Abstract
The building of heterocyclic systems containing hydrogenated fragments is an important step towards the creation of biologically-active compounds with a wide spectrum of pharmacological activity. Among the numerous methods for creating such systems, a special place is occupied by processes using N-substituted maleimides as the initial substrate. This molecule easily reacts in Diels-Alder/retro-Diels-Alder reactions, Michael additions with various nucleophiles, and co-polymerization processes, as have been described in numerous detailed reviews. However, information on the use of maleimides in cascade heterocyclization reactions is currently limited. This study is devoted to a review and analysis of existing literature data on the processes of recyclization of N-substituted maleimides with various C,N-/N,N-/S,N-di- and polynucleophilic agents, such as amidines, guanidines, diamines, aliphatic ketazines, aminouracils, amino- and mercaptoazoles, aminothiourea, and thiocarbomoyl pyrazolines, among others. The significant structural diversity of the recyclization products described in this study illustrates the powerful potential of maleimides as a building block in the organic synthesis of biologically-active compounds with hydrogenated heterocyclic fragments.
1. Introduction
Fragment-based drug discovery (FBDD) is a well-established method for creating new hits and leads [1,2,3,4,5,6]. This approach has been repeatedly confirmed in practice and it is an additional strategy to supplement other search methods, such as high-throughput screening [7].
A detailed evaluation of many existing “fragmentary” libraries indicates the predominance of (hetero)aromatic “planar” compounds and the very low diversity of chiral compounds rich in Csp3 atoms [8,9].
However, studies by Ritchieetal [10] and Loveringetal [11] demonstrated that an increase in the proportion of Csp3 atoms in a molecule or the limitation of the number of aromatic rings significantly increase the activity of compounds and their easy passage through barriers. In addition, it has been proven that mainly systems rich in Csp3 atoms are used in real clinical practice after human trials [12]. All these facts point to the necessity to build unsaturated condensed or linear coupled ensembles, both for the development of screening collections and in the subsequent development of hit to lead.
The number of studies aimed at synthesizing heterocyclic compound collections enriched in Csp3 atoms is very limited, and therefore access to new types of scaffolds is limited. There are only a few studies [13] devoted to the development of new approaches and methods for constructing fragments with several synthetically available three-dimensional growth vectors, which provide fast and efficient development of hit to lead after initial screening. Considering all the above points, an important task is the development of efficient synthetic routes for partially saturated bicyclic heteroaromatic (PSBH) frameworks with an increased content of Csp3 atoms compared to existing libraries.
The requirements for the efficiency of synthetic methods are constantly increasing due to the need to simultaneously increase the molecular complexity and minimize the number of steps in synthetic procedures. Cascade (tandem, domino) processes are a very promising methodology for organic synthesis, allowing the structure of the target molecule to complicate by combining a series of successive transformations in one synthetic operation. Among the developed methodologies for domino transformations, the most effective is the sequence of reactions, at the key stage of which the formation of a heterocyclic system occurs as a result of the recycling of the intermediate. With this approach, the chemo-, regio-, and stereoselectivity of processes usually increases significantly due to the greater determinism of the location of the reaction centres of the reagent and substrate.
The problem of searching for easily accessible, polyfunctional substrates that allow for directed cascade synthesis of various heterocyclic structures is one of the key ones. In this context, N-arylmaleimides deserve special attention [14,15]. Their interaction with various reagents, including the domino route, can lead to the formation of a large number of hydrogenated heterocyclic systems. However, only (retro-) Diels-Alder reactions [14,16,17], Michael additions [16,17] with various nucleophiles, and co-polymerization processes [16,18] have been studied in detail to date. This study is devoted to a review and analysis of existing literature data on the processes of recyclization of N-substituted maleimides with various linear and cyclic di- and polynucleophilic agents.
The analysis of the available literature data allowed us to draw a conclusion regarding the sequence of these reactions. The initial nucleophilic addition of a di- or polynucleophile according to the Michael reaction to the activated multiple bond of the imide and subsequent recyclization of the intermediate succinimide intermediate proceeds due to intramolecular nucleophilic substitution with the participation of another nucleophilic centre and one of the carbonyl groups. It should be noted that, depending on the structure of the dinucleophilic component and the selected conditions, the formation of various alternative products is possible, which is reflected in Scheme 1.
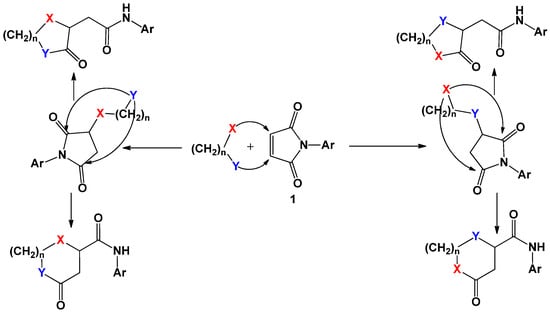
Scheme 1.
General interaction scheme of N-arylmaleimides with binucleophiles.
2. Reactions with N,N-dinucleophiles
Partially hydrogenated azoloazines represent a class of heterocyclic compounds with high biological and pharmacological activity [19,20,21]. In addition, these systems are often used as a simple model for studying such fundamental issues of medical and organic chemistry as conformational flexibility, tautomerism, electronic effects, etc. [10,11]. One of the most effective approaches to the building of partially hydrogenated azoloazines is the reactions of 1,3-N,N- and 1,4-N,N-dinucleophiles with maleic anhydride and its imides.
A typical example of recyclization reactions of maleimides 1 with 1,3-N,N-binucleophiles, is their interaction with carboxymidamides 2 (Scheme 2). Thus, Kh. S. Shikhaliev [22] and Yu. A. Kovygin [23] et al. found that the optimal conditions for these processes are boiling the mixture of reagents in acetone or chloroform. It was assumed that the mechanism of the process consists of two successive stages. During the first stage, the nucleophilic addition of the carboximidamide amino group at the double bond of the N-arylmaleimide molecule occurs. The resulting adduct undergoes subsequent tandem recycling of the succinimide moiety to form substituted 2-[4-oxo-4,5-dihydro-1H-imidazol-5-yl]-N-arylacetamides 4, the structure of which was proved using XRD analysis. The authors also noted that when using methanol, isopropanol, water, or dimethylformamide, mixtures which are difficult to separate were formed, which was most likely due to the solvolysis of maleimide that had been catalyzed by the highly basic carboxamide. In addition to the main target compound 4 (the yield fluctuated within 50%), the transamidation product 5 (yield up to 10%) was also isolated. The attempts for cyclization of this product were unsuccessful.
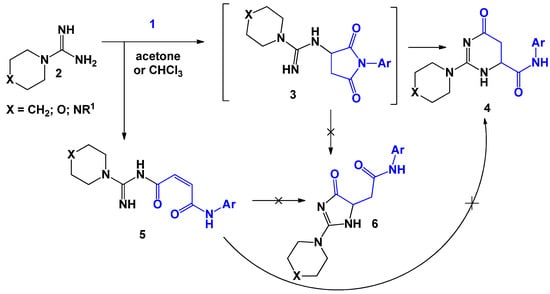
Scheme 2.
The interaction of N-arylmaleimides with carboximidamides.
We know about examples of substitution of aliphatic 1,3-binucleophiles in these reactions by their heterocyclic analogues containing a guanidine fragment [24]. Thus, for 2-aminobenzimidazole 7 and 2-aminotriazole 8 when they interact with 1 in dioxane medium, the corresponding 2-oxo-1,2,3,4-tetrahydrobenzo[4,5]imidazo[1,2-a]pyrimidine-4-carboxyanilides 9 and 7-oxo-5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-a]pyrimidine-5-carboxyanilides 13 were isolated. Taking into account the non-equivalence of nucleophilic centres in the initial heterocyclic matrices, the formation of several regioisomeric products during these processes (9–12) shown in Scheme 3 for 2-aminobenzimidazole is possible. The choice of the structure of the obtained compounds was carried out using the detailed analysis of the literature data [25] and obtained spectral [24,26] data.
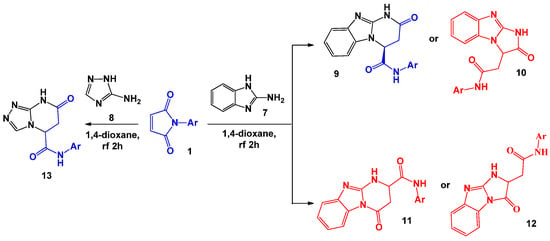
Scheme 3.
The interaction of N-arylmaleimides with aminoazoles.
Later, R.V. Rudenko et al. [26] showed that varying the solvent significantly changes the direction of this reaction. If in the case of 2-aminobenzimidazole the replacement of dioxane by dimethylformamide only reduced the reaction time, a completely different pattern would be observed for 2-aminotriazole 8. Thus, when the reaction was performed in the acetic acid or DMF medium, the regioisomer 14 was formed instead of the expected compound 13 (Scheme 4).
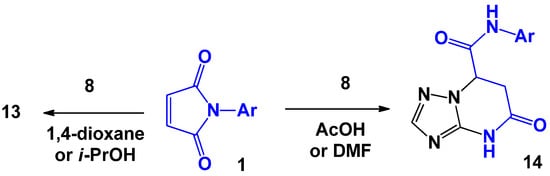
Scheme 4.
Specifics of the interaction of N-arylmaleimides with 2-aminotriazole.
However, in the case of the 5-amino-4-R-pyrazoles [27] 15 variation of aromatic substituents in 1, it allowed both tetrahydropyrazolo[1,5-a]pyrimidines 16 and dihydroimidazo[1,2-b]pyrazoles 17 to be isolated (Scheme 5, Table 1). The authors also noted that when the reaction was carried out in isopropyl alcohol, a linearly-bound intermediate 18 was formed. When this intermediate was boiled in acetic acid, the mixture of 16 and 17 was formed, and intermediate 16 was obtained as the result of boiling in dimethylformamide.
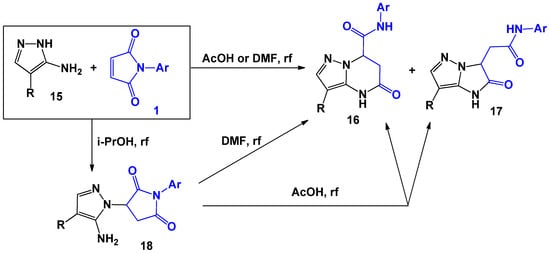
Scheme 5.
The interaction of N-arylmaleimides with 5-amino-4-R-pyrazoles.

Table 1.
The composition of the reaction masses.
