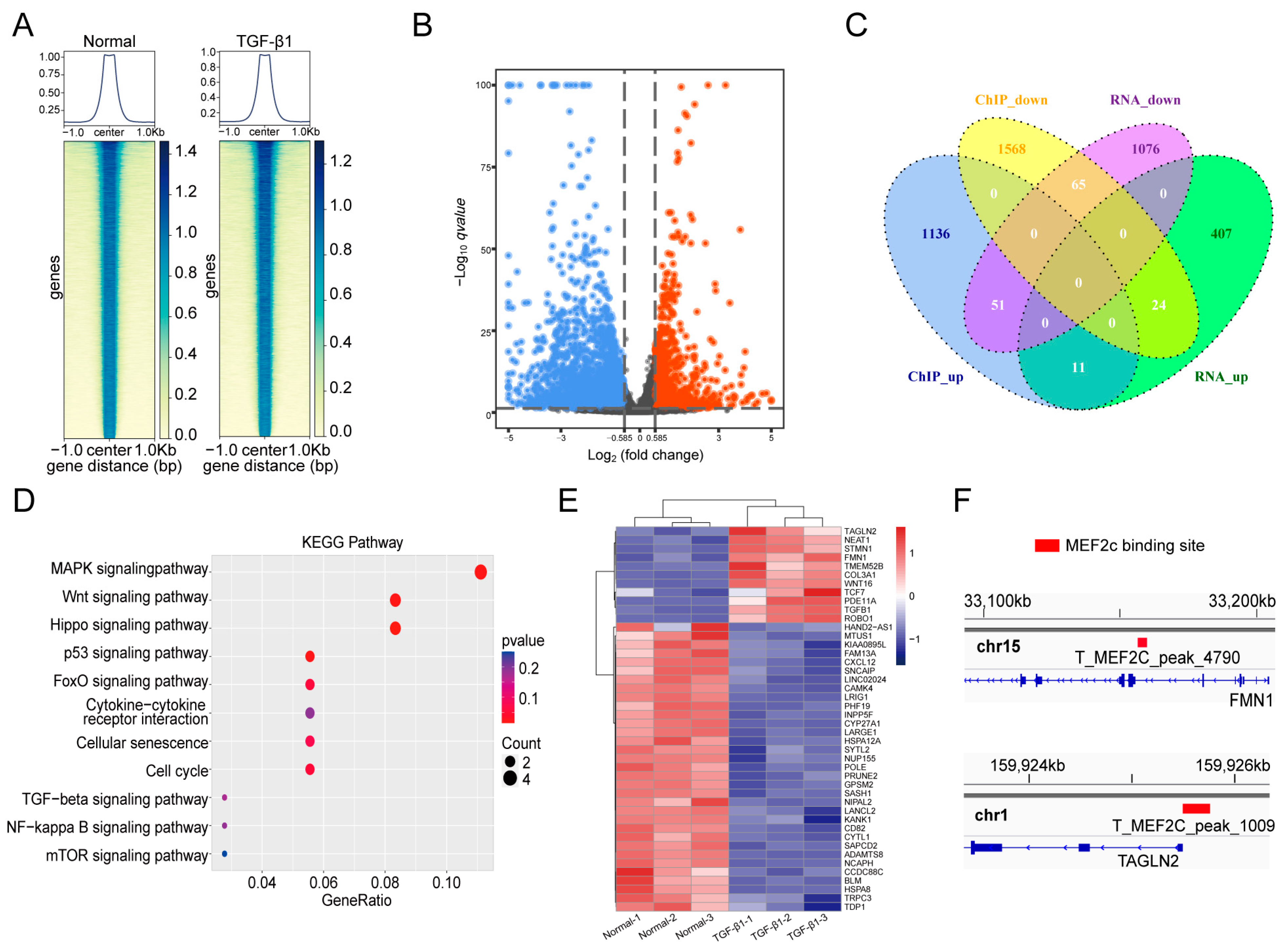
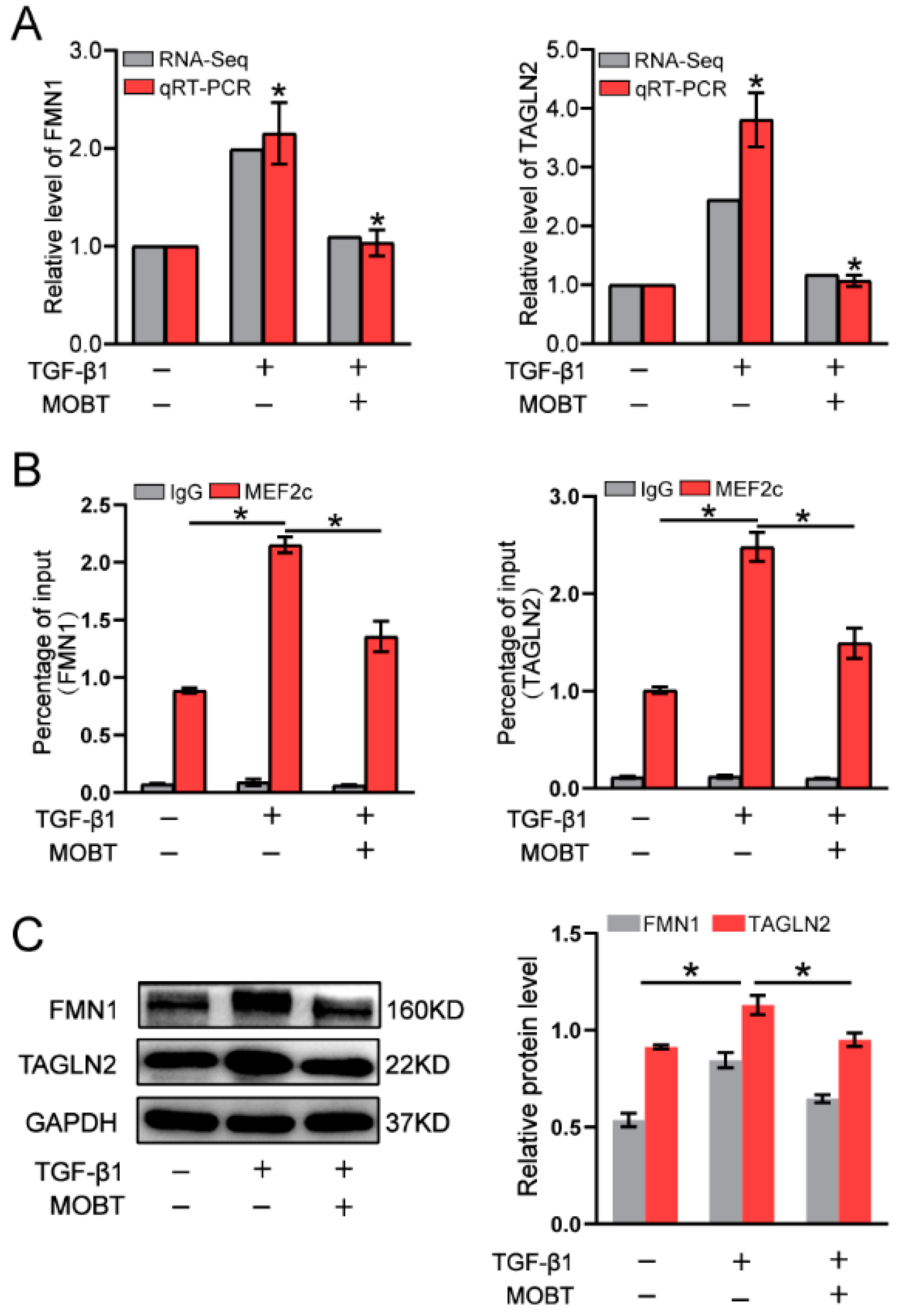
2.4. MOBT Downregulated the Target Genes of MEF2c
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Animal Model and Ethics Statement
4.3. Cell Model and Treatment
4.4. Western Blot
4.5. H&E and Masson Staining
4.6. Immunofluorescence Observation
4.7. Half-Life of lncITPF Analysis
4.8. ChIP-PCR
4.9. RNA-FISH
4.10. RTCA
4.11. RIP Analysis
4.12. RNA-Seq
4.13. ChIP-Seq
4.14. Statistical Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.; Yue, D.; Blackwell, T.S.; Lv, C.; Song, X. Long non-coding RNAs: Promising new targets in pulmonary fibrosis. J. Gene. Med. 2021, 23, e3318. [Google Scholar] [CrossRef] [PubMed]
- Guler, S.A.; Lindell, K.O.; Swigris, J.; Ryerson, C.J. What is idiopathic pulmonary fibrosis? IPF Part 1. Am. J. Respir. Crit. Care Med. 2021, 203, P5–P6. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Liu, Y.; Ma, S.; Yu, Z. Current advances in idiopathic pulmonary fibrosis: The pathogenesis, therapeutic strategies and candidate molecules. Future Med. Chem. 2019, 11, 2595–2620. [Google Scholar] [CrossRef]
- Spagnolo, P.; Kropski, J.A.; Jones, M.G.; Lee, J.S.; Rossi, G.; Karampitsakos, T.; Maher, T.M.; Tzouvelekis, A.; Ryerson, C.J. Idiopathic pulmonary fibrosis: Disease mechanisms and drug development. Pharmacol. Ther. 2021, 222, 107798. [Google Scholar] [CrossRef]
- Martinez, F.J.; Lederer, D. Focus on idiopathic pulmonary fibrosis: Advancing approaches to diagnosis, prognosis, and treatment. Chest 2018, 154, 978–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guler, S.A.; Lindell, K.O.; Swigris, J.; Ryerson, C.J. Medications for idiopathic pulmonary fibrosis: IPF Part 2. Am. J. Respir. Crit. Care Med. 2021, 203, P7–P8. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Zhang, J.; Zhao, X.; Xu, P.; Liu, X.; Li, M.; Lv, C.; Song, X. Crosstalk of mRNA, miRNA, lncRNA, and circRNA and Their Regulatory Pattern in Pulmonary Fibrosis. Mol. Ther. Nucleic Acids 2019, 18, 204–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Li, P.; Duan, J.X.; Liu, T.; Guan, X.X.; Mei, W.X.; Liu, Y.P.; Sun, G.Y.; Wan, L.; Zhong, W.J.; et al. Aucubin alleviates bleomycin-induced pulmonary fibrosis in a mouse model. Inflammation 2017, 40, 2062–2073. [Google Scholar] [CrossRef]
- Dong, Y.; Geng, Y.; Li, L.; Li, X.; Yan, X.; Fang, Y.; Li, X.; Dong, S.; Liu, X.; Li, X.; et al. Blocking follistatin-like 1 attenuates bleomycin-induced pulmonary fibrosis in mice. J. Exp. Med. 2015, 212, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, J.; Wang, M.; Liu, B.; Li, R.; Li, H.; Zhai, N.; Liu, W.; Lv, C.; Song, X. hnRNPL-activated circANKRD42 back-splicing and circANKRD42-mediated crosstalk of mechanical stiffness and biochemical signal in lung fibrosis. Mol. Ther. 2022, 30, 2370–2387. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Chang, M.W.; Pandey, P.R.; Tsitsipatis, D.; Yang, X.; Martindale, J.L.; Munk, R.; De, S.; Abdelmohsen, K.; Gorospe, M. Interaction of OIP5-AS1 with MEF2C mRNA promotes myogenic gene expression. Nucleic Acids Res. 2020, 48, 12943–12956. [Google Scholar] [CrossRef]
- Yu, B.; Qi, Y.; Li, R.; Shi, Q.; Satpathy, A.T.; Chang, H.Y. B cell-specific XIST complex enforces X-inactivation and restrains atypical B cells. Cell 2021, 184, 1790–1803.e17. [Google Scholar] [CrossRef]
- Song, X.; Xu, P.; Meng, C.; Song, C.; Blackwell, T.S.; Li, R.; Li, H.; Zhang, J.; Lv, C. lncITPF promotes pulmonary fibrosis by targeting hnRNP-L depending on its host gene ITGBL1. Mol. Ther. 2019, 27, 380–393. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Cao, G.; Jing, L.; Lin, S.; Wang, X.; Zhang, J.; Wang, M.; Liu, W.; Lv, C. Analysing the relationship between lncRNA and protein-coding gene and the role of lncRNA as ceRNA in pulmonary fibrosis. J. Cell. Mol. Med. 2014, 18, 991–1003. [Google Scholar] [CrossRef]
