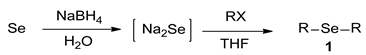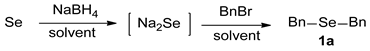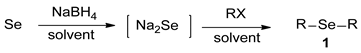Abstract
The studies on the selective synthesis of dialkyl selenide compounds 1 were presented. Overcoming the complexity and difficulty of selenides (R-Se-R) and/or multiselenides (R-Sen-R; n ≥ 2), we aimed to optimize the reaction condition for the tolerable preparation of sodium selenide (Na2Se) by reducing Se with NaBH4, and then to achieve selective syntheses of dialkyl selenides 1 by subsequently treating the obtained sodium selenide with alkyl halides (RX). Consequently, various dialkyl selenides 1 were efficiently synthesized in good-to-moderate yields. The investigations on reaction pathways and solvent studies were also described.
1. Introduction
Selenium (Se), one of the essential trace elements, belongs to group 16 elements, also known as the chalcogen family [1]. During the last 40 years, organoselenium compounds have attracted attention as important reagents and intermediates in organic synthesis as well as in medicine [2,3]. Selenium compounds seem to play important roles in biological systems as antioxidant, chemopreventive, anti-inflammatory, and antiviral agents [4]. Selenium is used to form selenoamino acids, selenocystein (Sec) and selenomethionine (SeMet) that are incorporated as surrogates to sulfur derivatives [5,6]. In many respects, selenium and sulfur have very similar physical and chemical properties, but at the same time may display keen differences [7]. In general, the strengths of selenium–carbon bonds (234 kJ/mol) are weaker than those of sulfur–carbon bonds (272 kJ/mol), and the bond length of selenium–carbon (198 pm) is longer than that of sulfur–carbon (C-S, 184 pm) [7]. Selenium compounds are more nucleophilic and more acidic (pKa is 3.74 for H2Se) than sulfur compounds (pKa is 7.0 for H2S) [8]. Despite these intriguing characters, organoselenium compounds have not been extensively studied for decades, because of their irregular reactivity and instability, biological toxicity, and lack of tolerable synthetic methods. However, currently organoselenium compounds have been used in many applications, including synthetic methodologies and medicinal purposes for antioxidant [9,10] and anticancer agents [11,12,13,14].
Among many organoselenium compounds, selenides (R-Se-R) and diselenides (R-Se-Se-R) are the most traditional classes of organoselenium compounds. Nevertheless, studies and derivatization of selenide compounds are less reported in comparison to diselenide compounds. The incorporated form of Se in selenoamino acids such as SeMet is the dialkyl selenide and so, we focused on the selective synthesis of dialkyl selenides. So far, several methods have been reported for the synthesis of dialkyl selenides [15,16,17,18,19,20]. Among them, reactions of sodium selenide (Na2Se) generated in situ and an organic halide in an appropriate solvent have been employed to prepare a small number of symmetrical dialkyl selenides. In these methods, selenium is reduced to selenide anion(s) by proper reducing agents. For this purpose, NaBH4 [18], NaH [19], Na [20], and Li(CH3CH2)3BH [2] have been used with corresponding reaction conditions. Most of the methodologies, however, suffer from various limitations, such as poor reproducibility, narrow tolerance according to structures, poor yields and product instability, side product formation, and tricky manipulations. In reality, we experienced poor reproducibility of previously known procedures, and severe difficulty in manipulating products due mainly to their chemical instabilities. In particular, we observed that, along with desired selenides, diselenides and multiselenides were generally formed as side products, and dark solids were also formed in storing for even a short period of time.
According to the previous reports [16,18] and our preliminary studies, we found that NaBH4 might be an appropriate reducing agent for this transformation; thus, we focused on the use of NaBH4. We here report the studies on the selective formation of sodium selenide and corresponding dialkyl selenides in a one-pot reaction, along with the studies on reaction pathways and solvent effects.
2. Results and Discussion
2.1. Optimization for the Reaction Conditions
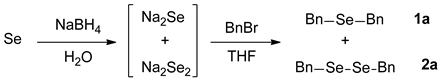
In order to achieve selective syntheses of dialkyl selenides (R-Se-R), we first aimed to investigate the reaction conditions for the selective formation of sodium selenide (Na2Se). Based on previous procedures for selenides [18], we attempted to apply these reaction conditions for various aliphatic selenides with proper modifications. For this purpose, we chose to employ selenium (Se) and NaBH4 as a reducing agent for the formation of sodium selenide. The sodium selenide generated in situ was treated with proper electrophiles such as alkyl halide (RX) to give desired dialkyl selenides 1. So, we conducted the reactions with benzyl bromide (BnBr) as a template alkyl halide, leading to the selective synthesis of dibenzyl selenide (1a), as shown in Scheme 1.