Catalysts play a special role in recyclization reactions involving N-arylmaleimides. T. Matviyuk [28] found that the interaction of 2-aminopyridines 19 with maleimides 1 in a dioxane medium with the involvement of lithium perchlorate led to the formation of 2-oxo-2,3-dihydroimidazo[1,2-a]pyridin-3-yl derivatives of succinmide 24 (Scheme 6). The authors suggested that during the first stage, 2-aminopyridine reacts with maleimide at the endocyclic nitrogen atom, forming a substituted succinimide, the subsequent recycling of which leads to intermediate compound 22. Alternatively, this reaction may proceed with an initial attack of the exocyclic nitrogen atom followed by the formation of adduct 23. However, the performed NOESY analysis demonstrates a strong correlation between the α-H of the pyridine ring and the proton of the methylene group in the side chain. Further, intermediate adduct 22, being a strong CH-acid, reacts with the second maleimide molecule, which leads to the final product 24.
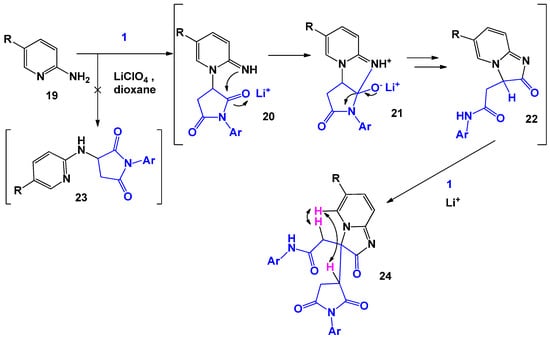
Scheme 6.
General scheme and possible mechanism of interaction of N-arylmaleimides with 2-aminopyridines.
A series of studies was devoted to the investigation of the interaction of maleimides with 1,4-binucleophiles. For 1,2-diaminoethane 25, N,N-dibenzylethane-1,2-diamine 26, and 12,-diaminocyclohexane 27, which are symmetrical dinucleophiles, A. V. Zorina [29] isolated the following compounds by boiling the mixture of reagents in methanol: 2-(3-oxopiperazin-2-yl)-acetanilides 28, 2-acet-4-methylanilido-1,4-dibenzyl-3-oxopiperazines 29, and 2-acetanylido-3-oxodecahydroquinoxalines 30 (Scheme 7).
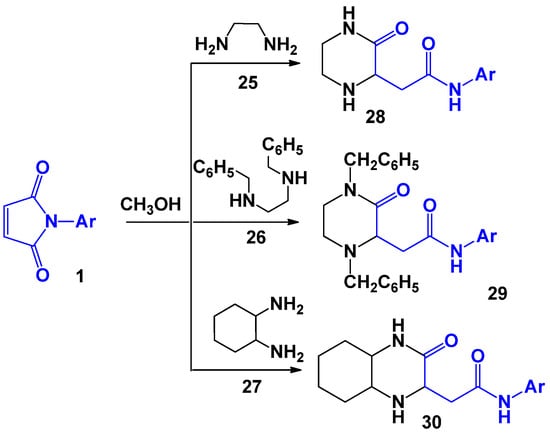
Scheme 7.
The interaction of N-arylmaleimides with various symmetrical 1,4-N,N-dinucleophiles.
M. M. Abelman [30] noted that when using unsymmetrical ethylenediamines, the reaction in ethanol at room temperature, depending on the radicals, can lead to various recyclization products (Scheme 8, Table 2).
Scheme 8.
Possible products of the interaction of N-arylmaleimides with ethylenediamines.

Table 2.
Interaction of substituted ethylenediamines with maleimides.
In the case of reaction 1 with o-phenylenediamine [31] (Scheme 9), regardless of the solvent used, the formation of only tetrahydroquinaxolinyl acetanilides 37 was noted. At the same time, the maximum yield of end products (67%) was achieved in aqueous ethanol.
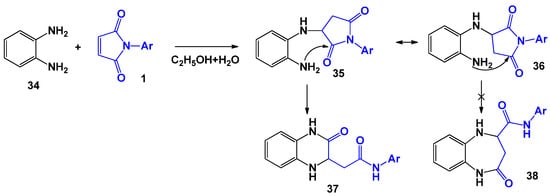
Scheme 9.
o-Phenylenediamine in the recyclization reaction with N-arylmaleimides.
3. Reactions with S- and O-containing Dinucleophiles
The thiazole molecule is a good pharmacophore nucleus due to its various pharmaceutical applications. Its derivatives possess a wide spectrum of biological activity, such as antioxidant, analgesic, antibacterial, anticancer, anti-allergic, antihypertensive, anti-inflammatory, antimalarial, antifungal, and antipsychotic effects [32,33,34,35,36,37].
For the first time, the possibility of using N-arylmaleimides to build a thiazole ring was described by M. Augustin [38] and D. Marrian [39]. Thiourea and N-phenylthiourea, which are examples of 1,3-N,S-dinucleophiles, were considered as initial substrates. The reaction was carried out in a dioxane medium (Scheme 10).
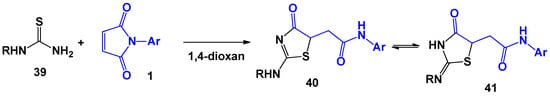
Scheme 10.
The interaction of N-arylmaleimides with unsubstituted and N-substituted thioureas.
The obtained results allowed the extension of this reaction to other systems containing a thioamide fragment (Scheme 11 and Scheme 12). Thus, interactions of primary and secondary thioamides with N-arylmaleimides were considered by T. Takido [40]. It has been established that, regardless of the structure of the thioamide component, the optimal conditions for the reaction are boiling the mixture of reagents in a dioxane medium. It was assumed that this reaction proceeds due to the nucleophilic attack by the sulphur atom of the thioamide 42 at the double bond of N-arylmaleimide 1 with the formation of succinimide 43. The intramolecular attack of the imine nitrogen on the nearest carbonyl group and subsequent recyclization of the imide ring leads to mesoionic intermediate 44. Further, due to the intramolecular rearrangement of the proton or alkyl (aryl) group from the nitrogen atom to the oxygen atom, the formation of final thiazoles 45 occurs.
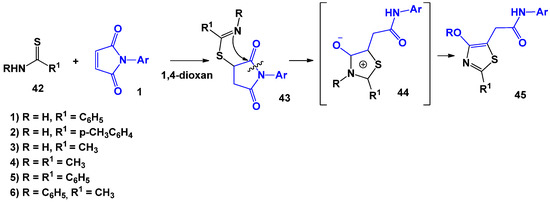
Scheme 11.
The interaction of primary and secondary thioamides with N-arylmaleimides.
Scheme 12.
The interaction of heterocyclic thioamides with N-arylmaleimides.
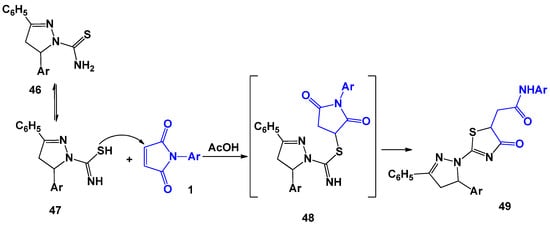
Later, D. Havrylyuk [41] et al. extended this process to heterocyclic systems containing a thioamide component, in particular, to 3-phenyl-5-aryl-1-thiocarbamoyl-2-pyrazolines (Scheme 12).
Interesting and contradictory results have been obtained in the case of heterocyclic matrices, in which the thioamide fragment was part of the cyclic system (Scheme 13). Lesyk R. et al. [42], as a result of boiling an equimolar mixture of reagents for 2 h in acetic acid, were able to isolate N-(R-phenyl)-(6-oxo-5,6-dihydro[1,3]thiazole[3,2-b][1,2,4]triazol-5-yl)acetamides 51 based on the example of 1,2,4-triazole-3(5)-thiol 50. However, S. Holota [43], when investigating the same interaction, showed that boiling an equimolar mixture in the range of 30 min–24 h and the use of such solvents as acetic acid, acetone, acetonitrile, benzene, and toluene in the presence or absence of sodium acetate only led to the formation of linearly-bound product 52. At the same time, attempts at the cyclization of 52 did not lead to success. Such a discrepancy in the obtained experimental results is due to the fact that in [42], the conclusion about the structure of the obtained compound was made only based on the interpretation of the spectral data. Thus, the signals in the 1H NMR spectra at δ = 13.8 and -14.3 ppm were treated as NH protons in amide group 51. However, a similar singlet in a similar magnetic field can belong to the signal of NH of the proton of triazole ring 52. This controversial issue was resolved in [43] by analyzing XRD data.
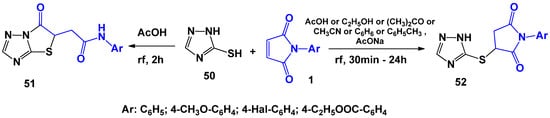
Scheme 13.
The interaction of N-arylmaleimides with mercaptotriazole.
Another direction in the chemistry of N-arylmaleimides, which is well represented in the literature, is the building of thiomorpholine rings when they interact with 1,4-N,S-binucleophiles [38,44] (Scheme 14). This framework is a common pharmacophoric element and it exhibits selective enzyme inhibition for many receptors and other types of molecular targets [45,46,47,48,49,50,51].
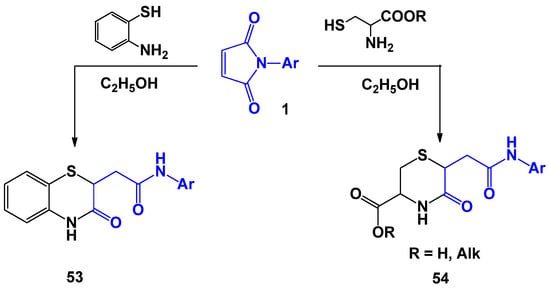
Scheme 14.
The reactions of N-arylmaleimides with 1,4-N,S-binucleophiles.
The reactions of maleimides with the involvement of 1,4-N,O-binucleophiles also proceed in a similar way. During these transformations, unsaturated oxazine (morpholine) cycles are formed, which are part of a number of compounds with a wide spectrum of biological activity [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66]. A. V. Zorina et al. [67,68] studied the interaction of 1 with aminophenol 55 and aminoethanols 56 in detail. It was noted that both linearly-bound adduct 57 and cyclic regioisomers 58 and 59 can be isolated when 55 was introduced into the reaction, which varies the solvents and catalysts (Scheme 15).