- Wu, Y.; Li, P.; Liu, L.; Goodwin, A.J.; Halushka, P.V.; Hirose, T.; Nakagawa, S.; Zhou, J.; Liu, M.; Fan, H. lncRNA Neat1 regulates neuronal dysfunction post-sepsis via stabilization of hemoglobin subunit beta. Mol. Ther. 2022, 30, 2618–2632. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Mohaqiq, M.; Majidpoor, J.; Moosavi, M.A.; Taheri, M. Exploring the role of non-coding RNAs in autophagy. Autophagy 2022, 18, 949–970. [Google Scholar] [CrossRef]
- Kumar, S.; Pan, C.C.; Shah, N.; Wheeler, S.E.; Hoyt, K.R.; Hempel, N.; Mythreye, K.; Lee, N.Y. Activation of Mitofusin2 by Smad2-RIN1 Complex during Mitochondrial Fusion. Mol. Cell 2016, 62, 520–531. [Google Scholar] [CrossRef] [Green Version]
- Kaur, K.; Hadas, Y.; Kurian, A.A.; Żak, M.M.; Yoo, J.; Mahmood, A.; Girard, H.; Komargodski, R.; Io, T.; Santini, M.P.; et al. Direct reprogramming induces vascular regeneration post muscle ischemic injury. Mol. Ther. 2021, 29, 3042–3058. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Cheng, S.J.; Ren, L.C.; Wang, Q.; Kang, Y.J.; Ding, Y.; Hou, M.; Yang, X.X.; Lin, Y.; Liang, N.; et al. An expanded landscape of human long noncoding RNA. Nucleic Acids Res. 2019, 47, 7842–7856. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.; Chung, H.; Basu, U. Regulation of long non-coding RNAs and genome dynamics by the RNA surveillance machinery. Nat. Rev. Mol. Cell Biol. 2020, 21, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Chekulaeva, M.; Rajewsky, N. Roles of long noncoding RNAs and circular RNAs in translation. Cold Spring Harb. Perspect. Biol. 2019, 11, a032680. [Google Scholar] [CrossRef] [PubMed]
- Shaath, H.; Vishnubalaji, R.; Elango, R.; Kardousha, A.; Islam, Z.; Qureshi, R.; Alam, T.; Kolatkar, P.R.; Alajez, N.M. Long non-coding RNA and RNA-binding protein interactions in cancer: Experimental and machine learning approaches. Semin. Cancer Biol. 2022, in press. [Google Scholar] [CrossRef]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Chen, H.; Li, H.; Xu, P.; Liu, B.; Zhang, Q.; Lv, C.; Song, X. ATF3—Activated accelerating effect of LINC00941/lncIAPF on fibroblast-to-myofibroblast differentiation by blocking autophagy depending on ELAVL1/HuR in pulmonary fibrosis. Autophagy 2022, in press. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Chen, M.; Wang, B.; Yang, X.; Yang, M.; Wang, Q.; Cui, Z.; Ge, F. Long noncoding RNA HOTAIR interacts with Y-Box Protein-1 (YBX1) to regulate cell proliferation. Life Sci. Alliance 2021, 4, e202101139. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Abak, A.; Talebi, S.F.; Shoorei, H.; Branicki, W.; Taheri, M.; Akbari Dilmaghani, N. Role of miRNA and lncRNAs in organ fibrosis and aging. Biomed. Pharmacother. 2021, 143, 112132. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Y.; Huan, L.; Zhao, J.; Zhou, Y.; Xu, L.; Hu, Z.; Liu, Y.; Chen, Z.; Wang, L.; et al. An LTR retrotransposon-derived long noncoding RNA lncMER52A promotes hepatocellular carcinoma progression by binding p120-Catenin. Cancer Res. 2020, 80, 976–987. [Google Scholar] [CrossRef]
- Sun, C.; Fu, Y.; Gu, X.; Xi, X.; Peng, X.; Wang, C.; Sun, Q.; Wang, X.; Qian, F.; Qin, Z.; et al. Macrophage-enriched lncRNA RAPIA: A novel therapeutic target for atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Saghafi, T.; Taheri, R.A.; Parkkila, S.; Emameh, R.Z. Phytochemicals as modulators of long non-coding RNAs and inhibitors of cancer-related carbonic anhydrases. Int. J. Mol. Sci. 2019, 20, 2939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lü, S.; Liu, Y.; Cui, J.; Yang, B.; Li, G.; Guo, Y.; Kuang, H.; Wang, Q. Mechanism of Caulophyllum robustum Maxim against rheumatoid arthritis using lncRNA-mRNA chip analysis. Gene 2020, 722, 144105. [Google Scholar] [CrossRef] [PubMed]
- Gueroussov, S.; Weatheritt, R.J.; O’Hanlon, D.; Lin, Z.Y.; Narula, A.; Gingras, A.C.; Blencowe, B.J. Regulatory expansion in mammals of multivalent hnRNP assemblies that globally control alternative splicing. Cell 2017, 170, 324–339.e23. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Chen, W.B.; Lv, D.J.; Yang, T.W.; Wu, K.H.; Zou, L.B.; Luo, J.; Zhou, X.M.; Liu, G.C.; Shu, F.P.; et al. LncRNA SNHG1 and RNA binding protein hnRNPL form a complex and coregulate CDH1 to boost the growth and metastasis of prostate cancer. Cell Death Dis. 2021, 12, 138. [Google Scholar] [CrossRef]