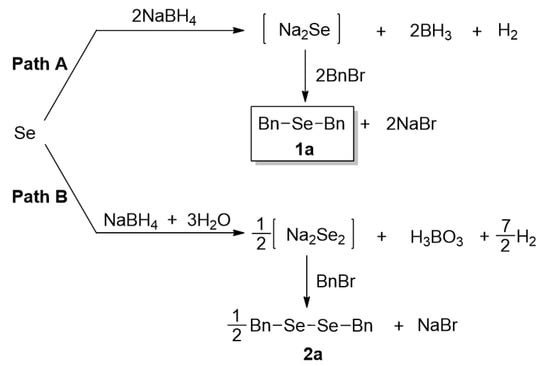
Scheme 1.
Formation of sodium selenide and dibenzyl selenide (1a).
We performed the reactions for the synthesis of sodium selenide under the condition of Se and NaBH4 in THF-H2O solvent. On the basis of the importance of reducing agent in these reactions, we optimized the reaction conditions by varying the amounts of NaBH4 and the reaction time at room temperature (25 °C). Then, the resulting sodium selenide was directly reacted with BnBr to give the product 1a. Regarding the amount of BnBr, we intended to provide a sufficient amount of it. Thus, we tested BnBr in the range of 2.0–3.0 eq, and found that 2.4 eq of BnBr is an adequate amount, unless otherwise mentioned (data not shown). As shown in Table 1, first we investigated the reaction conditions for the formation of sodium selenide. Based on our preliminary studies and the information that the theoretical amount of NaBH4 is 2.0 eq (vs. Se), we chose the range of 1.0–3.0 eq for NaBH4, and 25 °C for the reaction temperature. As expected, the amount of NaBH4 was found to be the most important factor, and 3.0 eq of this reagent gave the best yield in the given range. When we used a lower amount (e.g., 1.0 eq) of that, we observed the substantial formation of side product, dibenzyl diselenide 2a. As the amount of NaBH4 was increased, the formation of 2a was decreased, which is consistent with the suggested reaction mechanism (see below) and our previous observation [21]. Considering the required stoichiometric amount (2.0 eq) of the reducing agent, the use of 3.0 eq seemed to be excessive, but we believed that the excess amount of reducing agent could be required due to the presence of oxygen, incomplete workings of the reducing agent, and/or extraconsumption in cleaving side products 2, diselenides (Scheme 1). In addition, the reaction time for the formation of sodium selenide could affect the final results. Interestingly, as the reaction time is elongated, the product 1a (selenide) formation is decreased with the increased formation of side product 2a (diselenide). It is believed that when the reaction time for sodium selenide is getting longer, the sodium selenide could gradually oxidize to sodium diselenide, leading to the higher generation of 2a. Taken together, the best condition for the two-step reaction was: (1) Se 1.0 eq, NaBH4 3.0 eq and 1 h reaction time; (2) BnBr 2.4 eq and 2 h reaction time at room temperature.

Table 1.
Optimization of the reaction conditions for sodium selenide and dibenzyl selenide 1a a.
2.2. Reaction Pathways
According to the results so far, we wish to perform mechanistic studies on reaction pathways. Here, we propose reaction pathways for the formation of dialkyl selenides 1 and dialkyl diselenides 2, as shown in Scheme 2. At first, elemental Se could be reduced by NaBH4 to diselenide dianion (Se22−, oxidation state: —1), which could also be further reduced to selenide dianion (Se2−, oxidation state: —2). The two species (Se22− and Se2−) could equilibrate each other according to redox condition. Then, selenide dianion (Se2−) could react with alkyl halide to give the products, selenides 1, and similarly, diselenide dianion (Se22−), to give side products, diselenides 2. Notably, we could presume and indirectly verify the presence of the selenium species by capturing them with alkyl halide. As expected, the side products 2 were continuously formed in most of the reactions, and the control of selective synthesis of selenides 1 over diselenides 2 was a challenge. Therefore, we intended to employ sufficient amount of reducing agent to avoid the formation of diselenide dianion and dialkyl diselenides 2. Once 2 is formed, it could also undergo further reduction to give selenol 3, with further consumption of reducing agent. Taken together, we suggested the pathways for compounds 1–3 under reduction condition (e.g., NaBH4).
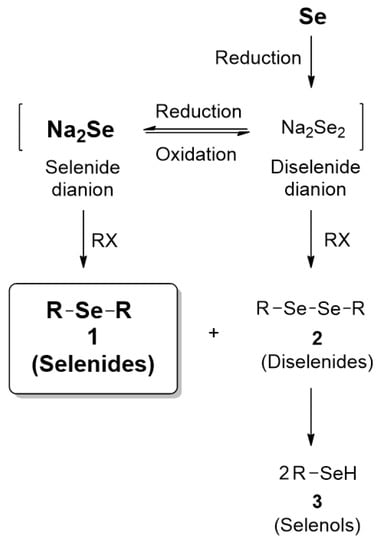
Scheme 2.
The proposed reaction pathways for products 1, and side products 2 and 3.
