Novel Organic Salts Based on Mefloquine: Synthesis, Solubility, Permeability, and In Vitro Activity against Mycobacterium tuberculosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methods
2.1.1. General Procedure for the Water Solubility Studies
2.1.2. General Procedure for the Permeability (P), Diffusion (D), and Partition Coefficient (Kd) Measurements
2.1.3. General Procedure for the Antimycobacterial Activity Studies
2.1.4. General Procedure for the Cytotoxicity Assays
3. Results and Discussion
3.1. Synthesis of the MFL Salts
3.2. Water Solubility and Permeability Studies
3.3. Biological Activity
3.4. Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| AOT | Dioctyl sulfosuccinate |
| API | Active Pharmaceutical Ingredient |
| API–OSILs | Active Pharmaceutical Ingredient Organic Salts and Ionic Liquids |
| BCS | Biopharmaceutics Classification System |
| CSA | Camsylate |
| D | diffusion |
| DSC | Differential Scanning Calorimetry |
| DMSO | Dimethyl sulfoxide |
| FTIR | Fourier Transform Infrared |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| Kd | partition coefficient |
| MFL | mefloquine |
| MFLH | mefloquine cation |
| MsO | mesylate |
| MABA | Micro-plate Alamar Blue assay |
| MDR | multidrug-resistant |
| MIC | Minimum inhibitory concentration |
| NMR | Nuclear Magnetic Resonance |
| OSILs | organic salts and ionic liquids |
| P | permeability |
| PES-U | polyethersulphone |
| RDIC | relative decrease in inhibitory concentrations |
| Sac | saccharinate |
| TsO | tosylate |
| TB | tuberculosis |
| Tg | glass transition temperature |
| Tm | melting temperature |
| US | United States |
| XRD | extensively drug-resistant |
References
- World Health Organization. Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Lange, C.; Dheda, K.; Chesov, D.; Mandalakas, A.M.; Udwadia, Z.; Horsburgh, C.R. Management of Drug-Resistant Tuberculosis. Lancet 2019, 394, 953–966. [Google Scholar] [CrossRef]
- Rao, M.; Ippolito, G.; Mfinanga, S.; Ntoumi, F.; Yeboah-Manu, D.; Vilaplana, C.; Zumla, A.; Maeurer, M. Improving Treatment Outcomes for MDR-TB—Novel Host-Directed Therapies and Personalised Medicine of the Future. Int. J. Infect. Dis. 2019, 80, S62–S67. [Google Scholar] [CrossRef] [PubMed]
- Nahid, P.; Mase, S.R.; Migliori, G.B.; Sotgiu, G.; Bothamley, G.H.; Brozek, J.L.; Cattamanchi, A.; Peter Cegielski, J.; Chen, L.; Daley, C.L.; et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am. J. Respir. Crit. Care Med. 2019, 200, e93–e142. [Google Scholar] [CrossRef] [PubMed]
- Palomino, J.C.; Martin, A. Is Repositioning of Drugs a Viable Alternative in the Treatment of Tuberculosis? J. Antimicrob. Chemother. 2013, 68, 275–283. [Google Scholar] [CrossRef]