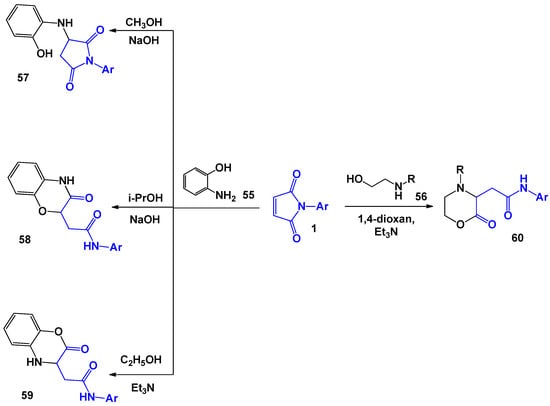
Scheme 15.
The reactions of N-arylmaleimides with 1,4-N,O-binucleophiles.
The scientific team led by I. Ito [69] obtained succinimide 62 in a similar interaction using unsubstituted maleimide and 2,4-diamino-5-hydroxy-6-methylpyrimidine (Scheme 16) in ethanol medium (the yield was 90%). It has been shown that boiling 62 in water resulted in hydrolysis with the formation of β-carbamoyl-(2,4-diamino-6methylpyridin-5-yl)hydroxyethylcarboxylic acid 63 and 64. If 63 was treated with sodium acetate in a mixture of acetic acid and ethanol, 2-acetamido-8-acetyl-4,6-dimethyl-6H-pyrimido[5,4-b][1,4]oxazin-7-one was formed, which can also be obtained via an alternative pathway (Scheme 17). The substance, 2-amino-6-carbamoylmethyl-4-methyl-6H-pyrimido[5,4-b][1,4]oxazin-7(8H)-one 66, was formed when succinimide 62 was heated in ethanol with the addition of catalytic amounts of triethylamine.
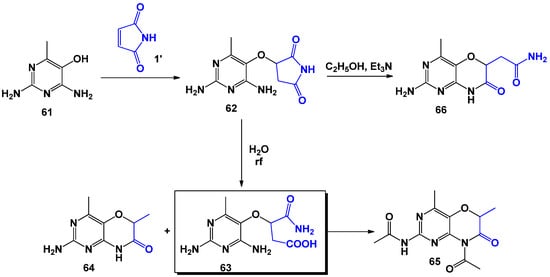
Scheme 16.
Specifics of the interaction of unsubstituted maleimide with 2,4-diamino-5-hydroxy-6-methylpyrimidine.
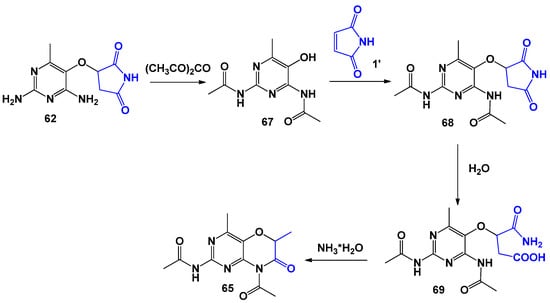
Scheme 17.
Modifications of succinimides based on 2,4-diamino-5-hydroxy-6-methylpyrimidine.
Interestingly, when using N-arylmaleimides, the process proceeds in a similar way, however, the authors were not able to isolate intermediate 71 (analogue 62) (Scheme 18).
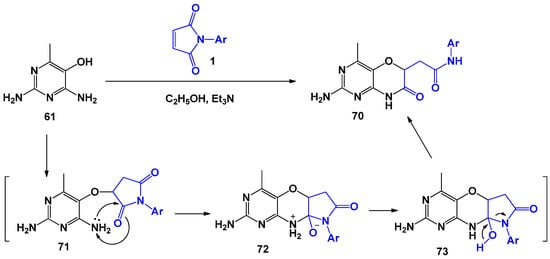
Scheme 18.
General scheme and possible mechanism of interaction of N-arylmaleimides with 2,4-diamino-5-hydroxy-6-methylpyrimidine.
4. Reactions with C,N-dinucleophiles
Recyclization reactions of maleimides during their interaction with C,N-binucleophiles occupy a special place in the literature. Depending on the structure of the binucleophilic component, such processes can form partially hydrogenated pyrrole, pyridazine, or pyridine fragments.
The 2-pyrrolidine core is one of the most abundant structural fragments in natural compounds and is also an important intermediate in the development of new drugs [70,71,72,73,74,75,76]. Among the entire array of data, a special place in the synthesis of this framework is occupied by the proposed Yu. A. Kovygin et al. [23] interaction of 1 with β-aminocrotonic acid methyl ester 74 as a representative of 1,3-C,N-binucleophiles. The process was carried out under various conditions: boiling in organic solvents (diethyl ether, alcohols, dioxane, dimethylformamide, acetic acid), including using acidic or basic catalysis. The authors found that, regardless of the medium used, in all cases the same major product of 5-oxo-4,5-dihydro-1H-pyrrol-3-carboxylate 76 is formed (Scheme 19). However, monitoring of the reaction showed that the maximum yield of 75–80% can be achieved by boiling the starting reagents in methanol with the addition of catalytic amounts of toluenesulfonic acid. In this case, the chemical route of the reaction assumes, as in the previous cases, the initial nucleophilic Michael addition of the CH-proton of the enamine molecule at double bond 1. Further, via the imino-enamine tautomerism stage, the amino group of 74 is attacked by carbonyl moiety 1, which is accompanied by the opening of the pyrrolidinone ring and the formation of a new pyrroline ring.
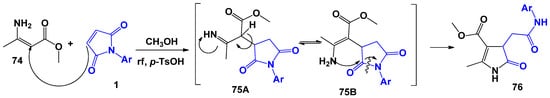
Scheme 19.
The interaction of N-arylmaleimides with β-aminocrotonic acid methyl ester.
Another promising direction in the chemistry of maleimides, which involves the use of 1,3-C,N-binucleophiles, is the building of subunits of 4,5-dihydropyridazin-3(2H)-one. It is known that this fragment is present in compounds with a significant spectrum of biological activity: phosphodiesterase 3/4 (PDE3/PDE4) inhibitors, [77] cyclooxygenase-2 (COX-2) inhibitors, [78] subtype-4 receptor agonists (MC4R), [79] platelet aggregation inhibitors, [80] adenosine-3′,5′-cyclic phosphate phosphodiesterase III (CAMPPDEIII) inhibitors, [81] p38 MAP kinase inhibitors, and [82] β-adrenergic antagonists, [83] and in compounds with antihypertensive, [84] positive inotropic, [85] cardiotonic, [86] antithrombotic, anti-inflammatory, and anti-ulcer effects [87]. A. Stepakov [88] showed that a simple and convenient way to build 4,5-dihydropyridazin-3(2H)-ones is the interaction of aliphatic and cyclic ketazines with arylmaleimides (Scheme 20).
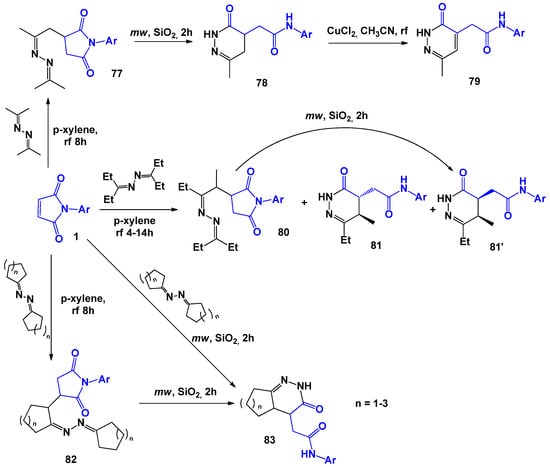
Scheme 20.
The synthesis of 4,5-dihydropyridazin-3(2H)-ones by the interaction of aliphatic and cyclic ketazines with N-arylmaleimides.
Although the mechanism of this reaction is not understood, the authors propose a probable route involving the 1,4-addition of the tautomeric form of the azine A to maleimide 1 with the formation of product B of the Michael addition. Further, adduct B can be converted into 4,5-dihydropyridazine-3(2H)-one D via N-substituted dihydropyridazinone C. Formation of dihydropyridazinone C occurs by intramolecular nucleophilic substitution with a carbonyl group (Scheme 21).
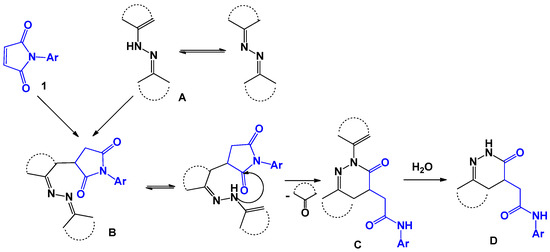
Scheme 21.
Possible mechanism of the reaction with ketazines.
Fused heterocyclic systems containing a partially hydrogenated pyrimidine ring have already proved to be efficient in medicinal chemistry and are promising objects for the creation of new drugs [89,90,91,92,93,94,95].
The authors of several studies [96,97,98] showed that Michael adducts are formed as a result of the interaction of 6-aminouracils 84 with N-arylmaleimides 1 in acetonitrile or isopropyl alcohol medium. However, the scientific team of R. Rudenko [99] succeeded in isolating the corresponding recycling products by changing the process conditions to boiling the reagents in acetic acid. It should be noted that, depending on the substituents in the structure of 6-aminouracils, the authors were able to isolate the corresponding succinimide 86, and fused pyridopyrimidines 85 and pyrrolidinopyrimidine 88 (Scheme 22). It was found that in the case of unsubstituted 84, the replacement of acetic acid—boiling of which led to the formation of mixture 85 and 86—with DMF led to the formation of single product 85. In this case, complete conversion was achieved after only 3 h of boiling of an equimolar mixture of reagents. Taking into account the fact that resulting systems 85′ and 88 have the same set of signals in 1H and 13C spectra, their structures were proved using NOE and XRD experiments.
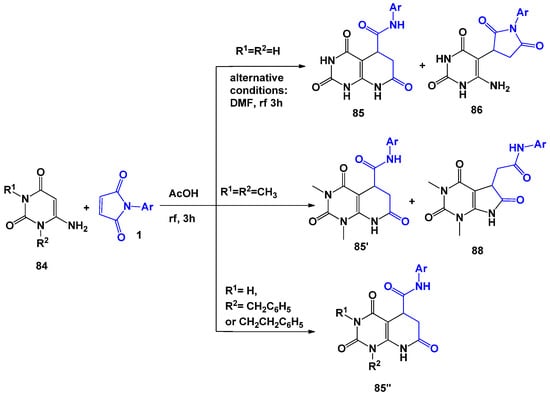
Scheme 22.