2.3. Synthesis of Dialkyl Selenides 1
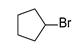
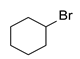
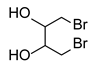
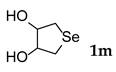
Using this optimized condition, we achieved the selective syntheses of various dialkyl selenides 1, expanding the scope of this reaction by applying different aliphatic halides, as shown in Table 2. We tried to employ primary, secondary, and tertiary alkyl bromides. At first, benzyl bromide, phenethyl bromide, and allyl bromide afforded good yields (Table 2, entries 1 to 3). As primary aliphatic bromides, n-butyl, n-pentyl, n-hexyl, and n-octyl bromide also gave good yields (entries 4 to 7). As secondary alkyl halides, c-pentyl, c-hexyl, 3-pentyl, and 4-heptyl bromide provided moderate-to-poor yields (entries 8 to 11). However, tertiary halides (e.g., t-butyl, t-amyl, and trityl bromide) did not afford an appreciable amount of product, due mainly to steric hindrance (data not shown). In addition, aromatic bromides generally gave poor results. Phenyl bromide, phenyl bromides with an electron-donating substituent (e.g., methyl group), and phenyl bromides with electron-withdrawing substituents (e.g., cyano or trifluoromethyl group) afforded poor yields (5–10%, data not shown) even at a high reaction temperature (e.g., 153 °C). In order to improve the results, we also tested the additional use of sodium iodide as a catalyst or tetrabutylammonium bromide as a phase-transfer catalyst (PTC), leading to no appreciable success. We also attempted to employ aromatic iodides instead of aromatic bromides under harsh reaction conditions (e.g., elongated reaction time at 120 °C), without appreciable improvement. Notably, we were also interested in the formation of cyclic selenides and so we tested the use of alkyl dibromides (e.g., α,α′-dibromoxylene and 1,4-dibromo-2,3-dihydroxybutane, entries 12 and 13, respectively). For these reactions, we applied half amount of alkyl dibromides and a low concentration of reagents (e.g., 64 mM for Se) in order to facilitate intramolecular cyclization reactions. As expected, these reactions worked, leading to the generation of cyclic selenides in modest yields (65 and 51%). Notably, for compound 1m, the used starting material ((±)-1,4–dibromo–2,3–butanediol) was an enantiomer mixture (two enantiomers (R,R)- and (S,S)-form) and thus the product 1m was also an enantiomer mixture ((R,R)- and (S,S)-form).

Table 2.
Synthesis of dialkyl selenides 1a a.
2.4. Solvent Studies
We performed the reactions under THF–H2O as a co-solvent system and the results were generally successful. Nevertheless, we conducted solvent studies to find out better and more convenient conditions. So, we chose ethanol (EtOH) as a volatile protic solvent and acetonitrile (MeCN), tetrahydrofuran (THF), and dimethoxyethane (DME) as volatile aprotic solvents. Using these solvent systems, we performed the reactions under similar conditions by using NaBH4 and BnBr as alkyl halide at room temperature (25 °C), which was summarized in Table 3. The reaction for the preparation of Na2Se under EtOH solvent seemed to be in a white suspension condition and selectively provided dibenzyl selenide 1a in a good yield (81%). The reactions under MeCN, THF, and DME solvents seemed to be in brown-to-reddish-brown suspension conditions with heterogeneous reaction media. The reaction under MeCN afforded dibenzyl selenide 1a in a good yield (80%) with a trace amount of dibenzyl diselenide. However, the other reactions under THF and DME solvents required longer reaction times (48 and 47 h, respectively) and provided modest yields (50 and 30%, respectively). Although the reaction under EtOH solvent gave a good result (81%), the reproducibility of the reaction was not so good, probably due to the undesired participation of EtOH as a nucleophile producing benzylethyl ether (BnOEt). As a result, MeCN was found to be a good choice as a non-aqueous, volatile, aprotic solvent.

Table 3.
Solvent test a.
Furthermore, inspired by the good results under MeCN solvent, we expanded test reactions under MeCN using several alkyl halides. We also compared the reactions of alkyl bromides with those of alkyl chlorides. In general, the results under MeCN solvent were similar or lower than those under the original solvent (H2O-THF), and sometimes the results fluctuated according to the conditions, as shown in Table 4. Although the results under MeCN were found to be variable, it seemed a milestone experiment that sodium selenide and then dialkyl selenides 1 could be obtained in good yield in MeCN as a non-aqueous, volatile, aprotic solvent. Nevertheless, the heterogeneity issue might still limit broad applications, and further studies for improvements are in progress.

Table 4.
Comparison of the results under H2O-THF and MeCN solvents a.