- Silva, D.R.; Dalcolmo, M.; Tiberi, S.; Arbex, M.A.; Munoz-Torrico, M.; Duarte, R.; D’Ambrosio, L.; Visca, D.; Rendon, A.; Gaga, M.; et al. New and Repurposed Drugs to Treat Multidrug and Extensively Drug-Resistant Tuberculosis. J. Bras. Pneumol. 2018, 44, 153–160. [Google Scholar] [CrossRef]
- Maitra, A.; Bates, S.; Kolvekar, T.; Devarajan, P.V.; Guzman, J.D.; Bhakta, S. Repurposing-a Ray of Hope in Tackling Extensively Drug Resistance in Tuberculosis. Int. J. Infect. Dis. 2015, 32, 50–55. [Google Scholar] [CrossRef]
- Talevi, A.; Bellera, C.L. Challenges and Opportunities with Drug Repurposing: Finding Strategies to Find Alternative Uses of Therapeutics. Expert Opin. Drug Discov. 2020, 15, 397–401. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug Repurposing: A Promising Tool to Accelerate the Drug Discovery Process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef]
- Rasheed, S.; Sánchez, S.S.; Yousuf, S.; Honoré, S.M.; Choudhary, M.I. Drug Repurposing: In-Vitro Anti-Glycation Properties of 18 Common Drugs. PLoS ONE 2018, 13, e0190509. [Google Scholar] [CrossRef]
- Simpkin, V.L.; Renwick, M.J.; Kelly, R.; Mossialos, E. Incentivising Innovation in Antibiotic Drug Discovery and Development: Progress, Challenges and next Steps. J. Antibiot. 2017, 70, 1087–1096. [Google Scholar] [CrossRef]
- Bahuguna, A.; Rawat, D.S. An Overview of New Antitubercular Drugs, Drug Candidates, and Their Targets. Med. Res. Rev. 2020, 40, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Lee, J.Y.; Kim, D.K.; Yoon, H.I.; Jeong, I.; Heo, E.Y.; Park, Y.S.; Jo, Y.S.; Lee, J.H.; Park, S.S.; et al. Substitution of Ethambutol with Linezolid during the Intensive Phase of Treatment of Pulmonary Tuberculosis: A Prospective, Multicentre, Randomised, Open-Label, Phase 2 Trial. Lancet Infect. Dis. 2019, 19, 46–55. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Outterson, K.; Engel, A.; Karlén, A. The Global Preclinical Antibacterial Pipeline. Nat. Rev. Microbiol. 2020, 8, 275–285. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Gottwalt, S.; Beyer, P.; Butler, M.; Czaplewski, L.; Lienhardt, C.; Moja, L.; Paul, M.; Paulin, S.; Rex, J.H. Analysis of the clinical antibacterial and antituberculosis pipeline. Lancet Infect. Dis. 2019, 19, e40–e50. [Google Scholar] [CrossRef]
- An, Q.; Li, C.; Chen, Y.; Deng, Y.; Yang, T.; Luo, Y. Repurposed Drug Candidates for Antituberculosis Therapy. Eur. J. Med. Chem. 2020, 192, 112175. [Google Scholar] [CrossRef]
- Ramharter, M.; Schwab, M.; Mombo-Ngoma, G.; Manego, R.Z.; Akerey-Diop, D.; Basra, A.; Mackanga, J.R.; Würbel, H.; Wojtyniak, J.G.; Gonzalez, R.; et al. Population Pharmacokinetics of Mefloquine Intermittent Preventive Treatment for Malaria in Pregnancy in Gabon. Antimicrob. Agents Chemother. 2019, 63, e01113-18. [Google Scholar] [CrossRef]