6-Aminouracil in reactions with N-arylmaleimides.
P. Romanov [100] showed that 2,4,6-triaminopyrimidines behave in a similar way (Scheme 23).
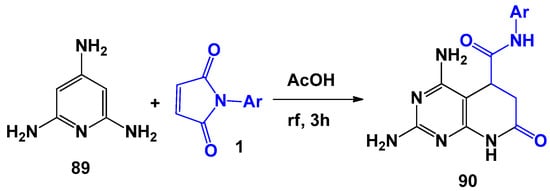
Scheme 23.
Triaminopyridines in reactions with N-arylmaleimides.
Among the large array of literature data on the building of bicyclic pyrrolidinones [101,102,103,104,105], the reactions of N-arylmaleimides with heterocyclic ketene amines (HKAs) deserve special attention [106] (Scheme 24). It was found that the optimal conditions for carrying out this process is a 20-min stirring of the mixture of reagents in an ethanol medium, while the yield of final bicyclic pyrrolidinones reached 85%. The mechanism of this reaction is similar to the interaction of 1 with β-aminocrotonic acid methyl ester 74 shown in Scheme 18.
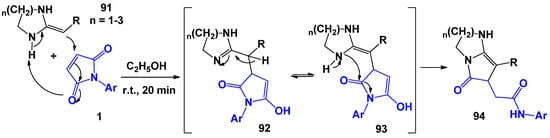
Scheme 24.
General scheme and possible mechanism of interaction of ketene amines with N-arylmaleimides.
Another example of the formation of pyrimidine and pyrrole rings is the interaction of N-arylitaconimides with 1,3-substituted 5-aminopyrazoles [27] 95 (Scheme 25). The process conditions were similar to those for 6-aminouracils. NOESY and XRD experiments were also used to establish the structure of compounds 96 and 97.
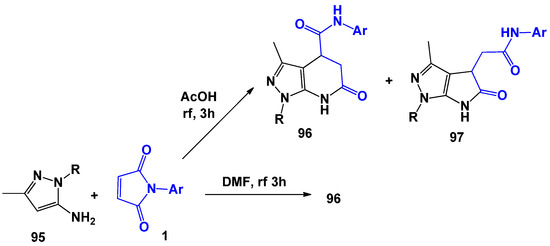
Scheme 25.
Interaction of N-substituted 5-aminopyrazoles with N-arylmaleimides.
5. Reactions with Polynucleophilic Reagents and Involvement in Multicomponent Processes
In conclusion, we should consider the interaction with polynucleophiles as the most promising and least studied direction currently in the recyclization reactions of maleimides. Now, such processes have been well studied using the example of N,N-, C,N- and N,S-containing polynucleophilic agents. In addition to the alternative opening of the maleimide ring, polynucleophiles with several non-equivalent reaction centres can contribute to the formation of various fused or linearly-linked systems, as well as their mixtures.
Despite the fact that aryl biguanidines 98 are polynucleophilic compounds, A.V. Zorina [107] and Yu.A. Kovygin [23] found that, when they interact with N-arylmaleimides, cyclization occurs during boiling in methanol with the involvement of the guanidine moiety only (Scheme 26). Thus, biguanidines behave like typical 1,3-N,N-dinucleophiles. It is also worth noting that attempts to change the conditions and the use of acidic or basic catalysis did not affect the change in the reaction route. The structure of the resulting 5-oxo-4,5-dihydroimidazol-4-yl-N-arylacetamides 99, for which the existence of a tautomeric form 99′ is also possible, was proved using the NOESY and XRD spectra. When polynucleophiles containing simultaneously competing 1,3-N,N- and 1,3-N,S-dinucleophilic centres were introduced into such reactions, the situation became more complicated. However, using the example of amidinothiourea 100 and thiosemicarbazone 101, it was established [107,108] that the reactions proceed at 1,3-N,S-dinucleophilic centres, forming the corresponding thiazoline cycles 102 and 103.
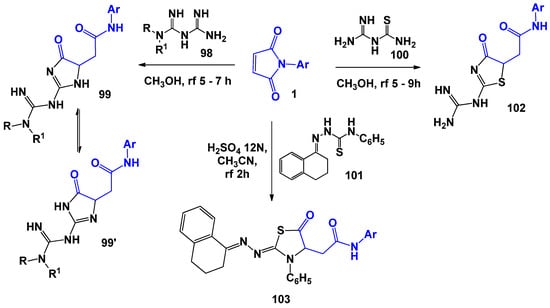
Scheme 26.
Biguanidines and amidinothioureas in reactions with N-arylmaleimides.
As mentioned earlier, the thiazoline ring can also be obtained from thioureas and N-phenylthioureas, the reaction of which with maleimides proceeded unambiguously. However, in the case of N,N-substituted thioureas, the reaction can proceed via several alternative routes (Scheme 27). Thus, the study of A. S. Pankova [109] showed that the reaction of N-alkylthioureas with maleimides at room temperature in ethanol can lead to the formation of mixture 104 and 105. In addition, the nature of the solvent, substituents, and the steric factor play an important role in the formation of specific regioisomers [110]. This result, according to the authors, is due to the fact that when using polar solvents, the reaction is subject to kinetic control, while non-polar solvents contribute to the formation of structure 108, which is more thermodynamically favourable.
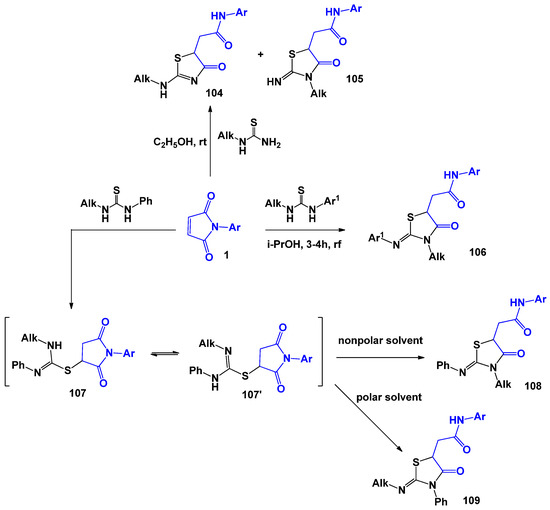
Scheme 27.
N,N-Substituted thioureas in recyclization reactions with N-arylmaleimides.
L. Salhi et al. [108] found that 2,3-diaminopyrimidine in the reaction with maleimides acts as a 1,3-N,N-dinucleophile, forming imidazo[1,2-a]pyridines with a wide range of biological activity [111,112,113,114]. As in [23], the authors suggested that the formation of 115 occurs first as a result of nucleophilic attack of the cyclic nitrogen atom of 2,3-diaminopyridine 110 of the double bond of maleimide 1. Further opening of fragment ring 1 occurs due to the intramolecular attack of the imine nitrogen on the carbonyl group, which was previously protonated with acid, with the formation of intermediate product 112. Next, second molecule 110 acts as a base and removes an acidic proton Hx with the formation of the final product according to Knoevenagel (Scheme 28).
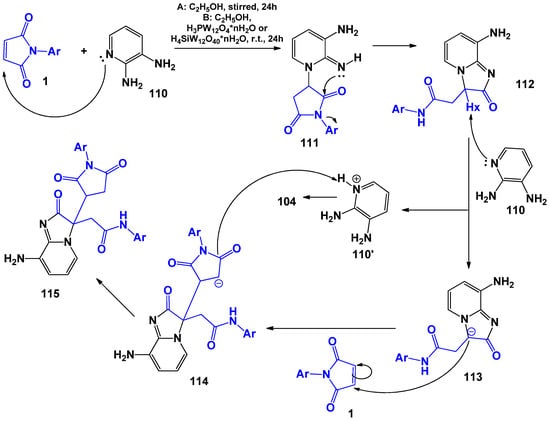
Scheme 28.
General scheme and possible mechanism of interaction of 2,3-diaminopyridine with N-arylmaleimides.
Later, it was shown [115] that 1,2-daiminobenzimidazole, which contains competing 1,4-N,N- and 1,3-N,N-dinucleophilic fragments, exclusively reacts with maleimides as 1,3-N,N-dinucleophile (Scheme 29). It should be noted that, unlike diaminopyridine, this reaction proceeds via the formation of succinimide by the aza-Michael reaction due to the exo-amino group in the second position.
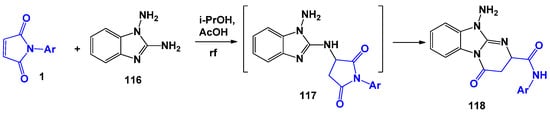
Scheme 29.
1,2-Diaminobenzimidazole in reactions with N-arylmaleimides.
The interactions of maleimides with aminoazoles also proceed ambiguously. R.V. Rudenko [27] showed that in the reaction of 5-amino-2-R-pyrazoles with 1, regardless of the solvent used, a mixture of pyrazolopyrimidines 119 and pyrazolopyridines 126 was formed (Scheme 30). However, for such polynucleophiles as 1,2-diamino-4-phenylimidazole [116], it was possible to isolate only one of the possible products—imidazodiazinon 122. In this case, the replacement of the solvent only affected the yield of the final product. The authors suggested that at the first stage, diaminoimidazole is added to the double bond of arylmaleimide 1 with the formation of linearly-bound products due to CH of the imidazole cycle or NH2 groups. Further, intramolecular cyclization of the resulting intermediates can lead to various alternative products. The exact structure of the resulting imidazopyridazine 122 was established by the step-by-step reaction with the release of succinimide 121.
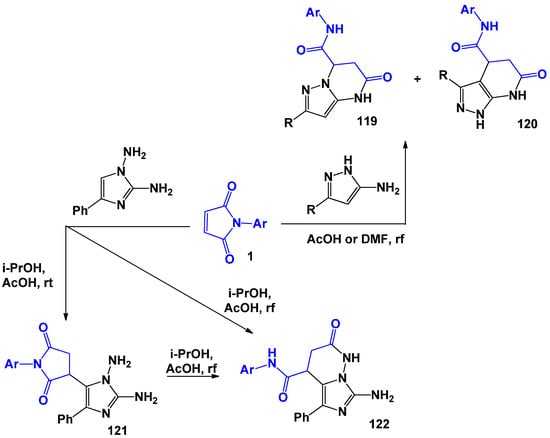
Scheme 30.
5-Amino-3-R-pyrazoles and 1,2-diamino-4-phenylimidazole in recyclization reactions involving N-arylmaleimides.
Other promising but poorly studied issues relating to the chemistry of maleimides are multicomponent processes. At the moment, there are only a few examples of such reactions.