3. Experimental
3.1. General
Most of the reagents were purchased at good commercial quality and used without further purification, unless otherwise noted. Reactions were continuously monitored by thin-layer chromatography (TLC) using glass plates (0.25 mm) coated with silica gel (20 × 20 cm2; Aldrich No. Z12272-6) and visualized by UV light and staining solutions such as cerium molybdate and phosphomolybdic acid, if necessary. Preparative TLC (PTLC) and column chromatography were mainly employed for purifications. PTLC separations were carried out on the same silica gel plates for TLC. Column chromatography was conducted using Merck silica gels (230–400 mesh). Measurement of melting points was carried out in open capillary tubes using Electrothermal IA9100 melting point apparatus, and the measurements were uncorrected. FT-IR spectra were obtained on a Thermo Fisher (Waltham, MA, USA) FT-IR spectrometer (Nicolet Summit) and frequencies (ν) are given in reciprocal centimeters (cm−1). 1H (300 MHz) and 13C (75 MHz) NMR spectra were obtained by a Bruker (Billerica, Massachusetts, USA) DRX 300 spectrometer and the chemical shift values (δ) are expressed as units relative to tetramethylsilane (TMS). Mass spectra were obtained using EI or ESI ionization method. HPLC analyses were conducted using the following Waters Associate Units: 1525 Binary HPLC Pump, 2998 Photodiode Array Detector, and C18 COSMOSIL column (4.6 × 250 mm2). The product analyses were performed using a linear gradient condition. Condition 1: from 70% A (H2O) and 30% B (MeCN) for 3 min (isocratic) to 10% A and 90% B in 30 min (gradient), then to 10% A and 90% B for 7 min (isocratic) and, finally, from 10% A and 90% B to 70% A and 30% B over 3 min (gradient), then to 70% A and 30% B for 5 min (isocratic). Condition 2: from 50% A (H2O) and 50% B (MeCN) for 3 min (isocratic) to 10% A and 90% B in 30 min (gradient), then to 10% A and 90% B for 7 min (isocratic) and, finally, from 10% A and 90% B to 50% A and 50% B over 3 min (gradient), then to 50% A and 50% B for 5 min (isocratic). The flow rate was 1 mL/min, and the eluent was monitored at 254 nm. The HPLC solvents were filtered (aqueous solution with Millipore HVLP, 0.45 mm; acetonitrile with Millipore HV, 0.45 mm) and degassed before utilization.
3.2. General Procedure for the Synthesis of Dialkyl Monoselenides 1
To a stirred mixture of NaBH4 (144 mg, 3.9 mmol, 3.0 eq) and H2O (2 mL), Se (100 mg, 1.3 mmol, 1.0 eq) was added under the nitrogen atmosphere. The resulting mixture was stirred for 1 h at room temperature turning white. Then, alkyl halides (3.1 mmol, 2.4 eq) in THF (8 mL) were slowly added and stirring was continued for 2–48 h at room temperature to 50 °C until completion of the reaction (Table 2). The reaction mixture was diluted with water (30 mL), extracted with CH2Cl2 (3 × 30 mL) and then washed with brine (30 mL). The combined organic layers were dried (anhydrous MgSO4), and concentrated in vacuo to give sticky residue. The residue was purified by column chromatography (1:50 → 1:1 EtOAc/n-hexanes) to give 1a–1m as a light yellow oil, unless otherwise noted. The NMR charts for all compounds 1 were provided in Supplementary Materials.
3.2.1. 1,2-Dibenzyl Selenide (1a) [2]
Use of benzyl bromide (0.36 mL, 3.1 mmol, 2.4 eq), 2 h reaction time at room temperature in general procedure, afforded the title compound 1a (310 mg, 87%) as a light yellow solid. Mp 44–45 °C; Rf 0.34 (1:20 EtOAc/n-hexanes); HPLC tR 31.17 min (condition 1); λmax = 215 nm; IR (KBr) 3033, 3030, 703, 696, 670, 667 cm−1; 1H-NMR (300 MHz, CDCl3) δ 7.32–7.18 (m, 10H, Ar), 3.72 (s, 4H, CH2); 13C-NMR (75 MHz, CDCl3) δ 139.37 (Ar), 129.17 (Ar), 128.69 (Ar), 27.75 (CH2); MS m/z 262 [M]+; HRMS (+ESI) calcd for C14H14Se [M]+ 262.0261, found 262.0264.
3.2.2. 1,2-Bis(2-phenylethyl) Selenide (1b) [2]
Use of phenethyl bromide (0.42 mL, 3.1 mmol, 2.4 eq), 5 h reaction time at room temperature, in general procedure afforded the title compound 1b (248 mg, 66%) as a light yellow solid. Mp 148–156 °C; Rf 0.30 (1:20 EtOAc/n-hexanes); HPLC tR 30.54 min (condition 1); λmax = 261 nm; IR (KBr) 3104, 1029, 705 cm−1; 1H-NMR (300 MHz, CDCl3) δ 7.36–7.10 (m, 10H, Ar), 2.95 (t, J = 7.8 Hz, 4H, SeCH2), 2.78 (t, J = 7.8 Hz, 4H, PhCH2); 13C-NMR (75 MHz, CDCl3) δ 141.42 (Ar), 128.66 (Ar), 128.55 (Ar), 126.52 (Ar), 37.36 (SeCH2), 25.19 (PhCH2); MS m/z 290 [M]+; HRMS (+ESI) calcd for C16H18Se [M]+ 290.0574, found 290.0570.