- Mansoor, R.; Dahal, P.; Humphreys, G.S.; Guerin, P.; Ashley, E.A.; Stepniewska, K. The Effect of Dose on the Antimalarial Efficacy of Artesunate-Mefloquine against Plasmodium falciparum Malaria: A Protocol for Systematic Review and Individual Patient Data (IPD) Meta-Analysis. BMJ Open 2019, 9, e027738. [Google Scholar] [CrossRef]
- Kitchen, L.W.; Vaughn, D.W.; Skillman, D.R. Reviews Of Anti-infective Agents: Role of US Military Research Programs in the Development of US Food and Drug Administration–Approved Antimalarial Drugs. Clin. Infect. Dis. 2006, 43, 67–71. [Google Scholar] [CrossRef]
- Nissani, E.; Ginsburg, H. Protonophoric Effects of Antimalarial Drugs and Alkylamines in Escherichia coli Membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 1989, 978, 293–298. [Google Scholar] [CrossRef]
- Zannoni, D. Mefloquine: An Antimalarial Drug Interacting with the b/c Region of Bacterial Respiratory Chains. FEBS Lett. 1985, 183, 340–344. [Google Scholar] [CrossRef]
- Danelishvili, L.; Wu, M.; Young, L.S.; Bermudez, L.E. Genomic Approach to Identifying the Putative Target of and Mechanisms of Resistance to Mefloquine in Mycobacteria. Antimicrob. Agents Chemother. 2005, 49, 3707–3714. [Google Scholar] [CrossRef] [PubMed]
- Kunin, C.M.; Ellis, W.Y. Antimicrobial Activities of Mefloquine and a Series of Related Compounds. Antimicrob. Agents Chemother. 2000, 44, 848–852. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Kolonoski, P.; Wu, M.; Aralar, P.A.; Inderlied, C.B.; Young, L.S. Mefloquine Is Active In Vitro and In Vivo against Mycobacterium avium Complex. Antimicrob. Agents Chemother. 1999, 43, 1870–1874. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Kolonoski, P.; Seitz, L.E.; Petrofsky, M.; Reynolds, R.; Wu, M.; Young, L.S. A Thiosemicarbazole, in Combination with Mefloquine and Moxifloxacin for Treatment of Murine Mycobacterium avium Complex Disease. Antimicrob. Agents Chemother. 2004, 48, 3556–3558. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, S.; Iso, Y.; Wan, B.; Franzblau, S.G.; Kozikowski, A.P. Design, Synthesis, and SAR Studies of Mefloquine-Based Ligands as Potential Antituberculosis Agents. ChemMedChem Chem. Enabling Drug Discov. 2006, 1, 593–597. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Meek, L. Mefloquine and Its Enantiomers Are Active against Mycobacterium tuberculosis In Vitro and in Macrophages. Tuberc. Res. Treat. 2014, 2014, 530815. [Google Scholar] [CrossRef]
- Gonalves, R.S.B.; Kaiser, C.R.; Loureno, M.C.S.; Bezerra, F.A.F.M.; De Souza, M.V.N.; Wardell, J.L.; Wardell, S.M.S.V.; Henriques, M.D.G.M.D.O.; Costa, T. Mefloquine-Oxazolidine Derivatives, Derived from Mefloquine and Arenecarbaldehydes: In Vitro Activity Including against the Multidrug-Resistant Tuberculosis Strain T113. Bioorg. Med. Chem. 2012, 20, 243–248. [Google Scholar] [CrossRef]