The first three-component synthesis involving maleimides was described by T. Takido [40] using the example of the interaction of 1 with thioamides and maleic anhydride 123 (Scheme 31), which led to rather unexpected tricyclic bridge systems. The optimal conditions for this process are the 3-h boiling of a mixture of reagents in dioxane. The mechanism proposed by the authors includes the first stage similar to that shown in Scheme 11, which consists of the formation of succinimides 124 and 43 due to nucleophilic attack by the sulphur atom of thioamide 42 at the double bond of N-arylmaleimide 1 or maleic anhydride 123. Further recycling of 124 and 43 lead to the formation of mesoionic intermediates 44 and 125, which easily enter into 1,3-dipolar cycloaddition reactions with 1- and 123-forming final products—tricyclic bridge systems 126–128.
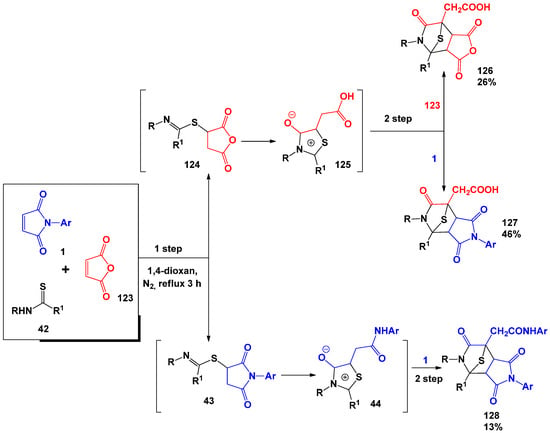
Scheme 31.
Possible interaction mechanism of N-arylmaleimides with thioamides and maleic anhydride.
The second example of a multicomponent process was proposed by J. Noth [117] and E. Fenster [118] for the production of γ-lactams (Scheme 32) as a result of the interaction of maleimides, aldehydes, and amines in the presence of reducing agents. The route of this cascade reaction includes the formation of formyl methylsuccinimide 130 intermediates during the first stage, which were obtained and characterized earlier by the authors [116,117]. Intermediate amino-succinimides 130 through the formation of Schiff bases 131 are converted into a bisamide product without the use of additional synthetic procedures under reductive amination conditions. Moreover, the process already proceeds at room temperature. However, lactam products 132 and 133 were obtained as a mixture of cis/trans isomers in the relative configuration of substituents on the lactam ring.
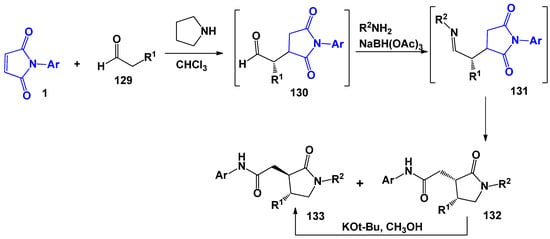
Scheme 32.
The building of γ-lactams involving N-arylmaleimides.
6. Conclusions
As can be seen from the presented review, heterocyclic systems based on N-arylmaleimides have attracted close attention from researchers for a long time. The interest in the chemical transformations of maleimides is determined by the presence of several reaction centres and the possibility for the synthesis of heterocyclic systems with a wide range of biological effects, including drugs based on them. In this review, we have tried to systematize all currently available data on the products and features of the interaction of N-arylmaleimides with various binucleophiles. However, it should be noted that most of the efficient synthetic pathways lead to partially saturated mono- and bicyclic heteroatomic (PSBH) frameworks with an increased content of Csp3 atoms. The presented data can help in understanding and expanding the chemistry of N-arylmaleimides, in particular, in identifying new directions for their application in the synthesis of various non-aromatic heterocyclic systems.
Author Contributions
Writing—preparation of the initial draft, D.Y.V.; writing—reviewing and editing, K.S.S.; conceptualisation, D.Y.V. and K.S.S.; obtaining funding, D.Y.V. and K.S.S.; guidance, K.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Russian Science Foundation grant No. 18-74-10097, https://rscf.ru/project/21-74-03011/ (accessed on 1 June 2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Murray, C.W.; Rees, D.C. The rise of fragment-based drug discovery. Nat. Chem. 2009, 1, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.E.; Murray, J.B. Experiences in fragment-based lead discovery. Methods Enzymol. 2011, 493, 509–531. [Google Scholar] [CrossRef] [PubMed]
- Erlanson, D.A. Introduction to fragment-based drug discovery. In Fragment-Based Drug Discovery and X-ray Crystallography; Davies, T.G., Hyvönen, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–32. [Google Scholar] [CrossRef]
- Zartler, E.R. Fragonomics: The -omics with real impact. ACS Med. Chem. Lett. 2014, 5, 952–953. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Congreve, M.; Chessari, G.; Tisi, D.; Woodhead, A.J. Recent developments in fragment-based drug discovery. J. Med. Chem. 2008, 51, 3661–3680. [Google Scholar] [CrossRef] [PubMed]
- Keserű, G.M.; Erlanson, D.A.; Ferenczy, G.G.; Hann, M.M.; Murray, C.W.; Pickett, S.D. Design Principles for Fragment Libraries:Maximizing the Value of Learnings from Pharma Fragment-Based Drug Discovery (FBDD) Programs for Use in Academia. J. Med. Chem. 2016, 59, 8189–8206. [Google Scholar] [CrossRef]
- Barelier, S.; Krimm, I. Ligand specificity, privileged substructures and protein druggability from fragment-based screening. Curr. Opin. Chem. Biol. 2011, 15, 469–474. [Google Scholar] [CrossRef]
- Hajduk, P.J.; Galloway, W.R.; Spring, D.R. Drug discovery: A question of library design. Nature 2011, 470, 42–43. [Google Scholar] [CrossRef]
- Hung, A.W.; Ramek, A.; Wang, Y.; Kaya, T.; Wilson, J.A.; Clemons, P.A.; Young, D.W. Route to three-dimensional fragments using diversity-oriented synthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 6799–6804. [Google Scholar] [CrossRef]
- Ritchie, T.J.; Macdonald, S.J. The impact of aromatic ring count on compound developability—Are too many aromatic rings a liability in drug design? Drug Discov. Today 2009, 14, 1011–1020. [Google Scholar] [CrossRef]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Morley, A.D.; Pugliese, A.; Birchall, K.; Bower, J.; Brennan, P.; Brown, N.; Chapman, T.; Drysdale, M.; Gilbert, I.H.; Hoelder, S.; et al. Fragment-based hit identification: Thinking in 3D. Drug Discov. Today 2013, 18, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.W.; Rees, D.C. Opportunity Knocks: Organic Chemistry for Fragment-Based Drug Discovery (FBDD). Angew. Chem. Int. Ed. Engl. 2016, 55, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, H.F.C.; Castro, M. Effects of the charge on the structural, electronic and reactivity properties of 43 substituted N–Phenylmaleimides. A DFT study. J. Mol. Struct. 2016, 1125, 79–92. [Google Scholar] [CrossRef]
- Panov, A.A.; Simonov, A.Y.; Lavrenov, S.N.; Lakatosh, S.A.; Trenin, A.S. 3,4-Disubstituted maleimides: Synthesis and biological activity. Chem. Heterocycl. Compd. 2018, 54, 103–113. [Google Scholar] [CrossRef]
- Dolci, E.; Froidevaux, V.; Joly-Duhamel, C.; Auvergne, R.; Boutevin, B.; Caillol, S. Maleimides As a Building Block for the Synthesis of High Performance Polymers. Polym. Rev. 2015, 56, 512–556. [Google Scholar] [CrossRef]
- Ma, Z.; Qiu, S.; Chen, H.C.; Zhang, D.; Lu, Y.L.; Chen, X.L. Maleimide structure: A promising scaffold for the development of antimicrobial agents. J. Asian Nat. Prod. Res. 2022, 24, 1–14. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Y.; Huang, Z.; Zhou, N.; Zhang, Z.; Zhu, X. Precise sequence regulation through maleimide chemistry. Polym. J. 2020, 52, 21–31. [Google Scholar] [CrossRef]
- Fischer, G. Chapter One—1,2,4-Triazolo[5,1-b]- and -[1,5-a]quinazolines and their hydro derivatives. In Advances in Heterocyclic Chemistry; Academic Press: Cambridge, MA, USA, 2021; Volume 135, pp. 1–55. [Google Scholar] [CrossRef]
- Rival, Y.; Grassy, G.; Michel, G. Synthesis and Antibacterial Activity of Some Imidazo [1, 2-a]pyrimidine Derivatives. Chem. Pharm. Bull. 1992, 40, 1170–1176. [Google Scholar] [CrossRef]
- Barlaam, B.; Casella, R.; Cidado, J.; Cook, C.; De Savi, C.; Dishington, A.; Donald, C.S.; Drew, L.; Ferguson, A.D.; Ferguson, D.; et al. Discovery of AZD4573, a Potent and Selective Inhibitor of CDK9 That Enables Short Duration of Target Engagement for the Treatment of Hematological Malignancies. J. Med. Chem. 2020, 63, 15564–15590. [Google Scholar] [CrossRef]
- Shikhaliev, K.S.; Kovygin, Y.A.; Potapov, A.Y.; Ssbynin, A.L.; Kosheleva, E.A. Recyclization of maleimides with N-carboximide amides. Russ. Chem. Bull. 2017, 66, 86–90. [Google Scholar] [CrossRef]
- Kovygin, Y.A.; Stolpovskaya, N.V.; Zorina, A.V.; Sabynin, A.L.; Vandyshev, D.Y.; Kruzhilin, A.A.; Krysin, M.Y.; Shikhaliev, K.S. Cascade recyclization of N-arilmaleimides at interaction with binucleophiles as a general method for construction of hydrogened heterocyclic systems. Vestnik VSU Ser. Chem. Biol. Pharm. 2018, 4, 15–24. (In Russian) [Google Scholar]
