Analytical Approaches for the Determination of Buprenorphine, Methadone and Their Metabolites in Biological Matrices
Abstract
:1. Introduction
2. Biological Matrices
3. Sample Pretreatment
4. Liquid Chromatography Hyphenated Techniques
5. Gas Chromatography Hyphenated Techniques
6. Other Techniques
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
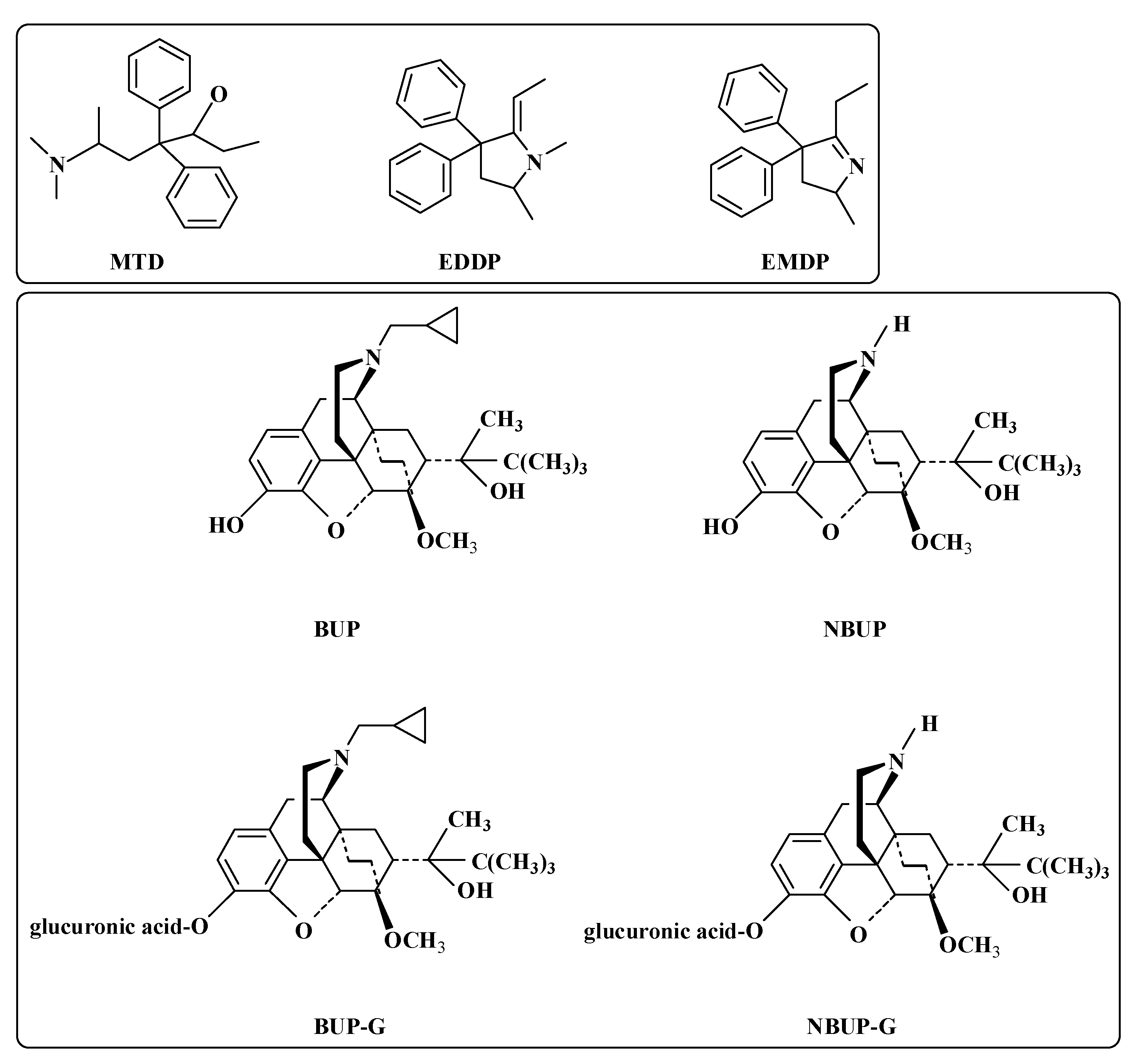
| OUD | Opioid use disorder |
| FDA | Food and Drug Administration |
| BUP | Buprenorphine |
| MTD | Methadone |
| NBUP | Norbuprenorphine |
| BUP-G | Buprenorphine-glucuronide |
| NBUP-G | Norbuprenorphine-glucuronide |
| EDDP | 2-Ethylidene-1, 5-dimethyl-3,3-diphenylpyrrolidine |
| EMDP | 2-Ethylidene-5-methyl-3,3-diphenylpyrrolidine |
| LC-MS/MS | Liquid chromatography tandem mass spectrometry |
| LC | Liquid chromatography |
| GC | Gas chromatography |
| CE | Capillary electrophoresis |
| VH | Vitreous humor |
| UHPLC-MS/MS | Ultra-high-performance liquid chromatography-tandem mass spectrometry |
| LLE | Liquid-liquid extraction |
| SPE | Solid-phase extraction |
| DLLME | Dispersive liquid-liquid microextraction |
| GC-MS | Gas chromatographymass spectrometry |
| NPs | Nanoparticles |
| MIPs | Molecularly imprinted polymers |
| Fe3O4@GO-DES | Fe3O4 nanoparticles/graphene oxide/deep eutectic solvent |
| GC-FID | Gas chromatography-flame ionization detector |
| PF | Preconcentration factor |
| UV | Ultraviolet |
| PAD | Photodiode array detector |
| FL | Fluorescence |
| EC | Electrochemical |
| LDR | Linear dynamic range |
| LOD | Limit of detection |
| DMSPE | Dispersive magnetic solid-phase extraction |
| LOQ | Limit of quantification |
| LLOQ | Lower limit of quantification |
| COC | Cocaine |
| MMT | Methadone maintenance treatment |
| PpPDA/Fe3O4 | Poly para-phenylenediamine modified Fe3O4 NPs |
| MS/MS | Tandem mass spectrometry |
| HRMS | High-resolution mass spectrometry |
| NLX | Naloxone; MTA: Methamphetamine |
| N6-WT | Nylon 6 modified wooden toothpick |
| UADLLME | Ultrasound-assisted DLLME |
| DSS | Dried saliva spots |
| SHS-HLLME | Switchable hydrophilicity solvent-based homogenous liquid-liquid microextraction |
| Fe@GO-DES | Magnetic graphene nanoparticles coated with a new deep eutectic solvent |
| CSDF-ME | Continuous sample drop flow microextraction |
| UA-SM-SFO-ME | Ultrasonic-assisted supramolecular based on solidification of floating organic drop microextraction |
| UA-SM-SFO-ME | Ultrasonic-assisted supramolecular based on solidification of floating organic drop microextraction |
| CS-MNCs | Citrate stabilized magnetic nanocrystals |
| CPE | Carbonpaste electrode |
| MWCNT | Multiwalled carbon nanotubes |
| ELISA | Enzymeimmunoassay |
| SERS | Surface-enhanced Raman spectroscopy |
| TRA | Tramadol |
References
- Ahmed, S.R.; Chand, R.; Kumar, S.; Mittal, N.; Srinivasan, S.; Rajabzadeh, A.R. Recent biosensing advances in the rapid detection of illicit drugs. TrAC Trend Anal. Chem. 2020, 131, 116006. [Google Scholar] [CrossRef]
- Dunn, K.E.; Finan, P.H.; Andrew Tompkins, D.; Strain, E.C. Frequency and correlates of sleep disturbance in methadone and buprenorphine-maintained patients. Addict. Behav. 2018, 76, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Strang, J.; Volkow, N.D.; Degenhardt, L.; Hickman, M.; Johnson, K.; Koob, G.F.; Marshall, B.D.L.; Walsh, S.L. Opioid use disorder. Nat. Rev. Dis. Primers 2020, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Blanco, C.; Volkow, N.D. Management of opioid use disorder in the USA: Present status and future directions. Lancet 2019, 393, 1760–1772. [Google Scholar] [CrossRef]
- Strand, M.C.; Vindenes, V.; Gjerde, H.; Mørland, J.G.; Ramaekers, J.G. A clinical trial on the acute effects of methadone and buprenorphine on actual driving and cognitive function of healthy volunteers. Br. J. Clin. Pharmacol. 2018, 85, 442–453. [Google Scholar] [CrossRef]
- Mariottini, C.; Gergov, M.; Ojanperä, I. Determination of buprenorphine, norbuprenorphine, naloxone, and their glucuronides in urine by liquid chromatography-tandem mass spectrometry. Drug Test. Anal. 2021, 13, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, I.I.; Nikolaou, P.D.; Athanaselis, S.A.; Pistos, C.M.; Spiliopoulou, C.A.; Maravelias, C.P. Development and validation of a highly sensitive GC/MS method for the determination of buprenorphine and nor-buprenorphine in blood. J. Pharm. Biomed. Anal. 2011, 54, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rosas, M.E.; Lofwall, M.R.; Strain, E.C.; Siluk, D.; Wainer, I.W. Simultaneous determination of buprenorphine, norbuprenorphine and the enantiomers of methadone and its metabolite (EDDP) in human plasma by liquid chromatography/mass spectrometry. J. Chromatogr. B 2007, 850, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Ayman Fareed MD, S.V.M.J. Effect of buprenorphine dose on treatment outcome. J. Addict. Dis. 2012, 31, 8–18. [Google Scholar]