3.2.3. 1,2-Diallyl Selenide (1c) [22]
Use of allyl bromide (0.26 mL, 3.1 mmol, 2.4 eq), 25 h reaction time at room temperature, in general procedure afforded the title compound 1c (135 mg, 66%). Bp 56–58 °C; Rf 0.51 (n-hexane); HPLC tR 33.86 min; λmax = 243 nm (condition 1); IR (KBr) 987, 739 cm−1; 1H-NMR (300 MHz, CDCl3) δ 5.89–5.80 (m, 2H, CH2=CH), 5.05–5.00 (m, 4H, CH2=CH), 3.15 (d, J = 7.6 Hz, 4H, SeCH2); 13C-NMR (75 MHz, CDCl3) δ 135.19 (CH2=CH), 116.61 (CH2=CH), 25.55 (SeCH2); MS m/z 162 [M]+.
3.2.4. 1,2-Di-n-butyl Selenide (1d) [19]
Use of n-butyl bromide (0.33 mL, 3.1 mmol, 2.4 eq), 3 h reaction time at room temperature, in general procedure afforded the title compound 1d (228 mg, 93%). Bp 72–74 °C; Rf 0.49 (1:50 EtOAc/n-hexanes); HPLC tR 32.73 min (condition 1); λmax = 222 nm; IR (KBr) 2953, 2872, 994, 741 cm−1; 1H-NMR (300 MHz, CDCl3) δ 2.56 (t, J = 7.5 Hz, 4H, SeCH2), 1.64 (quintet, J = 7.4 Hz, 4H, SeCH2CH2), 1.40 (sextet, J = 7.4 Hz, 4H, CH2CH3), 0.92 (t, J = 7.3 Hz, 6H, CH3); 13C-NMR (75 MHz, CDCl3) δ 32.99 (SeCH2), 23.84 (SeCH2CH2), 23.29 (CH2CH3), 13.83 (CH3); MS m/z 194 [M]+; HRMS (+ESI) calcd for C8H18Se [M]+ 194.0574, found 194.0575.
3.2.5. 1,2-Di-n-pentyl Selenide (1e) [2]
Use of n-pentyl bromide (0.38 mL, 3.1 mmol, 2.4 eq), 3 h reaction time at room temperature, in general procedure afforded the title compound 1e (230 mg, 82%). Bp 96–106 °C; Rf 0.50 (n-hexanes); HPLC tR 35.94 min (condition 2); λmax = 224 nm; IR (KBr) 2957, 2925, 1466, 1241, 728 cm−1; 1H-NMR (300 MHz, CDCl3) δ 2.55 (t, J = 7.5 Hz, 4H, SeCH2), 1.66 (quintet, J = 7.3 Hz, 4H, SeCH2CH2), 1.36–1.34 (m, 8H, (CH2)2CH3), 0.90 (t, J = 6.9 Hz, 6H, CH3); 13C-NMR (75 MHz, CDCl3) δ 32.39 (SeCH2), 30.59 (SeCH2CH2), 24.16 (CH2CH2CH3), 22.45 (CH2CH3), 14.18 (CH3); MS m/z 222 [M]+; HRMS (+ESI) calcd for C10H22Se [M]+ 222.0887, found 222.0889.
3.2.6. 1,2-Di-n-hexyl Selenide (1f) [19]
Use of n-hexyl bromide (0.43 mL, 3.1 mmol, 2.4 eq), 5 h reaction time at room temperature, in general procedure afforded the title compound 1f (269 mg, 85%). Bp 90–94 °C; Rf 0.50 (n-hexanes); HPLC tR 26.92 min (condition 2); λmax = 271 nm; IR (KBr) 3409, 2856, 1458, 743, 698 cm−1; 1H-NMR (300 MHz, CDCl3) δ 2.55 (t, J = 7.5 Hz, 4H, SeCH2), 1.65 (quintet, J = 7.4 Hz, 4H, SeCH2CH2), 1.43–1.28 (m, 12H, (CH2)3CH3), 0.89 (t, J = 6.8 Hz, 6H, CH3); 13C-NMR (75 MHz, CDCl3) δ 31.59 (SeCH2), 30.87 (SeCH2CH2), 29.90 (Se(CH2)2CH2), 24.20 (CH2CH2CH3), 22.78 (CH2CH3), 14.26 (CH3); MS m/z 250 [M]+; HRMS (+ESI) calcd for C12H26Se [M]+ 250.1200, found 250.1203.