- Rodrigues-Junior, V.S.; Villela, A.D.; Gonçalves, R.S.B.; Abbadi, B.L.; Trindade, R.V.; López-Gavín, A.; Tudó, G.; González-Martín, J.; Basso, L.A.; de Souza, M.V.N.; et al. Mefloquine and Its Oxazolidine Derivative Compound Are Active against Drug-Resistant Mycobacterium tuberculosis Strains and in a Murine Model of Tuberculosis Infection. Int. J. Antimicrob. Agents 2016, 48, 203–207. [Google Scholar] [CrossRef]
- Mao, J.; Wang, Y.; Wan, B.; Kozikowski, A.P.; Franzblau, S.G. Design, Synthesis, and Pharmacological Evaluation of Mefloquine-Based Ligands as Novel Antituberculosis Agents. ChemMedChem Chem. Enabling Drug Discov. 2007, 2, 1624–1630. [Google Scholar] [CrossRef]
- Mao, J.; Yuan, H.; Wang, Y.; Wan, B.; Pieroni, M.; Huang, Q.; Van Breemen, R.B.; Kozikowski, A.P.; Franzblau, S.G. From Serendipity to Rational Antituberculosis Drug Discovery of Mefloquine-Isoxazole Carboxylic Acid Esters. J. Med. Chem. 2009, 52, 6966–6978. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Yuan, H.; Wang, Y.; Wan, B.; Pak, D.; He, R.; Franzblau, S.G. Synthesis and Antituberculosis Activity of Novel Mefloquine-Isoxazole Carboxylic Esters as Prodrugs. Bioorg. Med. Chem. Lett. 2010, 20, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Montezano, D.; Meek, L.; Gupta, R.; Bermudez, L.E.; Bermudez, J.C.M. Flux Balance Analysis with Objective Function Defined by Proteomics Data-Metabolism of Mycobacterium tuberculosis Exposed to Mefloquine. PLoS ONE 2015, 10, e0134014. [Google Scholar] [CrossRef] [PubMed]
- Ridtitid, W.; Wongnawa, M.; Mahatthanatrakul, W.; Chaipol, P.; Sunbhanich, M. Effect of Rifampin on Plasma Concentrations of Mefloquine in Healthy Volunteers. J. Pharm. Pharmacol. 2000, 52, 1265–1269. [Google Scholar] [CrossRef]
- Welton, T. Ionic Liquids: A Brief History. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Vekariya, R.L. A Review of Ionic Liquids: Applications towards Catalytic Organic Transformations. J. Mol. Liq. 2017, 227, 44. [Google Scholar] [CrossRef]
- Dong, K.; Liu, X.; Dong, H.; Zhang, X.; Zhang, S. Multiscale Studies on Ionic Liquids. Chem. Rev. 2017, 117, 6636–6695. [Google Scholar] [CrossRef]
- Amarasekara, A.S. Acidic Ionic Liquids. Chem. Rev. 2016, 116, 6133–6183. [Google Scholar] [CrossRef]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are Ionic Liquids Chemically Stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef]