- Seldén, T.; Ahlner, J.; Druid, H.; Kronstrand, R. Toxicological and pathological findings in a series of buprenorphine related deaths. Possible risk factors for fatal outcome. Forensic Sci. Int. 2012, 220, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Tzatzarakis, M.N.; Vakonaki, E.; Kovatsi, L.; Belivanis, S.; Mantsi, M.; Alegakis, A.; Tsatsakis, A.M. Determination of buprenorphine, norbuprenorphine and naloxone in fingernail clippings and urine of patients under opioid substitution therapy. J. Anal. Toxicol. 2015, 39, 313–320. [Google Scholar] [CrossRef]
- Joshi, A.; Parris, B.; Liu, Y.; Heidbreder, C.; Gerk, P.M.; Halquist, M. Quantitative determination of buprenorphine, naloxone and their metabolites in rat plasma using hydrophilic interaction liquid chromatography coupled with tandem mass spectrometry. Biomed. Chromatogr. 2017, 31, e3785. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, M. Basic pharmacology of buprenorphine. Eur. J. Pain Suppl. 2007, 1, 69–73. [Google Scholar]
- Mueller, F.; Losacco, G.L.; Nicoli, R. Comparison of liquid chromatography and supercritical fluid chromatography coupled to mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2021, 1177, 122755. [Google Scholar] [CrossRef]
- Di Trana, A.; La Maida, N.; Tittarelli, R.; Huestis, M.A.; Pichini, S.; Busardò, F.P.; Carlier, J. Monitoring prenatal exposure to buprenorphine and methadone. Ther. Drug Monit. 2020, 42, 181–193. [Google Scholar] [CrossRef]
- Ribeiro, A.; Prata, M.; Vaz, C.; Rosado, T.; Restolho, J.; Barroso, M.; Gallardo, E. Determination of methadone and EDDP in oral fluid using the dried saliva spots sampling approach and gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 2177–2187. [Google Scholar] [CrossRef]
- Nowak, K.; Szpot, P.; Jurek, T.; Zawadzki, M. Quantification of methadone and its metabolites: EDDP and EMDP determined in autopsy cases using LC-MS/MS. J. Forensic Sci. 2021, 66, 1003–1012. [Google Scholar] [CrossRef]
- Ahmar, H.; Nejati-Yazdinejad, M.; Najafi, M.; Hasheminasab, K.S. Switchable hydrophilicity solvent-based homogenous liquid–liquid microextraction (SHS-HLLME) combined with GC-FID for the quantification of methadone and tramadol. Chromatographia 2018, 81, 1063–1070. [Google Scholar] [CrossRef]
- Jafarinejad, M.; Ezoddin, M.; Lamei, N.; Abdi, K.; Babhadi Ashar, N.; Pirooznia, N.; Akhgari, M. Effervescent tablet-assisted demulsified dispersive liquid–liquid microextraction based on solidification of floating organic droplet for determination of methadone in water and biological samples prior to GC-flame ionization and GC-MS. J. Sep. Sci. 2020, 43, 3266–3274. [Google Scholar] [CrossRef]
- Nedaei, M.; Abdi, K.; Ghorbanian, S.A.; Pirooznia, N. Ultrasonic-air-assisted solidification of settled organic drop microextraction using terpene-based deep eutectic solvents for the effectual enrichment of methadone in biological samples. Chromatographia 2020, 83, 1413–1421. [Google Scholar] [CrossRef]
- Forouzesh, M.; Valipour, R.; Shekari, A.; Barzegar, A.; Setareh, M. Comparison of methadone level measurement by enzyme immunoassay with gas chromatography-mass spectrometry. J. Adv. Pharm. Educ. Res. 2019, 9, 105–108. [Google Scholar]
- Muñoz-Muñoz, A.C.; Pekol, T.; Awad, A.; Hackett, P.; Sullivan, L.; Rodrigues, A.; Andrade, L. Norbuprenorphine interferences in urine drug testing LC–MS/MS confirmation methods from quetiapine metabolites. J. Anal. Toxicol. 2021, bkab113. [Google Scholar] [CrossRef] [PubMed]
- Da, C.K.; Rodrigues, L.C.; Huestis, M.A.; Costa, J.L. Miniaturized extraction method for analysis of synthetic opioids in urine by microextraction with packed sorbent and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1624, 461241. [Google Scholar]
- Dahlin, J.L.; Palte, M.J.; LaMacchia, J.; Petrides, A.K. A rapid dilute-and-shoot UPLC-MS/MS assay to simultaneously measure 37 drugs and related metabolites in human urine for use in clinical pain management. J. Appl. Lab. Med. 2019, 3, 974–992. [Google Scholar] [CrossRef]
- Agostini, M.; Renzoni, C.; Pierini, E.; Piergiovanni, M.; Termopoli, V.; Famiglini, G.; Cappiello, A. Rapid, hydrolysis-free, dilute-and-shoot method for the determination of buprenorphine, norbuprenorphine and their glucuronides in urine samples using UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2019, 166, 236–243. [Google Scholar] [CrossRef]
- Musile, G.; Cenci, L.; Piletska, E.; Gottardo, R.; Bossi, A.M.; Bortolotti, F. Development of an in-house mixed-mode solid-phase extraction for the determination of 16 basic drugs in urine by High Performance Liquid Chromatography-Ion Trap Mass Spectrometry. J. Chromatogr. A 2018, 1560, 10–18. [Google Scholar] [CrossRef]