3.2.7. 1,2-Di-n-octyl Selenide (1g) [23]
Use of n-octyl bromide (0.53 mL, 3.1 mmol, 2.4 eq), 5 h reaction time at room temperature, in general procedure afforded the title compound 1g (330 mg, 85%). Bp 132–148 °C; Rf 0.60 (1:100 EtOAc/n-hexanes); HPLC tR 28.82 min (condition 1); λmax = 297 nm; IR (KBr) 3418, 2914, 750, 696 cm−1; 1H-NMR (300 MHz, CDCl3) δ 2.55 (t, J = 7.5 Hz, 4H, SeCH2), 1.65 (quintet, J = 7.4 Hz, 4H, SeCH2CH2), 1.36–1.27 (m, 20H, (CH2)5CH3), 0.88 (t, J = 6.7 Hz, 6H, CH3); 13C-NMR (75 MHz, CDCl3) δ 32.05 (SeCH2), 30.90 (SeCH2CH2), 30.22 (Se(CH2)2CH2), 29.42 (Se(CH2)3CH2), 29.36 (CH2(CH2)2CH3), 24.19 (CH2CH2CH3), 22.88 (CH2CH3), 14.33 (CH3); MS m/z 306 [M]+; HRMS (+ESI) calcd for C16H34Se [M]+ 306.1826, found 306.1821.
3.2.8. 1,2-Di-c-pentyl Selenide (1h) [24]
Use of c-pentyl bromide (0.31 mL, 3.1 mmol, 2.4 eq), 3 h reaction time at 50 °C, in general procedure afforded the title compound 1h (138 mg, 50%). Bp 90–96 °C; Rf 0.47 (n-hexanes); HPLC tR 34.36 min (condition 1); λmax = 225; IR (KBr) 2956, 2866, 1448, 1217, 932 cm−1; 1H-NMR (300 MHz, CDCl3) δ 3.25 (quintet, J = 7.1 Hz, 2H, SeCH), 2.12–2.01 (m, 4H, SeCH(CHH)2), 1.80–1.51 (m, 12H, SeCH(CHH)2(CH2)2); 13C-NMR (75 MHz, CDCl3) δ 37.76 (SeCH), 35.06 (SeCH(CH2)2), 25.19 (SeCH(CH2)2(CH2)2); MS m/z 218 [M]+; HRMS (+ESI) calcd for C10H18Se [M]+ 218.0574, found 218.0576.
3.2.9. 1,2-Di-c-hexyl Selenide (1i) [25]
Use of c-hexyl bromide (0.38 mL, 3.1 mmol, 2.4 eq), 8 h reaction time at 50 °C, in general procedure afforded the title compound 1i (150 mg, 48%). Bp 104–106 °C; Rf 0.50 (n-hexanes); HPLC tR 35.65 min (condition 1); λmax = 228 nm; IR (KBr) 3422, 2849, 1724, 1447, 1258, 993, 699 cm−1; 1H-NMR (300 MHz, CDCl3) δ 2.96 (m, 2H, SeCH), 2.03–1.25 (m, 20H, CH2); 13C-NMR (75 MHz, CDCl3) δ 37.97 (SeCH), 35.40 (SeCH(CH2)2), 27.17 (SeCH(CH2)2(CH2)2), 26.08 (SeCH(CH2)4(CH2)2); MS m/z 246 [M]+; HRMS (+ESI) calcd for C12H22Se [M]+ 246.0887, found 246.0885.
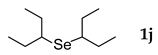
3.2.10. 1,2-Bis(3-pentyl) Selenide (1j) [22]
Use of 3-pentyl bromide (0.38 mL, 3.1 mmol, 2.4 eq), 5 h reaction time at 50 °C, in general procedure afforded the title compound 1j (87 mg, 31%). Bp 90–100 °C; Rf 0.50 (n–hexanes); HPLC tR 34.60 min (condition 1); λmax = 228 nm; IR (KBr) 2963, 2932, 1457, 1378, 1185, 861 cm−1; 1H-NMR (300 MHz, CDCl3) δ 2.69 (quintet, J = 6.3 Hz, 2H, CH), 1.75–1.60 (m, 8H, CH2), 0.99 (t, J = 7.3 Hz, 12H, CH3); 13C-NMR (75 MHz, CDCl3) δ 45.27 (CH), 28.63 (CH2), 12.29 (CH3); MS m/z 222 [M]+; HRMS (+ESI) calcd for C10H22Se [M]+ 222.0887, found 222.0883.
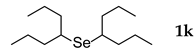
3.2.11. 1,2-Bis(4-heptyl) Selenide (1k)
Use of 4-heptyl bromide (0.48 mL, 3.1 mmol, 2.4 eq), 25 h reaction time at 50 °C, in general procedure afforded the title compound 1k (123 mg, 35%). Bp 118–120 °C; Rf 0.39 (n-hexanes); HPLC tR 32.85 min (condition 2); λmax = 250 nm; IR (KBr) 3398, 2932, 2872, 1458, 1378, 756, 698 cm−1; 1H-NMR (300 MHz, CDCl3) δ 2.81 (quintet, J = 6.4 Hz, 2H, CH), 1.65–1.36 (m, 16H, CH2), 0.91 (t, J = 7.2 Hz, 12H, CH3); 13C-NMR (75 MHz, CDCl3) δ 41.21 (CH), 38.83 (CHCH2), 21.00 (CH2CH3), 14.26 (CH3); MS m/z 278 [M]+; HRMS (+ESI) calcd for C14H30Se [M]+ 278.1513, found 278.1514.