- Hough, W.L.; Smiglak, M.; Rodríguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The Third Evolution of Ionic Liquids: Active Pharmaceutical Ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Al-Blewi, F.; Rezki, N.; Naqvi, A.; Qutb Uddin, H.; Al-Sodies, S.; Messali, M.; Aouad, M.R.; Bardaweel, S. A Profile of the In Vitro Anti-Tumor Activity and In Silico ADME Predictions of Novel Benzothiazole Amide-Functionalized Imidazolium Ionic Liquids. Int. J. Mol. Sci. 2019, 20, 2865. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.M.; Raposo, L.R.; Carrera, G.V.S.M.; Costa, A.; Dionísio, M.; Baptista, P.V.; Fernandes, A.R.; Branco, L.C. Ionic Liquids and Salts from Ibuprofen as Promising Innovative Formulations of an Old Drug. ChemMedChem 2019, 14, 907–911. [Google Scholar] [CrossRef]
- Shayanfar, S.; Shayanfar, A. Ionic Liquid Forms of Carvedilol: Preparation, Characterization, and Solubility Studies. J. Pharm. Innov. 2019, 14, 382–390. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Kelley, S.P.; Gurau, G.; Rogers, R.D. Chemistry: Develop Ionic Liquid Drugs. Nature 2015, 528, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Cojocaru, O.A.; Kelley, S.P.; Bica, K.; Wallace, S.P.; Gurau, G.; Rogers, R.D. Acyclovir as an Ionic Liquid Cation or Anion Can Improve Aqueous Solubility. ACS Omega 2017, 2, 3483–3493. [Google Scholar] [CrossRef]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic Liquids as a Potential Tool for Drug Delivery Systems. MedChemComm 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic Liquids in Pharmaceutical Applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef]
- Agatemor, C.; Ibsen, K.N.; Tanner, E.E.L.; Mitragotri, S. Ionic Liquids for Addressing Unmet Needs in Healthcare. Bioeng. Transl. Med. 2018, 3, 7–25. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Santos, M.M.; Alves, C.; Silva, J.; Florindo, C.; Costa, A.; Petrovski, Ž.; Marrucho, I.M.; Pedrosa, R.; Branco, L.C. Antimicrobial Activities of Highly Bioavailable Organic Salts and Ionic Liquids from Fluoroquinolones. Pharmaceutics 2020, 12, 694. [Google Scholar] [CrossRef]
- Florindo, C.; Costa, A.; Matos, C.; Nunes, S.L.; Matias, A.N.; Duarte, C.M.M.; Rebelo, L.P.N.; Branco, L.C.; Marrucho, I.M. Novel Organic Salts Based on Fluoroquinolone Drugs: Synthesis, Bioavailability and Toxicological Profiles. Int. J. Pharm. 2014, 469, 179–189. [Google Scholar] [CrossRef]
- Madeira, D.; Alves, C.; Silva, J.; Florindo, C.; Costa, A.; Petrovski, Ž.; Marrucho, I.M.; Pedrosa, R.; Santos, M.M.; Branco, L.C. Fluoroquinolone-Based Organic Salts and Ionic Liquids as Highly Bioavailable Broad-Spectrum Antimicrobials. Multidiscip. Digit. Publ. Inst. Proc. 2020, 78, 3. [Google Scholar] [CrossRef]
- Ferraz, R.; Teixeira, V.; Rodrigues, D.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.C. Antibacterial Activity of Ionic Liquids Based on Ampicillin against Resistant Bacteria. RSC Adv. 2014, 4, 4301–4307. [Google Scholar] [CrossRef]