- Suzuki, J.; Zinser, J.; Issa, M.; Rodriguez, C. Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst. Abus. 2017, 38, 504–507. [Google Scholar] [CrossRef]
- Hatefi-Mehrjerdi, A.; Boldaji, R.; Yaftian, M.R.; Shayani-Jam, H. Anion-doped overoxidized polypyrrole/multiwalled carbon nanotubes modified glassy carbon electrode as a new electrochemical sensing platform for buprenorphine opioid drug. Iran. J. Anal. Chem. 2021, 8, 56–64. [Google Scholar]
- Khorablou, Z.; Shahdost-Fard, F.; Razmi, H. Flexible and highly sensitive methadone sensor based on gold nanoparticles/polythiophene modified carbon cloth platform. Sens. Actuators B 2021, 344, 130284. [Google Scholar] [CrossRef]
- Akbari, S.; Jahani, S.; Foroughi, M.M.; Nadiki, H.H. Simultaneous determination of methadone and morphine at a modified electrode with 3D b-MnO2 nanoflowers: Application for pharmaceutical sample analysis. RSC Adv. 2020, 10, 38532–38545. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, N.; Chan, N.W.C.; Lee, W.E.; Jemere, A.B. Electrochemical determination of naloxone using molecularly imprinted poly(para-phenylenediamine) sensor. J. Electrochem. Soc. 2020, 167, 137508. [Google Scholar] [CrossRef]
- Yousefi, N.; Irandoust, M.; Haghighi, M. New and sensitive magnetic carbon paste electrode for voltammetry determination of morphine and methadone. J. Iran. Chem. Soc. 2020, 17, 2909–2922. [Google Scholar] [CrossRef]
- Alizadeh, T.; Atashi, F.; Akhoundian, M.; Ganjali, M.R. Highly selective extraction and voltammetric determination of the opioid drug buprenorphine via a carbon paste electrode impregnated with nano-sized molecularly imprinted polymer. Microchim. Acta 2019, 186, 654. [Google Scholar] [CrossRef]
- Karim Nezhad Ghasem, K.Z. Voltammetric Determination of methadone at stacked cysteic acid film and gold nanoparticles composite modified glassy carbon electrode. Anal. Bioanal. Electrochem. 2017, 9, 689–703. [Google Scholar]
- Hosseini, M.; Pur, M.; Norouzi, P.; Moghaddam, M.R.; Ganjali, M.R. An enhanced electrochemiluminescence sensor modified with a Ru(bpy)3(2+)/Yb2O3 nanoparticle/nafion composite for the analysis of methadone samples. Mater. Sci. Eng. C 2017, 76, 483–489. [Google Scholar] [CrossRef]
- Mohammadiazar, S.; Hasanli, F.; Maham, M.; Payami Samarin, S. Solid-phase microextraction of methadone in urine samples by electrochemically co-deposited sol-gel/Cu nanocomposite fiber. Biomed. Chromatogr. 2017, 31, e3926. [Google Scholar] [CrossRef]
- Rezaei, B.; Tajaddodi, A.; Ensafi, A.A. An innovative highly-sensitive sensor based on carbon quantum dots and multiwall carbon nanotubes for electrochemical purposes and determination of methadone hydrochloride in real samples. Anal. Methods 2020, 12, 5210–5218. [Google Scholar] [CrossRef]
- Akhgari, M.; Sani, N.M.; Mousavi, Z. Research Paper: Determination of methadone and tramadol in vitreous humor specimens using dispersive liquid liquid microextraction and ultra high performance liquid chromatography. Int. J. Med. Toxicol. Forensic Med. 2021, 11, 31530. [Google Scholar] [CrossRef]
- Mohammadi, F.; Shabani, A.M.H.; Dadfarnia, S.; Ansari, M.; Asgharinezhad, A.A. Dispersive solid-phase extraction of buprenorphine from biological fluids using metal-organic frameworks and its determination by ultra-performance liquid chromatography. J. Sep. Sci. 2020, 43, 3045–3052. [Google Scholar] [CrossRef]
- Abbasi, S.; Haeri, S.A.; Sajjadifar, S. Bio-dispersive liquid liquid microextraction based on nano rhamnolipid aggregates combined with molecularly imprinted-solid phase extraction for selective determination of paracetamol in human urine samples followed by HPLC. Microchem. J. 2019, 146, 106–114. [Google Scholar] [CrossRef]
- Ganjavi, F.; Ansari, M.; Kazemipour, M.; Zeidabadinejad, L. Computational design, synthesis and utilization of a magnetic molecularly imprinted polymer on graphene oxide nanosheets for highly selective extraction and determination of buprenorphine in biological fluids and tablets. Anal. Methods 2018, 10, 5214–5226. [Google Scholar] [CrossRef]
- Ganjavi, F.; Ansari, M.; Kazemipour, M.; Zeidabadinejad, L. Computer-aided design and synthesis of a highly selective molecularly imprinted polymer for the extraction and determination of buprenorphine in biological fluids. J. Sep. Sci. 2017, 40, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Adlnasab, L.; Shahdousti, P.; Ahmar, H. Layered double hydroxide intercalated with tyrosine for ultrasonic-assisted microextraction of tramadol and methadone from biological samples followed by GC/MS analysis. Microchim. Acta. 2020, 187, 265. [Google Scholar] [CrossRef] [PubMed]