3.2.12. 1,3-Dihydrobenzo[c]Selenophene (1l) [26]
Use of α,α′-dibromo-o-xylene (335 mg, 1.3 mmol, 1.0 eq), 24 h reaction time at room temperature, in general procedure afforded the title compound 1l (151 mg, 65%) as a light yellow solid. Mp 36–37 °C; Rf 0.39 (n-hexanes); HPLC tR 22.40 min (condition 1); λmax = 268 nm; IR (KBr) 3441, 3018, 1645, 1448, 834, 744 cm−1; 1H-NMR (300 MHz, CDCl3) δ 7.25–7.14 (m, 4H, Ar), 4.32 (s, 4H, CH2); 13C-NMR (75 MHz, CDCl3) δ 141.82 (Ar), 126.62 (Ar), 126.10 (Ar), 30.04 (CH2); MS m/z 184 [M]+; HRMS (+ESI) calcd for C8H8Se [M]+ 183.9791, found 183.9794.
3.2.13. Tetrahydro-3,4-Selenophenediol (1m) [27]
Use of 1.4-dibromo-2,3-butanediol (315 mg, 1.3 mmol, 1.0 eq), 48 h reaction time at room temperature, and then extraction with n-butanol in general procedure afforded the title compound 1m (108 mg, 51%) as a light yellow solid. Mp 77–80 °C; Rf 0.20 (3:1 EtOAc/n-hexanes); HPLC tR 28.12 min (condition 1); λmax = 297 nm; IR (KBr) 3378, 1637, 1492, 1015, 704 cm−1; 1H-NMR (300 MHz, MeOH-d4) δ 4.20 (m, 2H, CH), 3.09, 2.79 (each dd, J = 10.2 and 9.6 Hz, each 2H, CH2); 13C-NMR (75 MHz, MeOH-d4) δ 80.02 (CH), 27.90 (CH2); MS m/z 168 [M]+; HRMS (+ESI) calcd for C4H8O2Se [M]+ 167.9690, found 167.9691.
4. Conclusions
We reported the systematic studies on the synthesis of Na2Se and corresponding dialkyl selenide compounds 1. We established an optimized condition for these reactions as follows: (1) Se (1.3 mmol, 1.0 eq), NaBH4 (3.0 eq) in H2O for 1 h at room temperature; (2) alkyl bromide for 2–48 h with THF at room temperature to 50 °C. Applying this optimized condition using various alkyl halides, the desired dialkyl selenides 1 were successfully produced in good-to-moderate yields (31–93%). In particular, alkyl dihalide compounds were also employed to provide cyclic selenides, 1l and 1m, in 65% and 51% yields, respectively. Furthermore, solvent studies revealed that non-aqueous and volatile solvents (e.g., MeCN) other than THF-H2O also worked for these reactions. Taken together, we have completed systematic studies on the selective syntheses of Na2Se and the corresponding dialkyl selenide compounds 1 using NaBH4 as a reducing agent.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27165224/s1. The charts for 1H- and 13C-NMR spectroscopies are available online.
Author Contributions
Conceptualization, S.H.L. and H.C.; methodology, N.H.S., Y.J.L., C.K., Y.E.K., Y.R.J., H.C. and M.-S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) Grant (2021R1F1A1062059, 2016R1D1A1B03930981, and 2019M3E5D5066543), and by Priority Research Centers Program through NRF (2016R1A6A1A03007648) funded by the Ministry of Education, Science and Technology (MEST).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in insert article or Supplementary Materials here.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1a–1m are available from the authors.