- Ferraz, R.; Silva, D.; Dias, A.R.; Dias, V.; Santos, M.M.; Pinheiro, L.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.C. Synthesis and Antibacterial Activity of Ionic Liquids and Organic Salts Based on Penicillin g and Amoxicillin Hydrolysate Derivatives against Resistant Bacteria. Pharmaceutics 2020, 12, 221. [Google Scholar] [CrossRef]
- Frizzo, C.P.; Wust, K.; Tier, A.Z.; Beck, T.S.; Rodrigues, L.V.; Vaucher, R.A.; Bolzan, L.P.; Terra, S.; Soares, F.; Martins, M.A.P. Novel Ibuprofenate- and Docusate-Based Ionic Liquids: Emergence of Antimicrobial Activity. RSC Adv. 2016, 6, 100476–100486. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, S.H.; Cho, S.M.; Kang, W.H.; Nam, K.Y.; Jang, I.J.; Yu, K.S. Comparisons of the Pharmacokinetics and Tolerability of Fixed-Dose Combinations of Amlodipine Besylate/Losartan and Amlodipine Camsylate/Losartan in Healthy Subjects: Arandomized, Open-Label, Single-Dose, Two-Period, Two-Sequence Crossover Study. Drug Des. Dev. Ther. 2016, 10, 3021–3028. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.T.; da Silva Araújo, A.; Moraes, A.M.; de Souza, L.A.; Silva Lourenço, M.C.; de Souza, M.V.N.; Wardell, J.L.; Wardell, S.M.S.V. Synthesis and Biological Activities of Camphor Hydrazone and Imine Derivatives. Sci. Pharm. 2016, 84, 467–483. [Google Scholar] [CrossRef]
- Stavrakov, G.; Valcheva, V.; Philipova, I.; Doytchinova, I. Novel Camphane-Based Anti-Tuberculosis Agents with Nanomolar Activity. Eur. J. Med. Chem. 2013, 70, 372–379. [Google Scholar] [CrossRef]
- Alcalde, E.; Dinarès, I.; Ibáñez, A.; Mesquida, N. A General Halide-to-Anion Switch for Imidazolium-Based Ionic Liquids and Oligocationic Systems Using Anion Exchange Resins (A-Form). Chem. Commun. 2011, 47, 3266–3268. [Google Scholar] [CrossRef]
- Cole, M.R.; Li, M.; El-Zahab, B.; Janes, M.E.; Hayes, D.; Warner, I.M. Design, Synthesis, and Biological Evaluation of β-Lactam Antibiotic-Based Imidazolium- and Pyridinium-Type Ionic Liquids. Chem. Biol. Drug Des. 2011, 78, 33–41. [Google Scholar] [CrossRef]
- Psimadas, D.; Georgoulias, P.; Valotassiou, V.; Loudos, G. Molecular Nanomedicine Towards Cancer : 111In-labeled nanoparticles. J. Pharm. Sci. 2012, 101, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Crevoisier, C.; Handschin, J.; Barré, J.; Roumenov, D.; Kleinbloesem, C. Food increases the bioavailability of mefloquine. Eur. J. Clin. Pharmacol. 1997, 53, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Looareesuwan, S.; White, N.; Warrell, D.; Forgo, I.; Dubach, U.; Ranalder, U.; Schwartz, D. Studies of Mefloquine Bioavailability and Kinetics Using a Stable Isotope Technique: A Comparison of Thai Patients with Falciparum Malaria and Healthy Caucasian Volunteers. Br. J. Clin. Pharmacol. 1987, 24, 37–42. [Google Scholar] [CrossRef]