- Lamei, N.; Ezoddin, M.; Ardestani, M.S.; Abdi, K. Dispersion of magnetic graphene oxide nanoparticles coated with a deep eutectic solvent using ultrasound assistance for preconcentration of methadone in biological and water samples followed by GC–FID and GC–MS. Anal. Bioanal. Chem. 2017, 409, 6113–6121. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, E.; Fakhari, A.R. Simultaneous chiral separation of tramadol and methadone in tablets, human urine, and plasma by capillary electrophoresis using maltodextrin as the chiral selector. Chirality 2018, 30, 1161–1168. [Google Scholar] [CrossRef]
- Abedi, H. Solid-phase microextraction of methadone by using a chitosan nanocomposite incorporated with Polyoxomolibdate nanocluster/Graphene oxide. J. Sep. Sci. 2021, 44, 1969–1977. [Google Scholar] [CrossRef]
- Jamil, L. Optimization of New Sample preparation technique for the determination of methadone and codeine in plasma sample by GC-FID. J. Braz. Chem. Soc. 2020, 31, 580–588. [Google Scholar] [CrossRef]
- Metzger, I.F.; Thomas, A.E.; Evrard, C.A.; Jones, D.R.; Masters, A.R.; Haas, D.M.; Quinney, S.K. Stereoselective analysis of methadone and EDDP in laboring women and neonates in plasma and dried blood spots and association with neonatal abstinence syndrome. Am. J. Perinat. 2021, 38, 968–975. [Google Scholar] [CrossRef]
- Tikhomirov, M.; Poźniak, B.; Śniegocki, T. High-performance liquid chromatography-tandem mass spectrometry for buprenorphine evaluation in plasma—Application to pharmacokinetic studies in rabbits. Molecules 2021, 26, 437. [Google Scholar] [CrossRef]
- Kongstad, K.T.; Papathanasiou, T.; Springborg, A.D.; Lund, T.M.; Werner, M.U.; Staerk, D. Simultaneous quantification of high-dose naloxone and naloxone-3-β-d -glucuronide in human plasma by UHPLC–MS/MS. Bioanalysis 2019, 11, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Xu, A.; Nasser, A.F.; Heidbreder, C. Simultaneous determination of buprenorphine, norbuprenorphine and naloxone in human plasma by liquid chromatography/tandem mass spectrometry. J. Pharm. Biomed. Anal. 2016, 120, 142–152. [Google Scholar] [CrossRef]
- Theurillat, R.; Sandbaumhuter, F.A.; Gittel, C.; Larenza, M.M.; Braun, C.; Thormann, W. Enantioselective capillary electrophoresis for pharmacokinetic analysis of methadone and 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine in equines anesthetized with ketamine and isoflurane. Electrophoresis 2019, 40, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Gomar, F.; Afkhami, A.; Madrakian, T. Highly sensitive simultaneous quantification of buprenorphine and norbuprenorphine in human plasma by magnetic solid-phase extraction based on PpPDA/Fe3O4 nanocomposite and high-performance liquid chromatography. J. Iran. Chem. Soc. 2018, 15, 575–585. [Google Scholar] [CrossRef]
- Ezoddin, M.; Adlnasab, L.; Kaveh, A.A.; Karimi, M.A. Ultrasonically formation of supramolecular based ultrasound energy assisted solidification of floating organic drop microextraction for preconcentration of methadone in human plasma and saliva samples prior to gas chromatography—Mass spectrometry. Ultrason. Sonochem. 2019, 50, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Oliveto, A.; Mancino, M.J.; Hendrickson, H.P. Development and validation of a rapid liquid chromatography/tandem mass spectrometry method to quantitate gabapentin and buprenorphine in human serum. Rapid Commun. Mass Spectrom. 2021, 35, 9104. [Google Scholar] [CrossRef] [PubMed]
- Baghayeri, M.; Nabavi, S.; Hasheminejad, E.; Ebrahimi, V. Introducing an electrochemical sensor based on two layers of Ag nanoparticles decorated graphene for rapid determination of methadone in human blood serum. Top. Catal. 2022, 65, 623–632. [Google Scholar] [CrossRef]
- Jahanbakhshi, M. In situ synthesis of rhodium nanoparticles—Mesoporous carbon hybrid via a novel and facile nanocasting method for simultaneous determination of morphine and buprenorphine. Mater. Sci. Eng. C 2019, 97, 479–485. [Google Scholar] [CrossRef]
- Kokubun, H.; Takigawa, C.; Miyano, K.; Uezono, Y. A novel method for determination of methadone in the serum by high-performance liquid chromatography with electrochemical detection. Biol. Pharm. Bull. 2018, 41, 649–651. [Google Scholar] [CrossRef]
- Orfanidis, A.; Gika, H.G.; Theodoridis, G.; Mastrogianni, O.; Raikos, N. A UHPLC-MS-MS method for the determination of 84 drugs of abuse and pharmaceuticals in blood. J. Anal. Toxicol. 2021, 45, 28–43. [Google Scholar] [CrossRef]
- Feliu, C.; Konecki, C.; Binet, L.; Vautier, D.; Haudecoeur, C.; Oget, O.; Djerada, Z. Quantification of methadone, buprenorphine, naloxone, opioids, and their derivates in whole blood by liquid chromatography-high-resolution mass spectrometry: Analysis of their involvement in fatal forensic cases. J. Chromatogr. B 2020, 1152, 122226. [Google Scholar] [CrossRef] [PubMed]