- Kovygin, Y.A.; Krylski, D.V.; Zorina, A.V.; Shikhaliev, K.S. Novel Variant of Recyclization of N-Arylmaleimides when Reacted with Aminoazoles. Chem. Heterocycl. Compd. 2004, 40, 1222–1223. [Google Scholar] [CrossRef]
- Gordon, A.J.; Ford, R.A. The Chemist’s Companion; Mir: Moscow, Russia, 1976; p. 297. (In Russian) [Google Scholar]
- Rudenko, R.V.; Komykhov, S.A.; Musatov, V.I.; Konovalova, I.S.; Shishkin, O.V.; Desenko, S.M. Reactions of N-arylmaleimides with 3-amino-1,2,4-triazole and 2-aminobenzimidazole. J. Heterocycl. Chem. 2011, 48, 888–895. [Google Scholar] [CrossRef]
- Rudenko, R.V.; Komykhov, S.A.; Desenko, S.M.; Musatov, V.I.; Shishkin, O.V.; Konovalova, I.S.; Vashchenko, E.V.; Chebanov, V.A. Diverse Directions of Heterocyclizations Involving Derivatives of 5-Aminopyrazoles and N-Arylmaleimides. Synthesis 2011, 5, 783–793. [Google Scholar] [CrossRef]
- Matviiuk, T.; Gorichko, M.; Kysil, A.; Shishkina, S.; Shishkin, O.; Voitenko, Z. Catalysis by Lithium Perchlorate Enables Double-Conjugate Addition of Electron-Deficient Maleimides to 2- Aminopyridines and 2-Aminothiazoles. Synth. Commun. 2012, 42, 3304–3310. [Google Scholar] [CrossRef]
- Zorina, A.V.; Shikhaliev, K.S.; Kovygin, Y.A. 1,2-Binucleophiles in reactions with arylmaleimides. Vestnik VSU Ser. Chem. Biol. Pharm. 2005, 1, 39–41. (In Russian) [Google Scholar]
- Abelman, M.M.; Fisher, K.J.; Dorerffler, E.M.; Edwards, P.J. The synthesis of 2-ketopiperazine acetic acid esters and amides from ethylenediamines with maleates and maleimides. Tetrahedron Lett. 2003, 44, 1823–1826. [Google Scholar] [CrossRef]
- Romanenko, V.D.; Kulchitskaya, N.E.; Burmistrov, S.I. Condensed and bound quinoxalines. New pathway to arylamides of 1,2-dihydro-2-oxo-3-quinoxalineacetic acid. Khim. Geterotsikl. Soedin. 1973, 61, 264–266. [Google Scholar]
- Ye, J.; Liu, Q.; Wang, C.; Meng, Q.; Sun, H.; Peng, J.; Ma, X.; Liu, K. Benzylpenicillin inhibits the renal excretion of acyclovir by OAT1 andOAT3. Pharmacol. Rep. 2013, 65, 505–512. [Google Scholar] [CrossRef]
- Rahmutulla, B.; Matsushita, K.; Satoh, M.; Seimiya, M.; Tsuchida, S.; Kubo, S.; Shimada, H.; Ohtsuka, M.; Miyazaki, M.; Nomura, F. Alternative splicing of FBP-interacting repressor coordinates c-Myc, P27Kip1/cyclinE and Ku86/XRCC5 expression as a molecular sensor for bleomycin induced DNA damage pathway. Oncotarget 2014, 15, 2404–2417. [Google Scholar] [CrossRef]
- Popsavin, V. Synthesis and antiproliferative activity of two new tiazofurin analogues with 2′-amido functionalities. Bioorg. Med. Chem. Lett. 2006, 16, 2773–2776. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Cheng, J.; Meng, Y.; Ren, Y.; Deng, H.; Guo, Y. A novel formulation of thiamine dilaurylsulphate and its preservative effect on apple juice and sterilised milk. Food Chem. 2014, 1, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Sevrioukova, I.F.; Poulos, T.L. Dissecting cytochrome P450 3A4-ligand interactions using ritonavir analogues. Biochemistry 2013, 52, 4474–4481. [Google Scholar] [CrossRef] [PubMed]
- Novakova, I.; Subileau, E.A.; Toegel, S.; Gruber, D.; Lachmann, B.; Urban, E.; Chesne, C.; Noe, C.R.; Neuhaus, W. Transport rankings of nonsteroidal antiinflammatory drugs across blood-brain barrier in vitro models. PLoS ONE 2014, 9, e86806. [Google Scholar] [CrossRef]
- Augustin, M.; Muller, W. Synthese von N-Maleoyl-aminosäuren und -peptiden. J. Prakt. Chem. 1985, 327, 789–798. [Google Scholar] [CrossRef]
- Marrian, D.H. 384. The condensation of N-substituted maleimides with thioureas. J. Chem. Soc. 1949, 1949, 1797–1799. [Google Scholar] [CrossRef]
- Takido, T.; Tamura, S.; Sato, K.; Kamijo, H.; Nakazawa, T.; Hata, T.; Seno, M. The synthesis of hexahydrooxoepithiopyridinedicarboximides by the reaction of thioamides with N-substituted maleimides. J. Heterocycl. Chem. 1998, 35, 437–443. [Google Scholar] [CrossRef]
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Lesyk, R. Synthesis and Anticancer and Antiviral Activities of New 2-Pyrazoline-Substituted 4-Thiazolidinones. J. Heterocycl. Chem. 2013, 50, E55–E62. [Google Scholar] [CrossRef]
- Lesyk, R.; Vladzimirska, O.; Holota, S.; Zaprutko, L.; Gzella, A. New 5-substituted thiazolo[3,2-b][1,2,4]triazol-6-ones: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2007, 42, 641–648. [Google Scholar] [CrossRef]
- Holota, S.; Derkach, H.; Antoniv, O.; Slyvka, N.; Kutsyk, R.; Gzella, A.; Lesyk, R. Study of 1,2,4- triazole-3(5)-thiol Behavior in Reactions with 1-phenyl-1H-pyrrole- 2,5-dione Derivatives and 3-bromodihydrofuran-2(3H)-one and Antimicrobial Activity of Products. Chem. Proc. 2021, 3, 68. [Google Scholar] [CrossRef]
- Kanaoka, Y.; Machida, M.; Ban, Y.; Takamitsu, S. Fluorescence and structure of proteins as measured by incorporation of fluorophore. II. Synthesis of maleimide derivatives as fluorescence-labeled protein-sulfhydryl reagents. Chem. Pharm. Bull. 1967, 15, 1738–1743. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chung, M.C.; Malatesta, P.; Bosquesi, P.L.; Yamasaki, P.R.; Santos, J.L.; Vizioli, E.O. Advances in drug design based on the amino Acid approach: Taurine analogues for the treatment of CNS diseases. Pharmaceuticals 2012, 5, 1128–1146. [Google Scholar] [CrossRef] [PubMed]
- Biava, M.; Cesare Porretta, G.; Deidda, D.; Pompei, R.; Tafi, A.; Manetti, F. Importance of the thiomorpholine introduction in new pyrrole derivatives as antimycobacterial agents analogues of BM 212. Bioorg. Med. Chem. 2003, 11, 515–520. [Google Scholar] [CrossRef]
- Chabala, J.C.; Miller, M.W. Chemistry of Antiprotozoal Agents. In Chemotherapy of Parasitic Diseases; Campbell, W.C., Rew, R.S., Eds.; Springer: Boston, MA, USA, 1986; pp. 25–85. [Google Scholar] [CrossRef]
- Sisko, J.T.; Tucker, T.J.; Bilodeau, M.T.; Buser, C.A.; Ciecko, P.A.; Coll, K.E.; Fernandes, C.; Gibbs, J.B.; Koester, T.J.; Kohl, N.; et al. Potent 2-[(pyrimidin-4-yl)amine]-1,3-thiazole-5-carbonitrile-based inhibitors of VEGFR-2 (KDR) kinase. Bioorg. Med. Chem. Lett. 2006, 16, 1146–1150. [Google Scholar] [CrossRef]
- Karali, N.; Gürsoy, A.; Kandemirli, F.; Shvets, N.; Kaynak, F.B.; Ozbey, S.; Kovalishyn, V.; Dimoglo, A. Synthesis and structure-antituberculosis activity relationship of 1H-indole-2,3-dione derivatives. Bioorg. Med. Chem. 2007, 15, 5888–5904. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Stocks, P.A.; Sabbani, S.; Roberts, N.L.; Amewu, R.K.; Shore, E.R.; Aljayyoussi, G.; Angulo-Barturén, I.; Belén, M.; Jiménez-Díaz; et al. Synthesis and profiling of benzylmorpholine 1,2,4,5-tetraoxane analogue N205: Towards tetraoxane scaffolds with potential for single dose cure of malaria. Bioorg. Med. Chem. 2018, 26, 2996–3005. [Google Scholar] [CrossRef]
- Reddy, P.R.; Reddy, G.M.; Padmaja, A.; Padmavathi, V.; Kondaiah, P.; Krishna, N.S. Synthesis, antioxidant, and cytotoxic activities of N-azole substituted thiomorpholine derivatives. Arch. Pharm. 2014, 347, 221–228. [Google Scholar] [CrossRef]
- Badawneh, M.; Aljamal, J. Synthesis and antitubercular activity of piperidine and morpholine 1,8 naphthyridine analogues. Int. J. Pharm. Pharm. Sci. 2016, 8, 252–257. [Google Scholar] [CrossRef][Green Version]
- Bektaş, H.; Ceylan, S.; Demirbaş, N.; Alpay-Karaoğlu, S.; Sökmen, B.B. Antimicrobial and antiurease activities of newly synthesized morpholine derivatives containing an azole nucleus. Med. Chem. Res. 2013, 22, 3629–3639. [Google Scholar] [CrossRef]
- Matralis, A.N.; Kourounakis, A.P. Optimizing the Pharmacological Profile of New Bifunctional Antihyperlipidemic/Antioxidant Morpholine Derivatives. ACS Med. Chem. Lett. 2018, 10, 98–104. [Google Scholar] [CrossRef]
- Ermakova, T.; Vinokurova, N.G.; Zelenkova, N.F. Thiomorpholine transformation by the fungus Bjerkandera Adusta. Microbiology 2008, 77, 547–552. [Google Scholar] [CrossRef]
- Bespalova, G.; Lizak, I.; Sedavkina, V. Synthesis and antimicrobial activity of morpholine derivatives of 2-pyrrolidones and their thio analogs. Pharm. Chem. J. 1991, 25, 40–43. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Priya, M.G.; Kumar, N.R.; Saravanan, G. Synthesis and pharmacological evaluation of Schiff bases of 4-(2-aminophenyl)-morpholines. Indian J. Pharm. Sci. 2009, 71, 428–432. [Google Scholar] [CrossRef]