- Du Plessis, L.H.; Helena, C.; Van Huysteen, E.; Wiesner, L.; Kotzé, A.F. Formulation and Evaluation of Pheroid Vesicles Containing Mefloquine for the Treatment of Malaria. J. Pharm. Pharmacol. 2014, 66, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.L.; Tang, W.H.; Chen, W.C.; Diako, C.; Ross, C.F.; Li, S.D. Development of a Rapidly Dissolvable Oral Pediatric Formulation for Mefloquine Using Liposomes. Mol. Pharm. 2017, 14, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.V.; Dabke, A.P.; Shete, A.S. Crystal Engineering to Improve Physicochemical Properties of Mefloquine Hydrochloride. Drug Dev. Ind. Pharm. 2010, 36, 1036–1045. [Google Scholar] [CrossRef]
- Mbela, T.K.M.; Deharo, E.; Haemers, A.; Ludwig, A. Submicron Oil-in-Water Emulsion Formulations for Mefloquine and Halofantrine: Effect of Electric-Charge Inducers on Antimalarial Activity in Mice. J. Pharm. Pharmacol. 1998, 50, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Canetti, G.; Rist, N.; Grosset, J. Measurement of sensitivity of the tuberculous bacillus to antibacillary drugs by the method of proportions. Methodology, resistance criteria, results and interpretation. Rev. Tuberc. Pneumol. 1963, 27, 217–272. [Google Scholar]
- Franzblau, S.G.; Witzig, R.S.; Mclaughlin, J.C.; Torres, P.; Madico, G.; Hernandez, A.; Degnan, M.T.; Cook, M.B.; Quenzer, V.K.; Ferguson, R.M.; et al. Rapid, Low-Technology MIC Determination with Clinical Mycobacterium tuberculosis Isolates by Using the Microplate Alamar Blue Assay. J. Clin. Microbiol. 1998, 36, 362–366. [Google Scholar] [CrossRef]
- Reis, R.S.; Neves, I.; Lourenc, S.L.S.; Lourenc, M.C.S. Comparison of flow cytometric and Alamar Blue tests with the proportional method for testing susceptibility of Mycobacterium tuberculosis to rifampin and isoniazid. J. Clin. Microbiol. Soc. 2004, 42, 2247–2248. [Google Scholar] [CrossRef]
- Vanitha, J.D.; Paramasivan, C.N. Evaluation of Microplate Alamar Blue Assay for Drug Susceptibility Testing of Mycobacterium avium Complex Isolates. Diagn. Microbiol. Infect. Dis. 2004, 49, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.K.; Abramovitch, R.B. Macrophage Infection Models for Mycobacterium tuberculosis. In Mycobacteria Protocols, 3rd ed.; Parish, T., Roberts, D.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 1285. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of Phenotypic and Functional Stability of RAW 264.7 Cell Line through Serial Passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef] [PubMed]
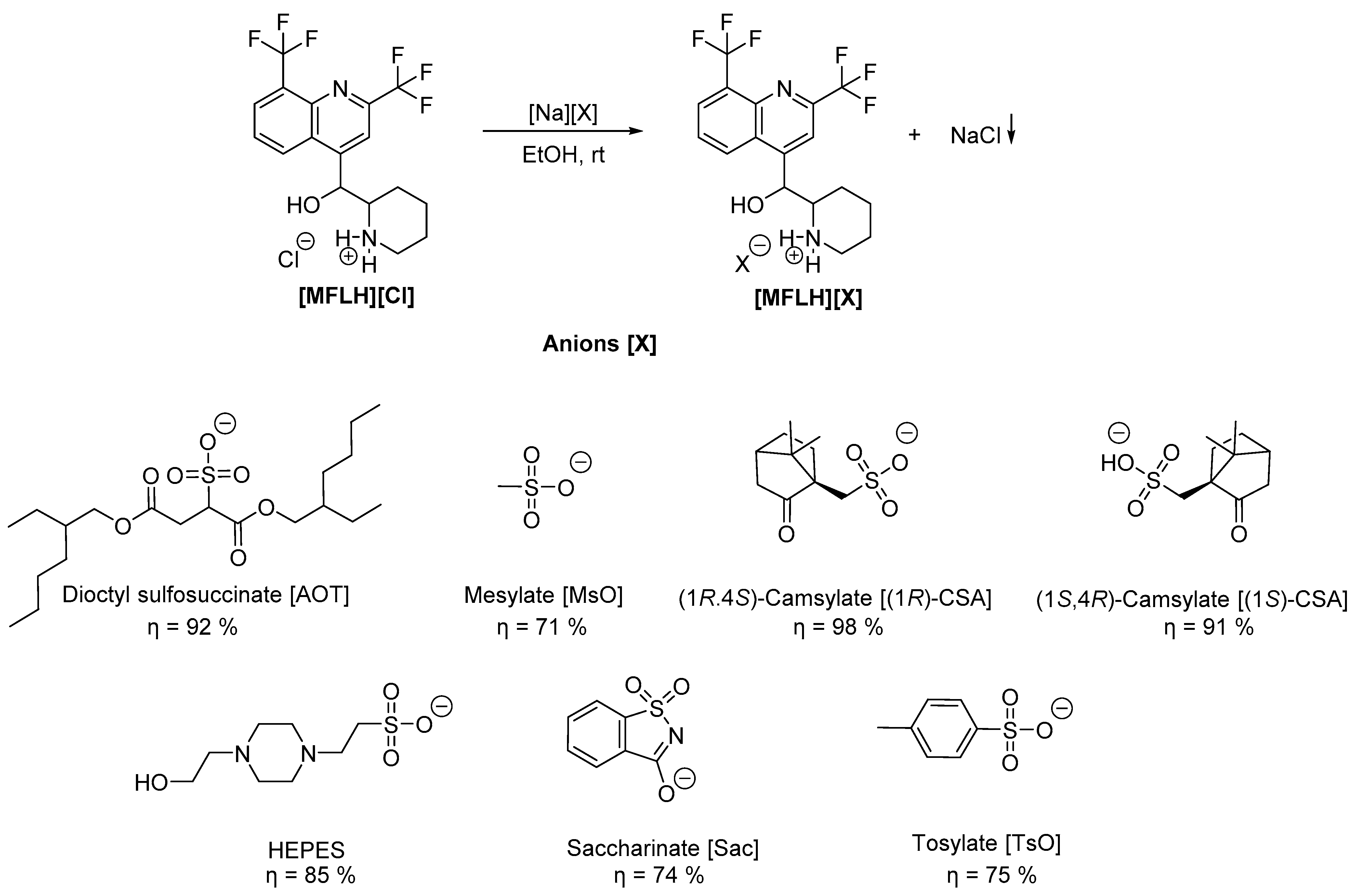
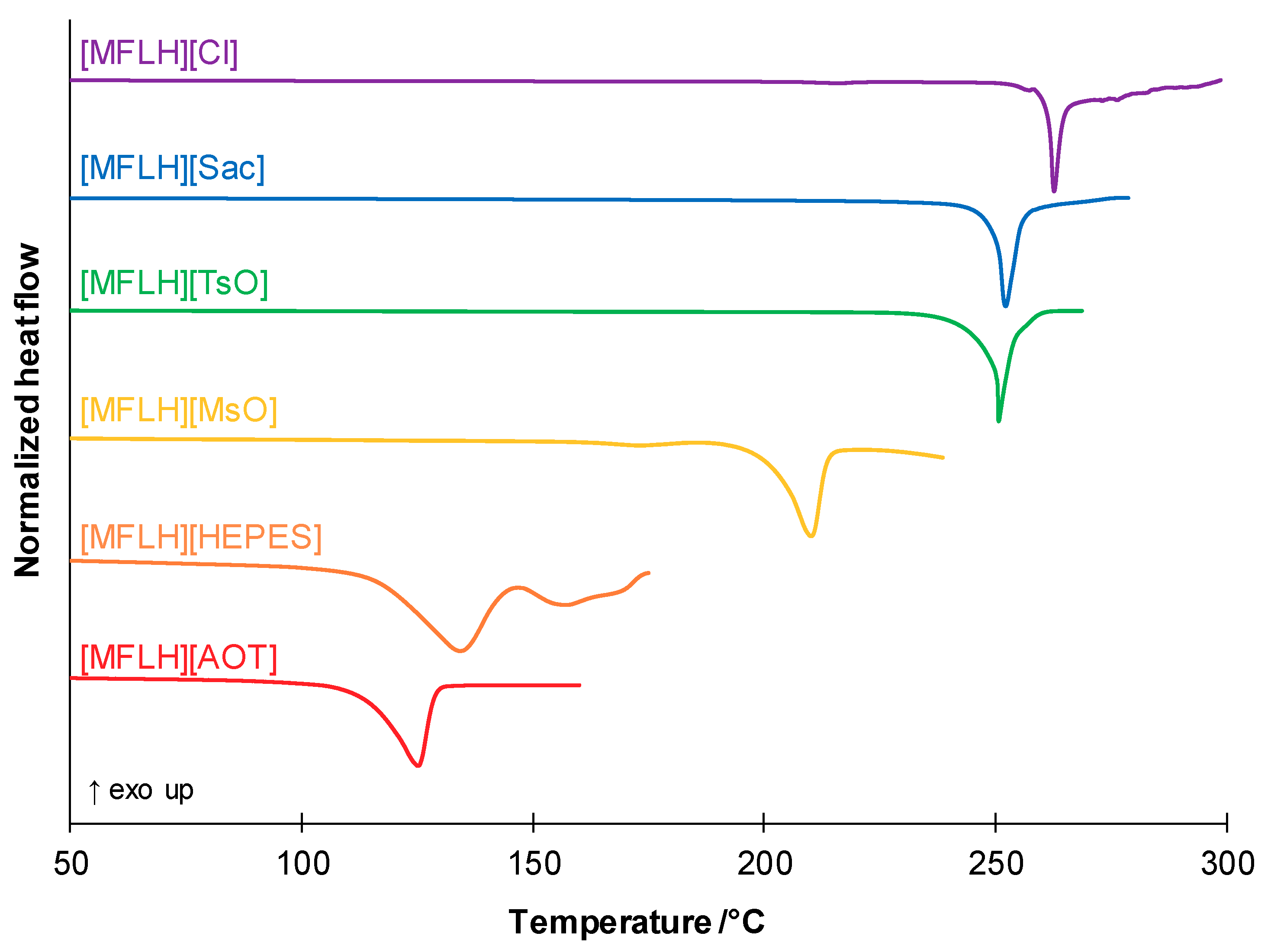
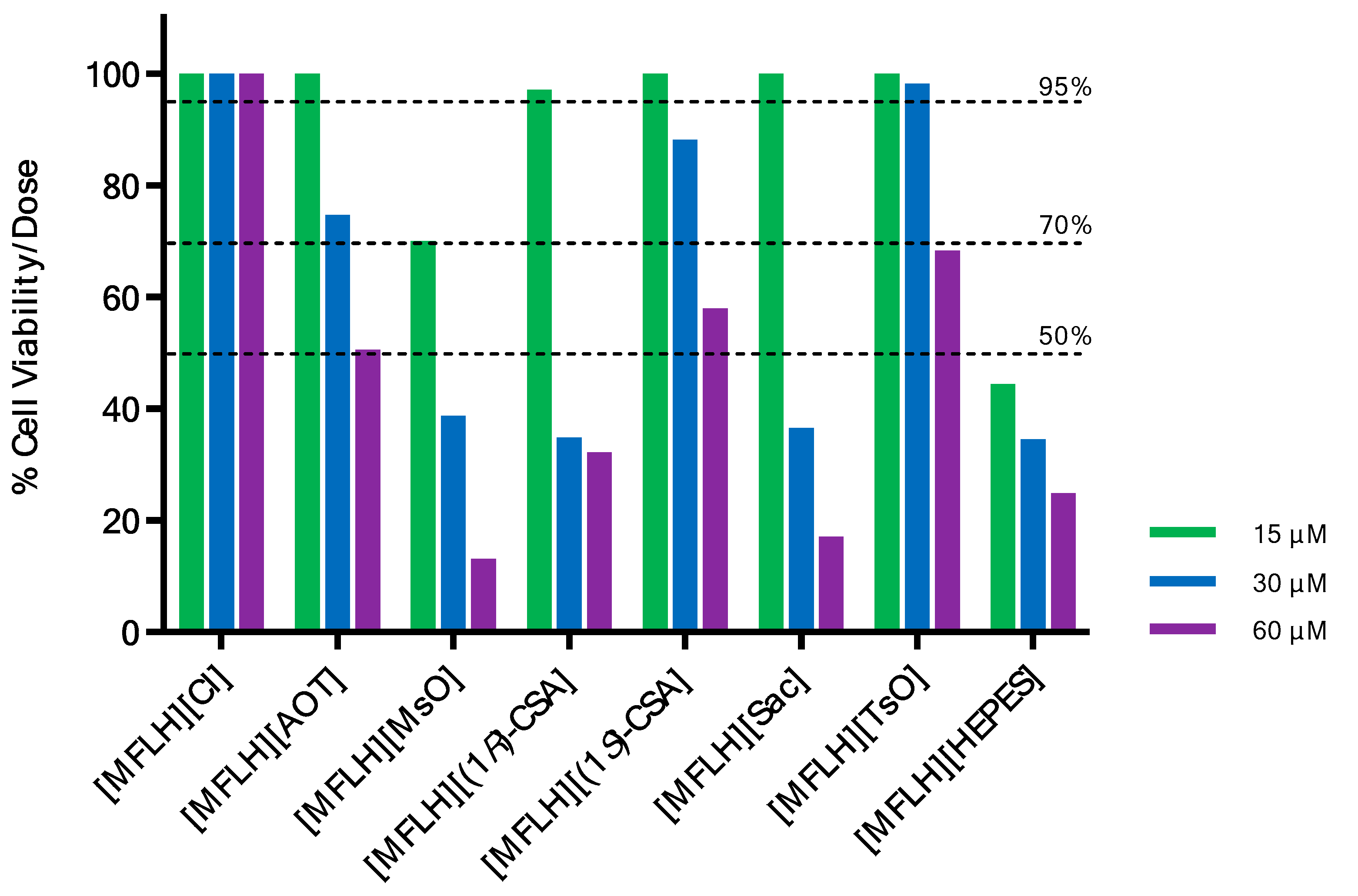
| MFL Salts | Physical State | Tm/°C a | Tg/°C b |
|---|---|---|---|