- He, X.; Chai, P.; Li, F.; Zhang, L.; Zhou, C.; Yuan, X.; Li, Y.; Yang, J.; Luo, Y.; Ge, S.; et al. A novel lncRNA transcript, RBAT1, accelerates tumorigenesis through interacting with HNRNPL and cis-activating E2F3. Mol. Cancer 2020, 19, 115. [Google Scholar] [CrossRef]
- Cosgrove, D.; Whitton, L.; Fahey, L.; Broin, P.Ó.; Donohoe, G.; Morris, D.W. Genes influenced by MEF2c contribute to neurodevelopmental disease via gene expression changes that affect multiple types of cortical excitatory neurons. Hum. Mol. Genet. 2021, 30, 961–970. [Google Scholar] [CrossRef]
- Pereira, A.; Cardoso, A.C.; Consonni, S.R.; Oliveira, R.R.; Saito, A.; Vaggione, M.; Matos-Souza, J.R.; Carazzolle, M.F.; Gonçalves, A.; Fernandes, J.L.; et al. MEF2c repressor variant deregulation leads to cell cycle re-entry and development of heart failure. EBioMedicine 2020, 51, 102571. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Yoshida, T.; Wu, L.; Maiti, D.; Cebotaru, L.; Duh, E.J. Transcription factor MEF2c suppresses endothelial cell inflammation via regulation of NF-κB and KLF2. J. Cell Physiol. 2015, 230, 1310–1320. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Ping, J.; Zhou, Y.; Chen, G.; Xu, L. Salvianolic Acid B Inhibits Activation of Human Primary Hepatic Stellate Cells Through Downregulation of the Myocyte Enhancer Factor 2 Signaling Pathway. Front. Pharmacol. 2019, 10, 322. [Google Scholar] [CrossRef]


| Antibody Name | Item Number | Brand of the Antibody |
|---|---|---|
| GAPDH | AF7021 | Affinity |
| a-SMA | Ab7817 | Abcam |
| Vimentin | AF7013 | Affinity |
| Collagen I | AF7001 | Affinity |
| MEF2c | Ab78888 | Abcam |
| hnRNP L | Ab6106 | Abcam |
| Smad2/3 | 8685 | Cell Signaling Technology |
| p-Smad2/3 | 8828 | Cell Signaling Technology |