- Wang, P.-F.; Qiu, H.-Y.; Ma, J.-T.; Yan, X.-Q.; Gong, H.-B.; Wang, Z.-C.; Zhu, H.-L. Dihydropyrazoles containing morpholine: Design, synthesis and bioassay testing as potent antimicrobial agents. RSC Adv. 2015, 5, 24997–25005. [Google Scholar] [CrossRef]
- Martínez-Aguilar, L.; Lezama-Martínez, D.; Orozco-Cortés, N.V.; González-Espinosa, C.; Flores-Monroy, J.; Valencia-Hernández, I. Antihypertensive Properties of a Novel Morphologic Derivative (4-tert-buthyl-2,6-bis(thiomorpholine-4-ilmethyl)phenol). J. Cardiovasc. Pharmacol. 2016, 67, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Fang, Z.; Huang, B.; Zhang, L.; Liu, H.; Pannecouque, C.; Naesens, L.; De Clercq, E.; Zhan, P.; Liu, X. Synthesis and Preliminary Antiviral Activities of Piperidine-substituted Purines against HIV and Influenza A/H1N1 Infections. Chem. Biol. Drug Des. 2015, 86, 568–577. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khalili, M.; Hajikhani, R.; Naserbakht, M. New morpholine analogues of phencyclidine: Chemical synthesis and pain perception in rats. Pharmacol. Biochem. Behav. 2011, 98, 227–233. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khalili, M.; Hajikhani, R.; Naserbakht, M. Synthesis and determination of acute and chronic pain activities of 1-[1-(4-methylphenyl)(cyclohexyl)] morpholine as a new phencyclidine derivative in rats. Arzneimittelforschung 2011, 61, 92–97. [Google Scholar] [CrossRef]
- Conaway, C.C.; Tong, C.; Williams, G.M. Evaluation of morpholine, 3-morpholinone, and N-substituted morpholines in the rat hepatocyte primary culture/DNA repair test. Mutat. Res. Gent. Toxicol. 1984, 136, 153–157. [Google Scholar] [CrossRef]
- Piotrowski, D.W.; Futatsugi, K.; Casimiro-Garcia, A.; Wei, L.; Sammons, M.F.; Herr, M.; Jiao, W.; Lavergne, S.Y.; Coffey, S.B.; Wright, S.W.; et al. Identification of Morpholino-2H-pyrido[3,2-b][1,4]oxazin-3(4H)-ones as Nonsteroidal Mineralocorticoid Antagonists. J. Med. Chem. 2018, 61, 1086–1097. [Google Scholar] [CrossRef]
- Goud, N.S.; Pooladanda, V.; Mahammad, G.S.; Jakkula, P.; Gatreddi, S.; Qureshi, I.A.; Alvala, R.; Godugu, C.; Alvala, M. Synthesis and biological evaluation of morpholines linked coumarin-triazole hybrids as anticancer agents. Chem. Biol. Drug Des. 2019, 94, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Pires Gouvea, D.; Vasconcellos, F.A.; Dos Anjos Berwaldt, G.; Neto, A.C.; Fischer, G.; Sakata, R.P.; Almeida, W.P.; Cunico, W. 2-Aryl-3-(2-morpholinoethyl)thiazolidin-4-ones: Synthesis, anti-inflammatory in vivo, cytotoxicity in vitro and molecular docking studies. Eur. J. Med. Chem. 2016, 118, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Zorina, A.V.; Shikhaliev, K.S. Recyclisation reactions of arylmaleimides in interaction with N,O-binucleophiles Vestnik VSU Ser. Chem. Biol. Pharm. 2006, 1, 39–42. (In Russian) [Google Scholar]
- Zorina, A.V.; Falaleev, A.V.; Shikhaliev, K.S. On peculiarities of interaction of N-arylmaleinimides with 2-aminophenol. Vestnik VSU Ser. Chem. Biol. Pharm. 2008, 2, 29–31. (In Russian) [Google Scholar]
- Ito, I.; Oda, N.; Kato, T. Synthesis of compounds related to antitumor agents. III. On the additions of acyl halide, dialkylacetylenedicarboxylate and N-substituted maleimides to 2,4-diamino-5-hydroxy-6-methylpyrimidine. Chem. Pharm. Bull. 1976, 24, 1189–1196. [Google Scholar] [CrossRef]
- Feling, R.H.; Buchanan, G.O.; Mincer, T.J.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Salinosporamide A: A highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus salinospora. Angew. Chem. Int. Ed. Eng. 2003, 42, 355–357. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, T.S.; Lee, H.S.; Park, J.Y.; Choi, I.K.; Kwon, H.J. Streptopyrrolidine, an angiogenesis inhibitor from a marine-derived Streptomyces sp. KORDI-3973. Phytochemistry 2008, 69, 2363–2366. [Google Scholar] [CrossRef]
- Reddy, P.A.; Hsiang, B.C.H.; Latifi, T.N.; Hill, M.W.; Woodward, K.E.; Rothman, S.M.; Ferrendelli, J.A.; Covey, D.F. 3,3-Dialkyl- and 3-Alkyl-3-Benzyl-Substituted 2-Pyrrolidinones: A New Class of Anticonvulsant Agents. J. Med. Chem. 1996, 39, 1898–1906. [Google Scholar] [CrossRef]
- Duan, J.J.; Chen, L.; Wasserman, Z.R.; Lu, Z.; Liu, R.Q.; Covington, M.B.; Qian, M.; Hardman, K.D.; Magolda, R.L.; Newton, R.C.; et al. Discovery of gamma-lactam hydroxamic acids as selective inhibitors of tumor necrosis factor alpha converting enzyme: Design, synthesis, and structure-activity relationships. J. Med. Chem. 2002, 45, 4954–4957. [Google Scholar] [CrossRef]
- Onyango, E.O.; Tsurumoto, J.; Imai, N.; Takahashi, K.; Ishihara, J.; Hatakeyama, S. Total synthesis of neooxazolomycin. Angew. Chem. Int. Ed. 2007, 46, 6703–6705. [Google Scholar] [CrossRef]
- Tekkam, S.; Alam, M.A.; Jonnalagadda, S.C.; Mereddy, V.R. Novel methodologies for the synthesis of functionalized pyroglutamates. Chem. Commun. 2011, 47, 3219–3221. [Google Scholar] [CrossRef] [PubMed]
- Kogure, N.; Ishii, N.; Kitajima, M.; Wongseripipatana, S.; Takayama, H. Four novel gelsenicine-related oxindole alkaloids from the leaves of Gelsemium elegans benth. Org. Lett. 2006, 8, 3085–3088. [Google Scholar] [CrossRef] [PubMed]
- Krier, M.; Araújo-Júnior, J.X.; Schmitt, M.; Duranton, J.; Justiano-Basaran, H.; Lugnier, C.; Bourguignon, J.J.; Rognan, D. Design of small-sized libraries by combinatorial assembly of linkers and functional groups to a given scaffold: Application to the structure-based optimization of a phosphodiesterase 4 inhibitor. J. Med. Chem. 2005, 48, 3816–3822. [Google Scholar] [CrossRef] [PubMed]
- Abouzid, K.; Bekhit, S.A. Novel anti-inflammatory agents based on pyridazinone scaffold; design, synthesis and in vivo activity. Bioorg. Med. Chem. 2008, 16, 5547–5556. [Google Scholar] [CrossRef] [PubMed]
- Ujjainwalla, F.; Warner, D.; Snedden, C.; Grisson, R.D.; Walsh, T.F.; Wyvratt, M.J.; Kalyani, R.N.; Macneil, T.; Tang, R.; Weinberg, D.H.; et al. Design and syntheses of melanocortin subtype-4 receptor agonists. Part 2: Discovery of the dihydropyridazinone motif. Bioorg. Med. Chem. Lett. 2005, 15, 4023–4028. [Google Scholar] [CrossRef]
- Thyes, M.; Lehmann, H.D.; Gries, J.; König, H.; Kretzschmar, R.; Kunze, J.; Lebkücher, R.; Lenke, D. 6-Aryl-4,5-dihydro-3(2H)-pyridazinones. A new class of compounds with platelet aggregation inhibiting and hypotensive activities. J. Med. Chem. 1983, 26, 800–807. [Google Scholar] [CrossRef]
- Robertson, D.W.; Jones, N.D.; Krushinski, J.H.; Pollock, G.D.; Swartzendruber, J.K.; Hayes, J.S. Molecular structure of the dihydropyridazinone cardiotonic 1,3-dihydro-3,3-dimethyl-5-(1,4,5,6-tetrahydro-6-oxo-pyridazinyl)-2H-indol-2-one, a potent inhibitor of cyclic AMP phosphodiesterase. J. Med. Chem. 1987, 30, 623–627. [Google Scholar] [CrossRef]
- Colletti, S.L.; Frie, J.L.; Dixon, E.C.; Singh, S.B.; Choi, B.K.; Scapin, G.; Fitzgerald, C.E.; Kumar, S.; Nichols, E.A.; O’Keefe, S.J.; et al. Hybrid-designed inhibitors of p38 MAP kinase utilizing N-arylpyridazinones. J. Med. Chem. 2003, 46, 349–352. [Google Scholar] [CrossRef]
- Howson, W.; Kitteringham, J.; Mistry, J.; Mitchell, M.B.; Novelli, R.; Slater, R.A.; Swayne, G.T. Synthesis and biological activity of the four stereoisomers of 6-[4-[3-[[2-hydroxy-3-[4-[2-(cyclopropylmethoxy)ethyl]phenoxy]propyl]amino]-propionamido] phenyl]-5-methyl-4,5-dihydro-3(2H)-pyridazinone, a combined vasodilator and beta-adrenoceptor antagonist. J. Med. Chem. 1988, 31, 352–356. [Google Scholar] [CrossRef]
- Curran, W.V.; Ross, A. 6-Phenyl-4,5-dihydro-3(2H)-pyridazinones. A series of hypotensive agents. J. Med. Chem. 1974, 17, 273–281. [Google Scholar] [CrossRef]