| [MFLH][Cl] | White solid | 216.3; 262.7 c | - d |
| [MFLH][AOT] | White solid | 125.1 | 21.8 |
| [MFLH][MsO] | White solid | 173.2; 210.1 | 64.6 |
| [MFLH][Sac] | White solid | 252.2 | 102.7 |
| [MFLH][TsO] | White solid | 250.6 | 92.2 |
| [MFLH][HEPES] | White solid | 134.2 a | - d |
| MFL Salts | Solubility (mg/mL) | D (×10−6 cm2/s) | P (×10−5 cm/s) | Kd |
|---|---|---|---|---|
| [MFLH][Cl] | 4.37 | 0.16 | 0.17 | 0.16 |
| [MFLH][AOT] | 0.03 | - a | - a | - a |
| [MFLH][MsO] | 7.71 | 0.62 | 0.79 | 0.19 |
| [MFLH][(1R)-CSA] | 0.59 | 1.67 | 1.95 | 0.18 |
| [MFLH][(1S)-CSA] | 0.77 | 0.22 | 0.36 | 0.25 |
| [MFLH][Sac] | 0.21 | 0.60 | 1.18 | 0.30 |
| [MFLH][TsO] | 0.09 | 1.21 | 4.23 | 0.52 |
| [MFLH][HEPES] | 0.64 | 1.75 | 1.13 | 0.10 |
| Compounds | MIC (µg/mL) | MIC (μM) | RDIC |
|---|---|---|---|
| [MFLH][Cl] | 12.5 | 30.1 | - a |
| [MFLH][AOT] | 25 | 31.2 | 0.96 |
| [MFLH][MsO] | 12.5 | 26.3 | 1.14 |
| [MFLH][(1R)-CSA] | 12.5 | 20.5 | 1.47 |
| [MFLH][(1S)-CSA] | 12.5 | 20.5 | 1.47 |
| [MFLH][Sac] | 12.5 | 22.4 | 1.35 |
| [MFLH][TsO] | 12.5 | 22.7 | 1.32 |
| [MFLH][HEPES] | 12.5 | 20.3 | 1.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.; Lopes, M.V.C.; Petrovski, Ž.; Santos, M.M.; Santos, J.P.; Yamada-Ogatta, S.F.; Bispo, M.L.F.; de Souza, M.V.N.; Duarte, A.R.C.; Lourenço, M.C.S.; et al. Novel Organic Salts Based on Mefloquine: Synthesis, Solubility, Permeability, and In Vitro Activity against Mycobacterium tuberculosis. Molecules 2022, 27, 5167. https://doi.org/10.3390/molecules27165167
Silva D, Lopes MVC, Petrovski Ž, Santos MM, Santos JP, Yamada-Ogatta SF, Bispo MLF, de Souza MVN, Duarte ARC, Lourenço MCS, et al. Novel Organic Salts Based on Mefloquine: Synthesis, Solubility, Permeability, and In Vitro Activity against Mycobacterium tuberculosis. Molecules. 2022; 27(16):5167. https://doi.org/10.3390/molecules27165167
Chicago/Turabian StyleSilva, Dário, Márcio V. C. Lopes, Željko Petrovski, Miguel M. Santos, Jussevania P. Santos, Sueli F. Yamada-Ogatta, Marcelle L. F. Bispo, Marcus V. N. de Souza, Ana Rita C. Duarte, Maria C. S. Lourenço, and et al. 2022. "Novel Organic Salts Based on Mefloquine: Synthesis, Solubility, Permeability, and In Vitro Activity against Mycobacterium tuberculosis" Molecules 27, no. 16: 5167. https://doi.org/10.3390/molecules27165167
APA StyleSilva, D., Lopes, M. V. C., Petrovski, Ž., Santos, M. M., Santos, J. P., Yamada-Ogatta, S. F., Bispo, M. L. F., de Souza, M. V. N., Duarte, A. R. C., Lourenço, M. C. S., Gonçalves, R. S. B., & Branco, L. C. (2022). Novel Organic Salts Based on Mefloquine: Synthesis, Solubility, Permeability, and In Vitro Activity against Mycobacterium tuberculosis. Molecules, 27(16), 5167. https://doi.org/10.3390/molecules27165167