- Davari, B.; Kotecha, N.; Clavijo, C.F.; Thomas, J.J.; Rzasa-Lynn, R.; Galinkin, J.L.; Christians, U.; Sempio, C. A sensitive LC-MS/MS assay for the quantification of methadone and its metabolites in dried blood spots: Comparison with plasma. Ther. Drug Monit. 2020, 42, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Yahyapour, M.; Ranjbar, M.; Mohadesi, A.; Rejaeinegad, M. Determination of buprenorphine (BUP) with molecularly imprinted polymer Zn/La3+ metal organic framework on modified glassy carbon electrode (GCE). Electroanalysis 2022, 34, 1012–1020. [Google Scholar] [CrossRef]
- Magalhaes, T.P.; Cravo, S.; Silva, D.; Dinis-Oliveira, R.J.; Afonso, C.; Lourdes, B.M.; Carmo, H. Quantification of Methadone and Main Metabolites in Nails. J. Anal. Toxicol. 2018, 42, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Millán-Santiago, J.; García-Valverde, M.T.; Lucena, R.; Cárdenas, S. Polyamide-coated wooden tips coupled to direct infusion mass spectrometry, a high throughput alternative for the determination of methadone, cocaine and methamphetamine in oral fluid. Microchem. J. 2021, 162, 105843. [Google Scholar] [CrossRef]
- Bassotti, E.; Merone, G.M.; D Urso, A.; Savini, F.; Locatelli, M.; Tartaglia, A.; de Grazia, U. A new LC-MS/MS confirmation method for the determination of 17 drugs of abuse in oral fluid and its application to real samples. Forensic Sci. Int. 2020, 312, 110330. [Google Scholar] [CrossRef]
- Fernández, P.; Regenjo, M.; Ares, A.; Fernández, A.M.; Lorenzo, R.A.; Carro, A.M. Simultaneous determination of 20 drugs of abuse in oral fluid using ultrasound-assisted dispersive liquid–liquid microextraction. Anal. Bioanal. Chem. 2019, 411, 193–203. [Google Scholar] [CrossRef]
- Truver, M.T.; Swortwood, M.J. Quantitative analysis of novel synthetic opioids, morphine and buprenorphine in oral fluid by LC–MS-MS. J. Anal. Toxicol. 2018, 42, 554–561. [Google Scholar] [CrossRef]
- Ares, A.M.; Fernández, P.; Regenjo, M.; Fernández, A.M.; Carro, A.M.; Lorenzo, R.A. A fast bioanalytical method based on microextraction by packed sorbent and UPLC–MS/MS for determining new psychoactive substances in oral fluid. Talanta 2017, 174, 454–461. [Google Scholar] [CrossRef]
- Vincenti, F.; Montesano, C.; Cellucci, L.; Gregori, A.; Fanti, F.; Compagnone, D.; Sergi, M. Combination of pressurized liquid extraction with dispersive liquid liquid micro extraction for the determination of sixty drugs of abuse in hair. J. Chromatogr. A 2019, 1605, 360348. [Google Scholar] [CrossRef]
- Rosado, T.; Gallardo, E.; Vieira, D.N.; Barroso, M. Microextraction by packed sorbent as a novel strategy for sample clean-up in the determination of methadone and EDDP in hair. J. Anal. Toxicol. 2020, 44, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Anzillotti, L.; Calò, L.; Giacalone, M.; Banchini, A.; Cecchi, R. Determination of methadone and eight new psychoactive substances in hair samples by gas chromatography/mass spectrometry. J. Forensic. Sci. Med. 2018, 4, 184. [Google Scholar] [CrossRef]
- Vandenbosch, M.; Somers, T.; Cuypers, E. Distribution of methadone and metabolites in skeletal tissue. J. Anal. Toxicol. 2018, 42, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Havig, S.M.; Vindenes, V.; Oiestad, A.; Rogde, S.; Thaulow, C.H. Methadone, buprenorphine, oxycodone, fentanyl, and tramadol in multiple postmortem matrices. J. Anal. Toxicol. 2022, 46, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Sandqvist, S.; Beck, O. A liquid chromatography and tandem mass spectrometry method to determine 28 non-volatile drugs of abuse in exhaled breath. J. Pharm. Biomed. Anal. 2018, 148, 251–258. [Google Scholar] [CrossRef]
- Hamidi, S.; Khoubnasabjafari, M.; Ansarin, K.; Jouyban-Gharamaleki, V.; Jouyban, A. Chiral separation of methadone in exhaled breath condensate using capillary electrophoresis. Anal. Methods 2017, 9, 2342–2350. [Google Scholar] [CrossRef]
- Pérez-Alcaraz, A.; Borrull, F.; Calull, M.; Aguilar, C. Cathinones in urine samples: A review of recent advances for their determination by chromatographic and related techniques. TrAC Trends Anal. Chem. 2021, 143, 116347. [Google Scholar] [CrossRef]
- Rivier, L. Techniques for analytical testing of unconventional samples. Best Pract. Res. Clin. Endocrinol. Metab. 2000, 14, 147–165. [Google Scholar] [CrossRef]
- Langel, K.; Gjerde, H.; Favretto, D.; Lillsunde, P.; Øiestad, E.L.; Ferrara, S.D.; Verstraete, A.G. Comparison of drug concentrations between whole blood and oral fluid. Drug Test. Anal. 2014, 6, 461–471. [Google Scholar] [CrossRef]
- Silveira, G.D.O.; Pego, A.M.F.; Pereira E Silva, J.; Yonamine, M. Green sample preparations for the bioanalysis of drugs of abuse in complex matrices. Bioanalysis 2019, 11, 295–312. [Google Scholar] [CrossRef]
- Dutkiewicz, E.P.; Urban, P.L. Quantitative mass spectrometry of unconventional human biological matrices. Philos. Trans. R. Soc. A 2016, 374, 20150380. [Google Scholar] [CrossRef] [PubMed]