- Mertens, A.; Friebe, W.G.; Müller-Beckmann, B.; Kampe, W.; Kling, L.; von der Saal, W. Nonsteroidal cardiotonics. 3. New 4,5-dihydro-6-(1H-indol-5-yl)pyridazin-3(2H)-ones and related compounds with positive inotropic activities. J. Med. Chem. 1990, 33, 2870–2875. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, Y.; Takai, H.; Ohno, T.; Nagashima, K.; Yao, K.; Yamada, K.; Kubo, K.; Ichimura, M.; Mihara, A.; Kase, H. Studies of cardiotonic agents. 8. Synthesis and biological activities of optically active 6-(4-(benzylamino)-7-quinazolinyl)-4,5-dihydro-5-methyl-3(2H)-pyridazinone (KF15232). J. Med. Chem. 1996, 39, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Sircar, I.; Steffen, R.P.; Bobowski, G.; Burke, S.E.; Newton, R.S.; Weishaar, R.E.; Bristol, J.A.; Evans, D.B. Cardiotonic agents. 9. Synthesis and biological evaluation of a series of (E)-4,5-dihydro-6-[2-[4-(1H-imidazol-1-yl)phenyl]ethenyl]-3 (2H)-pyridazinones: A novel class of compounds with positive inotropic, antithrombotic, and vasodilatory activities for the treatment of congestive heart failure. J. Med. Chem. 1989, 32, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Stepakov, A.V.; Kinzhalov, M.A.; Boitsov, V.M.; Stepakova, L.V.; Starova, G.L.; Vyazmin, S.Y.; Grinenko, E.V. A new approach to the synthesis of 4-(N-aryl)carbamoylmethyl-4,5-dihydropyridazin-3(2H)-ones. Tetrahedron Lett. 2011, 52, 3146–3149. [Google Scholar] [CrossRef]
- Alajarin, R.; Alvarez-Builla, J.; Vaquero, J.J.; Sunkel, C.; Fau de Casa-Juana, M.; Statkow, P.R.; Sanz-Aparicio, J. Synthesis and chromatographic separation of the stereoisomers of furnidipine. Tetrahedron Asymmetry 1993, 4, 617–620. [Google Scholar] [CrossRef]
- Bossert, F.; Vater, W. 1,4-Dihydropyridines—A basis for developing new drugs. Med. Res. Rev. 1989, 9, 291–324. [Google Scholar] [CrossRef]
- Triggle, D.J.; Langs, D.A.; Janis, R.A. Ca2+ channel ligands: Structure-function relationships of the 1,4-dihydropyridines. Med. Res. 1989, 9, 123–180. [Google Scholar] [CrossRef]
- Kappe, C.O. Biologically active dihydropyrimidones of the Biginelli-type—A literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef]
- Ram, V.J.; Goel, A.; Sarkhel, S.; Maulik, P.R. A convenient synthesis and hepatoprotective activity of imidazo[1,2-c]pyrimido[5,4-e]pyrimidine, tetraazaacenaphthene and tetraazaphenalene from cyclic ketene aminals through tandem addition-cyclization reactions. Bioorg. Med. Chem. 2002, 10, 1275–1280. [Google Scholar] [CrossRef]
- Tenser, R.B.; Gaydos, A.; Hay, K.A. Inhibition of herpes simplex virus reactivation by dipyridamole. Antimicrob. Agents Chemother. 2001, 45, 3657–3659. [Google Scholar] [CrossRef]
- Gebauer, M.G.; McKinlay, C.; Gready, J.E. Synthesis of quaternised 2-aminopyrimido[4,5-d]pyrimidin-4(3H)-ones and their biological activity with dihydrofolate reductase. Eur. J. Med. Chem. 2003, 38, 719–728. [Google Scholar] [CrossRef]
- Cobo, J.; Sánchez, A.; Nogueras, M. Reactions of 6-aminopyrimidin-4(3H)-ones with electron-deficient alkenyl derivatives. Easy preparation of heterocyclic analogues of Sangivamicine. Tetrahedron 1998, 54, 5753–5762. [Google Scholar] [CrossRef]
- Vel’chinskaya, E.V.; Kuz’menko, I.I.; Kulik, L.S. Synthesis of new substituted uracils and pyrimidines. Pharm. Chem. J. 1999, 33, 155–157. [Google Scholar] [CrossRef]
- Velchinskaya, E.; Petsushak, B.; Rogal, A. Investigation of the physicochemical characteristics and biological activity of new derivatives of N-substituted maleimides. Chem. Heterocycl. Compd. 2007, 43, 695–700. [Google Scholar] [CrossRef]
- Komykhov, S.; Chebanov, V.; Rudenko, R.; Desenko, S.; Sen’ko, Y.; Shishkin, O.; Shishkina, S. A Comprehensive Study of the Heterocyclizations of N-Arylmaleimides and 6-Aminouracils. Synthesis 2011, 19, 3161–3167. [Google Scholar] [CrossRef]
- Romanov, P.S.; Petrov, V.V.; Krysin, M.Y.; Zorina, A.V.; Shikhaliev, K.S. Reactions of 2,4,6-triaminopyrimidine with N-arylmaleimides. Synthesis of condensed diaminopyrimidopyrimidinones. Vestnik VSU Ser. Chem. Biol. Pharm. 2007, 1, 49–50. (In Russian) [Google Scholar]
- Hakimelahi, G.H.; Moosavi-Movahedi, A.A.; Tsay, S.C.; Tsai, F.Y.; Wright, J.D.; Dudev, T.; Hakimelahi, S.; Lim, C. Design, synthesis, and SAR of novel carbapenem antibiotics with high stability to Xanthomonas maltophilia oxyiminocephalosporinase type II. J. Med. Chem. 2000, 43, 3632–3640. [Google Scholar] [CrossRef]
- Morriello, G.J.; Devita, R.J.; Mills, S.G.; Young, J.R.; Lin, P.; Doss, G.; Chicchi, G.G.; Demartino, J.; Kurtz, M.M.; Tsao, K.L.; et al. Fused bicyclic pyrrolizinones as new scaffolds for human NK1 antagonists. Bioorg. Med. Chem. 2008, 16, 2156–2170. [Google Scholar] [CrossRef]
- Kazmierski, W.M.; Andrews, W.; Furfine, E.; Spaltenstein, A.; Wright, L. Discovery of potent pyrrolidone-based HIV-1 protease inhibitors with enhanced drug-like properties. Bioorg. Med. Chem. Lett. 2004, 14, 5689–5692. [Google Scholar] [CrossRef]
- Das Sarma, K.; Zhang, J.; Huang, Y.; Davidson, J.G. Amino Acid Esters and Amides for Reductive Amination of Mucochloric Acid: Synthesis of Novel γ-Lactams, Short Peptides and Antiseizure Agent Levetiracetam (Keppra®). Eur. J. Org. Chem. 2006, 2006, 3730–3737. [Google Scholar] [CrossRef]
- Ng, P.Y.; Tang, Y.; Knosp, W.M.; Stadler, H.S.; Shaw, J.T. Synthesis of diverse lactam carboxamides leading to the discovery of a new transcription-factor inhibitor. Angew. Chem. Int. Ed. 2007, 46, 5352–5355. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, H.-R.; Lin, X.-R.; Yan, S.-J.; Lin, J. Catalyst-free cascade reaction of heterocyclic ketene aminals with N-substituted maleimide to synthesise bicyclic pyrrolidinone derivatives. RSC Adv. 2014, 4, 27582–27590. [Google Scholar] [CrossRef]
- Zorina, A.V.; Stolpovskaya, N.V.; Shikhaliev, K.S.; Peregudova, A.S.; Ivonin, V.A. Recyclization Reactions of N-Arylmaleimides with Polynucleophilic Compounds. Chem. Heterocycl. Compd. 2015, 50, 1541–1546. [Google Scholar] [CrossRef]
- Salhi, L.; Achouche-Bouzroura, S.; Nechak, R.; Nedjar-Kolli, B.; Rabia, C.; Merazig, H.; Dunach, E. Synthesis of functionalized dihydroimidazo[1,2-a]pyridines and 4-thiazolidinone derivatives from maleimide, as new class of antimicrobial agents. Synth. Commun. 2020, 50, 412–422. [Google Scholar] [CrossRef]
- Pankova, A.S.; Golubev, P.R.; Khlebnikov, A.F.; Ivanov, A.Y.; Kuznetsov, M.A. Thiazol-4-one derivatives from the reaction of monosubstituted thioureas with maleimides: Structures and factors determining the selectivity and tautomeric equilibrium in solution. Beilstein J. Org. Chem. 2016, 12, 2563–2569. [Google Scholar] [CrossRef]
- Pankova, A.S.; Samartsev, M.A.; Shulgin, I.A.; Golubev, P.R.; Avdontceva, M.S.; Kuznetsov, M.A. Synthesis of thiazolidines via regioselective addition of unsymmetric thioureas to maleic acid derivatives. RSC Adv. 2014, 4, 51780–51786. [Google Scholar] [CrossRef]
- Marhadour, S.; Bazin, M.A.; Marchand, P. Prehistoric schistosomiasis parasite found in the Middle East. Tetrahedron Lett. 2012, 53, 297–300. [Google Scholar] [CrossRef]
- Almeida, G.M.; Rafique, J.; Saba, S.; Siminski, T.; Mota, N.S.R.S.; Filho, D.W.; Braga, A.L.; Pedrosa, R.C.; Ourique, F. Novel selenylated imidazo[1,2-a]pyridines for breast cancer chemotherapy: Inhibition of cell proliferation by Akt-mediated regulation, DNA cleavage and apoptosis. Biochem. Biophys. Res. Commun. 2018, 503, 1291–1297. [Google Scholar] [CrossRef]
- Hosseini, H.; Bayat, M. An efficient synthesis of new imidazo[1,2-a]pyridine-6-carbohydrazide and pyrido[1,2-a]pyrimidine-7-carbohydrazide derivatives via a five-component cascade reaction. Rsc. Adv. 2019, 9, 7218–7227. [Google Scholar] [CrossRef]
- Chitti, S.; Singireddi, S.; Santosh Kumar Reddy, P.; Trivedi, P.; Bobde, Y.; Kumar, C.; Rangan, K.; Ghosh, B.; Sekhar, K.V.G.C. Design, synthesis and biological evaluation of 2-(3,4-dimethoxyphenyl)-6 (1,2,3,6-tetrahydropyridin-4-yl)imidazo[1,2-a]pyridine analogues as antiproliferative agents. Bioorg. Med. Chem. Lett. 2019, 29, 2551–2558. [Google Scholar] [CrossRef]
- Vandyshev, D.Y.; Shikhaliev, K.S.; Potapov, A.Y. Interaction of 1,2-diaminobenzimidazole with N-arylimides. Eur. Chem. Bull. 2015, 4, 424–427. [Google Scholar] [CrossRef]
- Vandyshev, D.Y.; Shikhaliev, K.S.; Potapov, A.Y.; Krysin, M.Y. Condensation of 1,2-diamino-4-phenylimidazole and N-arylmaleimides with the formation of new tetrahydroimidazo[1,5-b]pyridazines. Chem. Heterocycl. Compd. 2015, 51, 829–833. [Google Scholar] [CrossRef]
- Nöth, J.; Frankowski, K.J.; Neuenswander, B.; Aubé, J.; Reiser, O. Efficient Synthesis of γ-Lactams by a Tandem Reductive Amination/Lactamization Sequence. J. Comb. Chem. 2008, 10, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Fenster, E.; Hill, D.; Reiser, O.; Aubé, J. Automated three-component synthesis of a library of γ-lactams. Beilstein J. Org. Chem. 2012, 8, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).