| ADAMTS8 | Bs-5859R | Bioss |
| TAGLN2 | 15508-1-AP | Proteintech |
| HSPA12A | Ab200838 | Abcam |
| FMN1 | 25982-1-AP | Proteintech |
| Experimental Type | Gene Name | Primer Sequence (5′ to 3′) |
|---|---|---|
| qRT-PCR | lncITPF | F: ACCGCCTAATACGACTCACTATAGGGACCC CACCATGGACTCAGTGATAGGAACAAAATGT R: TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTACT AACAT AAAACAGTGTGCTAATCA |
| qRT-PCR | TAGLN2 | F: CCTTCAAGCAGATGGAGCAG R: CTTCCCAGAGGTCCACAGTT |
| qRT-PCR | FMN1 | F: ATGTGGATGACTCCGTGGTT R: GATTATGCACTGGGCACGTT |
| ChIP-PCR | lncITPF | F: GAAAAAGCCCTCACAAAAGCCTCAC R: GGGGAGGTTACTTTGTGGAAGGATC |
| ChIP-PCR | TAGLN2 | F: CTCTGGAAGCACGCCTTTG R: ATCTATCTGTGAGGGTCCTGAG |
| ChIP-PCR | FMN1 | F: TTAGCTGGACATGGTGGTG R: TCACAATATTAGCCAAACACTG |
| RIP | pre-MEF2c | F: ACCGACATGGACAAAGTGCTTCTCA R: ATGTGTGTATGTGTGTGTGGCAGGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Zhang, H.; Li, H.; Liu, B.; Li, R.; Zhang, J.; Song, X.; Lv, C.; Li, H.; Chen, M. MOBT Alleviates Pulmonary Fibrosis through an lncITPF–hnRNP-l-Complex-Mediated Signaling Pathway. Molecules 2022, 27, 5336. https://doi.org/10.3390/molecules27165336
Xu P, Zhang H, Li H, Liu B, Li R, Zhang J, Song X, Lv C, Li H, Chen M. MOBT Alleviates Pulmonary Fibrosis through an lncITPF–hnRNP-l-Complex-Mediated Signaling Pathway. Molecules. 2022; 27(16):5336. https://doi.org/10.3390/molecules27165336
Chicago/Turabian StyleXu, Pan, Haitong Zhang, Huangting Li, Bo Liu, Rongrong Li, Jinjin Zhang, Xiaodong Song, Changjun Lv, Hongbo Li, and Mingwei Chen. 2022. "MOBT Alleviates Pulmonary Fibrosis through an lncITPF–hnRNP-l-Complex-Mediated Signaling Pathway" Molecules 27, no. 16: 5336. https://doi.org/10.3390/molecules27165336
APA StyleXu, P., Zhang, H., Li, H., Liu, B., Li, R., Zhang, J., Song, X., Lv, C., Li, H., & Chen, M. (2022). MOBT Alleviates Pulmonary Fibrosis through an lncITPF–hnRNP-l-Complex-Mediated Signaling Pathway. Molecules, 27(16), 5336. https://doi.org/10.3390/molecules27165336