- Boumba, V.; Ziavrou, K.; Vougiouklakis, T. Hair as a Biological Indicator of Drug Use, Drug Abuse or Chronic Exposure to Environmental Toxicants. Int. J. Toxicol. 2006, 25, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, T.; Ghimenti, S.; Biagini, D.; Bramanti, E.; Onor, M.; Bellagambi, F.; Fuoco, R.; Di Francesco, F. The effect of sampling procedures on the urate and lactate concentration in oral fluid. Microchem. J. 2018, 136, 255–262. [Google Scholar] [CrossRef]
- Bévalot, F.; Cartiser, N.; Bottinelli, C.; Fanton, L.; Guitton, J. Vitreous humor analysis for the detection of xenobiotics in forensic toxicology: A review. Forensic Toxicol. 2015, 34, 12–40. [Google Scholar] [CrossRef]
- Giordano, G.; Biehler-Gomez, L.; Seneci, P.; Cattaneo, C.; Di Candia, D. Detecting drugs in dry bone: A pilot study of skeletal remains with a post-mortem interval over 23 years. Int. J. Legal Med. 2021, 135, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Tufvesson, E.; Bjermer, L. Methodological improvements for measuring eicosanoids and cytokines in exhaled breath condensate. Resp. Med. 2006, 100, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Mastroianni, N.; Postigo, C.; Valcarcel, Y.; Gonzalez-Alonso, S.; Barcelo, D.; Lopez, D.A.M. Simultaneous LC-MS/MS determination of 40 legal and illegal psychoactive drugs in breast and bovine milk. Food Chem. 2018, 245, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Shekari, A.; Valipour, R.; Setareh, M.; Soltaninejad, K. Research Paper: Ultrasound-assisted liquid-liquid extraction for analyzing methadone in urine samples by gas chromatography-mass spectrometry. Int. J. Med. Toxicol. Forensic Med. 2020, 10, 29457. [Google Scholar] [CrossRef]
- Shekari, A.; Forouzesh, M.; Valipour, R.; Fallah, F.; Shojaei, P. Validation and optimization of ultrasound-assisted dispersive liquid-liquid microextraction as a preparation method for detection of methadone in saliva with gas chromatography-mass spectrometry technique. Adv. Pharm. Bull. 2020, 10, 329–333. [Google Scholar] [CrossRef]
- Chan, K.W.; Harun, H. Liquid chromatography tandem mass spectrometric method validation for the quantification of buprenorphine and norbuprenorphine in whole blood. Aust. J. Forensic Sci. 2017, 49, 186–200. [Google Scholar] [CrossRef]
- Lin, Z.; Li, J.; Zhang, X.; Qiu, M.; Huang, Z.; Rao, Y. Ultrasound-assisted dispersive liquid-liquid microextraction for the determination of seven recreational drugs in human whole blood using gas chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1046, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Farquharson, S.; Dana, K.; Shende, C.; Gladding, Z.; Newcomb, J.; Dascher, J.; Arias, A.J. Rapid identification of buprenorphine in patient saliva. J. Anal. Bioanal. Technol. 2017, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Prosen, H.; Fontanals, N.; Borrull, F.; Marcé, R.M. Determination of seven drugs of abuse and their metabolites in surface and wastewater using solid-phase extraction coupled to liquid chromatography with high-resolution mass spectrometry. J. Sep. Sci. 2017, 40, 3621–3631. [Google Scholar] [CrossRef] [PubMed]
- Habibi, B.; Rostamkhani, S.; Hamidi, M. Magnetic molecularly imprinted polymer nanoparticles for dispersive micro solid-phase extraction and determination of buprenorphine in human urine samples by HPLC-FL. J. Iran. Chem. Soc. 2018, 15, 1569–1580. [Google Scholar] [CrossRef]
- Farmany, A.; Shamsara, M.; Mahdavi, H. Enhanced electrochemical biosensing of buprenorphine opioid drugby highly stabilized magnetic nanocrystals. Sens. Actuators B 2017, 239, 279–285. [Google Scholar] [CrossRef]
- Cui, X.; Ni, C.; Liang, C.; Gong, F.; Wang, R.; Chen, G.; Zhang, Y. Screening and quantitation of forty-six drugs of abuse and toxic compounds in human whole blood by capillary electrophoresis: Application to forensic cases. Microchem. J. 2019, 144, 403–410. [Google Scholar] [CrossRef]
| Target Analytes | Matrices | Techniques | Extraction | Mobile Phase | LOD (LOQ or LLOQ) | Ref. |
|---|---|---|---|---|---|---|
| BUP, NAL, their metabolites | Urine | LC-MS/MS (Gemini-NX C18, 100 mm × 2.1 mm, 4 μm; C18 guard column, 4 mm × 2 mm) | SPE | Methanol and ammonium acetate buffer (both containing 0.1% formic acid) | BUP: 0.3 µg/L, NAL: 0.5 µg/L, NAL-G: 1 µg/L, NBUP: 1 µg/L, BUP-G: 0.3 µg/L, NBUP-G: 1 µg/L | [7] |
| BUP, NAL, their metabolites | Plasma | LC-MS/MS (Thermo HILIC, 100 mm × 2.1 mm, 3.5 μm) | LLE | 60% MeCN and 40% aqueous 25m M ammonium formate (pH 3.5) | / | [13] |
| MTD, EDDP | Post-mortem samples | LC-MS/MS SFC-MS/MS (AGP, 100 mm × 2.1 mm, 5 μm; 10 × 2.0 mm; 5 μm) | SPE | 10 mM Ammonium acetate(pH 5.8) and isopropanol | MTD: 2.5 µg/L in LC, 0.5 µg/L in SFC | [15] |