References
- Jain, V.K. An Overview of Organoselenium Chemistry: From Fundamentals to Synthesis. In Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments; Jain, V.K., Priyadarsini, K.I., Eds.; The Royal Society of Chemistry: London, UK, 2017; pp. 1–33. ISBN 978-1-78801-029-0. [Google Scholar]
- Gladysz, J.A.; Hornby, J.L.; Garbe, J.E. A Convenient One-Flask Synthesis of Dialkyl Selenides and Diselenides via Lithium Triethylborohydride Reduction of Sex. J. Org. Chem. 1978, 43, 1204–1208. [Google Scholar] [CrossRef]
- Ivanova, A.; Arsenyan, P. Rise of Diselenides: Recent Advances in the Synthesis of Heteroarylselenides. Coord. Chem. Rev. 2018, 370, 55–68. [Google Scholar] [CrossRef]
- Rayman, M.P. The Importance of Selenium to Human Health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Álvarez-Pérez, M.; Ali, W.; Marć, M.A.; Handzlik, J.; Domínguez-Álvarez, E. Selenides and Diselenides: A Review of their Anticancer and Chemopreventive Activity. Molecules 2018, 23, 628. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.-O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef]
- Wessjohann, L.A.; Schneider, A.; Abbas, M.; Brandt, W. Selenium in chemistry and biochemistry in comparison to sulfur. Biol. Chem. 2007, 388, 997–1006. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. Acs. Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Sies, H. Protection against reactive oxygen species by selenoproteins. Biochim. Biophys. Acta 2009, 1790, 1478–1485. [Google Scholar] [CrossRef]
- Nascimento, V.; Alberto, E.E.; Tondo, D.W.; Dambrowski, D.; Detty, M.R.; Nome, F.; Braga, A.L. GPx-Like Activity of Selenides and Selenoxides: Experimental Evidence for the Involvement of Hydroxy Perhydroxy Selenane as the Active Species. J. Am. Chem. Soc. 2012, 134, 138–141. [Google Scholar] [CrossRef]
- Abdulah, R.; Miyazaki, K.; Nakazawa, M.; Koyama, H. Chemical forms of selenium for cancer prevention. J. Trace Elem. Med. Biol. 2005, 19, 141–150. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, H.; Dong, Y.; Park, Y.-M.; Ip, C. Endoplasmic Reticulum Stress Signal Mediators are Targets of Selenium Action. Cancer Res. 2005, 65, 9073–9079. [Google Scholar] [CrossRef] [PubMed]
- Sonn, G.A.; Aronson, W.; Litwin, M.S. Impact of diet on prostate cancer: A review. Prostate Cancer Prostatic Dis. 2005, 8, 304–310. [Google Scholar] [CrossRef]
- Hodage, A.S.; Prabhu, C.P.; Phadnis, P.P.; Wadawale, A.; Priyadarsini, K.I.; Jain, V.K. Synthesis, characterization, structures and GPx mimicking activity of pyridyl and pyrimidyl based organoselenium compounds. J. Organomet. Chem. 2012, 720, 19–25. [Google Scholar] [CrossRef]
- Milton, M.D.; Khan, S.; Singh, J.D.; Mishra, V.; Khandelwal, B.L. A facile access to chalcogen and dichalcogen bearing dialkylamines and diols. Tetrahedron Lett. 2005, 46, 755–758. [Google Scholar] [CrossRef]
- Andrade, L.H.; Silva, A.V.; Milani, P.; Koszelewski, D.; Kroutil, W. ω-Transaminases as efficient biocatalysts to obtain novel chiral selenium-amine ligands for Pd-catalysis. Org. Biomol. Chem. 2010, 8, 2043–2051. [Google Scholar] [CrossRef]
- Klayman, D.L.; Griffin, T.S. Reaction of Selenium with Sodium Borohydride in Protic Solvents. A Facile Method for the Introduction of Selenium into Organic Molecules. J. Am. Chem. Soc. 1973, 95, 197–199. [Google Scholar] [CrossRef]
- Krief, A.; Derock, M. Synthesis of diselenides and selenides from elemental selenium. Tetrahedron Lett. 2002, 43, 3083–3086. [Google Scholar] [CrossRef]
- Rossi, R.A.; Penenory, A.B. Direct (One Pot) Synthesis of Organoselenium and Organotellurium Compounds from the Metals. J. Org. Chem. 1981, 46, 4580–4582. [Google Scholar] [CrossRef]
- Lim, Y.J.; Shin, N.H.; Kim, C.; Kim, Y.E.; Cho, H.; Park, M.-S.; Lee, S.H. An efficient and practical method for the selective synthesis of sodium diselenide and diorganyl diselenides through selenium reduction. Tetrahedron 2020, 76, 131720. [Google Scholar] [CrossRef]
- Ouchi, A.; Liu, S.; Li, Z.; Kumar, S.A.; Suzuki, T.; Hyugano, T.; Kitahara, H. Factors Controlling Photochemical Cleavage of the Energetically Unfavorable Ph−Se Bond of Alkyl Phenyl Selenides. J. Org. Chem. 2007, 72, 8700–8706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-B.; Ruan, M.-D.; Fan, W.-Q. Reactions of Selenobenzamide and Alkyl Halides. Synthesis of Dialkyl Selenides and Diselenides. Synth. Commun. 1996, 26, 4665–4670. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Tanaka, H.; Takami, N.; Ogawa, A.; Ryu, I.; Sonoda, N. Reduction of Elemental Selenium by Samarium Diiodide: Selective Synthesis of Diorganyl Selenides and Diselenides. Heteroat. Chem. 1991, 2, 427–430. [Google Scholar] [CrossRef]
- Arase, A.; Masuda, Y. A New Route to Dialkyl Selenide from Olefin via Hydroboration. Chem. Lett. 1975, 4, 419–422. [Google Scholar] [CrossRef]
- Magdesieva, N.N.; Vdovin, V.A. Synthesis of 1,3-Dihydrobenzo[c]selenophene. Chem. Heterocycl. Compd. 1972, 8, 22–23. [Google Scholar] [CrossRef]
- Phadnis, P.P.; Wadawale, A.; Priyadarsini, K.I.; Jain, V.K.; Iwaoka, M. Synthesis, characterization, and structure of trans-3,4-dihydroxy-1-selenolane {DHS(OH)2} substituted derivatives. Tetrahedron Lett. 2015, 56, 2293–2296. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).