| MTD, EDDP, EMDP | Postmortem Matrices | LC-MS/MS (Kinetex XB-C18, 150 mm × 2.1 mm, 2.6 μm) | LLE | / | LLOQ: MTD: 0.5 µg/L, EDDP: 0.5 µg/L, EMDP: 0.5 µg/L | [18] |
| BUP, NBUP, NAL | Urine | LC-MS/MS (CORTECS Phenyl, 50 mm × 2.1 mm, 1.6 μm) | SPE | 0.05% Formic acid in water and 0.1% formic acid in acetonitrile | LLOQ: NBUP: 5 µg/L | [23] |
| MTD, BUP and other durgs | Urine | UHPLC-MS/MS (HSS T3, 100 mm × 2.1 mm, 1.8 μm) | LLE | Methanol and 5 mM ammonium acetate containing 0.025% formic acid in water | BUP: 2 µg/L, NBUP: 2 µg/L, MTD: 1 µg/L, EDDP: 0.5 µg/L | [25] |
| BUP, NBUP and their metabolites | Urine | UHPLC-MS/MS (PFP, 50 mm × 2.1 mm, 1.9 μm) | / | 95% ACN with 0.1% formic acid and 5% formic acid | BUP: 0.5 µg/L, NBUP: 1.5 µg/L, NBUP-G: 0.5 µg/L, BUP-G: 1.0 µg/L | [26] |
| 16 Drugs | Urine | LC-MS (XDB C18, 150 mm × 2.1 mm, 5 μm) | SPE | Ultra-pure water/0.1% HCOOH and MeOH/0.1% HCOOH | MTD: 5 µg/L, EDDP: 20 µg/L | [27] |
| BUP, NBUP | Urine | LC-MS/MS | / | / | BUP: 0.5µg/L, NBUP: 0.5µg/L | [28] |
| MTD, tramadol | Vitreous Humor | UPLC-PDA (C18, 150 mm × 3 mm) | DLLME | Phosphate buffer (pH = 2.32) and acetonitrile | MTD: 3 µg/L | [39] |
| BUP | Serum | UPLC-PDA/UV (C18, 250 mm × 4.6 mm, 5 μm) | SPE | 95% Methanol and 5% deionized water containing 4 mM 1-octane sulfonic acid | 0.15 µg/L | [40] |
| BUP | Plasma, urine, tablets | LC-UV (ODS-H C18, 150 mm × 4.6 mm, 5 μm) | MSPE | 0.01 M Phosphate buffer (pH 3.1) and acetonitrile | 0.6 µg/L | [42] |
| BUP | Plasma, urine | LC-UV (ODS-H C18, 150 mm × 4.6 mm, 5 μm) | SPE | Acetonitrile and 0.01 M phosphate buffer with pH 3.1 | 3 µg/L | [43] |
| MTD, EDDP | Dried blood spots | LC-MS/MS (Chiral-AGP, 150 mm × 4.6 mm, 5 μm) | LLE | Acetonitrile (gradientfrom10 to34%) in 0.1% formic acid (pH 6.5) | / | [49] |
| BUP | Plasma | LC-MS/MS (XB-C18, 50 mm × 2.1 mm, 2.6 μm) | LLE | 0.1% Formic acid and methanol | 0.25 µg/L | [50] |
| BUP, NBUP | Plasma | LC-UV (Nova-pak C18, 250 mm × 4.6 mm, 5 μm) | MSPE | Phosphate buffer (pH 3.4) and acetonitrile | BUP: 0.8 µg/L, NBUP: 0.3 µg/L | [54] |
| BUP, gabapentin | Serum | LC-MS/MS (Biphenyl 100Å, 50 mm × 2.1 mm, 5 μm) | LLE | 10 mM Ammonium formate and methanol containing 0.1% formic acid | BUP: 1 µg/L | [56] |
| MTD | Serum | LC-ECD (RP18, 50 mm × 4.6 mm, 5 μm) | LLE | 10 mM Na2HPO4, CH3CN and CH3OH | 0.5 µg/L | [59] |
| MTD, BUP and their metabolites | Blood | UHPLC-MS-MS (BEH C18, 150 mm × 2.1 mm, 1.7 μm) | LLE | 0.1% Formic acid in water and 0.1% formic acid in methanol | MTD: 0.41 µg/L, EDDP: 1.41 µg/L, BUP: 0.59 µg/L, NBUP: 0.66 µg/L, | [61] |
| MTD, BUP, EDDP and other opioids | Whole blood | UPLC-HRMS (HSS T3, 50 mm × 2.1 mm, 1.8 μm) | LLE | 0.1% Formic acid in water and 0.1% formic acid in acetonitrile | BUP: 0.15 µg/L, NBUP: 0.1 µg/L, MTD: 0.5 µg/L, EDDP: 0.5 µg/L | [62] |
| MTD, EDDP | Dried blood spots and plasma | LC-MS/MS (Eclipse XDB, 12.5 mm × 4.6 mm, 5 μm) | / | 0.1% Formic acid in water and methanol | LLOQ: MTD: 0.1 µg/L, EDDP: 0.1 µg/L, EMDP: 0.1 µg/L | [63] |
| MTD, COC, methamphetamine | Oral fluid | LC-MS/MS | SPME | 0.1% Ammonium formate aqueous solution | MTD: 1.5 µg/L | [66] |
| MTD, EDDP and other 15 drugs | Oral fluid | LC-MS/MS (Hypersil PFP, 50 mm × 2.1 mm, 1.9 μm) | / | 0.1% Formic acid in water and 0.1% formic acid in methanol/AcN | / | [67] |
| 20 Drugs | Oral fluid | UPLC-MS/MS (RP 18, 100 mm × 2.1 mm, 1.7 μm) | UADLLME | 0.1% Formic acid in water and 0.1% formic acid in acetonitrile | BUP: 1 µg/L, MTD: 0.1 µg/L, EDDP: 0.5 µg/L | [68] |
| Novel synthetic opioids, morphine and BUP | Oral fluid | LC-MS/MS (EC-C18, 100 mm × 3.0 mm, 2.7 μm; 2.1 mm× 5.0 mm, 2.7 μm) | SPE | 0.05% Formic acid, 5mM ammonium formate in water and 0.1% formic acid in acetonitrile | BUP: 5 µg/L | [69] |
| 21 Drugs | Oral fluid | UHPLC-MS/MS (RP 18, 100 mm × 2.1 mm, 1.7 μm) | MEPS | 0.1% Formic acid in water and 0.1% formic acid in acetonitrile | LOQs: 0.5–1 µg/L | [70] |
| 60 Drugs | Hair | UHPLC-HRMS/MS (PFP, 100 mm × 2.1 mm, 2.6 μm) | DLLME | 0.1% Formic acid in water and 0.1% formic acid in ace tonitrile/methanol | BUP: 2 pg/mg, NBUP: 2 pg/mg, MTD: 0.2 µg/L, EDDP: 0.5 pg/mg | [71] |
| MTD, EDDP, EMDP | Skeletal tissue | LC-MS/MS (BEH C18, 50 mm × 2.1 mm, 1.7 μm) | LLE | An aqueous buffer (pH 4) and acetonitrile | MTD: 0.1 ng/g, EDDP: 0.17 ng/g, EMDP: 0.11 ng/g | [74] |
| BUP, MTD, oxycodone, fentanyl, tramadol | Postmortem Matrices | UPLC-MS/MS | LLE | / | LLOQ: MTD: 0.011µg/mL, BUP: 0.94 µg/L | [75] |
| 28 Drugs | Exhaled breath | LC-MS/MS (BEH phenyl, 100 mm × 2.1 mm, 1.7 μm) | LLE | 5% Methanol in water with 4 mM ammonium formate and 5% methanol in water with 0.1% ammonia | MTD: 1.2 pg/filter, EDDP: 0.5 pg/filter, BUP: 4 pg/filter, NBUP: 10 pg/filter | [76] |
| 40 Drugs | Breast milk | LC-MS/MS (RP18, 125 mm × 2.0 mm, 5 μm) | LLE | Acetonitrile and water containing 20 mM formic acid/ammonium formate buffer (pH 3.8) | MTD: 0.5 µg/L, EDDP: 0.2 µg/L | [88] |
| BUP, NBUP | Whole blood | LC-MS/MS | LLE | 0.1% Formic acid in acetonitrile, methanol and 0.1% formic acid in water | BUP: 4.4 µg/L, NBUP: 3.4 µg/L | [96] |
| Target Analytes | Matrices | Techniques | Extraction | LOD (LOQ or LLOQ) | Ref. |
|---|---|---|---|---|---|
| MTD, EDDP | Oral fluid | GC-MS/MS (30 m × 0.25 mm, 0.25 μm) | LLE | MTD: 5 µg/L EDDP: 5 µg/L | [17] |
| MTD, TRM | Urine | GC-FID (HP-5, 30 m × 0.25 mm, 0.25 μm) | LLME | MTD: 2.4 µg/L | [19] |
| MTD | Urine, plasma, saliva | GC-FID/MS (DB 5-ms, 30 m × 0.25 mm, 0.25 μm) | DLLME | GC-FID: Urine: 2.7 µg/L Plasma, saliva: 9.5 µg/L GC-MS: Urine: 0.06 µg/L Plasma, saliva: 0.2 µg/L | [20] |
| MTD | Plasma, urine, saliva | GC-FID (HP-5, 30 m × 0.32 mm, 0.25 μm) | / | Urine: 0.5 µg/L, Plasma: 0.7 µg/L Saliva: 1.5 µg/L | [21] |
| MTD, TRM | Urine, plasma, saliva | GC-MS (HP-5, 30 m × 0.25 mm, 0.25 μm) | SPE | Urine MTD: 0.45 µg/L MTD: 2.5 µg/L MTD: 0.8 µg/L | [44] |
| MTD | Urine, plasma | GC-FID/MS (HP-5, 30 m × 0.25 mm, 0.25 μm) | MSPE | GC-FID: MTD: 0.8 µg/L GC-MS: MTD: 0.03 µg/L | [45] |
| MTD, COD | Plasma | GC-FID (BP-5, 30 m × 0.25 mm, 0.25 μm) | LLE | MTD: 15 µg/L | [48] |
| MTD | Plasma and saliva | GC-MS (HP-5, 30 m × 0.32 mm, 0.25 μm) | UA-SM-SFO-ME | Plasma: 1.2 µg/L Saliva: 0.7 µg/L | [55] |
| MTD | Nail | GC-MS (VF-5ms, 30 m × 0.32 mm, 0.25 μm) | LLE and SPE | MTD: 3.3 ng/mg EDDP: 6.0 ng/mg EMDP: 6.0 ng/mg | [64] |
| MTD, EDDP | Hair | GC-MS/MS (Capillary Column, 30 m × 0.25 mm, 0.25 μm) | MEPS | LLOQ: MTD: 0.01 ng/mg EDDP: 0.01 ng/mg | [71] |
| MTD, EDDP, 8 new psychoactive substances (NPS) | Hair | GC-MS (DB-5, 30 m × 0.25 mm, 0.25 μm) | LLE | MTD: 0.2 ng/mg EDDP: 0.05 ng/mg | [72] |
| MTD | Urine | GC-MS (HP-5MS, 30 m × 0.25 mm, 0.25 μm) | UALLE | 2.1 µg/L | [88] |
| MTD | Saliva | GC-MS (HP-5MS, 30 m × 0.25 mm, 0.25 μm) | UADLLME | 50 µg/L | [89] |
| 7 recreational drugs | Whole blood | GC-MS (HP-5MS, 30 m × 0.25 mm, 0.25 μm) | UADLLME | MTD: 10 µg/L | [91] |
| Analytical Techniques | Target Analytes | Matrices | Sample Pretreatment | LOD (LOQ or LLOQ) | Ref. |
|---|---|---|---|---|---|
| Enzyme immunoassay (ELISA) | MTD | Serum | Alkaline extraction with ethyl acetate | 0.18 µg/L | [21] |
| Electrochemical sensor | BUP | Urine | Dilution with PBS | 28 nM | [29] |
| Electrochemical sensor | MTD | Blood serum, urine | Deproteinization with methanol | 14 nM | [30] |
| Electrochemical sensor | MTD, morphine | Blood, urine, saliva | Dilution with PBS | MTD: 5.6 nM | [31] |
| Electrochemical sensor | MTD, morphine | Urine | Dilution with buffer | MTD: 3 nM | [33] |
| Electrochemical sensor | BUP | Urine | Dilution with britton buffer | 0.6 nM | [34] |
| Electrochemical sensor | MTD | Serum, urine | Deproteinization with methanol | 14 nM | [35] |
| Electrochemical sensor | MTD | Urine | Direct immersion-solid phase microextraction | 0.2 µg/L | [37] |
| Electrochemical sensor | MTD | Blood serum, urine | Deproteinization with trichloroacetic acid | 0.03 µM | [38] |
| Capillary electrophoresis | MTD, TRM | Urine, plasma | Dilution with water | MTD: 1.5 µg/L | [46] |
| Capillary electrophoresis | MTD, EDDP | Plasma | LLE with dichloromethane | LOQ: MTD: 25 µg/L EDDP: 2.5 µg/L | [53] |
| Electrochemical sensor | MTD | Blood | Deproteinization with 0.5 M sulfuric acid | 0.12 µM | [57] |
| Surface-enhanced Raman spectroscopy (SERS) | BUP | Saliva | Liquid extraction with dichloromethane | / | [92] |
| Capillary electrophoresis | MTD | Exhaled breath Condensate, serum and urine | LLE with acetonitrile, | LLOQ: 0.15 µg/mL | [76] |
| Electrochemical sensor | BUP | Serum, urine | Deproteinization with methanol | 4.3 nM | [95] |
| Capillary electrophoresis | 46 drugs | Whole blood | SPE with an Oasis HLB column | MTD: 30 µg/L BUP: 30 µg/L | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, X.; Cao, C.; Yang, B. Analytical Approaches for the Determination of Buprenorphine, Methadone and Their Metabolites in Biological Matrices. Molecules 2022, 27, 5211. https://doi.org/10.3390/molecules27165211
Shan X, Cao C, Yang B. Analytical Approaches for the Determination of Buprenorphine, Methadone and Their Metabolites in Biological Matrices. Molecules. 2022; 27(16):5211. https://doi.org/10.3390/molecules27165211
Chicago/Turabian StyleShan, Xiaoyue, Chengjian Cao, and Bingsheng Yang. 2022. "Analytical Approaches for the Determination of Buprenorphine, Methadone and Their Metabolites in Biological Matrices" Molecules 27, no. 16: 5211. https://doi.org/10.3390/molecules27165211
APA StyleShan, X., Cao, C., & Yang, B. (2022). Analytical Approaches for the Determination of Buprenorphine, Methadone and Their Metabolites in Biological Matrices. Molecules, 27(16), 5211. https://doi.org/10.3390/molecules27165211






